Abstract
Allergic diseases, particularly respiratory allergies like asthma and allergic rhinitis, are a growing public health concern influenced by environmental factors such as climate change and air pollution. The exposome framework enables a comprehensive assessment of how lifelong environmental exposures shape immune responses and allergic sensitization. Peru’s diverse ecosystems and climates provide a unique setting to investigate regional variations in allergic sensitization. This study characterized these patterns in five Peruvian regions with distinct climatic, urbanization, and socioeconomic characteristics. A total of 268 individuals from Lima, Piura, Tarapoto, Arequipa, and Tacna were analysed for allergen-specific IgE responses using a multiplex IgE detection system. The results revealed significant geographical differences in sensitization frequencies and serodominance profiles, based on descriptive statistics and supported by Chi-square comparative analysis. House dust mites were predominant in humid regions, while Arequipa exhibited higher sensitization to cat allergens. In Tacna, olive pollen showed notable prevalence alongside house dust mites. Tarapoto’s high humidity correlated with increased fungal and cockroach allergen sensitization. Notably, some allergens traditionally considered minor, such as Der p 5 and Der p 21, reached sensitization prevalences close to or exceeding 50% in certain regions. These findings provide the most detailed molecular characterization of allergic sensitization in Peru to date, highlighting the importance of region-specific allergy management strategies. Understanding environmental influences on allergic diseases can support more effective diagnostic, therapeutic, and preventive approaches tailored to diverse geographical contexts.
1. Introduction
According to the World Health Organization (WHO) by 2025, approximately more than 50% of the world’s population will have had asthma and/or allergy at least once in their life [1]. Allergic diseases represent a growing public health concern particularly in middle and low-income countries such as Peru [2]. Respiratory sensitization and allergic asthma are defined as IgE-mediated immunological responses to aeroallergens (for example, dust mites, moulds, pollens, cockroaches, and epithelia) affecting the airways and show specific profiles according to geographical area and local environmental conditions [3].
In Peru, epidemiological research on respiratory allergies has primarily been conducted through two significant studies. The International Study of Asthma and Allergies in Childhood (ISAAC), focusing on rhinoconjunctivitis in Lima, reported a prevalence of 18.7% [4]. Building upon this knowledge, the Peru Urban versus Rural Asthma (PURA) study provided insights from different geographical settings, assessing 1441 individuals across both urban and rural areas, to evaluate asthma prevalence and its associations with environmental and socio-demographic factors. The PURA findings documented asthma prevalence rates of 12% in Lima and 3% in Tumbes, with severe persistent asthma affecting 5% and 14% of these cases, respectively [5].
These regional variations in disease prevalence can be better understood in the context of global environmental factors. Particularly, climate change and air pollution have emerged as significant modulators of allergic responses, especially in urban areas where pollutant exposure is higher [6]. Pollutants exacerbate oxidative stress, resulting in respiratory mucosal inflammation that facilitates sensitization to allergenic proteins [7]. In cities with high pollution levels, an increase in the prevalence of respiratory diseases such as asthma and allergic rhinitis has been observed [8].
Research highlights that climate change and air pollution accelerate plant growth, enhance pollen allergen potency, and extend the duration and intensity of flowering [9]. Moreover, they increase fungal spore production. Humid climates, in particular, foster conditions favourable for the proliferation of mites and fungi, heightening sensitization to these allergens [10].
The exposome—a comprehensive framework encompassing all environmental exposures an individual encounters throughout their life—is gaining traction in studying allergic sensitization. This concept integrates factors such as climate, air quality, and pollutants that influence immune responses [11]. Environmental conditions like temperature, humidity, and pollution levels can increase allergen exposure and modulate immune reactions [12,13,14]. Peru, with its diverse ecosystems and climates, presents unique patterns of allergic sensitization, varying across cities due to differences in local and autochthonous aeroallergens, and in relation to geography and altitude.
Understanding these environmental influences requires a structured approach to categorizing exposures. Within the exposome framework, environmental factors can be understood through three interconnected domains. The first domain encompasses the general external environment, including factors like climate, biodiversity, urbanization, and socioeconomic conditions. The second domain consists of the specific external environment, which includes direct exposures such as allergens, microbes, diet, tobacco smoke, pollutants, and toxic substances that directly impact health. The third domain involves the internal environment, comprising host-dependent physiological factors such as metabolism, inflammation, and oxidative stress, which influence individual responses to external exposures. These three domains collectively form the “meta-exposome,” reflecting the complex interplay between environmental exposures and human health [15].
Given this complex environmental framework, the aim of this study is to investigate Precision Allergy Molecular Diagnosis (PAMD@) and report regional patterns of allergic sensitization performed with a Multiplex test across five Peruvian regions with distinct climatic, urbanization, and socioeconomic characteristics.
2. Results
2.1. Demographic Features of Investigated Patients
All 268 subjects who met the ARIA or GINA criteria for allergic rhinitis (AR) and/or asthma (A) tested positive for one or more aeroallergens in a skin prick test (SPT). Most participants were female (64.45%) with a median age of 23 years (range: 3–84). None of the patients had previously or were currently receiving allergen immunotherapy or biologic treatment at the time of inclusion in the study (Table 1).

Table 1.
Descriptive statistics regarding basal comorbid conditions and associated clinical characteristics of the studied population (n = 268).
2.2. Prevalence, sIgE Reactivity and Individual Molecular Profile According to Atopic Disease and Geographical Location
The sensitization to aeroallergen extracts through SPT and the prevalence of the 268 patients who met the inclusion criteria are summarized in Table 2. Figure 1 represents Venn diagrams to grouped local aeroallergens by Skin Prick Test (n = 268)

Table 2.
Prevalence of sensitization to grouped local aeroallergens by Skin Prick Test (n = 268).
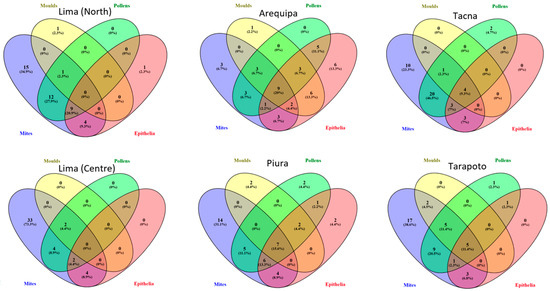
Figure 1.
Venn diagrams of grouped local aeroallergens by Skin Prick Test (n = 268).The study included a total of 268 patients, comprising 45 individuals from each city and 43 from Tacna. Figure 2 presents the profiles of the 20 most recognized molecular allergens in the six locations, highlighting the serodominance of each allergen.
Focusing on sIgE levels, overall, 211 out of 268 patients (78.73%) were sIgE-positive (≥0.35 kUA/L) to one or more of the 52 individual molecular aeroallergens included in the multiplex array (Figure 2). Despite having a positive SPT to at least one of the investigated aeroallergens, 57 patients (21.27%)—41 with AR (9 from Lima (Centre), 4 from Lima (North), 5 from Arequipa, 5 from Piura, 7 from Tacna and 11 from Tarapoto) and 16 with AA (6 from Lima (Centre), 2 from Arequipa, 2 from Piura, 4 from Tacna and 2 from Tarapoto)—did not exhibit any sIgE levels > 0.35 kUA/L to any of the tested allergens in the multiplex array.
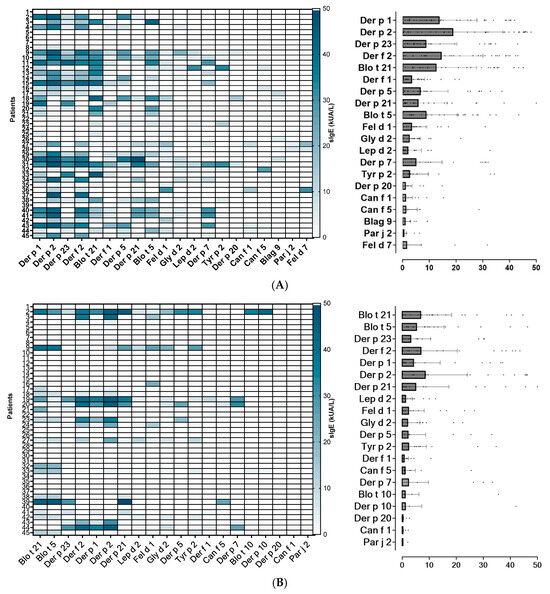
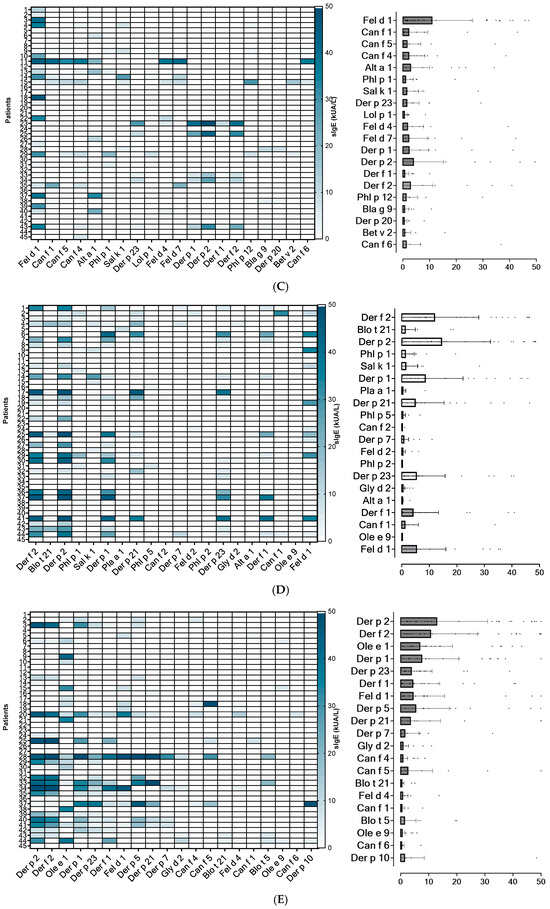
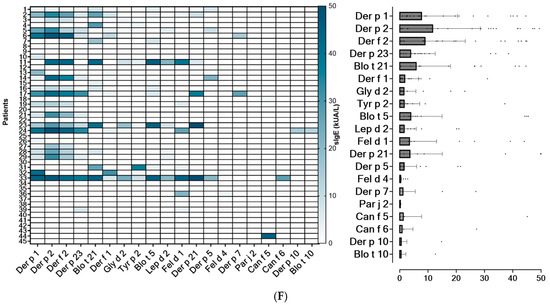
Figure 2.
Heatmap of the 20 most frequently identified molecular allergens in each region, along with their serodominance. (A): Lima North; (B): Lima Centre; (C): Arequipa. (D): Piura; (E): Tacna; (F): Tarapoto.
Based on sIgE profiles, mites were identified as the most prevalent source of sensitizing airborne allergens in the Lima (North and Centre), Piura and Tarapoto populations, regardless of the subjects’ underlying atopic condition. In Tacna, the main sensitizing allergens were olive pollen together with mites, followed by cat dander. Interestingly, Arequipa was the only city where mites were not the serodominant allergens. In this location, cat dander is the most prevalent sensitizing source, followed by moulds, pollens and mites (Figure 2).
Regarding symptomatology, allergic rhinitis (AR) was the predominant symptom across all age groups, except in the infant population (0–15 years old), where asthma was more prevalent in most locations (Table 3).

Table 3.
Number of patients with exclusive AR symptoms or AA (with or without AR) symptoms, by age and geographical location.
2.3. Mites
Sensitization to one or more of the 17 investigated mite molecular allergens was observed in 158 (58.95%) subjects. The remainder 108 patients—68 with AR (7 from Lima (North), 11 from Lima (Centre), 17 from Arequipa, 8 from Piura, 12 from Tacna, 13 from Tarapoto) and 40 with AA (7 from Lima (Centre), 18 from Arequipa, 4 from Piura, 8 from Tacna, 3 from Tarapoto)—were not sensitized to any of the mite allergens tested.
The results showed in each area Der p 1, Der p 2, Der p 5, Der p 7, Der p 21, Der p 23, Der f 1, Der f 2, Blo t 5, Blo t 21, Gly d 2, Lep d 2, and Tyr p 2 as major (>50%) and/or mid-tier allergens (20–50% prevalence) (Table 4 and Table 5). Certain allergens, such as Der p 5 or Der p 21, which are often classified as minor or mid-tier allergens, showed a prevalence of 48.9% across the entire study population in Lima (North) (Table 4). When focusing exclusively on the mite-sensitized population, this prevalence increased to 57.9% (Table 5). Similarly, the prevalence of sensitization to Der p 5 across the mite-sensitized population was 48.4% in Piura and 43.5% in Tarapoto. Minor allergens, with a prevalence below 20%, were Der p 10, Der p 11, Der p 20, and Blo t 10, except for Arequipa, where Der p 20 had a prevalence of 40% and Blo t 10 a 20%.

Table 4.
Frequency (%) of sensitization to individual mite allergens in the general allergic population studied, in each area.

Table 5.
Frequency (%) of sensitization to individual mite allergens in allergic patients showing IgE to one or more mite allergens. in each area.
The overall proportion of subjects with sIgE to group 1 allergens—Der p 1 and/or Der f 1—was 41.04%, which was similar to group 2 allergens—Der p 2 and/or Der f 2—at 43.28%, and to Der p 23 at 38.1%. In this context, group 1 and group 2 refer to major mite allergen categories commonly used in molecular allergy diagnostics, based on their structural and functional characteristics. Among the individual molecular allergens, Der p 2 (42.91%) was the most frequently identified with sIgE ≥ 0.35 kUA/L, closely followed by Der f 2 (41.79%), Der p 1 (41.04%), Der p 23 (38.06%), and Der f 1 (28.73%). Among the 110 subjects sensitized to group 1 allergens, 77 (28.73%) were positive for both Der f 1 and Der p 1, and 33 subjects were exclusively sensitized to Der p 1. Among the subjects sensitized to group 2 allergens (n = 116), 111 (41.42%) had sIgE to both Der p 2 and Der f 2 (three subjects were exclusively sensitized to Der p 2 and one subject to Der f 2).
In North Lima, 53.33% of patients recognized five or more HDM allergens, while in Lima Centre, 22.22% of patients recognized between five and nine HDM allergens. In the rest of the cities, the percentage of patients sensitized to five or more HDM allergens was 11.11% in Arequipa, 40.00% in Piura, 32.56% in Tacna and 20.00% in Tarapoto.
Sensitization to a single mite allergen was rare (4.47%), with three patients monosensitized to Der p 1 (1.12%), two to Der p 2 (0.74%), two to Der p 20 (0.74%), two to Der p 23 (0.74%), one to Blo t 10 (0.37%), one to Blo t 21 (0.37%) and one to Lep d 2 (0.37%).
None of the 268 patients showed sIgE to Der p 11.
2.3.1. Mite sIgE Reactivity Profiles and Basal Respiratory Allergic Diseases by Geographic Location
Patients with AR and AA displayed the same mite sIgE profile, showing sensitization to different combinations of 16 out of the 17 molecules analysed—Der p 1, Der p 2, Der p 5, Der p 7, Der p 10, Der p 20, Der p 21, Der p 23, Der f 1, Der f 2, Blo t 5, Blo t 10, Blo t 21, Gly d 2, Lep d 2, and Tyr p 2 (Table 6).

Table 6.
sIgE mean, median and frequency of sensitization for each mite allergen in the AR and AA Peruvian population. (Darker colors indicate higher values; lighter colors indicate lower values).
Table 6 shows the frequency of sensitization to each mite allergen and the mean and median sIgE levels in the Peruvian population studied. While the overall frequency of molecular sensitization was quantitatively higher in patients with AR than in AA (Table 6 and Table S1), the mean sIgE levels for each allergen was a mean of 1.98 ± 0.37 times higher in AA patients.
2.3.2. Age, sIgE Reactivity Profiles, and Symptoms
In the Peruvian population studied, the frequency of the sensitization pattern to the different mite allergens was similar in all age ranges. Nevertheless, frequency of sensitization to mite allergens was higher in younger patients (0–15 years old, n = 96, 95 AR, 52 AA), mainly to Der p 1, Der p 2 and Der p 23 (with frequencies between 15 and 20%) and, Der p 5, Der p 21, Der f 1, Der f 2 and Blo t 21 (with frequencies ranging from 10 to 15%), followed by adolescents and young adults (16–30 y.o., n = 62, 62 AR, 18 AA), with lower frequencies. Older adults (31–45 y.o., n = 58, 58 AR, 14 AA and 46–60 y.o., 38 AR, 11 AA) showed frequencies under 5% and patients over 60 y.o. (n = 14, 14 AR, 6 AA). The results showed that, as the age of the patients studied increases, the frequency of the sensitization decreases (Table 7).

Table 7.
sIgE Mean (kU/L), median (kU/L) and frequency of sensitization for each mite allergen by age. Values for median and frequency are highlighted in bold.
2.4. Cat and Dog Dander
In the studied population from Lima North, 21 out of 45 patients (46.7%) exhibited sensitization to at least one of the four investigated cat molecular allergens. Similarly, 13 out of 45 patients (28.9%) were sensitized to at least one of the six evaluated dog molecular allergens. Among these, nine patients demonstrated exclusive (among the epithelia allergens studied) sensitization to cat allergens, while four patients were exclusively sensitized to dog allergens. Notably, all patients sensitized to cat allergens exhibited reactivity to Fel d 1. In contrast, sensitization to the other three studied cat allergens was observed in fewer than 10% of patients. Regarding dog allergens, the primary sensitizing molecules were Can f 1 and Can f 5, each with a sensitization frequency of 15.6%. Additionally, two patients exhibited sensitization to Can f 4, whereas no sensitization was detected for Can f 2, Can f 3, or Can f 6.
In Lima Centre, 10 out of 45 patients (22.2%) were sensitized to one or more of the four investigated cat molecular allergens and 7 out of 45 patients (15.6%) were sensitized to at least one of the six evaluated dog molecular allergens. Eight patients demonstrated exclusive sensitization (among the epithelia allergens studied) to cat allergens, whereas five were exclusively sensitized to dog allergens. Similar to Lima North, all patients sensitized to cat allergens reacted to Fel d 1. However, only one patient showed sensitization to Fel d 4 and one to Fel d 7, while no patients were sensitized to Fel d 2. For dog allergens, the primary sensitizing molecules in Lima Centre were Can f 5 (13.3%) and Can f 1 (4.44%). Additionally, one patient exhibited sensitization to Can f 4, which acted as the major allergen in that case. No sensitization was detected for Canf 2, Can f 3 or Can f 6.
In Arequipa, 71.1% (32/45) of patients exhibited sensitization to at least one of the four investigated cat molecular allergens, while 40% (18/45) of patients were sensitized to at least one of the six evaluated dog molecular allergens. Among these, 17 patients (37.8%) demonstrated exclusive sensitization (among the epithelia allergens studied) to cat allergens, whereas three patients (6.7%) were exclusively sensitized (among the epithelia allergens studied) to dog allergens. All cat-sensitized patients exhibited reactivity to Fel d 1, confirming its role as the dominant cat allergen. Sensitization to Fel d 4 and Fel d 7 was observed in 13.3% of cases, while Fel d 2 sensitization was not detected in this cohort. Regarding dog allergens, the most frequently recognized molecules were Can f 1 (26.7%), Can f 5 (24.4%), and Can f 4 (22.2%). Sensitization to Can f 6 (8.89%) and Can f 2 (4.4%) was less frequent, while no patients exhibited reactivity to Can f 3.
In Piura, 35.6% (16/45) of patients exhibited sensitization to at least one of the four investigated cat molecular allergens, while 22.2% (10/45) of patients were sensitized to at least one of the six evaluated dog molecular allergens. Among these, seven patients (15.6%) demonstrated exclusive sensitization (among the epithelia allergens studied) to cat allergens, whereas one patient (2.2%) was exclusively sensitized (among the epithelia allergens studied) to dog allergens. All cat-sensitized patients reacted to Fel d 1. Sensitization to Fel d 7 and Fel d 2 was observed in 8.9% and 2.2% of cases, respectively, while no patients exhibited reactivity to Fel d 4. Regarding dog allergens, the most frequently recognized molecules were Can f 1 (13.3%), Can f 4 (6.7%), and Can f 5 (6.7%). Sensitization to Can f 2, Can f 3, and Can f 6 was detected in 2.2% of cases each.
In Tacna, 14 out of 43 patients (32.6%) exhibited sensitization to at least one of the four investigated cat molecular allergens, while 22.2% (10/45) of patients were sensitized to at least one of the six evaluated dog molecular allergens. Among these, seven patients (16.3%) demonstrated exclusive sensitization (among the epithelia allergens studied) to cat allergens, whereas three patients (6.7%) were exclusively sensitized (among the epithelia allergens studied) to dog allergens. All cat-sensitized patients reacted to Fel d 1 (30.2%), confirming its primary role. Sensitization to Fel d 4 was observed in 9.3% of cases, while Fel d 2 and Fel d 7 showed a lower prevalence (2.3% each). Regarding dog allergens, the most frequently recognized molecules were Can f 4 (11.6%), Can f 5 (11.6%), and Can f 1 (9.3%). Sensitization to Can f 6 was detected in 9.9% of cases, while Can f 2 and Can f 3 exhibited the lowest frequencies (2.3% each).
In Tarapoto, 8 out of 45 patients (17.8%) of patients exhibited sensitization to at least one of the four investigated cat molecular allergens, while 11.1% (5/45) of patients were sensitized to at least one of the six evaluated dog molecular allergens. Among these, two patients (4.4%) were exclusively sensitized to cat allergens, and two patients (4.4%) were exclusively sensitized to dog allergens. All cat-sensitized patients exhibited reactivity to Fel d 1 (17.8%), confirming its dominance. Additionally, three patients showed sensitization to Fel d 4, though with low sIgE levels. For dog allergens, the most frequently recognized molecules were Can f 5 (4.4%), Can f 6 (4.4%), and Can f 1 (2.2%).
Overall, 109 patients (40.7%) exhibited sensitization to at least one of the four studied cat allergens, while 71 patients (26.5%) were sensitized to at least one of the six dog allergens. Arequipa had the highest sensitization rates for both cat and dog allergens, and Tarapoto showed the lowest overall sensitization rates.
Among cat-allergic individuals, Fel d 1 was the predominant allergen across all cities, with 100% of sensitized patients reacting to it. Frequency of sensitization to Fel d 4 and Fel d 7 was observed in less than the 13.3% of cases, while Fel d 2 sensitization was rare, appearing in only 2.2% of patients in Piura and 2.3% in Tacna. Regional variations were notable, with the highest cat allergen sensitization rates observed in Arequipa (71.1%), followed by Lima North (46.7%). In contrast, Tarapoto exhibited the lowest rate (17.8%). Exclusive sensitization to cat allergens (without dog allergen sensitization) ranging from 8.9% in Piura to 37.8% in Arequipa.
Regarding dog allergens, the most frequently recognized allergens, with moderate regional variations, were Can f 1, Can f 5, and Can f 4, with sensitization frequencies reaching 26.7% (Can f 1) and 24.4% (Can f 5) in Arequipa. Sensitization to Can f 6 remained below 10% in all cities, while Can f 2 and Can f 3 were rarely detected (less than 4.4%). The highest prevalence of dog allergen sensitization was recorded in Arequipa (40%), followed by Lima North (28.9%). The lowest sensitization rate was observed in Tarapoto (11.1%). Exclusive sensitization to dog allergens ranged from 2.2% in Piura to 11.1% in Lima Centre.
2.5. Pollens
Overall, the frequency of pollen sensitization was low. In Lima, 8 out of 90 patients showed sIgE to pollens (Lima North: four to Paritaria judaica, two to Olea europaea, and two to Phleum pratense; Lima Centre: one to Par j 2), displaying low sIgE levels. In Arerquipa, 12 out 45 patients showed sensitization to a variety of pollens (P. pratense, Salsola kali, P. judaica, O. europaea, Betula verrucosa). Among the P. pratense allergens studied, Phl p 1 and Phl p 12 showed the highest prevalence of sensitization (15.6% and 11.1%, respectively), showing Phl p 12 higher levels of sIgE than Phl p 1 in one patient, or being the only Phleum-sensitizing allergen in the other patient. Pollen sensitization in Piura was anecdotic, with 1 out of 45 patients showing sIgE to Phl p 12, Ole e 7 and Bet v 2 and one patient sensitized to Par j 2. Similarly occurs in Tarapoto, with 1 out of 45 patients positive to Pla a 1 (1.73 kU/L).
The most relevant city regarding sensitization to pollens was Tacna, where olive pollen (O. europaea) showed a frequency of sensitization of 44.18%, with Ole e 1 as the main sensitizing allergen (41.9%, media sIgE levels 5.9 kU/L) with patients reaching sIgE levels of >30–40 kU/L. One out of the forty-three patients studied showed exclusive sensitization to Ole 7 (20.82 kU/L).
2.6. Cockroaches and Moulds
Sensitization to the mould Alternaria and the cockroach Blatella varied across the Peruvian population. The highest Alt a 1 frequency of sensitization was observed in Arequipa (20%), with a mean sIgE level of 2.68 kU/L (maximum: 34.24 kU/L). In other regions, Alt a 1 sensitization was detected in isolated cases, with levels ranging from 2.92 kU/L in Lima Centro to 1.31 kU/L in Tacna.
For cockroach, the highest percentage of sensitization was observed in Lima North (13.3%) and Arequipa (11.1%), and the highest sIgE levels were found in Piura (3/45 patients sensitized, maximum IgE level 39.76 kU/L). Isolated cases were reported in Tacna (2/43, 6.99 kU/L and 5.4 kU/L), Lima Centre (1/45, 0.47 kU/L), and Tarapoto (1/45, 0.37 kU/L).
3. Discussion
3.1. Molecular Characterization of Allergic Sensitization
The present study provides an extensive molecular characterization of allergic sensitization across five distinct regions in Peru, revealing significant geographical, climatic, and demographic variations in allergen-specific IgE responses. Our findings demonstrate notable differences in sensitization frequencies and serodominance profiles, with particular relevance to allergic rhinitis (AR) and asthma (AA). Furthermore, our comparative analysis with González-Pérez et al. (2025) [15], examining similar patterns in Lima and Tenerife (Spain), reinforces the importance of considering environmental influences, socioeconomic disparities, and urbanization levels in shaping allergic sensitization patterns, a phenomenon previously highlighted by Platts-Mills et al. [16] in epidemiological studies.
The specific environmental conditions of each region, including temperature, humidity, and atmospheric pollutant levels, may influence allergen exposure and modulate the immune response [17]. This variability is particularly reflected in the distribution of “indoor” and “outdoor” allergens, whose prevalence varies significantly according to geographical location [12,18]. The characterization of these patterns in the Peruvian context acquires special relevance given the country’s remarkable diversity of ecosystems, with native pollens and fungi not yet identified or studied, and altitudinal variations, which can directly influence the observed sensitization profiles.
These findings can be interpreted within the broader framework of the exposome, a concept described by Pawankar et al. [2], which considers the set of environmental exposures throughout an individual’s lifetime [12]. This perspective complements our understanding of how region-specific environmental factors and local aeroallergens may contribute to the different patterns of allergic sensitization observed in the Peruvian population.
3.2. Molecular Sensitization Profiles: Regional Characterization and Specific Patterns
Delving into the molecular characterization of these sensitization patterns, our analysis revealed specific allergen distributions that reflect the previously described geographic and environmental diversity.
House dust mites (HDMs) emerged as the predominant source of sensitizing aeroallergens in most cities, particularly in Lima, Piura, and Tarapoto, which aligns with global trends in humid and tropical environments [16]. Among these, Der p 1, Der p 2, and Der p 23 stood out as the most frequently recognized components, consistent with their well-established role as major allergens. Interestingly, certain allergens, such as Der p 5 or Der p 21, which are often categorized as minor or mid-tier allergens in other regions [19], exhibited prevalence rates exceeding 40% and 50% in some regions of our study. These rates align with thresholds typically associated with major allergens, suggesting that the allergenic significance of Der p 5 and Der p 21 may be region-specific. This underscores the importance of considering geographic and population-specific variations when defining the clinical relevance of allergens. Furthermore, this detailed characterization of molecular profiles provides a solid foundation for understanding the complex interaction between the regional environment and the population’s immune response. Similarly, Smejda et al. [20] have demonstrated that sensitization to specific molecular components of pet allergens, such as Fel d 1, Fel d 2, and Fel d 4 in cats, and Can f 1, Can f 2, and Can f 5 in dogs, is closely associated with distinct clinical symptoms in children, particularly respiratory and dermatological manifestations. These findings highlight the relevance of molecular characterization in identifying sensitization patterns to better predict the type and severity of allergic responses.
In contrast to these findings, Arequipa showed a predominance of sensitization to feline epithelium allergens (Fel d 1), even surpassing HDM allergens. This result could be explained by the city’s lower humidity levels, which hinder mite proliferation, and by a higher prevalence of indoor pet ownership [20]. In Tacna, while house dust mites remained the primary sensitizing agent, olive pollen (Olea europaea) emerged as the second most prevalent allergen; this latter finding is likely due to the region’s semi-arid climate and the presence of extensive olive groves, factors that favor high pollen concentrations [21,22]. Conversely, in Tarapoto, high humidity conditions correlated with a particular sensitization profile, characterized by an increased response to fungi, particularly Alternaria alternata, and cockroach allergens (Bla g 9), in contrast to regions with drier climates [23]. This high humidity also favors a greater abundance of mites in Tarapoto. Previous studies have evidenced the presence of species in the rain forest, other than Dermatophagoides, Blomia or Tyrophagus, such as Malayoglyphus intermedius and Tarsonemus sp., the latter being the predominant species in the area [24], as well as Mesoplophora quasigaveae and Arphthicarus simplex [25].
The characteristic environmental conditions of different regions in Peru help explain the observed sensitization patterns (Figure 3) [26]. In Tarapoto, a city located in the Peruvian rain forest and known for its high humidity and elevated temperatures, high sensitization rates to Der p 1 and Der p 2 (both above 40%) were found. These findings are consistent with previous studies by Arlian et al. [27], who established that relative humidity above 73% significantly favors dust mite proliferation, reaching densities of 500–1000 mites per gram of dust. This pattern is also reflected in Peruvian coastal cities, where characteristic environmental humidity is associated with high sensitization rates to Der p 1 and Der p 2. In contrast, the particular climatic conditions of Arequipa, an Andean city known for its low humidity and moderate to low temperatures, correlate with a distinctive sensitization profile, where Fel d 1 sensitization predominates over mite allergens. This pattern could be explained by the city’s characteristically low temperatures, which traditionally lead to a lifestyle with greater indoor permanence, thus increasing exposure to pet allergens, while environmental conditions are less favorable for mite development.
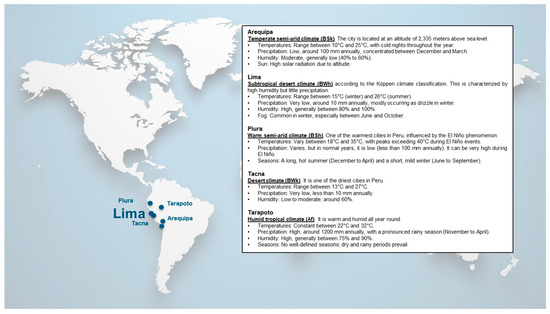
Figure 3.
Climatology, external contaminants, and socio-economic status among the six Peruvian cities studied.
These results, demonstrating marked variability in allergen sensitization prevalence and distribution, could be modulated by factors such as climate, altitude, and urbanization level, which aligns with research findings that have documented geographical variability in aeroallergen exposure. The detailed molecular characterization also allowed for the identification of significant differences in serodominance profiles, as 78.73% of patients were sIgE-positive to one or more of the 52 molecular allergens studied, a finding that Buters et al. [22] related to the complexity of allergic immune responses. Considering the significant heterogeneity of molecular profiles identified in the Peruvian population and their possible association with specific geoclimatic factors, it is methodologically pertinent to establish a comparative analysis between the Peruvian population from Lima and the Spanish population from Tenerife, as a tropical European reference, which will allow for contextualizing our findings within the broader framework of the literature on allergenic sensitization patterns across different latitudes.
3.3. Comparative Analysis with Reference Populations
To contextualize our findings within a broader framework, we conducted a comparative analysis with a study examining similar sensitization profiles. The analysis with González-Pérez et al. [15] reveals significant differences in molecular sensitization profiles between the populations of Tenerife, Spain, and Lima, Peru—two regions with similar climates but distinct socio-economic conditions.
In the Spanish population, 85% of participants showed reactivity to at least one mite allergen, presenting a broader spectrum of molecular recognition. This pattern contrasts with our Peruvian population, where more specific and focused sensitization was observed. Thomas et al. [28] have suggested that differences in sensitization profiles might be related to age-associated factors, such as early-life exposure to dust mites and cumulative exposure to allergens like pollens and pet dander in later stages. Furthermore, Spanish patients, particularly those with asthma, exhibited higher levels of specific IgE for a broader range of allergens, which could be linked to prolonged exposure to various environmental allergens.
In this context, Posa et al. [19] have reported that cumulative exposure to house dust mite allergens (Dermatophagoides pteronyssinus) is associated with greater IgE responses, particularly against components such as Der p 1, Der p 2, and Der p 23, as well as with a higher frequency of polysensitization to multiple mite molecules. Additionally, the study emphasizes that factors such as early exposure to these allergens and atopic predisposition (for example, parental history of hay fever) significantly contribute to the expansion of the sensitization profile, which could increase the risk of developing allergic rhinitis and asthma during childhood and adolescence.
3.4. Clinical and Therapeutic Implications
The molecular characterization of sensitization patterns identified in this study has fundamental implications for the diagnostic and therapeutic approach to allergic diseases. Research by Resch et al. [29] has been decisive in demonstrating how the precise identification of specific molecular components, such as Der p 18, optimizes diagnostic accuracy in patients allergic to house dust mites, particularly due to its capacity to induce significant IgE responses and basophil activation. In this context, studies by Konradsen et al. [30] on the sensitization to animal dander allergens, specifically Fel d 1 and Can f 5 have proven crucial for identifying patients with a greater predisposition to developing asthma and other allergic respiratory pathologies.
The differential geographical distribution of sensitization profiles has also revealed region-specific therapeutic considerations. For example, in dry climate zones like Tacna, where Ole e 1 sensitization predominates, there is evidence for the need to develop immunotherapy protocols focused on olive allergens for those patients who present seasonal symptoms associated with olive exposure.
Detailed molecular characterization can not only improve diagnostic accuracy but also allow for risk stratification regarding progression toward more severe respiratory conditions. This information is crucial for developing preventive interventions and for the appropriate selection of targeted biological therapies. Additionally, the simultaneous analysis of multiple allergenic components facilitates the implementation of public health strategies that consider both regional environmental particularities and potential cross-reactivities between aeroallergens and other environmental or food sensitizers.
3.5. Cross-Reactivity Between Aeroallergens and Food Allergens
The detailed molecular characterization described in previous sections suggests that certain components, such as Ole e 7, may be involved in cross-reactivity patterns with food allergens, an aspect with important clinical implications. For instance, Durham and Shamji [31] documented that sensitization to minor olive pollen allergens (e.g., Ole e 7) increases clinical severity and the risk of adverse reactions during immunotherapy in areas where olive pollen is prevalent, a scenario of interest in regions like Tacna. Given the high prevalence of sensitization to Olea europaea in this region, understanding its potential cross-reactivity with homologous plant-derived food allergens could be relevant in patient management. Other cases of cross-reactivity between aeroallergens and food allergens have also been described, particularly in pollen–food syndromes.
In this context, in the European population, several well-documented pollen–food syndromes illustrate how sensitization to airborne allergens can lead to allergic reactions to specific foods. Among them, sensitization to Art v 1 from mugwort pollen has been linked to allergic reactions to chamomile and celery, while sensitization to Amb a 1 from ragweed pollen may induce cross-reactivity with melon and banana [32]. The role of protein homology in cross-sensitization is also evident in other allergen families. For instance, tropomyosins, which are present in both indoor aeroallergens (dust mites and cockroaches) and shellfish, have demonstrated cross-sensitization potential between these sources [33]. Additional patterns of cross-reactivity have been documented in syndromes such as cat–pork or bird–egg, produced by serum albumins [32].
3.6. Study Limitations and Future Directions
After presenting the geographical variability of sensitization and potential cross-reactivity described in previous sections, it is important to note some limitations of this study. First, a direct assessment of climatic factors’ impact on the observed sensitization patterns was not performed, which prevents establishing more precise relationships between environmental variables and allergen distribution. Furthermore, discrepancies emerged between skin test results and molecular analysis, particularly in Arequipa, where notably different percentages of mite sensitization were observed depending on the methodology employed. The skin test is a valid tool to identify allergic sensitization to allergens that are not included in the multiplex PAMD@.
A significant methodological limitation of this study relates to the molecular diagnostic platform used. While Precision Allergy Molecular Diagnosis (PAMD@) enables the identification of individual IgE reactivity profiles [15], its effectiveness in specific regions depends on the incorporation of local aeroallergens to which the population is exposed in order to ensure accurate diagnoses and personalized treatments. However, the allergenic proteins from pollens and fungi included in the multiplex array were primarily developed based on European aerobiology, which may not fully represent the Peruvian and Latin American context. For instance, several prevalent aeroallergens in the Peruvian region, such as tropical grass pollens (Panicoideae, Chloridoideae) [34] and fungi like Fusarium spp., Nigrospora spp., Stemphylium spp., Dreschlera/Helmintosporium spp., Curvularia spp., reported in Lima aerobiology studies [35] are not examined in these allergy multiplex tests. This geographical and ecological mismatch in allergen panels highlights the need for more regionally specific molecular diagnostics that better reflect Peruvian and Latin American aerobiology.
In future research, it would be valuable to incorporate more detailed climatic classifications and consider historical environmental condition data to correlate them with molecular sensitization profiles. Furthermore, investigating cross-reactivity patterns between local aeroallergens and region-specific food allergens would be crucial for understanding the complete sensitization landscape in Peru and Latin America. Additionally, it would be advisable to integrate genetic and socioeconomic factors, as well as aspects related to lifestyle and urban infrastructure, with the aim of understanding the complex interaction underlying the development of allergic sensitization.
Moreover, conducting longitudinal studies would allow evaluation of how sensitization patterns evolve in response to changes in environmental and social conditions. The use of emerging technologies could also facilitate the identification of epidemiological trends and the development of robust predictive models, contributing to a more proactive approach in allergic sensitization surveillance.
4. Conclusions
This study provides a molecular characterization of allergic sensitization across different Peruvian regions to date. Through the integration of clinical, geographical, and molecular data, and with the limitations of the research, we identified regional patterns in IgE reactivity.
House Dust Mites (HDMs) were the most dominant allergens in most cities, particularly in Lima, Piura, and Tarapoto, which aligns with global trends in humid and tropical environments. The findings underscore the necessity of personalized allergy management strategies, tailored to local aeroallergen exposure and clinical phenotypes, to enhance diagnosis and treatment effectiveness.
5. Materials and Methods
5.1. Subjects
The study was conducted in 5 different Peruvian cities: Arequipa, Lima, Piura, Tacna and Tarapoto. Patients aged 0 to 85 years who attended outpatient allergy or respiratory clinics between January and May 2024 were screened for participation. Individuals presenting with symptoms compatible with allergic rhinitis (AR) and/or asthma (A), and for whom allergy testing was clinically indicated, were considered eligible. Recruitment followed a consecutive sampling strategy until reaching the predetermined target of approximately 45 patients per region, ensuring a balanced geographical representation. Informed consent was obtained from all participants prior to inclusion in the study, and for those under 18 years of age, consent was provided by their parents or legal guardians.
A total of 268 allergic patients were recruited according to predefined selection criteria. Eligibility required a confirmed diagnosis of allergy, determined through a positive skin prick test (SPT) to one or more relevant aeroallergens (pollen, mites, moulds and/or pet dander). To meet the inclusion criteria, patients were required to have experienced clinical symptoms for at least three years after establishing local residency. The severity and stage of allergic diseases were assessed through clinical evaluation following specific guidelines to ensure standardized and accurate classification [25,26,27]. Clinical data collected from patients’ medical records included clinical history and details about their medications. Patients who had undergone past or current allergen immunotherapy or treatment with monoclonal antibodies (biologics) were excluded, as well as pregnant and breastfeeding women. This investigation was reviewed and approved by the local Ethical Committee (code number 22333-23).
5.2. Skin Prick Test
Skin prick tests (SPT) were conducted following European guidelines [36], using standardized allergen extracts provided by Inmunotek (Madrid, Spain). The panel included extracts of house dust mites Dermatophagoides pteronyssinus (D. pteronyssinus), Dermatophagoides farinae (D. farinae), Lepidoglyphus destructor (L. destructor), Blomia tropicalis (B. tropicalis), mould extracts from Alternaria alternata (A. alternata), Cladosporium herbarum (C. herbarum), Penicillium notatum (P. notatum), Aspergillus fumigatus (A. fumigatus), cockroach extract from Periplaneta americana (P. americana), grass pollens extracts from Phleum pratense (P. pratense), Dactylis glomerata (D. glomerata), Lolium perenne (L. perenne), Olea europaea (O. europaea), Artemisia vulgaris (A. vulgaris), Ambrosia elatior (A. elatior), Cynodon dactylon (C. dactylon), and extracts from cat and dog dander. Histamine (10 mg/mL) and saline were used as positive and negative controls, respectively. Antihistamine use was discontinued at least one week before testing. Reactions were measured 20 min post-application, and a wheal size > 3 mm was considered positive.
5.3. Serological Analysis
All participants had blood samples collected, which were stored at −40 °C and processed using the ALEX MacroArray diagnostic system (MacroArray Diagnostics, Vienna, Austria). Total IgE was expressed in IU/mL and specific IgE (sIgE) in kUA/L, with a threshold of ≥0.35 kUA/L for positivity.
The array included 282 reagents, including 157 whole extracts and 125 molecular allergens. Among the allergens tested were 17 mite molecules (e.g., Der p 1, Der f 2, Blo t 5), 10 animal dander allergens (e.g., Fel d 1, Can f 5), 22 pollen allergens (e.g., Phl p 1, Ole e 1, Art v 1), 2 mould allergens (Alt a 1, Alt a 6), and 1 cockroach allergen (Bla g 9).
The assay included 17 mite molecular allergens: Der p 1, Der p 2, Der p 5, Der p 7, Der p 10, Der p 11, Der p 20, Der p 21, Der p 23, Der f 1, Der f 2, Blo t 5, Blo t 10, Blo t 21, Lep d 2, Gly d 2, and Tyr p 2. Additionally, 10 cat and dog epithelial allergens were tested: Fel d 1, Fel d 2, Fel d 4, Fel d 7, Can f 1, Can f 2, Can f 3, Can f 4, Can f 5, and Can f 6. A total of 22 pollen allergens were included: Bet v 1, Bet v 2, Bet v 6, Cup a 1, Pla a 1, Pla a 2, Pla a 3, Ole e 1, Ole e 7, Ole e 9, Phl p 1, Phl p 2, Phl p 5, Phl p 6, Phl p 7, Phl p 12, Lol p 1, Sal k 1, Pla l 1, Par j 2, Art v 1, and Art v 3. To evaluate mould sensitization, Alt a 1 and Alt a 6 were assessed, while Bla g 9 was tested for cockroach sensitization.
5.4. Statistical Analysis
Demographic characteristics were summarized using medians and standard deviations for continuous variables, and percentages for categorical variables. Comparative analyses of sensitization patterns between regions were performed using the Chi-square test to assess significant differences. All statistical analyses were performed using GraphPad Prism version 10.0.0 for Windows (GraphPad Software, La Jolla, CA, USA).
Generative AI (ChatGPT, developed by OpenAI) was used to assist with the following tasks:-Refinement of language and grammar in the manuscript to ensure clarity and adherence to British English conventions.- Assisting with summarisation, formatting, and improving the readability of the content while maintaining the integrity of the scientific findings and data.The authors confirm that all scientific content, data interpretation, and conclusions were independently determined and verified by the authors. Generative AI was utilised strictly as a tool to streamline the writing process and ensure professional presentation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/allergies5030023/s1, Figure S1: Heatmap illustrating the sensitization profile to the allergens in Perú, accompanied by their serodominance levels. A. Lima (North); B. Lima (Centre); C. Arequipa; D. 28 Piura; E. Tacna; F. Tarapoto. AR, allergic rhinitis; A, asthma Table S1: Number of identified mite molecular allergens, geographical location, and corresponding 3 basal atopic disease (allergic rhinitis (AR), and allergic asthma (AA)) in 268 patients studied with 4 microarray.; Table S2: Serological analysis—Mean (frequency) of specific IgE responses (kU/L) to mite molecular 21 allergens in patients with allergic rhinitis (AR, n = 135) and allergic asthma (AA, n = 75). Bold figures 22 indicate significant differences (p < 0.05) in mean sIgE levels to mite molecular allergens between the 23 two atopic conditions.
Author Contributions
Conceptualization, F.P. and R.G.-P.; Formal analysis, E.A.-F.; Investigation, O.M.C.-L., C.A.G. and M.J.M.; Visualization, E.A.-F.; Writing—original draft, O.M.C.-L., C.A.G. and E.A.-F.; Writing—review and editing, O.M.C.-L., C.A.G., R.G.-P., E.A.-F. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was reviewed and approved by the Ethics Committee of HONADOMANI San Bartolomé, under approval number 22333-23.
Informed Consent Statement
Written informed consent was obtained from all participants prior to the collection of serum samples and their inclusion in the study.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to confidentiality and privacy restrictions, but may be available from the corresponding author upon reasonable request and with appropriate approvals.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. María José Martínez, Eva Abel-Fernández and Fernando Pineda are employees of Inmunotek S. L., who provided the prick tests for patient inclusion in this study. Inmunotek also conducted the analyses using the microarray platform (ALEX). César Galván has given talks about ALEX 2 for Pharmedic International SAC, the distributor of ALEX 2 in Perú.
References
- Annesi-Maesano, I.; Maesano, C.N.; Biagioni, B.; D’Amato, G.; Cecchi, L. Call to action: Air pollution, asthma, and allergy in the exposome era. J. Allergy Clin. Immunol. 2021, 148, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacheco, S.E.; Guidos-Fogelbach, G.; Annesi-Maesano, I.; Pawankar, R.; d’Amato, G.; Latour-Staffeld, P.; Urrutia-Pereira, M.; Kesic, M.J.; Hernandez, M.L. Climate change and global issues in allergy and immunology. J. Allergy Clin. Immunol. 2021, 148, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Aït-Khaled, N.; Pearce, N.; Anderson, H.R.; Ellwood, P.; Montefort, S.; Shah, J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy 2009, 64, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.L.; Baumann, L.M.; Gilman, R.H.; Romero, K.; Combe, J.M.; Cabrera, L.; Hansel, N.N.; Barnes, K.; Gonzalvez, G.; Wise, R.A.; et al. The Peru Urban versus Rural Asthma (PURA) Study: Methods and baseline quality control data from a cross-sectional investigation into the prevalence, severity, genetics, immunology and environmental factors affecting asthma in adolescence in Peru. BMJ Open 2012, 2, e000421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Regan, A.C.; Nyhan, M.M. Towards sustainable and net-zero cities: A review of environmental modelling and monitoring tools for optimizing emissions reduction strategies for improved air quality in urban areas. Environ. Res. 2023, 231, 116242. [Google Scholar] [CrossRef] [PubMed]
- Skevaki, C.; Nadeau, K.C.; Rothenberg, M.E.; Alahmad, B.; Mmbaga, B.T.; Masenga, G.G.; Sampath, V.; Christiani, D.C.; Haahtela, T.; Renz, H. Impact of climate change on immune responses and barrier defense. J. Allergy Clin. Immunol. 2024, 153, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.M.; Tsai, F.J.; Lee, Y.L.; Chang, J.H.; Chang, L.T.; Chang, T.Y.; Chung, K.F.; Kuo, H.P.; Lee, K.Y.; Chuang, K.J.; et al. The impact of air pollution on respiratory diseases in an era of climate change: A review of the current evidence. Sci. Total Environ. 2023, 898, 166340. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Chong-Neto, H.J.; Monge Ortega, O.P.; Vitale, C.; Ansotegui, I.; Rosario, N.; Haahtela, T.; Galan, C.; Pawankar, R.; Murrieta-Aguttes, M.; et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy 2020, 75, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Zakzuk, J.; Caraballo, L. House Dust Mite Allergy Under Changing Environments. Allergy Asthma Immunol. Res. 2019, 11, 450–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gevaert, P.; Wong, K.; Millette, L.A.; Carr, T.F. The Role of IgE in Upper and Lower Airway Disease: More Than Just Allergy! Clin. Rev. Allergy Immunol. 2022, 62, 200–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amini, H.; Amini, M.; Wright, R.O. Climate Change, Exposome Change, and Allergy: A Review. Immunol. Allergy Clin. N. Am. 2024, 44, 1–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinmuth-Selzle, K.; Kampf, C.J.; Lucas, K.; Lang-Yona, N.; Fröhlich-Nowoisky, J.; Shiraiwa, M.; Lakey, P.S.J.; Lai, S.; Liu, F.; Kunert, A.T.; et al. Air Pollution and Climate Change Effects on Allergies in the Anthropocene: Abundance, Interaction, and Modification of Allergens and Adjuvants. Env. Sci. Technol. 2017, 51, 4119–4141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabrera, M.; Garzón García, B.; Moreno Grau, S.; Subiza, J. Association Between Seasonal Allergic Rhinitis and Air Pollution, Meteorological Factors, and Grass Pollen Counts in Madrid (1996 and 2009). J. Investig. Allergol. Clin. Immunol. 2019, 29, 371–377. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, R.; Galván-Calle, C.A.; Galán, T.; Poza-Guedes, P.; Sánchez-Machín, I.; Enrique-Calderón, O.M.; Pineda, F. Molecular Signatures of Aeroallergen Sensitization in Respiratory Allergy: A Comparative Study Across Climate-Matched Populations. Int. J. Mol. Sci. 2024, 26, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Platts-Mills, T.A.; Vervloet, D.; Thomas, W.R.; Aalberse, R.C.; Chapman, M.D. Indoor allergens and asthma: Report of the Third International Workshop. J. Allergy Clin. Immunol. 1997, 100 Pt 1, S2–S24. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.M.; Kaplan, A.N.; Järvinen, K.M. Environmental Exposures may Hold the Key; Impact of Air Pollution, Greenness, and Rural/Farm Lifestyle on Allergic Outcomes. Curr. Allergy Asthma Rep. 2023, 23, 77–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charpin, D.; Ramadour, M.; Lavaud, F.; Raherison, C.; Caillaud, D.; de Blay, F.; Pauli, G.; Annesi-Maesano, I. Climate and Allergic Sensitization to Airborne Allergens in the General Population: Data from the French Six Cities Study. Int. Arch. Allergy Immunol. 2017, 172, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Posa, D.; Perna, S.; Resch, Y.; Lupinek, C.; Panetta, V.; Hofmaier, S.; Rohrbach, A.; Hatzler, L.; Grabenhenrich, L.; Tsilochristou, O.; et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J. Allergy Clin. Immunol. 2017, 139, 541–549.e548. [Google Scholar] [CrossRef] [PubMed]
- Smejda, K.; Jerzynska, J.; Podlecka, D.; Brzozowska, A. Sensitization to cat and dog components and prediction of symptoms in cat-sensitized children. Asian Pac. J. Allergy Immunol. 2024, 42, 165–170. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; van Cauwenberge, P. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Buters, J.T.M.; Thibaudon, M.; Smith, M.; Kennedy, R.; Rantio-Lehtimäki, A.; Albertini, R.; Reese, G.; Weber, B.; Galan, C.; Brandao, R.; et al. Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos. Environ. 2012, 55, 496–505. [Google Scholar] [CrossRef]
- Cooper, P.J.; Rodrigues, L.C.; Cruz, A.A.; Barreto, M.L. Asthma in Latin America: A public heath challenge and research opportunity. Allergy 2009, 64, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, S.; Calderón, O.; Sastre, J.; Quirce, S.; Iraola, V. Exposure and Sensitization to Dust Mites in Peruvian Cities. J. Allergy Clin. Immunol. 2015, 135, AB190. [Google Scholar] [CrossRef]
- Niedbała, W.; Adamski, Z.; Laniecki, R.; Magowski, W.L. Ptyctimous Mites (Acari, Oribatida) of Peru with the Description of an Extraordinary New Phthiracaroid Mite from the Peruvian Andes. Animals 2023, 13, 2403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aybar, C.; Fernandez-Palomino, C.; Huerta, A.; Lavado, W.; Vega, F.; Felipe-Obando, O. Construction of a high-resolution gridded rainfall dataset for Peru from 1981 to the present day. Hydrol. Sci. J. 2019, 65, 770–785. [Google Scholar] [CrossRef]
- Arlian, L.G.; Morgan, M.S.; Neal, J.S. Dust mite allergens: Ecology and distribution. Curr. Allergy Asthma Rep. 2002, 2, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R.; Hales, B.J.; Smith, W.A. House dust mite allergens in asthma and allergy. Trends Mol. Med. 2010, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Resch, Y.; Blatt, K.; Malkus, U.; Fercher, C.; Swoboda, I.; Focke-Tejkl, M.; Chen, K.W.; Seiberler, S.; Mittermann, I.; Lupinek, C.; et al. Molecular, Structural and Immunological Characterization of Der p 18, a Chitinase-Like House Dust Mite Allergen. PLoS ONE 2016, 11, e0160641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konradsen, J.R.; Fujisawa, T.; van Hage, M.; Hedlin, G.; Hilger, C.; Kleine-Tebbe, J.; Matsui, E.C.; Roberts, G.; Rönmark, E.; Platts-Mills, T.A. Allergy to furry animals: New insights, diagnostic approaches, and challenges. J. Allergy Clin. Immunol. 2015, 135, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.; Moreno, C.; Ledesma, A.; Serrano, P.; Galán, A.; Villalba, M.; Guerra, F.; Lombardero, M.; Rodríguez, R. Degree of olive pollen exposure and sensitization patterns. Clinical implications. J. Investig. Allergol. Clin. Immunol. 2007, 17 (Suppl. S1), 11–16. [Google Scholar] [PubMed]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francis, O.L.; Wang, K.Y.; Kim, E.H.; Moran, T.P. Common food allergens and cross-reactivity. J. Food Allergy 2020, 2, 17–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramon, G.D.; Barrionuevo, L.B.; Viego, V.; Vanegas, E.; Felix, M.; Cherrez-Ojeda, I. Sensitization to subtropical grass pollens in patients with seasonal allergic rhinitis from Bahia Blanca, Argentina. World Allergy Organ. J. 2019, 12, 100062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calderón-Llosa, O.M. Identification and sensitization to environmental fungal spores in Lima City, Peru. Rev. Alerg. Mex. (Tecamachalco Puebla Mex. 1993) 2024, 71, 82. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
