Abstract
Affecting around 30–40% of the population worldwide, allergic disorders including asthma, rhinitis, eczema, and food allergies, are relatively common. Environmental factors, such as air pollution and climate change, which aggravate allergic reactions, contribute to the growth of these diseases. Although conventional treatments such as antihistamines and immunotherapy remain the standard for symptom management, growing interest in natural remedies highlights the potential value of medicinal plants as complementary therapies. Commonly present in plants, vitamins and antioxidants have strong anti-inflammatory and antioxidant actions that can control immune responses, lower oxidative stress, and thus reduce inflammation, which is the main element in allergic reactions. By focusing on the fundamental causes of inflammation and immunological dysregulation, phytochemicals have shown encouraging effects in reducing allergic symptoms. This review investigates the role of plant flavonoids, polyphenols, and vitamins in lowering allergic symptoms and inflammation, and suggests their potential in allergy management. It also aims to provide a short review of various plant species that are used in folk medicine for allergy treatment. The inclusion of plant-based compounds in allergy therapy could provide more complete and environmentally friendly remedies to enhance patients’ quality of life.
1. Introduction
Allergic reactions are one of the most prevalent chronic disorders. The incidence of allergic illnesses is increasing worldwide [1]. The frequency of allergic disorders has been increasing over the past few decades, and an estimated 30–40% of the global population now lives with at least one allergy [2]. Statistics from the World Health Organization (WHO) indicate that over 300 million people suffer from asthma, while hundreds of millions suffer from rhinitis [1,2]. Allergy cases are expected to worsen with increasing temperatures and air pollution. Pollen counts, stinging insect populations, and mold populations linked to allergic illnesses are affected by these environmental changes [2].
Dealing with this major worldwide issue, which threatens health and economies, requires a global action strategy with alliances among several stakeholders [1,3]. To have a higher quality of life, everyone should cooperate to lower the effect of allergic diseases, find innovative and reasonably priced ways to prevent them, and treat patients more holistically [1].
Asthma, allergic rhinitis, eczema, food allergies, and other allergies all arise from an overactive immune reaction to typically harmless molecules, sometimes referred to as allergens, which cause inflammation and oxidative damage [4]. Although conventional treatments, such as antihistamines, corticosteroids, and immunotherapy are widely used to control allergic reactions [5,6], natural remedies from plants have attracted increasing attention as a way to combat allergic inflammation [7] and provide antioxidant protection [8].
Plants rich in vitamins and antioxidants have many health advantages, including reduced inflammation [9], neutralizing oxidative stress [10], and modulating immune responses [11]. By addressing the fundamental causes of inflammation and oxidative damage [10], many phytochemicals, including vitamin C [12], vitamin D [13], vitamin E [14], minerals such as zinc, iron, selenium [13], flavonoids [15], and polyphenols [16] have shown promise in reducing allergic symptoms.
Considering the above, this study highlights the role of plant flavonoids, polyphenols, and vitamins in reducing allergic symptoms and inflammation, suggesting their potential in allergy management. It also aims to provide a short review of various plant species that are used in folk medicine as treatments for allergies. By harnessing the natural benefits of plant-based compounds, conventional therapies can be improved to provide alternative solutions to those seeking holistic methods to prevent and manage allergies.
2. Mechanisms of Allergies
The underlying mechanisms of allergies are primarily driven by the immune system, particularly involving immunoglobulin E (IgE) antibodies, mast cells, and various pro-inflammatory molecules [17]. Made by B cells in response to allergens, IgE is a fundamental antibody involved in allergic reactions. Through the high-affinity FcεRI receptor, it binds to mast cells and basophils, sensitizing them. Re-exposure to the allergen causes cross-linking of IgE, which releases histamine and other inflammatory mediators, thereby producing symptoms, including itching, swelling, and anaphylaxis. Allergy disorders, such as food allergies, hay fever, and asthma, are associated with increased levels of IgE [17].
Allergic reactions are primarily mediated by allergen-specific T helper (Th) cells; therefore, they are important in the pathophysiology of allergic hypersensitivity events. These Th cells initiate an effective immune response that produces strong mediators and attracts inflammatory cells, therefore causing the clinical signs of allergic disorders [18]. The inappropriate response of the immune system to allergens is often due to a loss of immune tolerance, which is normally maintained by T regulatory cells. These cells suppress Th2-mediated inflammation and promote immune tolerance by inducing suppressive cytokines and inhibiting allergen-specific IgE [19]. However, in allergic individuals, loss of immune tolerance may lead to inappropriate immune activation. The sensitization phase begins with the first allergen encounter, which causes the immune system to identify the allergen as a threat. T lymphocytes, especially T helper 2 (Th2) cells, which are fundamental in organizing allergic reactions, are activated during this phase. Th2 cells cause B cells to generate IgE antibodies particular to the allergen [20]. IgE antibodies attach the receptors on their surfaces by priming mast cells and basophils for future allergen exposure [21]. As shown by epicutious sensitization, which is associated with food allergies and other allergic diseases [22], sensitization can occur through multiple pathways, including the skin.
Re-exposure to an allergen sets up the effector phase. The allergen degranulates IgE antibodies on the surface of mast cells and basophils during this phase by cross-linking them [23]. This release causes immediate symptoms of allergic reactions, such as itching, swelling, and redness. Studies employing monoclonal antibodies to prevent mast cell activation [24] have shown the importance of the effector phase in the expression of allergy symptoms, and how one may target it for therapeutic approaches.
If an allergic reaction cannot be controlled, it can enter a chronic phase marked by continuous inflammation and tissue remodeling. This phase consists of the recruitment and activation of eosinophils and other immune cells, which help sustain inflammation and tissue destruction [21]. The constant presence of eosinophils and mast cells, which interact in what is known as the “allergic effector unit”, marks chronic allergic inflammation [21]. Long-term disorders, including allergic rhinitis and asthma, in which the immune system stays hyperactive to allergens [25], might result from this chronic phase.
Figure 1 illustrates the stages of the allergy mechanism.
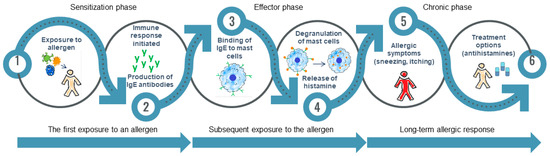
Figure 1.
Main phases of allergy development. (1–2) Sensitization phase: Initial exposure to an allergen leads the immune system to identify it as a threat. Th2 cells play an essential role in activating B cells, which then produce IgE. These antibodies attach to mast cells and basophils, preparing them for future reactions; (3–4) Effector phase: When exposed again, the allergen connects with IgE antibodies, leading to mast cell degranulation and the release of inflammatory mediators. This results in rapid allergic reactions such as itching, swelling, and redness; (5–6) Chronic phase: Uncontrolled allergic reactions lead to lasting inflammation and changes in tissue structure, mostly caused by eosinophils and mast cells. This phase contributes to chronic allergic conditions, such as asthma and allergic rhinitis.
Environmental exposure and genetic predispositions shape the evolution of allergic disorders. Rising allergic illnesses have been linked to modern lifestyles, marked by increased urbanization and antibiotic use. These environmental changes enhance pollution exposure and other risk factors that interact with the immune system, thereby influencing disease development [26]. Furthermore, epithelial barrier malfunction and changes in the microbiome are important factors that predispose individuals to allergic disorders [27]. The neurological system can also be involved in allergic reactions, which produce symptoms, including sneezing, nasal congestion, and bronchoconstriction. Mediators released during allergic reactions interact with sensory nerves and alter neuronal excitability and transmission, aggravating symptoms [28].
3. Inflammation and Oxidative Stress in Allergic Reactions
Inflammation and oxidative stress are closely associated with the development and progression of allergic reactions. By modulating both inflammation and oxidative stress through dietary antioxidants, pharmacological treatments, and environmental changes, it may be possible to better manage and reduce allergic symptoms and their long-term impact on health [29].
The overproduction of reactive oxygen species (ROS), which can damage proteins, lipids, and DNA, drives most of the oxidative stress in allergic responses. A characteristic of allergy diseases, this oxidative damage is usually aggravated by environmental elements, including pollen exposure and air pollution [29]. The oxidative stress response in allergic rhinitis is linked to the Nrf2/NF-κB signaling pathway; particulate matter (PM2.5) exposure increases allergic inflammation by blocking Nrf2 activity and activating NF-κB, thereby increasing inflammatory cytokine generation. Through NAD(P)H oxidase activity, pollen grains can also cause oxidative stress, therefore aggravating allergic reactions by raising intracellular ROS levels [30].
A Th2-dominant immune response, marked by a higher production of IL-4, IL-5, and IL-13 cytokines, drives allergic reactions. Oxidative stress further aggravates this cytokine imbalance by promoting inflammatory cells and worsening symptoms [31]. This stress can activate the NLRP3 inflammasome, a protein complex that triggers the release of proinflammatory cytokines and amplifies the inflammatory response. In allergic diseases, this process worsens inflammation and symptoms because NLRP3 activation contributes to increased inflammatory cell recruitment and tissue damage [32]. Inhibiting NLRP3 can alleviate allergy symptoms by encouraging mitophagy, thereby reducing oxidative stress and inflammation [33], and providing therapeutic benefits.
Exposure to PM2.5 has been linked to higher oxidative stress and inflammation in allergic rhinitis. In allergic conjunctivitis, pollen grains can produce oxidative stress and instantaneous hypersensitivity reactions that increase Th2-dominant inflammation and create redox imbalance, thus aggravating allergic symptoms [31,34]. The oxidative burst of pollen stimulates inflammatory responses and inflammatory cell recruitment [35].
By lowering ROS levels and balancing redox balance, N-acetylcysteine (NAC) has been demonstrated to reduce oxidative stress and inflammation in allergy models. This implies possible therapeutic advantages in the control of allergic diseases aggravated by oxidative stress [22,26]. Targeting oxidative stress-related signaling pathways, such as blocking the NLRP3 inflammasome or boosting antioxidant defenses, may provide new approaches for treating allergic disorders [33].
4. Effects of Vitamins and Antioxidants on Allergies
Vitamins and antioxidants are essential for maintaining human health and can play a significant role in modulating allergic reactions (Figure 2). They function by reducing inflammation, neutralizing oxidative stress, and regulating immune system responses [36], all of which are critical for managing allergic conditions such as asthma, rhinitis, eczema, and food allergies.
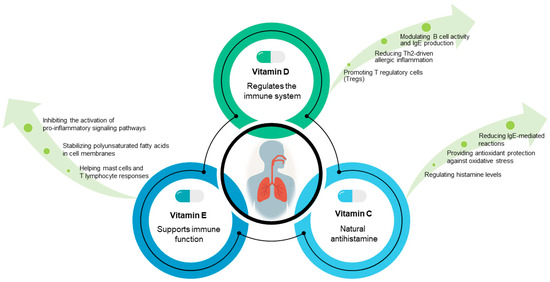
Figure 2.
Immunomodulatory roles of vitamins D, C, and E in allergy prevention and management. Vitamin D is linked to a lower risk of allergic disorders owing to its effects on immune response regulation and inflammation. Vitamin C helps to control allergic reactions due to its antioxidant properties and the regulation of histamine and inflammation. Vitamin E appears to have a complex function in allergy control through its antioxidative and anti-inflammatory effects, as well as its impact on immunological responses.
4.1. Vitamins and Their Role on Immune Regulation
4.1.1. Vitamin D
Allergic disorders are greatly modulated by vitamins, especially vitamin D. Studies show that vitamin D affects the immune system and might influence the initiation and development of disorders including allergic rhinitis, atopic dermatitis, and asthma [37,38,39].
Vitamin D primarily exists in two forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Although vitamin D3 is mostly produced in the skin by sunlight exposure, vitamin D2 is derived from some fungi and yeasts, including irradiated mushrooms [40]. Plant sources usually provide low quantities of vitamin D; thus dietary supplementation or sun exposure is required as an alternative, particularly for individuals at risk of deficiency [40].
Vitamin D has significant regulatory functions in the immune system. It influences T cell development and balances Th1 and Th2 responses, which are important in the context of allergies [41]. A healthy immune system depends on well-regulated Th1/Th2 balance. An imbalance favoring Th2 responses is a feature of allergic diseases including food allergies, atopic dermatitis, and asthma, with excessive Th2 activity causing IgE production and inflammation. Restoring this balance using pharmaceutical methods, probiotic supplements, or dietary changes, can increase immunological tolerance and lower allergy severity [42]. Research conducted in animal models has revealed that vitamin D deficiency may alter immune responses towards Th2 dominance, therefore aggravating allergy reactions [43]. Furthermore, vitamin D promotes the synthesis of antimicrobial peptides and improves the activity of regulatory T cells, which are essential for immunological tolerance [44]. The association between low serum vitamin D levels and various allergic conditions, including asthma and food allergies, highlights its critical functional role [45].
The immunomodulatory actions of vitamins have been well investigated. It has been observed to reduce IgE-mediated responses and modulate the maturation of dendritic cells, which are vital components of the immune response to allergens [46]. Furthermore, research has indicated that maternal vitamin D deficiency during pregnancy corresponds to higher allergic sensitivity in children [43]. This association between early vitamin D levels and the development of atopy or asthma implies that vitamin D can control the cause of allergic illnesses early in life [47]. Although vitamin D supplements do not always decrease asthma episodes, children with low baseline vitamin D levels may benefit. It also seems to lower allergic rhinitis symptoms and atopic dermatitis severity [37].
Active studies on the therapeutic possibilities of vitamin D supplements for preventing and controlling allergic disorders are still in progress. With conflicting results, some clinical trials have investigated the effects of vitamin D supplementation on immunological responses in children at risk [48]. Although there is suggestive evidence that vitamin D supplementation may confer protective effects against allergic diseases, randomized controlled trials have produced conflicting results, indicating that further research is needed to identify ideal dosage strategies and the cohorts most likely to benefit from supplementation [49]. Although further long-term studies are needed to support statements about the efficacy of vitamin D in allergy prevention, current recommendations call for vitamin D supplementation, especially in high-risk individuals [48,50].
4.1.2. Vitamin C
Vitamin C (ascorbic acid), a water-soluble vitamin, is widely found in plants. Fruits and vegetables, such as citrus fruits, strawberries, kiwi, bell peppers, and leafy greens abound in it [51]. As these plant foods are the main dietary sources, public health campaigns aimed at controlling vitamin C levels usually emphasize the consistent consumption of these foods. Moreover, accepted analytical techniques for measuring ascorbic acid in plant matrices assist in continuous nutritional monitoring that relates food intake to clinical outcomes, including immune function and related allergic processes [51].
Well known for its antioxidant properties, vitamin C protects DNA, proteins, and lipids against oxidative damage. This protective role is vital in the context of inflammation and immune cell activation, where reactive oxygen species may be produced in response to allergens. In addition to its antioxidant properties, vitamin C is crucial for collagen formation and participates in several cell signaling pathways supporting normal immune system operation [51]. The pharmacological profile described by Levine et al. highlighted that under normal concentrations, vitamin C capabilities extend to modulating inflammatory responses, which is an important factor considering its role in allergic illnesses [52].
The immunomodulatory power of vitamin C in allergic disorders has been progressively underlined. Its antioxidant properties help to reduce oxidative stress that can cause persistent inflammatory reactions usually seen in allergic diseases [51]. Vitamin C has been investigated as an adjuvant treatment in clinical settings aimed at lowering inflammatory mediators and regulating immune responses. For example, a randomized trial showing corticosteroid-sparing effects of daily oral vitamin C in asthmatic patients revealed its immunomodulatory ability [53]. Furthermore, studies examining its impact on respiratory outcomes in at-risk populations indicate that vitamin C’s ability to neutralize free radicals is thought to be advantageous in reducing IgE-mediated reactions [54]. Moreover, vitamin C, possibly in combination with other immunomodulators, may prevent the IgE-mediated anaphylactic cascade in food allergies, thus extending its function beyond basic antioxidant activity to targeted immunomodulation [55].
Although vitamin C has general health benefits, its direct application as a medicinal agent in allergy therapy or prevention is still under investigation. Intravenous vitamin C administration shows possible advantages based on clinical data derived from observational research [53]. In individuals with allergic rhinitis, asthma, and dermatitis, such therapies have been linked to improvements in the general symptom scores. Studies examining maternal supplementation during pregnancy, on the other hand, show conflicting results; some studies note no appreciable effect on respiratory or allergic outcomes in early childhood [56], whereas others show improvements in pulmonary function and a lowered risk of wheezing among newborns born to supplemented mothers [54]. Furthermore, although very rare, hypersensitivity to vitamin C has been recorded in isolated cases involving both topical formulations and oral challenges [57,58]. These cases underline the importance of customized therapeutic approaches and remind clinicians that hypersensitivity reactions are rare. Although more randomized controlled trials and future studies are required to clarify ideal dosages and identify subpopulations that might benefit most from supplementation, the present data support the hypothesis that vitamin C helps to modulate immune responses in allergy.
Indicating it is possible to minimize allergy symptoms, vitamin C has been demonstrated to drastically lower the production of histamine and other inflammatory mediators in allergic reactions, both in vitro and in vivo [59]. By increasing the Th1/Th2 cytokine production ratio, high doses of vitamin C can alter the immune response and hence aid in lowering allergic inflammation [60,61]. As an antioxidant, vitamin C lowers oxidative stress, which is a major component of the pathophysiology of allergic illnesses [53] and may increase glucocorticoid action, thereby modulating immunological responses and reducing allergic symptoms [62]. By lowering oxidative stress and inflammation, vitamin C supplements have been shown to alleviate allergic rhinitis symptoms. Moreover, higher plasma vitamin C levels correlate with symptom improvement [63].
4.1.3. Vitamin E
Owing to its antioxidant and immunomodulatory properties, vitamin E could have certain advantages in controlling allergies. The key to the pathophysiology of allergy disorders is that oxidative stress is partially reduced by vitamin E. This decrease in oxidative stress could help redirect immune reactions away from pathways aggravating allergies [64]. Studies have shown that vitamin E may help treat allergic rhinitis and asthma symptoms. When combined with selenium [65], it lowers mucus secretion, tightness, and inflammation of the airways. It has been demonstrated that vitamin E suppress IgE reactions to allergens, thus lowering serum IgE levels and reducing allergic sensitivity [66]. Higher doses of vitamin E supplements have been shown to cause quiet nasal allergic reactions, thereby lowering symptoms, including nasal congestion and sneezing. Although it did not greatly diminish the requirement for further treatment, vitamin E supplementation has been linked to reduced nasal symptoms scores in patients with seasonal allergic rhinitis [53].
Mostly occurring in nature as tocopherols and tocotrienols, vitamin E is synthesized only by plants and cyanobacteria. Key dietary sources of vitamin E include a wide spectrum of edible oils, including those derived from soybean, sunflower, corn, wheat germ, walnut, cereals, legume seeds, and even underutilized edible sources such as wild plants and edible flowers [67,68,69]. Research on plant biofortification has underlined the importance of crops such as maize in increasing vitamin E content, especially with regard to increasing α-tocopherol concentrations, given their maximum biological activity [68]. Furthermore, studies on the tocopherol concentration in nuts (e.g., hazelnut oils) support the perspective that, taken as part of a varied diet, even foods with modest vitamin E amounts contribute greatly to daily intake when eaten together [69].
Vitamin E’s main use is as an antioxidant which neutralizes reactive oxygen species and free radicals, therefore shielding cell membranes from lipid degradation. This antioxidant action helps preserve cellular homeostasis in different organs, thereby protecting the integrity of immune cells, especially those prone to oxidative stress. By stabilizing polyunsaturated fatty acids in cell membranes, thereby reducing oxidative damage and indirectly modulating cellular signaling pathways linked to inflammation, tocopherols and tocotrienols perform their protective roles [70]. These acts are essential not only for maintaining cellular health but also for controlling persistent inflammatory processes linked to allergy disorders.
Vitamin E plays an essential role in modulating the immune system, largely through its antioxidant properties. By mitigating oxidative stress, vitamin E helps regulate immune responses in allergic conditions, leading to a reduction in the activation of pro-inflammatory transcription factors and cytokine cascades. Its immunomodulatory effects are believed to involve modulation of inflammatory cell signaling pathways and a decrease in oxidative damage that contributes to hypersensitivity reactions. Mendelian randomization studies further support a causal relationship between vitamin E and reduced risk of atopic dermatitis, underscoring its potential therapeutic relevance [71]. Thought to be important in the pathophysiology of allergy disorders, these immunomodulatory systems help mast cells and T lymphocyte responses to be stabilized.
Vitamin E has been investigated as a possible therapeutic agent for the treatment and prevention of allergy disorders based on its antioxidant and immunomodulating actions. By balancing inflammation and oxidative stress, clinical and observational research implies that higher dietary intake of vitamin E supplements may lower the incidence or severity of atopic dermatitis and other allergic diseases [71]. Increasing the intake of biofortified crops and vitamin E-rich plant oils is attracting interest as a non-pharmacological means to reduce allergic reactions. Although the data are encouraging, further well-designed clinical studies are needed to establish standard dosages and ascertain the efficacy and safety of vitamin E supplementation in various populations with allergic diseases.
4.1.4. Other Vitamins
Other vitamins, notably vitamin A and folic acid, have less obvious and occasionally contradictory effects on allergies. For example, vitamin A supplements have been linked to a higher risk of atopy, especially in young women, but have no appreciable effect on wheezing [72]. Reduced folic acid consumption by mothers has been linked to higher rates of respiratory allergies in newborns and children [73]. Clarifying these links and optimizing vitamin supplementation plans for allergy control and treatment require further study.
4.2. Role of Antioxidants in Allergy Management
Antioxidants are a double-edged sword in allergies, potentially offering protective effects against oxidative stress and inflammation, whereas excessive intake might increase the risk of allergy [74]. Dietary patterns, genetic factors, and environmental factors affect the complex relationship between antioxidants and allergies. Figure 3 shows the effects of antioxidants on the modulation of allergic diseases.
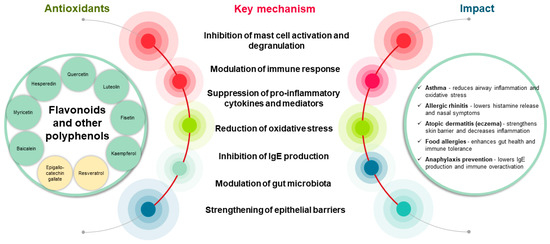
Figure 3.
Impact of flavonoids and polyphenols on the development and modulation of allergic diseases: key mechanisms and therapeutic potential. These bioactive compounds mitigate allergic inflammation by inhibiting mast cell degranulation, thereby reducing the release of histamine and proinflammatory cytokines. Additionally, flavonoids can suppress the expression of the high-affinity IgE receptor (FcεRI) on basophils, thereby decreasing allergen sensitivity. Polyphenols contribute to allergy prevention by lowering IgE levels and modulating cytokine production, thereby preventing allergic sensitization. Their antioxidant properties further limit oxidative stress and reduce the inflammation and tissue damage associated with allergic responses. Collectively, these mechanisms highlight the therapeutic potential of flavonoids and polyphenols as complementary agents for allergy management.
Table 1 summarizes the mechanisms underlying the anti-allergic activity of flavonoids and polyphenols, highlighting their molecular targets and mouse model evidence.

Table 1.
Flavonoids and polyphenols in allergy modulation.
4.2.1. Flavonoids
Because of their anti-inflammatory, anti-allergic, and immunomodulatory effects, naturally occurring chemicals found in plants, flavonoids, have shown promise in controlling allergies [78]. These compounds are under increasing research as they help avoid allergic illnesses and relieve symptoms. Most importantly, flavonoids prevent mast cell degranulation and Th2 cytokine generation in allergic reactions. By suppressing the release of inflammatory mediators such as histamine and cytokines, they also help lower airway hyperresponsiveness and inflammation [79]. Essential for the control of immune responses in allergies, flavonoids alter the Th1/Th2 cytokine balance. They control immune cell activities, including those of T cells, macrophages, and dendritic cells, thereby lowering allergic inflammation [80]. Flavonoids have been demonstrated to reduce chemokine activity, which helps to attract inflammatory cells during allergic responses [81]. According to epidemiological research, a high consumption of flavonoids is linked to a reduced prevalence of asthma and other allergic disorders, thereby stressing their possible dietary intervention value [82]. Although the quality of the evidence varies, a systematic evaluation of clinical studies has shown that flavonoid supplementation can help individuals with allergic rhinitis, asthma, and atopic dermatitis [15].
The strong antioxidant properties of flavonoids, including apigenin and quercetin, help to reduce the negative effects of oxidative stress on immune cells [83]. This result is important because oxidative stress has been linked to the pathophysiology of several allergic diseases, including food allergies and asthma [84]. The substantial anti-inflammatory effects of flavonoids help to stop the synthesis of pro-inflammatory cytokines and mediators that cause allergic reactions. Flavonoids have been found, for example, to prevent the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a fundamental transcription factor that regulates the inflammatory response. Important mediators of Th2 responses in allergens, such as cytokines IL-4 and IL-13, express themselves less because of this reduction [85,86]. Moreover, studies have shown that flavonoids can downregulate the expression of many surface molecules linked to mast cell activation, thereby decreasing the release of histamine and other allergic mediators [87].
Flavonoids have been found to affect immune cell behavior and produce a balanced immunological response. For instance, flavonoids can lower mast cell activation and degranulation, which are fundamental in the initial stages of allergic reactions [88]. Their possible use as therapeutic agents against allergies is improved by their capacity to inhibit mast cell histamine release. Furthermore, flavonoids may control T cell differentiation, thereby favoring the growth of regulatory T cells (Tregs) over effector T cells, which are sometimes involved in aggravating allergy. Flavonoids can help reduce strong immunological responses by increasing the activity of Tregs, thereby fostering tolerance to allergens [89].
Maintaining gut barrier integrity is another function of flavonoids, which is essential for avoiding allergic sensitization. The gut microbiome plays an important role in immune regulation. Changes in gut microbiota have been associated with the development of allergic diseases such as food allergies and asthma [90]. It produces metabolites, such as short-chain fatty acids, which help maintain immune balance and may protect against food allergies by regulating immune pathways [91]. Polyphenols influence gut microbiota composition, promote the production of helpful metabolites that support immune health, and may help reduce allergic reactions [92]. Their interaction with gut bacteria boosts antioxidant and anti-inflammatory effects, which can play a role in preventing diseases linked to oxidative stress, including allergies [93]. Maintaining balanced gut microbiota, known as microbiota homeostasis, is essential for immune function and disease prevention. Food allergies, asthma, and atopic dermatitis are immune-mediated disorders linked to dysbiosis, which is an imbalance in this microbial population [94]. Flavonoids reportedly strengthen tight junctions in intestinal epithelial cells, thereby lowering their permeability to pro-inflammatory compounds and allergens [95]. This property is especially essential for food allergies, and other allergic sensitivities are associated with higher intestinal permeability. Furthermore, flavonoids affect the gut flora, which may indirectly affect immune regulation. Certain flavonoids could help balance the gut flora, thereby enhancing an immunological profile that is less sensitive to allergic reactions [89]. This implies that a major path for future allergy therapy research could be the interaction of the microbiome with flavonoids.
Flavonoids have been associated with the modification of several signaling systems connected to allergic reactions. They can therefore block the mitogen-activated protein kinase (MAPK) cascade [96] and phosphoinositide 3-kinase (PI3K/-Akt) pathway. Flavonoids may lower inflammatory cytokine generation and promote an anti-inflammatory state by altering these pathways, thereby helping to control allergies.
Moreover, flavonoids can interact with Toll-like receptors (TLRs), which are fundamental components of the innate immune system, thereby affecting the identification of allergens and the subsequent adaptive immunological response. This modification can alter cytokine profiles and lower allergy sensitivity [97].
4.2.2. Polyphenols
One significant mechanism is the control of chemokine production and the consequent immune cell recruitment. For instance, blackcurrant anthocyanin-rich extracts have been shown to greatly reduce CCL11 (eotaxin), a chemokine key to recruiting eosinophils to inflamed tissues [98]. These polyphenols reduce eosinophilic inflammation in the airways by lowering CCL11 levels, thereby reducing the typical airway inflammation in allergic asthma. The results showed that polyphenols serve to reduce important elements of the allergic inflammatory cascade by directly linking the antioxidant qualities of anthocyanins with a reduction in chemokine-mediated inflammatory cell migration [98].
Polyphenols have also been demonstrated to control immunological responses by adjusting the balance between pro-allergic and regulatory mediators and by controlling cytokine generation [99]. Investigation of polyphenols extracted from areca nuts revealed that oral supplementation of ovalbumin-sensitized mice produced lower levels of IgE, less intestinal inflammation, and less mast cell activation [100]. By upregulating cytokines like interleukin-10 and lowering Th2 cytokines like interleukin-4, the study found that such polyphenols create an anti-inflammatory environment. This immunomodulatory effect implies that polyphenols can both stop allergen sensitization and reduce allergic reactions by modulating the hyperactive immune systems that cause allergic reactions [100].
Furthermore, polyphenolic substances like rosmarinic acid, plentiful in Perilla frutescens extracts, have demonstrated direct reductions in clinical symptoms of allergic rhinitis and conjunctivitis. Implicating rosmarinic acid as the main active component responsible for the anti-allergic effects, research has shown that rosmarinic acid-enriched extracts offer a level of symptom relief similar to that obtained with Perilla decoctions [101]. The fundamental processes most likely include the suppression of IgE-mediated mast cell activation and a consequent decrease in the production of pro-inflammatory mediators. This result supports the idea that particular polyphenols can have focused actions on a series of events that cause allergic inflammation [101].
Moreover, thorough studies on dietary polyphenols offer an integrated understanding of the anti-allergic mechanisms of polyphenols [76]. Polyphenols have lower protein allergenicity by binding to allergens and may change their structure, thereby lowering their immunogenicity. Important for the synthesis of pro-inflammatory cytokines and chemokines, major transcription factors (e.g., NF-κB and MAPK) are inhibited by additional mechanisms. Polyphenols help restore immunological homeostasis and, hence, moderate the inflammatory signaling pathways shared by allergy disorders. Their therapeutic potential in lowering allergen sensitivity and consequent allergic responses is further enhanced by their capacity to change the gut microbiota and strengthen gut barrier integrity [76].
The synthesis of the molecular activity of polyphenols in allergies is complex. By modifying chemokine and cytokine release (such as CCL11, IL-4, and others), mast cells are stabilized to prevent degranulation, alter immune responses in favor of regulatory pathways, and function as strong antioxidants that reduce oxidative stress and suppress inflammatory signaling. Taken together, these activities help reduce allergic inflammation and imply that dietary or pharmacological treatments employing polyphenol-rich extracts may present interesting approaches for the prevention and therapy of allergic diseases.
Through modulation of immune responses, reduction of protein allergenicity, and alteration of gut microbiota, polyphenols from plants show promising anti-allergic effects. By altering immune responses, they can lower allergic inflammation and symptoms related to disorders, such as food allergies, asthma, atopic dermatitis, and allergic rhinitis [102]. By interacting with proteins, these molecules can lessen their allergenicity and either hide or eliminate allergenic epitopes, thereby lowering immune response and recognition [103]. Polyphenols can change the composition of gut bacteria, which influences immune system control and might help clarify their anti-allergic properties [102].
Resveratrol, curcumin, quercetin, and catechins have been highlighted for their capacity to reduce allergic reactions and prevent their development [76]. Reducing the immunogenicity and allergenicity of wheat gliadins, a common allergen, cranberry, and apple extracts have been very successful [104]. Strong anti-allergic effects in animal models have been established for extracts of vegetables including perilla and chives [105].
Including foods high in polyphenols, such as berries, green tea, turmeric, citrus fruits, and dark chocolate in the diet might provide a natural and efficient way to control allergic reactions [102].
4.2.3. Selenium
Selenium is known to improve immune system control, which can help prevent food allergies, among other immune-related disorders [106]. Selenoproteins, including selenium, are essential for preventing dysregulation that can cause allergies [107]. Key components of allergic reactions, particularly antibody responses and mast cell activation, may be reduced. For example, selenium has been shown to alter allergic reactions to whey protein in a mouse model, suggesting that it is possible to reduce food allergy symptoms [108]. Selenium nanoparticles have been produced to treat allergic dermatitis by inducing antioxidative pathways, thereby reducing inflammatory reactions. By reducing the release of histamine and inflammatory cytokines, these nanoparticles show therapeutic benefits in allergic diseases [109]. Although selenium alone did not help manage allergy symptoms in asthma and rhinitis, its combination with vitamin E showed promise in lowering inflammation and allergic mediators, thereby implying a synergistic effect in managing these disorders [110].
5. Medicinal Plants for Natural Anti-Inflammatory and Antihistamine Therapies
The cultivation of medicinal plants that produce anti-inflammatory and antihistamine compounds is a promising natural approach for the management of allergies. Advances in breeding, genetic engineering, and optimized agricultural practices can enhance the production of bioactive compounds in these plants, making them more effective for therapeutic use.
5.1. Medicinal Plants with Natural Antihistamine Compounds
Natural antihistamines derived from plants such as Asystasia gangetica, Cassia sophera, Clerodendrum serratum, Euphorbia hirta, Myrica esculenta, and many other species used in traditional medicine, offer a promising alternative to synthetic drugs for managing allergies. These plant-based compounds can alleviate allergic symptoms by inhibiting histamine release and modulating immune responses, providing a safer and potentially more effective treatment option [111]. Many plant-derived compounds function by blocking histamine receptors, especially H1R, thereby preventing the usual allergic reaction [112,113]. Some plants, including those used in traditional medicine for allergic rhinitis, reduce the synthesis of immunoglobulin E and cytokines, which are the main contributors to allergic reactions. This modulation reduces the severity of allergic symptoms [114].
Plants such as garlic (Allium sativum) and chamomile (Matricaria recutita) are frequently utilized in areas like Brazil for their antihistamine qualities; they are sometimes passed down through family customs rather than official medical guidance [115]. Using plants such as Tephrosia purpurea for their antihistaminic and smooth muscle relaxant properties, Indian traditional medicine highlights the long-standing use of natural medicines for controlling allergies [116]. Phyllanthus amarus shows antihistamine action by blocking histamine 1 receptor (H1R) activation. The hypophyllanthin molecule identified in P. amarus displays good binding to the H1R, implying a possible anti-allergic action [112]. Well-known for its therapeutic qualities, extracts from the barberry fruit (Berberis vulgaris) have competitive antihistaminic and anticholinergic actions, thereby helping to control reactions generated by histamine [117]. The significant antihistamine activity of 5-hydroxy-1-methylpiperidin-2-one, isolated from Tragia involucrata, provides muscle relaxant and bronchodilating effects, which are advantageous for the control of allergic reactions [113]. Different Garcinia species have bioactive chemicals with antihistaminic effects, which make them therapeutically valuable in treating allergies and other inflammatory diseases [118]. Petasin, found in butterbur (Petasites hybridus), is known to lower histamine-related inflammation in allergic rhinitis and asthma [119]. Perilla frutescens decoction significantly suppressed the passive cutaneous anaphylactic reaction in mice induced by rosmarinic acid [120].
Although many plants have antihistamine effects, scientific confirmation of their safety and efficacy depends on clinical investigations [111,114]. Identifying bioactive chemicals from plants provides a route for developing new antihistamine medications that might offer safer and more effective alternatives to the present synthetic choices [121,122]. Natural antihistamine chemicals found in plants offer a great basis for creating alternative allergy remedies. To fully realize their possibilities and include them in regular medical practice, ongoing research and clinical validation are important.
5.2. Medicinal Plants with Anti-Inflammatory Effects
Plants with anti-inflammatory effects can be beneficial in managing allergies because of their ability to modulate immune responses and reduce inflammation [86]. Several studies have highlighted the potential of plant-based compounds in alleviating allergic symptoms.
Astragalus membranaceus, Nigella sativa, and Perilla frutescens were traditionally utilized for their immunomodulating and anti-allergic effects. By altering the immunological responses, these herbs can reduce allergy symptoms [123]. Propolis is rich in flavonoids and exhibits anti-allergic, anti-inflammatory, and antioxidative properties. It can affect interactions between microbe-immune systems, which helps control allergies [86]. The Lamiaceae family, which includes mint among other species, has long been used to treat allergic inflammatory diseases. The anti-allergic properties of these plants can be attributed, in part, to the presence of flavonoids and terpenoids [99]. Recognized for their anti-allergic and anti-histaminic qualities, certain herbs, including Solanum xanthocarpum, Ocimum tenuiflorum, and Piper longum, offer safer alternatives for traditional allergy remedies [124]. Plant extracts, including turmeric (Curcuma longa) [125], green tea (Camellia sinensis) [126], licorice root (Glycyrrhiza glabra) [127], stinging nettle (Urtica dioica) [128], and butterbur (Petasites hybridus) [119], have shown anti-inflammatory and antihistaminic qualities, thus helping to effectively reduce allergic symptoms [121].
In particular, those high in polyphenols and flavonoids, plants with anti-inflammatory qualities have great promise for alleviating allergies by helping to reduce inflammation and control immunological reactions.
5.3. Medicinal Plant Cultivation for Healthier and More Resilient Growth
Selective breeding methods, biotechnological developments, and sustainable agriculture methods will help produce better medicinal plants when used together [129] for allergy treatment. These techniques can improve the quality, safety, and efficacy of medicinal plants. While biotechnological developments, such as genetic modification and tissue culture, maximize plant compounds with anti-allergic effects, sustainable agricultural practices assist in maintaining soil health and biodiversity. Furthermore, focused breeding initiatives have chosen and improved plant varieties with greater therapeutic value [129]. Combining these techniques allows for the creation of premium medicinal plants that support allergy therapy as well as environmental and financial sustainability.
Reducing pesticide exposure and increasing biodiversity helps organic farming become a sustainable approach that improves the quality and safety of medicinal plants. This method also increases the commercial value of medicinal plants because of its organic premium and ecological stability support [130]. The inclusion of medicinal plants in agroforestry projects can help encourage their growth and protection. Smallholders may find this approach economically advantageous because it entails producing shade-tolerant medicinal plants either as intercrops or under forest cover [131]. Compared with plants cultivated in soil, hydroponics can increase the content of secondary metabolites in medicinal plants. This approach enables the choice of systems to optimize the yield of desired plant organs and metabolites [132]. Plant development can be improved, and the phytochemical content of medicinal plants may be modified using plant growth-boosting rhizobacteria. This microbiological method reduces abiotic stress and is ecologically beneficial, thereby enhancing the quality of bioactive molecules [133].
Breeding techniques concentrate on selecting characteristics that enhance both medicinal and agricultural value [134]. Tissue culture and genetic modifications can improve the production and yield of medicinal plants. These methods allow exact genome editing [135,136] and help overcome the challenges linked to regeneration resistance.
5.4. Gene-Edited Plants for Allergy Management: The Role of CRISPR-Cas9
One promising method to inactivate genes in plants that cause allergenic proteins is gene editing using CRISPR-Cas9 technology [137,138]. This approach provides an exact and effective way to reduce allergens in food crops, thereby possibly minimizing food allergies and related medical problems.
Plants have specific genes responsible for allergenic proteins that are targeted and edited successfully using CRISPR-Cas9. For durum wheat (Triticum durum), for instance, this technique was used to alter the α-amylase/trypsin inhibitor (ATI) genes, particularly the WTAI-CM3 and WTAI-CM16 subunits, which are connected to wheat allergies, including baker asthma and, perhaps, non-coeliac wheat sensitivity. Wheat lines resulting from this modification have a lower allergenic potential [138].
Similar to brown mustard (Brassica juncea), the main allergen Bra j I, a seed storage protein, was targeted using CRISPR-Cas9. Large deletions or frameshift mutations in the Bra j I homeologs, resulting from editing, greatly reduce or eliminate allergenic proteins in the seeds [137].
CRISPR-Cas9 has shown great efficiency in the production of targeted mutations. Regarding brown mustard, most mutations resulting from CRISPR/Cas9 were heritable to the T1 progeny, suggesting the possibility of hereditary transmission of the edited traits over generations [137]. The editing technique in durum wheat was completed in less time than in conventional breeding techniques, therefore underlining the efficacy of CRISPR technology in generating allergen-reduced crops [138]. Although CRISPR-Cas9 effectively lowered allergenic proteins, it may cause unintended pleiotropic effects. For instance, in durum wheat, inadvertently induced ATI 0.28 pseudogene editing results in unexpected effects on plant physiology or allergenicity [138].
Sometimes, gene editing can influence plant survival and development. Certain modified lines in brown mustard showed decreased seed production and viability; some seeds showed accelerated embryo development, leading to testa rupture [137]. Careful evaluation of these effects will help guarantee the general health and output of transformed plants.
With its successful use in crops, such as durum wheat and brown mustard, CRISPR-Cas9 technology is a potent tool for lowering allergenic proteins in plants. Although this technology promises to produce safer food crops, practical application of gene editing in agriculture depends on the rigorous evaluation of possible pleiotropic effects and consequences on plant viability [137,138]. Safe and successful application in allergy management depends on ongoing research and regulatory controls.
Another way of using CRISPR as a tool for allergy management is to improve the antioxidant content of plants. Targeted gene editing using this technology allows the modification, insertion, or deletion of specific genes that control antioxidant biosynthesis. Targeting genes linked to the synthesis of polyphenolic compounds or carotenoids allows CRISPR to increase the antioxidant content without adding foreign DNA, thus preserving the inherent traits of the plant. For instance, plants have been modified to boost antioxidant content by editing the lycopene β-cyclase (LCYB) gene responsible for the synthesis of carotenoids in tomatoes (Solanum lycopersicum) [139] or the CHS gene, which regulates the synthesis of flavonoids in hardy oranges (Poncirius trifoliate) [140].
Black rice (Oryza sativa ssp. japonica) is an uncommon variety of rice with health benefits owing to its anthocyanin pigment. Using CRISPR-Cas9, researchers have altered genes linked to anthocyanin production in rice. Editing genes alters the production of anthocyanins, affecting also pigment variation. Furthermore, a major regulator of anthocyanin production in black rice is the OsMYB3 transcription factor [141].
One major component of the bioactive molecules in red sage (Salvia miltiorrhiza) is phenolic acids, including rosmarinic and salvianolic acids. Using CRISPR-Cas9, the bZIP2 gene was silenced, which led to an increase in the phenolic acid content in Salvia miltiorrhiza [142].
6. Conclusions and Future Perspectives on Using Vitamins and Herbal Antioxidants in the Prevention and Control of Allergies
The rising frequency of allergies has encouraged studies on natural remedies for prevention and aid in their management [7]. Vitamin and herbal antioxidants are valuable for allergy control and have anti-inflammatory, immunomodulatory, and antihistamine effects [8,111]. Including these phytochemicals in treatments and preventive plans helps people with allergies control their symptoms, reduce their drug use, and lead to better lives. These natural substances might become essential components of individualized allergy treatment and combined health solutions as studies have advanced [7]. A diet high in colorful fruits, vegetables, and whole foods provides essential nutrients, thereby supporting a strong and healthy immune system [102]. New advances in biotechnology, customized medicine, and nutraceuticals are needed to improve their potency. Future research pathways for allergy management are summarized in Figure 4.
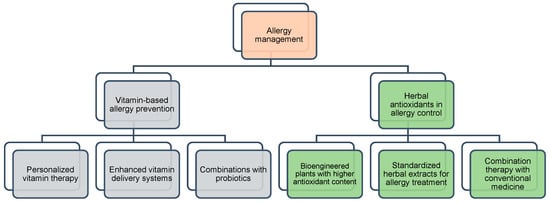
Figure 4.
Research pathways on phytochemicals for allergy prevention and control. Orange color represents the central concept of allergy management. Grey colors indicate strategies based on vitamin interventions. Green colors represent herbal antioxidant approaches. These colors help differentiate the main categories and clarify their respective roles in allergy control.
More individualized and powerful treatments are possible with the development of vitamin-based allergy prevention. Nutrigenomics and genetic profiling will allow customized vitamin intake [143] for those susceptible to allergies, hence maximizing immune responses [144]. While sustained-release formulations will guarantee ongoing anti-inflammatory effects, enhanced vitamin delivery systems such as liposomal and nanoparticle-based formulations will boost bioavailability [145]. Through microbiome modification, synergistic combinations of vitamins and probiotics, such as Vitamin D with Lactobacillus, may also improve gut-immune interactions and lower food allergies [146]. In addition to vitamins, herbal antioxidants have great potential for allergy control. Strong natural anti-allergic agents could come from bioengineered plants with increased polyphenol and flavonoid content [147,148] attained by genetic engineering, selective breeding, and modern agricultural technologies (e.g., hydroponics [132]). For focused allergy relief, pharmaceutical-grade herbal extracts, such as curcumin, quercetin, and rosmarinic acid, could be mixed into nasal sprays and inhalers [76]. Moreover, combining herbal antioxidants with conventional treatments may enhance therapeutic efficacy, lower dependence on corticosteroids [149], and offer a more sustainable way of allergy control [150].
Future research on genetic and microbiome-based dietary interventions may lead to personalized supplementation strategies tailored to an individual’s allergy susceptibility [151]. Fortified food, bioavailable antioxidant formulations, and pharmaceutical holistic therapies (such as antihistamines and corticosteroids) could have synergistic effects, reducing inflammation and allergy symptoms. Antioxidants, in particular, may serve as adjunct therapies for chronic allergic conditions that are resistant to conventional treatments [152]. The future of allergy prevention and treatment is moving toward precision medicine [153], providing safer, more effective, and sustainable solutions for people with allergies worldwide, including modern scientific advances with natural therapeutic approaches [151].
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Pawankar, R. Allergic Diseases and Asthma: A Global Public Health Concern and a Call to Action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Allergies. Available online: https://gaapp.org/diseases/allergies/ (accessed on 26 January 2025).
- Sánchez-Borges, M.; Martin, B.L.; Muraro, A.M.; Wood, R.A.; Agache, I.O.; Ansotegui, I.J.; Casale, T.B.; Fleisher, T.A.; Hellings, P.W.; Papadopoulos, N.G.; et al. The Importance of Allergic Disease in Public Health: An iCAALL Statement. World Allergy Organ. J. 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M. Allergic Diseases: A Comprehensive Review on Risk Factors, Immunological Mechanisms, Link with COVID-19, Potential Treatments, and Role of Allergen Bioinformatics. Int. J. Environ. Res. Public Health 2021, 18, 12105. [Google Scholar] [CrossRef]
- Larsen, J.N.; Broge, L.; Jacobi, H. Allergy Immunotherapy: The Future of Allergy Treatment. Drug Discov. Today 2016, 21, 26–37. [Google Scholar] [CrossRef]
- Wu, A.Y.-Y. Immunotherapy—Vaccines for Allergic Diseases. J. Thorac. Dis. 2012, 4, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jeong, I.; Cho, J.; Shin, C.; Kim, K.-I.; Shim, B.-S.; Ko, S.-G.; Kim, B. The Natural Products Targeting on Allergic Rhinitis: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 1524. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-Inflammatory and Anti-Allergic Potential of Dietary Flavonoids: A Review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef]
- Ghalibaf, M.H.E.; Kianian, F.; Beigoli, S.; Behrouz, S.; Marefati, N.; Boskabady, M.; Boskabady, M.H. The Effects of Vitamin C on Respiratory, Allergic and Immunological Diseases: An Experimental and Clinical-Based Review. Inflammopharmacology 2023, 31, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients 2023, 15, 3683. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory Role of Vitamin E in the Immune System and Inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Brasiel, P.G.; Guimarães, F.V.; Rodrigues, P.M.; Bou-Habib, D.C.; de Carvalho, V.F. Therapeutic Efficacy of Flavonoids in Allergies: A Systematic Review of Randomized Controlled Trials. J. Immunol. Res. 2022, 2022, 8191253. [Google Scholar] [CrossRef]
- Simões, R.; Ribeiro, A.C.; Dias, R.; Freitas, V.; Soares, S.; Pérez-Gregorio, R. Unveiling the Immunomodulatory Potential of Phenolic Compounds in Food Allergies. Nutrients 2024, 16, 551. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Engels, F.; Knippels, L.M.; Garssen, J.; Nijkamp, F.P.; Redegeld, F.A. Mechanisms of Allergy and Asthma. Eur. J. Pharmacol. 2008, 585, 354–360. [Google Scholar] [CrossRef]
- Soyer, O.U.; Akdis, M.; Ring, J.; Behrendt, H.; Crameri, R.; Lauener, R.; Akdis, C.A. Mechanisms of Peripheral Tolerance to Allergens. Allergy 2013, 68, 161–170. [Google Scholar] [CrossRef]
- León, B.; Ballesteros-Tato, A. Modulating Th2 Cell Immunity for the Treatment of Asthma. Front. Immunol. 2021, 12, 637948. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Pahima, H.; Puzzovio, P.G.; Levi-Schaffer, F. Update on Eosinophil Interaction with Mast Cells: The Allergic Effector Unit. Methods Mol. Biol. 2021, 2241, 221–242. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous Sensitization in the Development of Food Allergy: What Is the Evidence and How Can This Be Prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef] [PubMed]
- Minai-Fleminger, Y.; Levi-Schaffer, F. Mast Cells and Eosinophils: The Two Key Effector Cells in Allergic Inflammation. Inflamm. Res. 2009, 58, 631–638. [Google Scholar] [CrossRef]
- Suber, J.; Zhang, Y.; Ye, P.; Guo, R.; Burks, A.W.; Kulis, M.D.; Smith, S.A.; Iweala, O.I. Novel Peanut-Specific Human IgE Monoclonal Antibodies Enable Screens for Inhibitors of the Effector Phase in Food Allergy. Front. Immunol. 2022, 13, 974374. [Google Scholar] [CrossRef]
- Rosenwasser, L. New Insights into the Pathophysiology of Allergic Rhinitis. Allergy Asthma Proc. 2007, 28, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental Exposures and Mechanisms in Allergy and Asthma Development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef]
- Krempski, J.W.; Dant, C.; Nadeau, K.C. The Origins of Allergy from a Systems Approach. Ann. Allergy Asthma Immunol. 2020, 125, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Undem, B.J.; Taylor-Clark, T. Mechanisms Underlying the Neuronal-Based Symptoms of Allergy. J. Allergy Clin. Immunol. 2014, 133, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.P.; Crapo, J.D. Oxidative Stress in Allergic Respiratory Diseases. J. Allergy Clin. Immunol. 2002, 110, 349–356. [Google Scholar] [CrossRef]
- Bacsi, A.; Dharajiya, N.; Choudhury, B.K.; Sur, S.; Boldogh, I. Effect of Pollen-Mediated Oxidative Stress on Immediate Hypersensitivity Reactions and Late-Phase Inflammation in Allergic Conjunctivitis. J. Allergy Clin. Immunol. 2005, 116, 836–843. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Zhang, R.; Han, Z.; Huang, Y.; Deng, C.; Dong, W.; Zhuang, G. Effects of N-Acetylcysteine on Oxidative Stress and Inflammation Reactions in a Rat Model of Allergic Rhinitis after PM2.5 Exposure. Biochem. Biophys. Res. Commun. 2020, 533, 275–281. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lu, X.; Wang, H.; Chen, W.; Niu, B. NLRP3 Inflammasome Deficiency Alleviates Inflammation and Oxidative Stress by Promoting PINK1/Parkin-Mediated Mitophagy in Allergic Rhinitis Mice and Nasal Epithelial Cells. J. Asthma Allergy 2024, 17, 717–731. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Shin, H.S.; Kim, H.T.; Song, C.H.; Chai, O.H. PM2.5 Exacerbates Oxidative Stress and Inflammatory Response through the Nrf2/NF-κB Signaling Pathway in OVA-Induced Allergic Rhinitis Mouse Model. Int. J. Mol. Sci. 2021, 22, 8173. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Aguirre, L.; Hao, W.; Pan, L.; Li, X.; Saavedra-Molina, A.; Bacsi, A.; Radak, Z.; Sur, S.; Brasier, A.R.; Ba, X.; et al. Pollen-Induced Oxidative DNA Damage Response Regulates miRNAs Controlling Allergic Inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L1058–L1068. [Google Scholar] [CrossRef] [PubMed]
- Khadim, R.M.; Al-Fartusie, F.S. Antioxidant Vitamins and Their Effect on Immune System. J. Phys. Conf. Ser. 2021, 1853, 012065. [Google Scholar] [CrossRef]
- Li, Q. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36235600/ (accessed on 23 February 2025).
- Zhang, P.; Xu, Q.; Zhu, R. Vitamin D and Allergic Diseases. Front. Immunol. 2024, 15, 1420883. [Google Scholar] [CrossRef]
- Noriega, D.B.; Savelkoul, H.F.J. Vitamin D and Allergy Susceptibility during Gestation and Early Life. Available online: https://www.mdpi.com/2072-6643/13/3/1015 (accessed on 23 February 2025).
- Zeng, R.; Li, Y.; Shen, S.; Qiu, X.; Chang, C.-L.; Koplin, J.J.; Perrett, K.P.; Dharmage, S.C.; Lodge, C.J.; Lowe, A.J. Is Antenatal or Early-Life Vitamin D Associated with Eczema or Food Allergy in Childhood? A Systematic Review. Clin. Exp. Allergy 2023, 53, 511–525. [Google Scholar] [CrossRef]
- Benson, A.A.; Toh, J.A.; Vernon, N.; Jariwala, S.P. The Role of Vitamin D in the Immunopathogenesis of Allergic Skin Diseases. Allergy 2012, 67, 296–301. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, Y.; Huang, S.; Lv, D.; Yang, F.; Lou, L.; Zheng, Y.; Zhang, J.; Liu, S.; Zhang, N.; et al. Immunomodulatory Effect of Bifidobacterium Breve on Experimental Allergic Rhinitis in BALB/c Mice. Exp. Ther. Med. 2018, 16, 3996–4004. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, Y.; Shen, X.; Yang, K.; Cai, W. Maternal and Early-Life Vitamin D Deficiency Enhances Allergic Reaction in an Ovalbumin-Sensitized BALB/c Mouse Model. Food Nutr. Res. 2018, 62, 1401. [Google Scholar] [CrossRef]
- Rosendahl, J.; Pelkonen, A.S.; Helve, O.; Hauta-alus, H.; Holmlund-Suila, E.; Valkama, S.; Enlund-Cerullo, M.; Viljakainen, H.; Hytinantti, T.; Mäkitie, O.; et al. High-Dose Vitamin D Supplementation Does Not Prevent Allergic Sensitization of Infants. J. Pediatr. 2019, 209, 139–145.e1. [Google Scholar] [CrossRef]
- Bener, A.; Ehlayel, M.S.; Tulic, M.K.; Hamid, Q. Vitamin D Deficiency as a Strong Predictor of Asthma in Children. Int. Arch. Allergy Immunol. 2012, 157, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hollams, E.M.; Hart, P.H.; Holt, B.J.; Serralha, M.; Parsons, F.; de Klerk, N.H.; Zhang, G.; Sly, P.D.; Holt, P.G. Vitamin D and Atopy and Asthma Phenotypes in Children: A Longitudinal Cohort Study. Eur. Respir. J. 2011, 38, 1320–1327. [Google Scholar] [CrossRef]
- Mullins, R.J.; Clark, S.; Katelaris, C.; Smith, V.; Solley, G.; Camargo, C.A., Jr. Season of Birth and Childhood Food Allergy in Australia. Pediatr. Allergy Immunol. 2011, 22, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; De Koker, C.; Dziubak, R.; Skrapac, A.-K.; Godwin, H.; Reeve, K.; Chebar-Lozinsky, A.; Shah, N. A Practical Approach to Vitamin and Mineral Supplementation in Food Allergic Children. Clin. Transl. Allergy 2015, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Panjari, M.; Koplin, J.J.; Ponsonby, A.-L.; Vuillermin, P.; Gurrin, L.C.; Greaves, R.; Carvalho, N.; Dalziel, K.; Tang, M.L.K.; et al. VITALITY Trial: Protocol for a Randomised Controlled Trial to Establish the Role of Postnatal Vitamin D Supplementation in Infant Immune Health. BMJ Open 2015, 5, e009377. [Google Scholar] [CrossRef]
- Wegienka, G.; Havstad, S.; Zoratti, E.M.; Kim, H.; Ownby, D.R.; Johnson, C.C. Association between Vitamin D Levels and Allergy-Related Outcomes Vary by Race and Other Factors. J. Allergy Clin. Immunol. 2015, 136, 1309–1314.e4. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef]
- Vollbracht, C.; Raithel, M.; Krick, B.; Kraft, K.; Hagel, A.F. Intravenous Vitamin C in the Treatment of Allergies: An Interim Subgroup Analysis of a Long-Term Observational Study. J. Int. Med. Res. 2018, 46, 3640–3655. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Forno, E.; Holguin, F.; Celedón, J.C. Diet and Asthma: An Update. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Han, M.; Qiao, S.; He, P.; Li, D.; Li, N.; Ma, X. Soybean Antigen Proteins and Their Intestinal Sensitization Activities. Curr. Protein Pept. Sci. 2015, 16, 613–621. [Google Scholar] [CrossRef]
- West, C.E.; Dunstan, J.; McCarthy, S.; Metcalfe, J.; D’Vaz, N.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; Prescott, S.L. Associations between Maternal Antioxidant Intakes in Pregnancy and Infant Allergic Outcomes. Nutrients 2012, 4, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Belhadjali, H.; Giordano-Labadie, F.; Bazex, J. Contact Dermatitis from Vitamin C in a Cosmetic Anti-Aging Cream. Contact Dermat. 2001, 45, 317. [Google Scholar] [CrossRef]
- Love, M.; Imran, M.; Irum, A.; Fraga, G.; Gierer, S. Oral Vitamin C (Ascorbic Acid) Allergy and Avoidance Leading to Severe Hypovitaminosis C. Kans. J. Med. 2016, 9, 69–70. [Google Scholar] [CrossRef]
- Li, Q.; Tang, X.; Huang, L.; Wang, T.; Huang, Y.; Jiang, S. Anti-Allergic Effect of Vitamin C through Inhibiting Degranulation and Regulating T1/T2 Cell Polarization. J. Sci. Food Agric. 2024, 104, 5955–5963. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, D.; Dong, B.; Qiao, S.; Ma, X.; Chen, X. Vitamin C: An Immunomodulator That Attenuates Anaphylactic Reactions to Soybean Glycinin Hypersensitivity in a Swine Model. Food Chem. 2009, 113, 914–918. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chen, C.-S.; Lin, J.-Y. High Dose Vitamin C Supplementation Increases the Th1/Th2 Cytokine Secretion Ratio but Decreases Eosinophilic Infiltration in Bronchoalveolar Lavage Fluid of Ovalbumin-Sensitized and Challenged Mice. J. Agric. Food Chem. 2009, 57, 10471–10476. [Google Scholar] [CrossRef]
- Kodama, M.; Kodama, T. Vitamin C and the Genesis of Autoimmune Disease and Allergy (Review). In Vivo 1995, 9, 231–238. [Google Scholar]
- Munjal, M.; Singh, A.; Khurana, A.S.; Bajwa, N.; Munjal, S.; Dhawan, N.; Waraich, G. Study of Vitamin C Therapy in Allergic Rhinitis. Int. J. Otorhinolaryngol. Head Neck Surg. 2020, 6, 1951–1955. [Google Scholar] [CrossRef]
- Shams, M.-H.; Jafari, R.; Eskandari, N.; Masjedi, M.; Kheirandish, F.; Ganjalikhani Hakemi, M.; Ghasemi, R.; Varzi, A.-M.; Sohrabi, S.-M.; Baharvand, P.A.; et al. Anti-Allergic Effects of Vitamin E in Allergic Diseases: An Updated Review. Int. Immunopharmacol. 2021, 90, 107196. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, B.B.M.; Jáuregui-Renaud, K.; Arias, A.d.C.B.; Ayala, J.C.; Martínez, M.D.M.; Navarrete, R.C.; Rosalia, I.S.V.; Salazar, M.d.R.C.; Serrano, H.A.C.; Mondragón, A.O.; et al. Vitamin E Effects on Nasal Symptoms and Serum Specific IgE Levels in Patients with Perennial Allergic Rhinitis. Ann. Allergy Asthma Immunol. 2006, 96, 45–50. [Google Scholar] [CrossRef]
- Fogarty, A.; Lewis, S.; Weiss, S.; Britton, J. Dietary Vitamin E, IgE Concentrations, and Atopy. Lancet 2000, 356, 1573–1574. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, S.P.; Kostić, A.Ž.; Pešić, M.B. Nutritional Behavior of Students during COVID-19 Quarantine. Hrana Ishr. 2020, 61, 36–43. [Google Scholar] [CrossRef]
- Alves, M.L.; Bento-Silva, A.; Carbas, B.; Gaspar, D.; Paulo, M.; Brites, C.; Mendes-Moreira, P.; Brites, C.M.; do Rosario Bronze, M.; Malosetti, M.; et al. Alleles to Enhance Antioxidant Content in Maize—A Genome-Wide Association Approach. J. Agric. Food Chem. 2020, 68, 4051–4061. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. The Comparison of Properties of the Oil and Kernels of Various Hazelnuts from Germany and Turkey. Eur. J. Lipid Sci. Technol. 2012, 114, 801–806. [Google Scholar] [CrossRef]
- Raila, J.; Rohn, S.; Schweigert, F.J.; Abraham, G. Increased Antioxidant Capacity in the Plasma of Dogs after a Single Oral Dosage of Tocotrienols. Br. J. Nutr. 2011, 106, S116–S119. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, Y.; Yuan, Y. Causal Role of Vitamin E in Atopic Dermatitis Risk: A Mendelian Randomization Study. Food Sci. Nutr. 2024, 12, 4981–4988. [Google Scholar] [CrossRef]
- Su, J.; Li, T.; Pan, H. Association of Vitamin A Supplementation with Immune-Related Allergic Diseases: A Meta-Analysis. Front. Nutr. 2022, 9, 984161. [Google Scholar] [CrossRef]
- Chen, Z.; Xing, Y.; Yu, X.; Dou, Y.; Ma, D. Effect of Folic Acid Intake on Infant and Child Allergic Diseases: Systematic Review and Meta-Analysis. Front. Pediatr. 2021, 8, 615406. [Google Scholar] [CrossRef]
- Moreno-Macias, H.; Romieu, I. Effects of Antioxidant Supplements and Nutrients on Patients with Asthma and Allergies. J. Allergy Clin. Immunol. 2014, 133, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and Related Compounds as Anti-Allergic Substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef]
- Dębińska, A.; Sozańska, B. Dietary Polyphenols—Natural Bioactive Compounds with Potential for Preventing and Treating Some Allergic Conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, B.-K.; Lee, Y.-C. Antiasthmatic Effects of Hesperidin, a Potential Th2 Cytokine Antagonist, in a Mouse Model of Allergic Asthma. Mediat. Inflamm. 2011, 2011, 485402. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of mTOR Pathway Signaling Activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Siddiqui, M.A.; Gupta, A. Recent Advancement and Novel Application of Natural Polyphenols for the Treatment of Allergy Asthma: From Phytochemistry to Biological Implications. Crit. Rev. Immunol. 2023, 43, 29–41. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Neta, M.T.S.L.; Sathiyabama, R.G.; Quintans, J.d.S.S.; de Oliveira e Silva, A.M.; de Araújo, A.A.S.; Narain, N.; Júnior, L.J.Q.; Gurgel, R.Q. Flavonoids as Th1/Th2 Cytokines Immunomodulators: A Systematic Review of Studies on Animal Models. Phytomedicine 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Oja, A.E. The Inhibitory Effects of Flavonoids on Chemokine Function in Allergic Asthma and Food Allergy. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2014. [Google Scholar]
- Tanaka, T. Flavonoids for Allergic Diseases: Present Evidence and Future Perspective. Curr. Pharm. Des. 2014, 20, 879–885. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary Polyphenols in the Prevention and Treatment of Allergic Diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Shu, J.; Cui, X.; Liu, X.; Yu, W.; Zhang, W.; Huo, X.; Lu, C. Licochalcone A Inhibits IgE-Mediated Allergic Reaction through PLC/ERK/STAT3 Pathway. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221135462. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Allergic Inflammation: Effect of Propolis and Its Flavonoids. Molecules 2022, 27, 6694. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, R. Flavonoids and Asthma. Nutrients 2013, 5, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Solekha, R.; Purnobasuki, H.; Puspaningsih, N.N.T.; Setiyowati, P.A.I. Secondary Metabolites and Antioxidants Activity from Citronella Grass Extract (Cymbopogon nardus L.). Indian. J. Pharm. Educ. Res. 2024, 58, 298–305. [Google Scholar] [CrossRef]
- Feng, X.; Yan, Z.; Ren, X.; Jia, Y.; Sun, J.; Guo, J.; Gao, Z.; Li, H.; Long, F. Sea Buckthorn Flavonoid Extracted with High Hydrostatic Pressure Alleviated Shrimp Allergy in Mice through the Microbiota and Metabolism. J. Agric. Food Chem. 2024, 72, 25094–25102. [Google Scholar] [CrossRef]
- Prince, B.T.; Mandel, M.J.; Nadeau, K.; Singh, A.M. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr. Clin. N. Am. 2015, 62, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, C.; Zhang, K.; Xue, W. The Role of Gut Microbiota and Its Metabolites Short-Chain Fatty Acids in Food Allergy. Food Sci. Hum. Wellness 2023, 12, 702–710. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Januszkiewicz, E.; Mierzejewski, M.; Biniszewska, O.; Szczygieł, M.; Sepczuk, E.; Kleniewska, P.; Pawliczak, R. The Importance of the Gut Microbiome in the Development of Allergic Diseases. Alergol. Pol. Pol. J. Allergol. 2023, 10, 202–209. [Google Scholar] [CrossRef]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential Effects of Flavonoids on Barrier Integrity in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, F.J.; Massot-Cladera, M.; Rodríguez-Lagunas, M.J.; Castell, M. Flavonoids Affect Host-Microbiota Crosstalk through TLR Modulation. Antioxidants 2014, 3, 649–670. [Google Scholar] [CrossRef]
- Shaw, O.M.; Nyanhanda, T.; McGhie, T.K.; Harper, J.L.; Hurst, R.D. Blackcurrant Anthocyanins Modulate CCL11 Secretion and Suppress Allergic Airway Inflammation. Mol. Nutr. Food Res. 2017, 61, 1600868. [Google Scholar] [CrossRef]
- Sim, L.Y.; Abd Rani, N.Z.; Husain, K. Lamiaceae: An Insight on Their Anti-Allergic Potential and Its Mechanisms of Action. Front. Pharmacol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Lin, Y.-R.; Liao, M.-H.; Jan, T.-R. Oral Supplementation with Areca-Derived Polyphenols Attenuates Food Allergic Responses in Ovalbumin-Sensitized Mice. BMC Complement. Altern. Med. 2013, 13, 154. [Google Scholar] [CrossRef]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.; Yoshikawa, T. Extract of Perilla Frutescens Enriched for Rosmarinic Acid, a Polyphenolic Phytochemical, Inhibits Seasonal Allergic Rhinoconjunctivitis in Humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A.; Aatif, M.; Muteeb, G.; Khan, K.; Siddiqui, F.A. Dietary Polyphenols, Plant Metabolites, and Allergic Disorders: A Comprehensive Review. Pharmaceuticals 2024, 17, 670. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Cheng, J.; Fu, G.; Guo, M. A Review on Polyphenols and Their Potential Application to Reduce Food Allergenicity. Crit. Rev. Food Sci. Nutr. 2023, 63, 10014–10031. [Google Scholar] [CrossRef]
- Pérot, M.; Lupi, R.; Guyot, S.; Delayre-Orthez, C.; Gadonna-Widehem, P.; Thébaudin, J.-Y.; Bodinier, M.; Larré, C. Polyphenol Interactions Mitigate the Immunogenicity and Allergenicity of Gliadins. J. Agric. Food Chem. 2017, 65, 6442–6451. [Google Scholar] [CrossRef]
- Takemoto, K.; Ganlin, T.; Iji, M.; Narukawa, T.; Koyama, T.; Hao, L.; Watanabe, H. Vegetable Extracts as Therapeutic Agents: A Comprehensive Exploration of Anti-Allergic Effects. Nutrients 2024, 16, 693. [Google Scholar] [CrossRef] [PubMed]
- Sadler, R.A.; Mallard, B.A.; Shandilya, U.K.; Hachemi, M.A.; Karrow, N.A. The Immunomodulatory Effects of Selenium: A Journey from the Environment to the Human Immune System. Nutrients 2024, 16, 3324. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, S.; Li, T.; Huang, X.; Dong, Y.; Wang, P.; Huang, J. Advances in the Study of the Mechanism by Which Selenium and Selenoproteins Boost Immunity to Prevent Food Allergies. Nutrients 2022, 14, 3133. [Google Scholar] [CrossRef]
- Zhao, X.; Thijssen, S.; Chen, H.; Garssen, J.; Knippels, L.M.J.; Hogenkamp, A. Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow’s Milk Allergy. Nutrients 2021, 13, 2479. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zeng, D.; Yang, M.; Tang, Z.; He, L.; Chen, T. Translational Selenium Nanoparticles to Attenuate Allergic Dermatitis through Nrf2-Keap1-Driven Activation of Selenoproteins. ACS Nano 2023, 17, 14053–14068. [Google Scholar] [CrossRef]
- Jiang, J.; Mehrabi Nasab, E.; Athari, S.M.; Athari, S.S. Effects of Vitamin E and Selenium on Allergic Rhinitis and Asthma Pathophysiology. Respir. Physiol. Neurobiol. 2021, 286, 103614. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Zayed, A.; Ezzat, S.M. 9—Important Antihistaminic Plants and Their Potential Role in Health. In Phytochemistry, the Military and Health; Mtewa, A.G., Egbuna, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–191. ISBN 978-0-12-821556-2. [Google Scholar]
- Abd Rani, N.Z.; Lam, K.W.; Jalil, J.; Mohamad, H.F.; Mat Ali, M.S.; Husain, K. Mechanistic Studies of the Antiallergic Activity of Phyllanthus Amarus Schum. & Thonn. and Its Compounds. Molecules 2021, 26, 695. [Google Scholar] [CrossRef]
- Alagar Yadav, S.; Ramalingam, S.; Jabamalai Raj, A.; Subban, R. Antihistamine from Tragia Involucrata L. Leaves. J. Complement. Integr. Med. 2015, 12, 217–226. [Google Scholar] [CrossRef]
- Rahim, N.A.; Jantan, I.; Said, M.M.; Jalil, J.; Abd Razak, A.F.; Husain, K. Anti-Allergic Rhinitis Effects of Medicinal Plants and Their Bioactive Metabolites via Suppression of the Immune System: A Mechanistic Review. Front. Pharmacol. 2021, 12, 660083. [Google Scholar] [CrossRef]
- Da Silva, E.S.; da Silva, J.F.; de Lins, S.R.O. Plantas com atividade anti-histamínica/Plants with anti-histaminic activity. Braz. J. Dev. 2020, 6, 95873–95884. [Google Scholar] [CrossRef]
- Sharma, A.; Srivastava, P.; Mavi, G.S.; Kaur, S.; Kaur, J.; Bala, R.; Singh, T.P.; Sohu, V.S.; Chhuneja, P.; Bains, N.S.; et al. Resurrection of Wheat Cultivar PBW343 Using Marker-Assisted Gene Pyramiding for Rust Resistance. Front. Plant Sci. 2021, 12, 570408. [Google Scholar] [CrossRef] [PubMed]
- Shamsa, F.; Ahmadiani, A.; Khosrokhavar, R. Antihistaminic and Anticholinergic Activity of Barberry Fruit (Berberis Vulgaris) in the Guinea-Pig Ileum. J. Ethnopharmacol. 1999, 64, 161–166. [Google Scholar] [CrossRef]
- Do Espirito Santo, B.L.S.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.O.; Bogo, D.; de Freitas, K.C.; de Guimarães, R.C.A.; Hiane, P.A.; Pott, A.; de Filiú, W.F.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.M.; Lee, D.K.C.; Lipworth, B.J. The Effects of Butterbur on the Histamine and Allergen Cutaneous Response. Ann. Allergy Asthma Immunol. 2004, 92, 250–254. [Google Scholar] [CrossRef]
- Makino, T.; Furuta, Y.; Fujii, H.; Nakagawa, T.; Wakushima, H.; Saito, K.; Kano, Y. Effect of Oral Treatment of Perilla Frutescens and Its Constituents on Type-I Allergy in Mice. Biol. Pharm. Bull. 2001, 24, 1206–1209. [Google Scholar] [CrossRef]
- Gupta, P.; Pathak, R.; Bisht, S.; Deep, P. Natural Allergens and Herbal Remedies Possessing Antiallergic Activity. J. Mt. Res. 2023, 18, 217–224. [Google Scholar] [CrossRef]
- Shaik, A.R.; Avula, P.R.; Mamillapalli, V. Antiasthmatic Activity of 2-Piperidone by Selective Animal Models. JRP 2020, 24, 334–340. [Google Scholar] [CrossRef]
- Bival Štefan, M. Astragalus Membranaceus, Nigella Sativa, and Perilla Frutescens as Immunomodulators—Molecular Mechanisms and Clinical Effectiveness in Allergic Diseases. Curr. Issues Mol. Biol. 2024, 46, 9016–9032. [Google Scholar] [CrossRef]
- Saini, R.; Dhiman, N.K. Natural Anti-Inflammatory and Anti-Allergy Agents: Herbs and Botanical Ingredients. Antiinflamm. Antiallergy Agents Med. Chem. 2022, 21, 90–114. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Wu, S.Y.; Silverberg, J.I.; Joks, R.; Durkin, H.G.; Smith-Norowitz, T.A. Green Tea (Camelia Sinensis) Mediated Suppression of IgE Production by Peripheral Blood Mononuclear Cells of Allergic Asthmatic Humans. Scand. J. Immunol. 2012, 76, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Bakhshaee, M.; Mohammad Pour, A.H.; Esmaeili, M.; Jabbari Azad, F.; Alipour Talesh, G.; Salehi, M.; Noorollahian Mohajer, M. Efficacy of Supportive Therapy of Allergic Rhinitis by Stinging Nettle (Urtica Dioica) Root Extract: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Iran. J. Pharm. Res. 2017, 16, 112–118. [Google Scholar]
- Ona, A.D.; Muntean, L.; Berindean, I.; Costin, A.D.; Racz, I.; Popa, M. Modern Insights into Breeding of Medicinal Plants for Health and Industry. Hop. Med. Plants 2024, 32, 119–138. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Y.; Wang, X.; Guo, W.; Bi, Y.; Zhang, C.; Wang, J.; Li, M. New Insights Explain That Organic Agriculture as Sustainable Agriculture Enhances the Sustainable Development of Medicinal Plants. Front. Plant Sci. 2022, 13, 959810. [Google Scholar] [CrossRef]
- Rao, M.R.; Palada, M.C.; Becker, B.N. Medicinal and Aromatic Plants in Agroforestry Systems. Agrofor. Syst. 2004, 61, 107–122. [Google Scholar] [CrossRef]
- Atherton, H.R.; Li, P. Hydroponic Cultivation of Medicinal Plants—Plant Organs and Hydroponic Systems: Techniques and Trends. Horticulturae 2023, 9, 349. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; El-Beltagi, H.S.; Umar, S.; Lee, J. Bioprospecting Plant Growth Promoting Rhizobacteria for Enhancing the Biological Properties and Phytochemical Composition of Medicinally Important Crops. Molecules 2022, 27, 1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and Challenges in Medicinal Plant Breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef]
- Bennur, P.L.; O’Brien, M.; Fernando, S.C.; Doblin, M.S. Improving Transformation and Regeneration Efficiency in Medicinal Plants: Insights from Other Recalcitrant Species. J. Exp. Bot. 2025, 76, 52–75. [Google Scholar] [CrossRef]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing Medicinal Plants into Cultivation: Opportunities and Challenges for Biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Assou, J.; Zhang, D.; Roth, K.D.R.; Steinke, S.; Hust, M.; Reinard, T.; Winkelmann, T.; Boch, J. Removing the Major Allergen Bra j I from Brown Mustard (Brassica Juncea) by CRISPR/Cas9. Plant J. 2022, 109, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Camerlengo, F.; Frittelli, A.; Sparks, C.; Doherty, A.; Martignago, D.; Larré, C.; Lupi, R.; Sestili, F.; Masci, S. CRISPR-Cas9 Multiplex Editing of the α-Amylase/Trypsin Inhibitor Genes to Reduce Allergen Proteins in Durum Wheat. Front. Sustain. Food Syst. 2020, 4, 104. [Google Scholar] [CrossRef]
- Diretto, G.; Frusciante, S.; Fabbri, C.; Schauer, N.; Busta, L.; Wang, Z.; Matas, A.J.; Fiore, A.; Rose, J.K.C.; Fernie, A.R.; et al. Manipulation of Β-carotene Levels in Tomato Fruits Results in Increased ABA Content and Extended Shelf Life. Plant Biotechnol. J. 2019, 18, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheng, S.; Liu, X.-Q.; Kuča, K.; Hashem, A.; Al-Arjani, A.-B.F.; Almutairi, K.F.; Abd_Allah, E.F.; Wu, Q.-S.; Zou, Y.-N. Cloning of a CHS Gene of Poncirus Trifoliata and Its Expression in Response to Soil Water Deficit and Arbuscular Mycorrhizal Fungi. Front. Plant Sci. 2022, 13, 1101212. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhao, M.; Yang, Z.; Zhou, Z.; Guo, Y.; Lin, Y.; Chen, H. OsMYB3 Is a R2R3-MYB Gene Responsible for Anthocyanin Biosynthesis in Black Rice. Mol. Breed. 2021, 41, 51. [Google Scholar] [CrossRef]
- Shi, M.; Du, Z.; Hua, Q.; Kai, G. CRISPR/Cas9-Mediated Targeted Mutagenesis of bZIP2 in Salvia miltiorrhiza Leads to Promoted Phenolic Acid Biosynthesis. Ind. Crops Prod. 2021, 167, 113560. [Google Scholar] [CrossRef]
- Lagoumintzis, G.; Afratis, N.A.; Patrinos, G.P. Editorial: Nutrigenomics and personalized nutrition: Advancing basic, clinical, and translational research. Front. Nutr. 2024, 11, 1435475. [Google Scholar] [CrossRef]
- Robinson, A. Nutrigenomics in Personalized Nutrition and Health Optimization. J. Nutraceuticals Food Sci. 2024, 9, 70. [Google Scholar]
- Koirala, P.; Bhandari, Y.; Nirmal, N. Liposome Encapsulation: Innovative Vitamin Delivery in Fortified Foods. In Fortified Foods; Sarkar, T., Ed.; Springer: New York, NY, USA, 2025; pp. 29–60. ISBN 978-1-07-164346-4. [Google Scholar]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and Microbiota: Is There a Link with Allergies? Int. J. Mol. Sci. 2021, 22, 4288. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Wróbel-Kwiatkowska, M.; Starzycki, M.; Szopa, J. Engineering Flax with Increased Flavonoid Content and Thus Fusarium Resistance. Physiol. Mol. Plant Pathol. 2007, 70, 38–48. [Google Scholar] [CrossRef]
- Zhu, C.; Sanahuja, G.; Yuan, D.; Farré, G.; Arjó, G.; Berman, J.; Zorrilla-López, U.; Banakar, R.; Bai, C.; Pérez-Massot, E.; et al. Biofortification of Plants with Altered Antioxidant Content and Composition: Genetic Engineering Strategies. Plant Biotechnol. J. 2013, 11, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lin, Y.-H.; Wu, J.-C.; Hu, S.; Yang, S.; Chen, J.-L.; Chen, Y.-C.; Lo, S.-S. Use of Traditional Chinese Medicine Reduces Exposure to Corticosteroid among Atopic Dermatitis Children: A 1-Year Follow-up Cohort Study. J. Ethnopharmacol. 2015, 159, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Solleti, S.K.; Singh, H.; Verma, S.; Sharma, N.; Nain, P.; Varshney, A. Herbal Decoction Divya-Swasari-Kwath Attenuates Airway Inflammation and Remodeling through Nrf-2 Mediated Antioxidant Lung Defence in Mouse Model of Allergic Asthma. Phytomedicine 2020, 78, 153295. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J. Toward Precision Medicine and Health: Opportunities and Challenges in Allergic Diseases. J. Allergy Clin. Immunol. 2016, 137, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.V.; Khazaei, H.; Morovati, M.R.; Joshi, T.; Aneva, I.Y.; Farzaei, M.H.; Echeverría, J. Phytochemicals as Treatment for Allergic Asthma: Therapeutic Effects and Mechanisms of Action. Phytomedicine 2024, 122, 155149. [Google Scholar] [CrossRef]
- Proper, S.P.; Azouz, N.P.; Mersha, T.B. Achieving Precision Medicine in Allergic Disease: Progress and Challenges. Front. Immunol. 2021, 12, 720746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).