Smart Bio-Nanocoatings with Simple Post-Synthesis Reversible Adjustment
Abstract
1. Introduction
2. Materials and Methods
2.1. Atomic Force Microscopy (AFM)
2.2. Simulations
2.3. Wettability Test
2.4. Mass-Spectrometry
2.5. Protein Purification
2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Western Blotting
2.8. Wax Emulsion Preparation
2.9. Circular Dichrosim
2.10. ThT Fluorescence Measurement
2.11. Nanocoating Generation
2.12. Statistical Analysis
3. Results
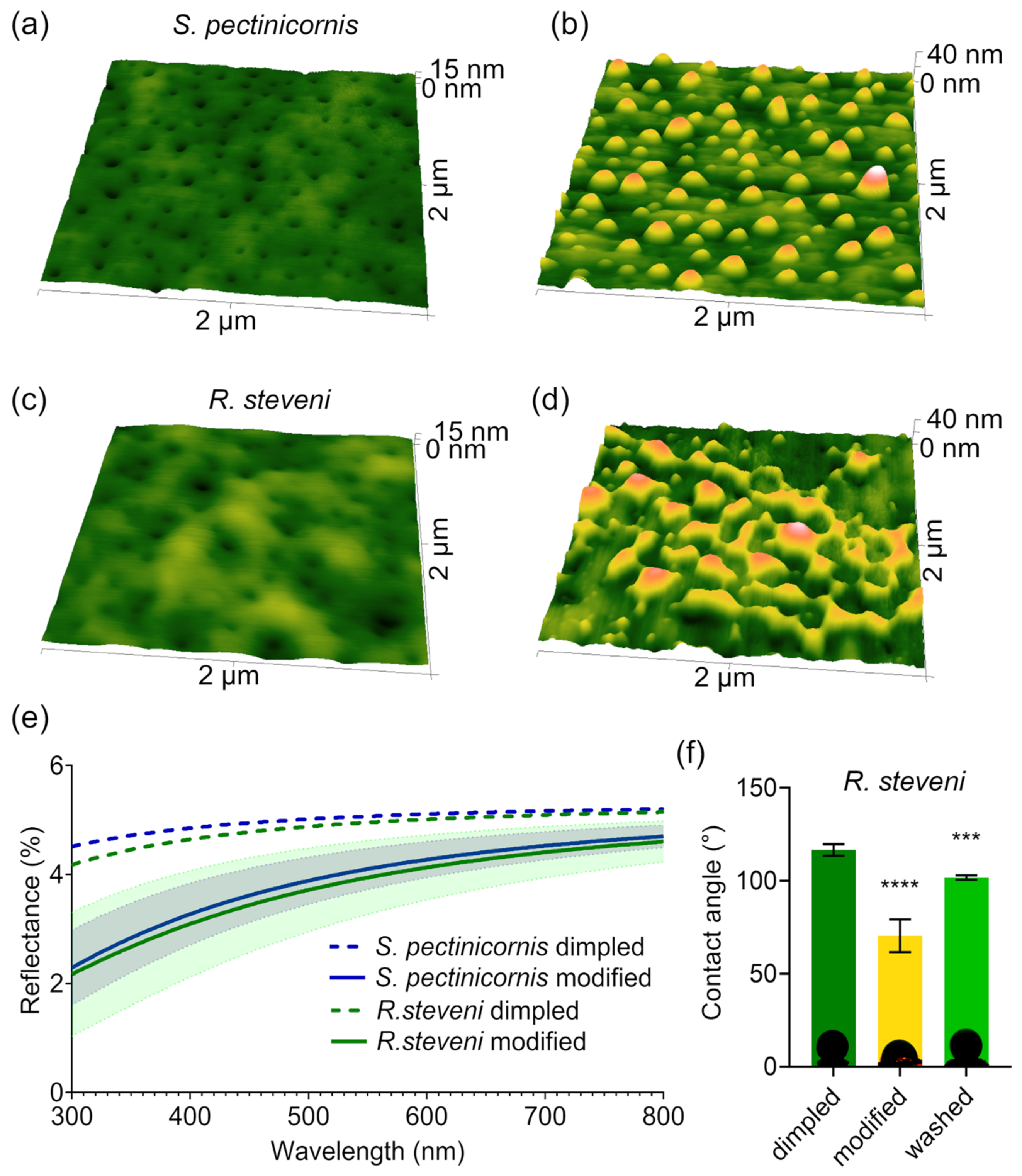
3.1. Two Types of Nanostructures and Their Functionality
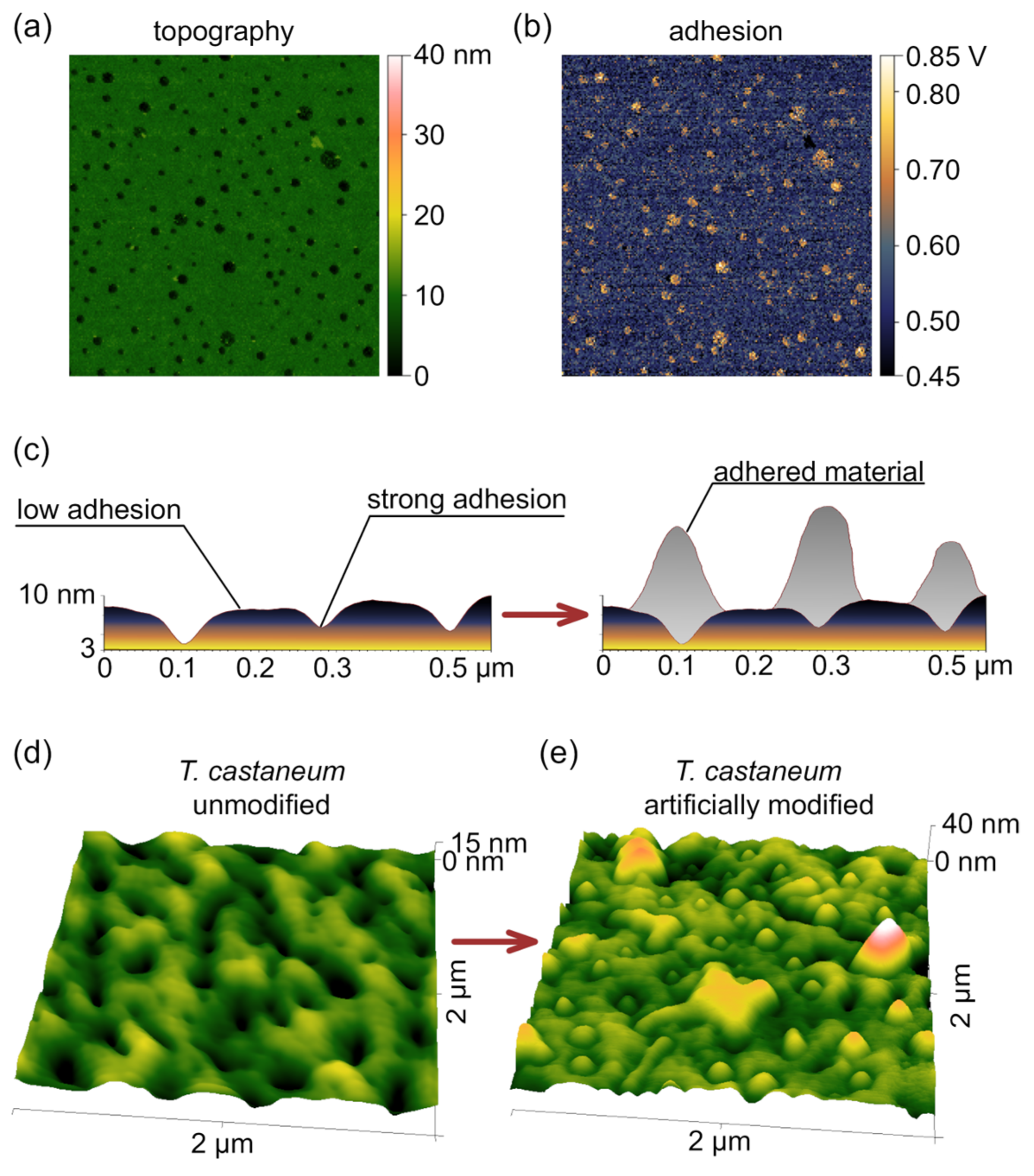
3.2. Switching Mechanism
3.3. Reverse Engineering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlian, A.; Vanya, K. Nanotopography in directing osteogenic differentiation of mesenchymal stem cells: Potency and future perspective. Future Sci. OA 2021, 8, FSO765. [Google Scholar] [CrossRef] [PubMed]
- Muneekaew, S.; Wang, M.-J.; Chen, S.-Y. Control of stem cell differentiation by using extrinsic photobiomodulation in conjunction with cell adhesion pattern. Sci. Rep. 2022, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Casanellas, I.; Lagunas, A.; Vida, Y.; Pérez-Inestrosa, E.; Andrades, J.A.; Becerra, J.; Samitier, J. Matrix Nanopatterning Regulates Mesenchymal Differentiation through Focal Adhesion Size and Distribution According to Cell Fate. Biomimetics 2019, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.A.; Yan, Y. Current understanding of biological identity at the nanoscale and future prospects. Nat. Nanotechnol. 2021, 16, 229–242. [Google Scholar] [CrossRef]
- Kállai-Szabó, N.; Farkas, D.; Lengyel, M.; Basa, B.; Fleck, C.; Antal, I. Microparticles and multi-unit systems for advanced drug delivery. Eur. J. Pharm. Sci. 2024, 194, 106704. [Google Scholar] [CrossRef]
- Chen, Z.; Lan, Y.; Ling, S.-D.; Dong, Y.-H.; Wang, Y.-D.; Xu, J.-H. Microfluidic-Generated Biopolymer Microparticles as Cargo Delivery Systems. Adv. Mater. Technol. 2022, 7, 2100733. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Muraleedharan, A.; Acharya, S.; Kumar, R. Recent Updates on Diverse Nanoparticles and Nanostructures in Therapeutic and Diagnostic Applications with Special Focus on Smart Protein Nanoparticles: A Review. ACS Omega 2024, 9, 42613–42629. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, N.; Lyu, Z.; Zhu, W.; Chang, Y.-C.; Hu, X.; Du, D.; Lin, Y. Protein-based nanomaterials and nanosystems for biomedical applications: A review. Mater. Today 2021, 43, 166–184. [Google Scholar] [CrossRef]
- Vardaxi, A.; Kafetzi, M.; Pispas, S. Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers 2022, 14, 777. [Google Scholar] [CrossRef]
- Moxey, M.; Johnson, A.; El-Zubir, O.; Cartron, M.; Dinachali, S.S.; Hunter, C.N.; Saifullah, M.S.; Chong, K.S.; Leggett, G.J. Fabrication of Self-Cleaning, Reusable Titania Templates for Nanometer and Micrometer Scale Protein Patterning. ACS Nano 2015, 9, 6262–6270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Cao, Y.; Marelli, B.; Xia, X.; Tao, T.H. Engineering the Future of Silk Materials through Advanced Manufacturing. Adv. Mater. 2018, 30, 1706983. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Stührenberg, M.; Chen, S.; Rhee, D.; Lee, W.K.; Odom, T.W.; Heilshorn, S.C.; Enejder, A. Micro- and nano-patterned elastin-like polypeptide hydrogels for stem cell culture. Soft Matter 2017, 13, 5665–5675. [Google Scholar] [CrossRef] [PubMed]
- Shadish, J.A.; Strange, A.C.; DeForest, C.A. Genetically Encoded Photocleavable Linkers for Patterned Protein Release from Biomaterials. J. Am. Chem. Soc. 2019, 141, 15619–15625. [Google Scholar] [CrossRef]
- Humenik, M.; Scheibel, T. Nanomaterial Building Blocks Based on Spider Silk–Oligonucleotide Conjugates. ACS Nano 2014, 8, 1342–1349. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Panda, A.K. Recent advances in nano-engineered approaches used for enzyme immobilization with enhanced activity. J. Mol. Liq. 2021, 338, 116602. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Patek, S.N. Biomimetics and evolution. Science 2014, 345, 1448–1449. [Google Scholar] [CrossRef]
- Kim, E.S. Directed Evolution: A Historical Exploration into an Evolutionary Experimental System of Nanobiotechnology, 1965–2006. Minerva 2008, 46, 463–484. [Google Scholar] [CrossRef]
- Agheli, H.; Malmström, J.; Larsson, E.M.; Textor, M.; Sutherland, D.S. Large Area Protein Nanopatterning for Biological Applications. Nano Lett. 2006, 6, 1165–1171. [Google Scholar] [CrossRef]
- Schroeder, T.B.H.; Houghtaling, J.; Wilts, B.D.; Mayer, M. It’s Not a Bug, It’s a Feature: Functional Materials in Insects. Adv. Mater. 2018, 30, 1705322. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mao, Q.; Ye, Z.; Liao, Z.; Shan, H.-W.; Li, J.-M.; Zhang, C.-X.; Chen, J.-P. Brochosomes as an Antireflective Camouflage Coating for Leafhoppers. eLife 2024, 13, RP99639. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Bilousov, O.; Lehmann, J.; Fiebig, M.; Katanaev, V.L. Reverse and forward engineering of Drosophila corneal nanocoatings. Nature 2020, 585, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkov, M.; Jobin, M.; Savitsky, V.; Smirnov, S.; Katanaev, V.L. Evolutionary bet-hedging at the nanoscale. Res. Sq. 2024. preprint. [Google Scholar] [CrossRef]
- Madsen, J.S.M.; Jensen, S.A.; Nygård, J.; Hansen, P.E. Replacing libraries in scatterometry. Opt. Express 2018, 26, 34622–34632. [Google Scholar] [CrossRef]
- Wang, Z.; Valnohova, J.; Kolesnichenko, K.; Baba, A.; Sun, H.; Mao, X.; Kryuchkov, M.; Katanaev, V.L. Chemically Hydrophobic and Structurally Antireflective Nanocoatings in Papilio Butterflies. ACS Appl. Bio Mater. 2025, 8, 784–791. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Lehmann, J.; Schaab, J.; Cherepanov, V.; Blagodatski, A.; Fiebig, M.; Katanaev, V.L. Alternative moth-eye nanostructures: Antireflective properties and composition of dimpled corneal nanocoatings in silk-moth ancestors. J. Nanobiotechnol. 2017, 15, 61. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Savitsky, V.; Wilts, B.D.; Gray, E.; Katanaev, V.L. Light Polarization by Biological Nanocoatings. ACS Appl. Mater. Interfaces 2021, 13, 23481–23488. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J. Nanotechnol. 2012, 2012, 270383. [Google Scholar] [CrossRef]
- Rodina, N.P.; Sulatsky, M.I.; Sulatskaya, A.I.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Photophysical Properties of Fluorescent Probe Thioflavin T in Crowded Milieu. J. Spectrosc. 2017, 2017, 2365746. [Google Scholar] [CrossRef]
- Ando, T.; Sekine, S.; Inagaki, S.; Misaki, K.; Badel, L.; Moriya, H.; Sami, M.M.; Itakura, Y.; Chihara, T.; Kazama, H.; et al. Nanopore Formation in the Cuticle of an Insect Olfactory Sensillum. Curr. Biol. 2019, 29, 1512–1520.e1516. [Google Scholar] [CrossRef]
- Varija Raghu, S.; Thamankar, R. A Comparative Study of Crystallography and Defect Structure of Corneal Nipple Array in Daphnis nerii Moth and Papilio polytes Butterfly Eye. ACS Omega 2020, 5, 23662–23671. [Google Scholar] [CrossRef]
- Bernhard, C.G.; Miller, W.H.; Moller, A.R. The insect corneal pipple array. A Biologica, Broad-band impedance transformer that acts as an antireflection coating. Acta Physiol. Scand. Suppl. 1965, 63 (Suppl. 243), 241–279. [Google Scholar]
- Wilson, S.J.; Hutley, M.C. The Optical Properties of ‘Moth Eye’ Antireflection Surfaces. Opt. Acta Int. J. Opt. 1982, 29, 993–1009. [Google Scholar] [CrossRef]
- Deinega, A.; Valuev, I.; Potapkin, B.; Lozovik, Y. Minimizing light reflection from dielectric textured surfaces. J. Opt. Soc. Am. A 2011, 28, 770–777. [Google Scholar] [CrossRef]
- Raut, H.K.; Ganesh, V.A.; Nair, A.S.; Ramakrishna, S. Anti-reflective coatings: A critical, in-depth review. Energy Environ. Sci. 2011, 4, 3779–3804. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Foletti, S.; Palasantzas, G.; Arikawa, K. Light on the moth-eye corneal nipple array of butterflies. Proc. R. Soc. B Biol. Sci. 2005, 273, 661–667. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Blagodatski, A.; Cherepanov, V.; Katanaev, V.L. Arthropod Corneal Nanocoatings: Diversity, Mechanisms, and Functions. In Functional Surfaces in Biology III: Diversity of the Physical Phenomena; Gorb, S.N., Gorb, E.V., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–52. [Google Scholar] [CrossRef]
- Sun, T.L.; Feng, L.; Gao, X.F.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Devi, A.L.L.; Nongthomba, U.; Bobji, M.S. Quantitative characterization of adhesion and stiffness of corneal lens of Drosophila melanogaster using atomic force microscopy. J. Mech. Behav. Biomed. 2016, 53, 161–173. [Google Scholar] [CrossRef]
- Zhukovskaya, M.; Yanagawa, A.; Forschler, B.T. Grooming Behavior as a Mechanism of Insect Disease Defense. Insects 2013, 4, 609–630. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review. Coatings 2020, 10, 233. [Google Scholar] [CrossRef]
- Christman, K.L.; Enriquez-Rios, V.D.; Maynard, H.D. Nanopatterning proteins and peptides. Soft Matter 2006, 2, 928–939. [Google Scholar] [CrossRef]
- Anderson, M.S.; Gaimari, S.D. Raman-atomic force microscopy of the ommatidial surfaces of Dipteran compound eyes. J. Struct. Biol. 2003, 142, 364–368. [Google Scholar] [CrossRef]
- Kryuchkov, M.; Adamcik, J.; Katanaev, V.L. Bactericidal and Antiviral Bionic Metalized Nanocoatings. Nanomaterial 2022, 12, 1868. [Google Scholar] [CrossRef]
- Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Brown, S.J.; Denell, R.; Beeman, R.W.; Gibbs, R.; Beeman, R.W.; Brown, S.J.; Bucher, G.; et al. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef]
- Polilov, A.A.; Makarova, A.A. The scaling and allometry of organ size associated with miniaturization in insects: A case study for Coleoptera and Hymenoptera. Sci. Rep. 2017, 7, 43095. [Google Scholar] [CrossRef]
- Malebary, S.J.; Alromema, N. iDLB-Pred: Identification of disordered lipid binding residues in protein sequences using convolutional neural network. Sci. Rep. 2024, 14, 24724. [Google Scholar] [CrossRef]
- Deryusheva, E.; Nemashkalova, E.; Galloux, M.; Richard, C.A.; Eléouët, J.F.; Kovacs, D.; Van Belle, K.; Tompa, P.; Uversky, V.; Permyakov, S. Does Intrinsic Disorder in Proteins Favor Their Interaction with Lipids? Proteomics 2019, 19, e1800098. [Google Scholar] [CrossRef]
- Katuwawala, A.; Zhao, B.; Kurgan, L. DisoLipPred: Accurate prediction of disordered lipid-binding residues in protein sequences with deep recurrent networks and transfer learning. Bioinformatics 2021, 38, 115–124. [Google Scholar] [CrossRef]
- Knyazeva, E.L.; Grishchenko, V.M.; Fadeev, R.S.; Akatov, V.S.; Permyakov, S.E.; Permyakov, E.A. Who is Mr. HAMLET? Interaction of human alpha-lactalbumin with monomeric oleic acid. Biochemistry 2008, 47, 13127–13137. [Google Scholar] [CrossRef]
- Van der Goot, F.G.; González-Mañas, J.M.; Lakey, J.H.; Pattus, F. A ‘molten-globule’ membrane-insertion intermediate of the pore-forming domain of colicin A. Nature 1991, 354, 408–410. [Google Scholar] [CrossRef]
- Nemashkalova, E.L.; Kazakov, A.S.; Khasanova, L.M.; Permyakov, E.A.; Permyakov, S.E. Structural Characterization of More Potent Alternatives to HAMLET, a Tumoricidal Complex of α-Lactalbumin and Oleic Acid. Biochemistry 2013, 52, 6286–6299. [Google Scholar] [CrossRef]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Sudhof, T.C. A broken α-helix in folded α-Synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Kerimova, İ.; Huseynova, E. Species Composition of Chortobiont Beetles (Coleoptera: Lycidae, Lampyridae, Cantharidae, Oedemeridae) Less Studied in Azerbaijan. Artvin Çoruh Üniversitesi Orman. Fakültesi Derg. 2014, 15, 1–8. [Google Scholar] [CrossRef]
- Pushkin, S. Аннатирoванный списoк видoв класса насекoмых (Insecta) oкрестнoстей села Садoвoе (Грачи) Ахтубинскoгo Райoна Астраханскoй Области. 2016. Available online: https://www.researchgate.net/publication/299619254_ANNATIROVANNYJ_SPISOK_VIDOV_KLASSA_NASEKOMYH_INSECTA_OKRESTNOSTEJ_SELA_SADOVOE_GRACI_AHTUBINSKOGO_RAJONA_ASTRAHANSKOJ_OBLASTI (accessed on 4 March 2025).
- Shapovalov, M.; Zamotajlov, A.; Saprykin, M. Coleopterous Insects (Insecta, Coleoptera) of Republic of Adygheya (Annotated Catalogue of Species); Fauna Conspecta of Adygheya No. 1; Zamotajlov, A.S., Nikitsky, N.B., Eds.; Adyghei State University Publishers: Maykop, Russia, 2010; 404p. [Google Scholar]
- Horák, J. Niche partitioning among dead wood-dependent beetles. Sci. Rep. 2021, 11, 15178. [Google Scholar] [CrossRef]
- Alexander, K.; Anderson, R. The Beetles of Decaying Wood in Ireland; A Provisional Annotated Checklist of Saproxylic Coleoptera; National Parks and Wildlife Service: Dublin, Ireland, 2012. [Google Scholar]
- Bagge, L.E.; Osborn, K.J.; Johnsen, S. Nanostructures and Monolayers of Spheres Reduce Surface Reflections in Hyperiid Amphipods. Curr. Biol. 2016, 26, 3071–3076. [Google Scholar] [CrossRef]
- Wolff, J.O.; Schwaha, T.; Seiter, M.; Gorb, S.N. Whip spiders (Amblypygi) become water-repellent by a colloidal secretion that self-assembles into hierarchical microstructures. Zool. Lett. 2016, 2, 23. [Google Scholar] [CrossRef]
- Nickerl, J.; Tsurkan, M.; Hensel, R.; Neinhuis, C.; Werner, C. The multi-layered protective cuticle of Collembola: A chemical analysis. J. R. Soc. Interface 2014, 11, 20140619. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gokan, N. Anatomy and ultrastructural details of the eye of the passalid beetle Ceracupes yui Okano 1988 (Scarabaeoidea; Passalidae). Arthropod Struct. Dev. 2024, 80, 101361. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Yu, Q.; Erb, U. Mesostructure of Ordered Corneal Nano-nipple Arrays: The Role of 5–7 Coordination Defects. Sci. Rep. 2016, 6, 28342. [Google Scholar] [CrossRef] [PubMed]
- Urca, T.; Lehmann, F.-O.; Gorb, E.V.; Gorb, S.N. Nanoscale mesh acts as anti-adhesive surface against particulate contamination in eyes of whiteflies. Sci. Rep. 2024, 14, 18267. [Google Scholar] [CrossRef] [PubMed]
- Gajdzik, J.; Lenz, J.; Natter, H.; Hempelmann, R.; Kohring, G.-W.; Giffhorn, F.; Manolova, M.; Kolb, D.M. Enzyme immobilisation on self-organised nanopatterned electrode surfaces. Phys. Chem. Chem. Phys. 2010, 12, 12604–12607. [Google Scholar] [CrossRef]
- Willner, I.; Willner, B. Biomolecule-Based Nanomaterials and Nanostructures. Nano Lett. 2010, 10, 3805–3815. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Nelson, C.M. Nanopatterned Surfaces for Exploring and Regulating Cell Behavior. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 2598–2606. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryuchkov, M.; Wang, Z.; Valnohova, J.; Savitsky, V.; Karamehmedović, M.; Jobin, M.; Katanaev, V.L. Smart Bio-Nanocoatings with Simple Post-Synthesis Reversible Adjustment. Biomimetics 2025, 10, 163. https://doi.org/10.3390/biomimetics10030163
Kryuchkov M, Wang Z, Valnohova J, Savitsky V, Karamehmedović M, Jobin M, Katanaev VL. Smart Bio-Nanocoatings with Simple Post-Synthesis Reversible Adjustment. Biomimetics. 2025; 10(3):163. https://doi.org/10.3390/biomimetics10030163
Chicago/Turabian StyleKryuchkov, Mikhail, Zhehui Wang, Jana Valnohova, Vladimir Savitsky, Mirza Karamehmedović, Marc Jobin, and Vladimir L. Katanaev. 2025. "Smart Bio-Nanocoatings with Simple Post-Synthesis Reversible Adjustment" Biomimetics 10, no. 3: 163. https://doi.org/10.3390/biomimetics10030163
APA StyleKryuchkov, M., Wang, Z., Valnohova, J., Savitsky, V., Karamehmedović, M., Jobin, M., & Katanaev, V. L. (2025). Smart Bio-Nanocoatings with Simple Post-Synthesis Reversible Adjustment. Biomimetics, 10(3), 163. https://doi.org/10.3390/biomimetics10030163







