Abstract
Peanut allergies, driven by sensitization to key allergens Ara h1, Ara h2, and Ara h3, present significant health risks, particularly in food processing and consumer settings where accidental exposure is frequent. To mitigate this risk, we developed AYA22AR321, a novel aptamer with selective, high-affinity binding to these allergens (Kd values: 0.5 nM for Ara h1, 14.5 nM for Ara h2, and 6.6 nM for crude peanut extract). Functional assays using RBL-2H3 (rat basophilic leukemia cell line) cells showed that AYA22AR321 significantly reduces IgE-mediated degranulation, indicating its potential to attenuate allergic responses. To translate these findings into practical use, we formulated an allergen-neutralizing spray, FISTOQ, containing AYA22AR321, which effectively neutralized peanut allergens on peanut-butter-contaminated surfaces. Stability tests confirmed that FISTOQ, comprising eco-friendly surfactant and preservative, maintains its allergen-neutralizing efficacy over time. Comprehensive safety assessments, including immunogenicity, cytotoxicity in human PBMCs, and mutagenicity via the Ames test, demonstrated that AYA22AR321 is non-immunogenic, non-cytotoxic, and non-mutagenic. This study establishes AYA22AR321 as a promising, targeted strategy for allergen control, providing a significant advancement in allergen mitigation and food safety for high-risk environments.
1. Introduction
Peanut allergy is a significant public health concern, marked by IgE-mediated type I hypersensitivity reactions that can lead to life-threatening anaphylaxis. Seventeen peanut allergenic proteins have been identified, Ara h1, Ara h2, Ara h3, and Ara h6 are the most prevalent and potent due to their high concentrations in peanuts. Despite the availability of treatments such as Palforzia and Xolair to mitigate severe allergic reactions, along with strict avoidance strategies, the risk of accidental exposure remains a persistent concern. Cross-contamination in food processing and public space significantly increases this risk. Many food businesses utilize precautionary allergy labels (PAL) to inform consumers about the potential unintended allergen presence. However, studies indicated that PAL does not always accurately reflect the presence of allergens nor does the absence of such labeling guarantee a low or nonexistent risk of contamination [1,2]. In some cases, peanut has been detected in prepacked foods regardless of whether PAL was present [3,4]. Furthermore, studies demonstrated that peanut allergens, such as Ara h1, can persist on surfaces for up to 110 days without adequate cleaning [5]. Studies show that accidental peanut exposures, particularly in home environments, occur in 12.4% of peanut-allergic individuals annually. Of these incidental exposures, 57% result in moderate to severe reactions [6]. Therefore, implementing effective allergen management strategies is crucial to minimize accidental exposure to peanut allergens in schools, homes, and public spaces, especially for highly sensitive individuals.
Several methods have been explored to reduce the allergenicity of peanut proteins and mitigate food contamination. Techniques such as pulsed ultraviolet light (PUL), genetic and chemical modification, oiling, pressure cooking, and enzymatic treatment have shown potential in reducing the allergenicity of peanut proteins. SDS-PAGE analysis in PUL has shown a significant decrease in the protein band intensity of major peanut allergens (Ara h1, Ara h2, and Ara h3) at energy levels ranging from 111.6 to 2232 J/cm2 [7]. Acylation, alkylation, and polymerization using glutaraldehyde have shown efficacy in decreasing the allergenic response in both pea [8] and peanut proteins [9]. Boiling and pressure cooking, have also been effective at reducing peanut allergenicity in vitro [10,11]. Peroxidase and proteolytic hydrolysis have been investigated for allergen reduction as well. Non-thermal methods, like cold argon plasma jet treatment, have shown promise, with a reduction in Ara h1 antigenicity by 38% after 3 min and up to 66% after approximately 15 min of treatment [12]. Other potential methods for allergen management in food processing include zonation [13], thermal processing, gamma irradiation [14], and ultrasonication [15]. However, many of these methods have limitations, including the presence of residual chemicals that are not food-grade, limited reduction in allergenicity, and altering the taste of foods. Thus, a need persists for effective, safe, and food-compatible methods to mitigate peanut allergenicity.
Effective surface allergen cleaning is another critical strategy to reduce cross-contamination and accidental exposure to peanut allergens. Cleaning methods can be broadly categorized as either wet or dry. Dry cleaning removes allergens without water, while wet cleaning utilizes water or combines it with detergents, such as alkaline, acidic, enzyme-based, or alcohol-saturated wipes, to remove food allergens. Bedford’s study showed that wet cloths and wipes are more effective than dry wipes at removing allergens from surfaces [16]. Watson’s research demonstrated that after cleaning with common household and hospital wipes, no detectable levels of peanut allergen (Ara h1) remained on contaminated surfaces [17]. Dr. Perry’s study further showed that Ara h1 was undetectable on hands after washing with liquid soap or commercial wipes, while plain water and antibacterial hand sanitizer did not completely remove the allergen [18]. However, some concerns persist; a study by Da Silva showed that household use of green cleaning products or disinfecting wipes was associated with uncontrolled asthma in adults [19].
Currently, there is no commercial product specifically designed to remove peanut allergens from surfaces. Thus, there is a need to develop a product that effectively and safely eliminates peanut allergenic proteins. In a previous work, we developed and validated the AYA22AR321 aptamer, which specifically binds to multiple allergenic proteins, including Ara h1 and Ara h2, and effectively inhibits allergen-induced degranulation, demonstrating potential in peanut allergy prevention [20].
In this study, we evaluate the effectiveness of AYA22AR321 in neutralizing peanut allergen from contaminated surfaces, offering a novel approach to improve allergen decontamination in food handling, manufacturing environments, and public spaces. Our aptamer has potential for development into a commercial cleaning product specifically targeting peanut proteins, thereby reducing the risk of allergic reactions in peanut-sensitive individuals due to contamination. Additionally, safety evaluations, including immunogenicity, cytotoxicity in human PBMCs, and mutagenicity via the Ames test, confirmed AYA22AR321 as non-immunogenic, non-cytotoxic, and non-mutagenic, supporting its suitability as an allergen-specific cleaning agent. Unlike general-purpose cleaners, this aptamer-based product could significantly reduce allergenic exposure risks in sensitive environments such as schools, restaurants, hospitals, and food processing facilities.
2. Materials and Methods
2.1. AYA22AR321 Generation
The AYA22AR321 aptamer was developed using an established computational biology platform, with its design, in silico docking, and initial characterization previously reported in our earlier work. The AYA22AR321 aptamer was computationally optimized for high binding specificity to key peanut allergens, Ara h1 and Ara h2. This aptamer has been shown to effectively inhibit degranulation in vitro, utilizing RBL-2H3 cells (rat basophilic leukemia cell line). For comprehensive details on the development, docking analysis, binding specificity, and functional validation of AYA22AR321, please refer to our earlier publication [20].
2.2. Cell Lines
Rat basophilic leukemia cell line RBL-2H3 was purchased from the American Type Culture Collection (ATCC, #CRL-2256). The cells were cultured with Eagle’s Minimum Essential Medium (EMEM) (ATCC, #30-2003) supplemented with 10% HyClone standard fetal bovine serum (FBS) (Cytiva life Sciences, Burlington, NJ, USA SH30088.03HI) and 50 U/mL penicillin/streptomycin (Gibco, New York, NY, USA). The cells were cultured in a humidified incubator at 37 °C in 5% CO2, as previously described [21]. The materials and key resources used in the methods are mentioned in detail in Supplementary Materials, Table S1.
2.3. ELISA-Based Binding Assay of Biotinylated AYA22AR321 Aptamer to Peanut Allergenic Proteins
The binding of AYA22AR321 to peanut allergenic proteins, Ara h1, Ara h2, Ara h3, and Ara h6, as well as crude peanut extract (CPE), was assessed by direct binding as well as competition ELISA, as previously described [20]. Briefly, overnight, a MaxiSorp plate was coated with 2 µg/mL of Ara h1, Ara h2, Ara h3, Ara h6, and CPE prepared in a 50 mM carbonate–bicarbonate solution. Following incubation, the plate underwent blocking for 1 h using 2% BSA diluted in PBS supplemented with 1 mM MgCl2 and 0.05% Tween-20. Subsequently, after washing twice with 1X PBS/Tween-20 solution, biotinylated AYA22AR321 aptamer was added at a concentration of 100 nM in duplicate and incubated at room temperature for 1 h. In the competition assay, unlabeled AYA22AR321 was added along with the biotinylated AYA22AR321, with the unlabeled aptamer being present in a 100-fold excess compared to the biotinylated aptamers. Following three washing steps with wash buffer (20 mM Tris, 150 mM NaCl pH 7.5, 0.1% BSA, and 0.1% Tween-20), streptavidin-HRP (ThermoFisher Scientific, cat# 21130, Waltham, MA, USA) was added at a dilution of 1:4000 in the incubation buffer. The plate was washed again three times to remove any unbound reagents. The bound biotinylated aptamers were then detected using 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (1-Step Ultra TMB-ELISA from ThermoFisher Scientific, cat# 34028, Waltham, MA, USA) following the manufacturer’s instructions. The reaction was terminated using 1M H2SO4, and absorbance was measured using a spectrophotometer at 450 nm.
2.4. Isolation of Crude Peanut Protein (CPE)
To isolate crude peanut extract (CPE) protein from ground peanut material, we prepared an extraction buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, and 1 mM EDTA (pH 8.0) (Sigma-Aldrich, St. Louis, MO, USA; G-Biosciences, St. Louis, MO, USA; Invitrogen, Grand Island, NY, USA) in a sterile 50 mL centrifuge tube. A 1 g sample of ground peanuts was combined with 10 mL of this cold extraction buffer. The mixture was vortexed vigorously to ensure even dispersion and further homogenized with a tissue homogenizer to enhance protein release. The homogenate was incubated on ice for 30 min, with periodic vortexing to optimize extraction efficiency. Post-incubation, the mixture was centrifuged at 7000× g for 40 min at 4 °C to separate the soluble protein fraction. The supernatant was carefully collected into a fresh 50 mL centrifuge tube and mixed with ice-cold acetone in a 1:4 ratio, facilitating protein precipitation. This mixture was incubated at −20 °C for 2 h, followed by centrifugation at 7000× g for 30 min at 4 °C to pellet the proteins. After removing the acetone supernatant, the protein pellet was allowed to air dry for 15 min, then resuspended in 1X PBS to ensure solubilization. The CPE concentration was quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) following the manufacturer’s instructions, and absorbance was measured at 562 nm with a spectrophotometer. This standardized extraction yielded a consistent CPE preparation for subsequent allergenicity assays.
2.5. RBL-2H3 Cells Degranulation and -Hexosaminidase Assay
The release of -hexosaminidase was measured to assess degranulation of RBL-2H3 cells, as previously described [20]. Briefly, RBL-2H3 cells (0.5 × 106 cells/well) were cultured in EMEM media without FBS. The cells were sensitized with 0.25 µg/mL of mouse anti-crude peanut extract lgE monoclonal antibody [Clone 2G11G7, Cat # 3070 (mouse anti-Ara h3 lgE monoclonal antibody)] for 30 min at 37 °C in 5% CO2. The sensitized cells were then stimulated with or without allergenic peanut proteins such as CPE or Ara h1, Ara h2, Ara h3, and Ara h6, as well as extract from the peanut-contaminated surface in the presence or absence of AYA22AR321 aptamer and non-specific aptamer, for 1 h at 37 °C in 5% CO2. Degranulation was detected spectroscopically by measuring the activity of the granule-stored enzyme -hexosaminidase secreted into the supernatant. As a positive control, to detect the total -hexosaminidase in the cytoplasm, the cells were lysed with 1% Triton X-100. After 1 h, 30 µL of the cell culture supernatants were incubated with 1 mM p-nitrophenyl-N-acetyl--d-glucosamine in 0.05 M citrate buffer (pH 4.5) for 1 h at 37 °C, followed by a stopping solution [200 µL of 0.05 M sodium carbonate buffer (pH 10.0)]. The -hexosaminidase activity was determined by measuring the difference in absorbance at 405 nm and 630 nm, and the percentage of degranulation was calculated as follows: percent (%) degranulation = (experimental -hexosaminidase release − vehicle control -hexosaminidase release)/(Triton-X-100 -hexosaminidase release − vehicle control -hexosaminidase release) × 100. All the degranulation assays were repeated in triplicate.
2.6. Flow-Cytometry-Based Cytotoxicity Assay
Human PBMCs (1 × 106 cells/100 µL), suspended in cRPMI media, were stimulated with or without the AYA22AR321 aptamer at varying concentrations (1, 2, 5, and 10 µM) and Tween-20 at concentrations of 0.005%, 0.01%, 0.02%, 0.5%, and 1%. The cells were incubated for 30 min at room temperature. Following incubation, the cells were washed with cold BioLegend’s cell staining buffer, resuspended in Annexin V binding buffer, and stained with 5 µL of FITC-conjugated Annexin V and 5 µL of 7-AAD viability staining solution. The samples were gently mixed and incubated for 15 min at room temperature in the dark. The stained cells were analyzed using a Navios EX flow cytometer (Beckman Coulter Inc., Pasadena, CA, USA). Data acquisition and analysis were performed using FlowJoTM software version 10.8.1 (Becton Dickinson Life Sciences, Franklin Lakes, NJ, USA).
2.7. Exploring the Immunogenic Reaction of AYA22AR321 on Human Peripheral Blood Mononuclear Cells (hPBMCs)
Human PBMCs were isolated from buffy coats provided by Carter BloodCare (Bedford, TX, USA). Isolation was carried out through density-gradient centrifugation utilizing Ficoll-Paque Plus from GE Healthcare. The isolated human PBMCs suspended in cRPMI media were subjected to stimulation with or without the AYA22AR321 aptamer and a control aptamer. Various concentrations (1, 5, and 10 µM) of these aptamers were used, along with positive control stimuli such as LPS (200 ng/mL), ODN 1826 (20 µM), or a combination of LPS (100 ng/mL) and ODN 1826 (10 µM). Following incubation periods of 24 h, the cell supernatant was collected via centrifugation at 200 g for 3 min. Cytokine levels secreted into the media were evaluated using the LEGENDplex Human Inflammation Panel 1 from BioLegend (cat# 740808, San Diego, CA, USA) following the manufacturer’s protocol, soluble analytes were acquired using a Navios EX flow cytometer (Beckman Coulter Inc., Pasadena, CA, USA), and subsequent analysis was performed using BioLegend’s LEGENDplexTM system (San Diego, CA, USA).
2.8. Ames Test
The mutagenic potential of AYA22AR321 was evaluated using the Ames test with the Xenometrix Ames MPF PENTA I kit, including S9 and Positive Controls (Aniara Diagnostica, code: AC01-512-S2-P; manufacturer’s part number: C01 512-S2-P, West Chester Township, OH, USA). The assay procedure was performed according to the manufacturer’s protocol, as outlined in our previous publication [22].
2.9. Statistical Analysis
Significance was determined in Prism 5.0 (GraphPad Software) using a non-parametric (Mann–Whitney) test or unpaired Student’s t test for two-group comparisons. Data are expressed as mean ± SD. p < 0.05 was considered statistically significant. * denotes p < 0.05, ** denotes p < 0.01, *** denotes p< 0.001, and **** denotes p < 0.0001.
3. Results
3.1. Characterization of Aptamer AYA22AR321 for Targeting Peanut Allergens
Using an in silico approach, we developed aptamers (AYA22A) that specifically target peanut allergens [20], binding to major peanut allergenic proteins and effectively neutralizing crude peanut extract (CPE)-induced degranulation in RBL-2H3 cells. The aptamer AYA22AR321, one of the most promising candidates, was further validated for its specificity to native peanut allergens, including Ara h1, Ara h2, Ara h3, and Ara h6, as well as crude peanut extract (CPE). Direct binding and competition ELISA assays confirmed that AYA22AR321 binds specifically to Ara h1, Ara h2, and Ara h3 (Supplementary Figure S1A), but does not bind to Ara h6. Additionally, AYA22AR321 demonstrated strong binding to both commercially available CPE (Chondrex, Inc., Woodinville, WA, USA) and in-house isolated CPE (CPE-ABS) (Supplementary Figure S1B,C). We demonstrated that the binding affinity of the native AYA22AR321 aptamer to CPE is comparable to that of its PEG- or TEG-modified counterparts. This finding indicates that chemical modifications introduced to enhance the aptamer’s stability do not compromise its ability to bind to the target protein.
To quantify the binding affinity of AYA22AR321, competition ELISAs were performed with Ara h1, Ara h2, and CPE proteins. The dissociation constants (Kd) were determined by incubating biotinylated AYA22AR321 with immobilized peanut proteins (Ara h1, Ara h2, and CPE) in the presence or absence of a 100-fold excess of non-biotinylated AYA22AR321. The binding was detected using streptavidin-horseradish peroxidase and a TMB substrate. The estimated Kd values for AYA22AR321 were 0.5 nM for Ara h1, 14.5 nM for Ara h2, and 6.6 nM for CPE, Figure 1A–C. These results validate AYA22AR321 as a highly specific aptamer with a strong affinity for peanut allergens, positioning it as a promising candidate for the development of an aptamer-based peanut allergen neutralization and decontamination agent. Such a decontaminant would be particularly valuable for mitigating peanut allergen contamination on surfaces in food processing and consumer environments, where accidental exposure is a frequent concern.
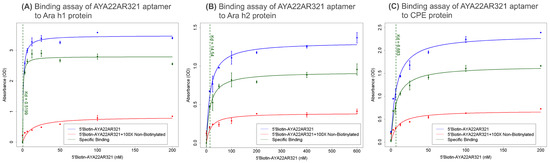
Figure 1.
Binding specificity and affinity of AYA22AR321 aptamer to peanut allergens. ELISA-based competition assays measuring binding of 5’Biotin-AYA22AR321 to (A) purified Ara h1, (B) purified Ara h2, and (C) crude peanut extract (CPE). The indicated concentrations of 5’Biotin-AYA22AR321 were incubated with proteins immobilized on a 96-well ELISA plate in the absence or presence of 100-fold excess non-biotinylated AYA22AR321. Absorbance was measured after incubation with streptavidin horseradish peroxidase (HRP) bound to the biotinylated aptamer in the presence of TMB substrate. Each bar shows the average of duplicate measurements. The binding affinity of AYA22AR321 to Ara h1, Ara h2, and CPE is estimated to be 0.5 nM, 14.5 nM, and 6.6 nM, respectively.
3.2. Aptamer AYA22AR321 Efficiently Neutralizes Peanut-Allergen-Induced Degranulation and Surface Contamination
To elucidate mechanisms of IgE-mediated degranulation in response to peanut allergens, RBL-2H3 cells were sensitized with a mouse anti-crude peanut extract (CPE) IgE antibody. Following sensitization, the cells were challenged with (a) CPE and various distinct allergens, including walnut, sesame, almond, red birch, wheat grain, egg white, soya bean, mixed shellfish, cow milk, cashew nut, and hazel nut (Supplementary Figure S2A), and (b) in-house purified crude peanut extract (CPE-ABS) and other nuts (almond-ABS, cashew-ABS, and hazelnut-ABS) (Supplementary Figure S2B) to quantify allergen-induced degranulation. In parallel, we assessed the neutralization capacity of the aptamer AYA22AR321. RBL-2H3 cells were exposed to peanut allergens in the presence or absence of AYA22AR321, with control aptamers included to confirm the specificity of this response. Our findings reveal that AYA22AR321 significantly attenuates allergen-induced degranulation, illustrating its potential to mitigate CPE- or purified peanut-allergen-driven IgE responses as compared to allergen alone or allergen+control aptamer groups. (Supplementary Figure S2).
To extend these findings in a practical context, a neutralizing spray incorporating AYA22AR321 was applied to surfaces contaminated with peanut allergens, specifically with peanut butter. Cells exposed to these treated surfaces displayed a marked significant reduction in degranulation (Figure 2A), affirming the potent allergen-neutralizing efficacy of AYA22AR321 when deployed in a spray format. Notably, the control product, lacking AYA22AR321, failed to neutralize allergens from treated surfaces (Figure 2A). Furthermore, we observed that the spray containing AYA22AR321 effectively neutralized peanut allergen-induced degranulation by contaminated surfaces as compared to allergen alone or allergen+control aptamer groups, even with one (Figure 2B), two (Figure 2C), or three applications (Figure 2D). Interestingly, we observed that wiping with water or alcohol after each treatment spray showed a differential effect on peanut protein stability. Alcohol wiping, compared to water, appeared to stabilize peanut proteins without interfering with their allergenic functionality (Figure 2D). This suggests that alcohol-based solutions are not suitable for mitigating or neutralizing the allergenic potential of peanut proteins.
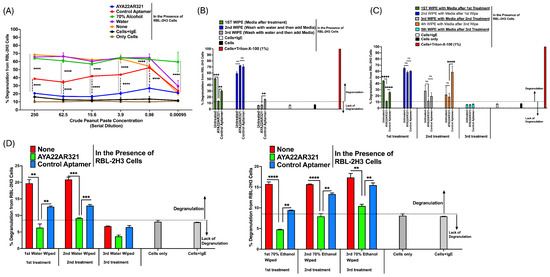
Figure 2.
AYA22AR321 effectively neutralizes degranulation induced by peanut-contaminated surfaces. RBL-2H3 cells (0.5 × 106 cells per well) were seeded into a 96-well plate in 100 µL of EMEM media without FBS. Cells were sensitized with 0.25 µg/mL of anti-crude-peanut-extract (CPE) IgE monoclonal antibody or left unsensitized and incubated at 37 °C for 1 h. After sensitization, cells were washed twice with EMEM media (without FBS) to remove unbound antibodies. The cells were then exposed to experimental conditions for 1 h at 37 °C in 200 µL of media. The experimental conditions included the following: (A) Neutralization of crude peanut paste: Serially diluted crude peanut paste was preincubated for 30 min at room temperature with or without AYA22AR321 (5 µM), control unrelated aptamer (5 µM), 70% ethanol, or water. Contaminated surfaces were wiped using clean Kimwipes, scraped in the presence of 200 µL EMEM media (without FBS), and tested for degranulation. (B) Peanut-butter-contaminated surfaces: Surfaces contaminated with peanut butter were treated with or without AYA22AR321 (5 µM) for 30 min. The surface was scraped in 200 µL media after the first wipe, followed by washing with water and subsequent scraping after the second and third wipes. (C) Sequential applications of aptamer treatment: Contaminated surfaces were treated with AYA22AR321 (5 µM) or control aptamer (5 µM) for 30 min (first treatment). The surface was scraped with 200 µL media (first wipe) and then subjected to additional aptamer applications (second and third treatments). Scrapes were collected in 200 µL media after each wipe, generating samples for a total of five wipes. (D) Comparison of aptamer treatment with ethanol wipes: Contaminated surfaces were treated with AYA22AR321 (5 µM) or control aptamer (5 µM) for three consecutive applications, followed by wiping with water or 70% ethanol. After each wipe, surfaces were scraped in 200 µL media to evaluate the residual allergen presence. Degranulation was assessed by measuring the release of the granule-stored enzyme -hexosaminidase into the supernatant. This was quantified spectroscopically using the p-nitrophenyl-N-acetyl--d-glucosamine substrate. Percent (%) degranulation was calculated using the following formula: (Experimental -hexosaminidase release − vehicle control -hexosaminidase release)/(Triton-X-100 -hexosaminidase release − vehicle control -hexosaminidase release) × 100. All data are representative of at least two independent experiments. Error bars represent mean ± standard deviation (SD). Statistical significance was determined using an unpaired Student’s t test. ** denotes p < 0.01, *** p < 0.001, and **** p < 0.0001, and “ns”denotes non-significant. The dashed line indicates the results of the control condition. Results above the line indicate degranulation, while results below it indicate a lack of degranulation.
Collectively, these results provide compelling evidence that the AYA22AR321-based decontaminating spray efficiently neutralizes peanut allergens on contaminated surfaces, offering a robust approach to preventing IgE-mediated cell activation and allergic responses in environments with peanut allergen contamination.
3.3. Functional Efficacy and Stability of Aptamer AYA22AR321 for Neutralizing Peanut Allergens on Contaminated Surfaces
To assess the functional efficacy and stability of the aptamer AYA22AR321 in neutralizing peanut allergens, we evaluated its allergen-neutralization activity in the presence or absence of various commercially available cleaning agents, including Lime-A-Way, Lysol, Sprayway Glass Cleaner, Germ-X Hand Sanitizer, Clorox, Microban, Allersearch ADMS, ALPET D2 Surface Sanitizing Wipes, and Nozin (Supplementary Figure S3). Our results demonstrated that none of these cleaning agents alone affected the stability or activity of peanut allergens. However, when mixed with AYA22AR321, these agents significantly neutralized peanut allergens on surfaces contaminated with peanut products, such as peanut butter (insoluble peanut allergens are present in peanut butter, contributing to allergen persistence), without compromising the aptamer’s functional stability compared to the allergen alone or allergen+control aptamer groups (Supplementary Figure S3).
To optimize AYA22AR321 as a stable, effective peanut allergen neutralizer, AYA22AR321 was mixed with these mild concentrations of Triton-X-100 and applied as a spray to surfaces contaminated with crude peanut extract (CPE) or peanut butter. Our results showed that AYA22AR321 combined with mild Triton-X-100 effectively neutralized peanut allergens on contaminated surfaces compared to allergen alone (CPE, a soluble peanut allergen) or allergen+control aptamer groups (Figure 3A).
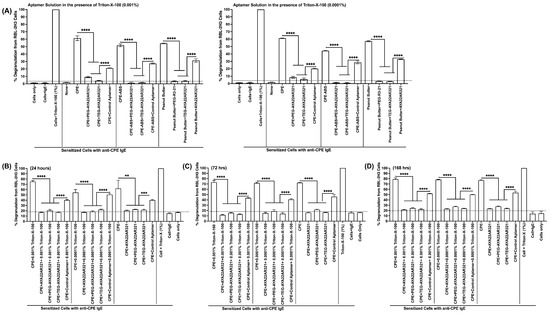
Figure 3.
Stability and Functionality of AYA22AR321 Neutralizer Spray in the Presence of Mild Detergent for Neutralizing Peanut Proteins from Various Sources. The stability and neutralization efficacy of AYA22AR321 and its modified forms (PEG- and TEG-conjugated AYA22AR321) were evaluated in a mild detergent solution. The neutralizer spray was formulated with Triton-X-100 at concentrations of 0.001% and 0.0001%, and its allergen-neutralizing activity was assessed. For functional assays, RBL-2H3 cells (0.5 × 106 cells per well) were seeded into 96-well plates in 100 µL of EMEM medium without FBS. The cells were sensitized with 0.25 µg/mL of anti-crude-peanut-extract (CPE) IgE monoclonal antibody or left unsensitized (control) and incubated at 37 °C for 1 h. Following sensitization, the cells were washed twice with EMEM medium (without FBS) to remove unbound antibodies. The experimental setup involved pre-incubating CPE with the neutralizer spray formulated with AYA22AR321, PEG-AYA22AR321, or TEG-AYA22AR321 for 30 min at room temperature to assess: (A) the aptamer functionality in freshly prepared neutralizer spray and (B–D) the functional stability of the neutralizer spray over time. The treated CPE samples were then added to the RBL-2H3 cells and incubated for 1 h at 37 °C in 200 µL of medium. Degranulation was quantified by measuring the release of -hexosaminidase, a granule-stored enzyme, into the supernatant. The enzymatic activity was determined spectroscopically using the p-nitrophenyl-N-acetyl--d-glucosamine substrate. Percent (%) degranulation was calculated using the following formula: (Experimental -hexosaminidase release − vehicle control -hexosaminidase release)/(Triton-X-100 -hexosaminidase release − vehicle control -hexosaminidase release) × 100. All data are representative of at least two independent experiments. Error bars represent mean ± standard deviation (SD). Statistical significance was determined using an unpaired Student’s t test. ** denotes p < 0.01, *** p < 0.001, and **** p < 0.0001. The dashed line indicates the results of the control condition. Results above the line indicate degranulation, while results below it indicate a lack of degranulation.
Furthermore, the stability of native and modified forms of AYA22AR321 was assessed when spiked into a mild Triton-X-100 solution and stored for 24 h (Figure 3B), 72 h (Figure 3C), and 168 h (Figure 3D). Remarkably, both the native and modified AYA22AR321 maintained stable functionality over these time points, effectively neutralizing peanut allergens consistently across all tested conditions compared to allergen alone or allergen+control aptamer groups (Figure 3).
Collectively, these findings demonstrate that AYA22AR321, in its native or modified form (PEG-AYA22AR321 or TEG-AYA22AR321), remains functionally stable and effective in neutralizing peanut allergens, even when incorporated into a range of cleaning agents. This highlights its potential as a robust solution for allergen decontamination on peanut-contaminated surfaces.
3.4. Immunogenicity, Cell Cytotoxicity, and Mutagenicity Assessments of AYA22AR321Aptamer
To assess the potential immunogenicity of AYA22AR321, we utilized an in vitro human model by measuring the release of inflammatory cytokines and chemokines from cultured peripheral blood mononuclear cells (PBMCs) in response to varying doses of AYA22AR321. The cytokines and chemokines measured included IL-1, IFN-2, IFN-, TNF-, MCP-1 (CCL2), IL-6, IL-8 (CXCL8), IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33, as illustrated in Figure 4. PBMCs were treated with AYA22AR321 at concentrations of 1, 5, or 10 µM, and the culture supernatants were collected 24 h post-treatment for analysis.
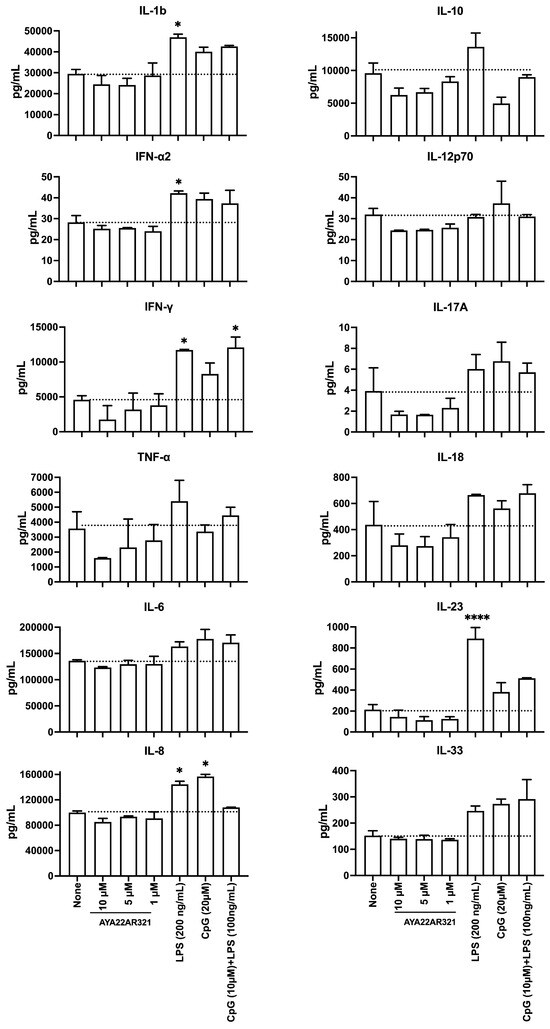
Figure 4.
Immunogenicity Assessment of AYA22AR321 Aptamer. Immunogenic responses of AYA22AR321. Human PBMCs were stimulated with various dilutions of AYA22AR321 (10 µM, 5 µM, and 1 µM), LPS, ODN 1826, and LPS + ODN 1826 at 37 °C for 24 h. The levels of cytokines secreted by the hPBMCs were measured using the LEGENDplexTM Human Inflammation Kit. The soluble analytes were quantified by flow cytometry and analyzed with BioLegend’s LEGENDplexTM software (https://www.biolegend.com/en-ie/immunoassays/legendplex/support/software (accessed on 22 November 2024)). Each bar represents the mean ± SD (n = 4). Statistical significance was determined using an unpaired Student’s t test. * denotes p < 0.05, and **** p < 0.0001. The dashed line indicates the results of the control condition.
The results indicated that PBMCs treated with AYA22AR321 displayed cytokine and chemokine secretion levels comparable to mock-treated cells across all measured concentrations. Specifically, the levels of IL-1, IFN-2, IFN-, TNF-, IL-6, IL-8 (CXCL8), IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33 remained unchanged, highlighting a lack of pro-inflammatory response under these conditions. In contrast, positive control samples treated with LPS (100 ng/mL) demonstrated a robust inflammatory response, marked by significantly elevated levels of IL-1, IFN-2, IFN-, TNF-, IL-6, IL-8 (CXCL8), IL-10, IL-17A, IL-18, IL-23, and IL-33 compared to mock-treated cells, thus confirming assay sensitivity and responsiveness. Notably, MCP-1 (CCL2) was undetectable in all experimental conditions.
These findings collectively suggest that AYA22AR321 does not elicit immune activation or inflammatory cytokine release in human PBMCs, indicating an absence of immunogenic properties in this in vitro model. These observations align with the findings of Thiel et al. [23], who reported a similar lack of immune activation by an RNA aptamer in human PBMCs. Based on this analysis, AYA22AR321 appears to have a favorable immunological safety profile, supporting its potential utility in clinical applications without unintended immune activation.
Next, we examined the cytotoxicity of AYA22AR321 by treating human PBMCs with varying concentrations and assessing cell viability via Annexin V/7-ADD staining and flow cytometry (Supplementary Figure S4) [24,25]. Tween-20, a biodegradable non-ionic detergent, was included as a positive control for cytotoxicity. AYA22AR321 treatment did not induce apoptosis or necrosis in PBMCs at any concentration. However, Tween-20 at higher concentrations (1% and 0.5%) resulted in late apoptosis/necrosis, while concentrations between 0.02% and 0.005% exhibited no cytotoxicity (Supplementary Figure S4). These results further confirm that AYA22AR321 is non-cytotoxic to human PBMCs.
To evaluate the mutagenicity of AYA22AR321, we conducted an Ames test, a widely accepted bacterial reverse mutation assay that assesses the mutagenic potential of chemical compounds. This assay utilizes various bacterial strains, including Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537, as well as Escherichia coli strains WP2 [pKM101] and WP2 uvrA [26]. These bacterial strains were exposed to increasing concentrations of AYA22AR321, ranging from 0.5 µM to 10 µM, and mutagenic events were quantified by selecting for reversion mutations. Across all tested concentrations, AYA22AR321-treated strains consistently displayed fewer revertant colonies than the baseline established by the positive control, indicating no mutagenic activity (Supplementary Figure S5).
Taken together, these findings demonstrate that AYA22AR321 does not induce inflammatory responses or cytotoxicity in human PBMCs and does not exhibit mutagenicity in bacterial models. While these results suggest a favorable safety profile, further in vivo studies, including assessments of gastrointestinal effects, are necessary to fully evaluate its safety for human use. Nonetheless, these data support AYA22AR321’s potential for environmental and consumer applications as a decontamination agent.
3.5. Compatibility and Efficacy of AYA22AR321 Aptamer for Eco-Friendly Peanut Allergen Neutralization
To assess the suitability of AYA22AR321 for integration into eco-friendly solution solutions, we evaluated its allergen-neutralizing performance when combined with EPA-approved green solutions and mild surfactants. Specifically, we examined two eco-friendly cleaning solutions, 7th Generation and ECOS, alongside Tween-20, a biodegradable, non-cytotoxic detergent commonly used in biological applications.
Incorporating AYA22AR321 into 7th Generation and ECOS sprays did not impair its allergen-neutralizing function. Both formulations containing AYA22AR321 achieved a 1.7-fold reduction in CPE-induced degranulation in RBL-2H3 cells, comparable to the 3.5-fold reduction observed with AYA22AR321 in standard activation buffer alone as compared to CPE alone or CPE+control aptamer groups (Supplementary Figure S6A,B). These findings suggest that while eco-friendly solutions are compatible with AYA22AR321, they may slightly reduce its allergen-neutralizing efficacy compared to the standard activation buffer.
To ensure cytocompatibility, we next tested various concentrations of Tween-20 to establish its non-toxic range for RBL-2H3 cells. Tween-20 concentrations from 0.02% to 0.005% did not induce degranulation or cytotoxicity, confirming the detergent’s safety and suitability (Supplementary Figure S6C). At concentrations between 0.1% and 0.005%, Tween-20 effectively maintained cell viability and did not stimulate degranulation, consistent with its established biocompatibility in cellular assays [27].
Combining AYA22AR321 with Tween-20 at these optimal concentrations resulted in substantial peanut allergen neutralization. The aptamer’s activity was preserved across all Tween-20 concentrations tested, with a 3.5-fold reduction in CPE-induced degranulation, equivalent to its performance in activation buffer alone (Supplementary Figure S6C). Based on these findings, Tween-20 at 0.01% was selected as the optimal surfactant for formulating an aptamer-based peanut allergen neutralizer.
To further stabilize the formulation, we tested the compatibility of AYA22AR321 in Tween-20 with various preservatives, including potassium sorbate (PS), 2-Methyl-4-isothiazolin-3-one (2MI), and 2-Octyl-isothiazolin-3-one (2OI), to assess their impact on aptamer functionality. PS (0.01%) in 0.01% Tween-20 retained AYA22AR321 activity, whereas 2MI and 2OI combinations showed reduced efficacy as well as interference with the degranulation assay (Supplementary Figure S7). Therefore, PS was selected as the preservative for the final AYA22AR321-Tween-20 formulation.
Finally, we identified the optimal aptamer concentration for allergen neutralization by testing a range from 1 µM to 5 µM in the 0.01% Tween-20 and 0.01% PS formulation. AYA22AR321 at 1 µM, compared to allergen alone or allergen+control aptamer groups, achieved effective mitigation of CPE-induced degranulation in RBL-2H3 cells compared to CPE alone or CPE+control aptamer groups, demonstrating comparable performance to higher concentrations while offering cost advantages (Figure 5). Consequently, a 1 µM concentration of AYA22AR321 was selected for an effective and economically viable peanut allergen neutralizing spray.
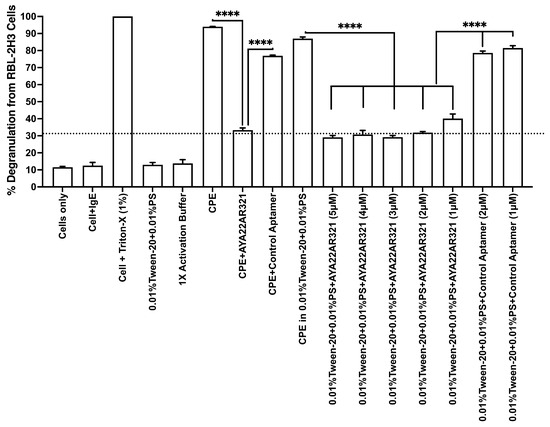
Figure 5.
AYA22AR321 neutralizer spray effectively shields peanut product-contaminated surfaces at various concentrations. RBL-2H3 cells (0.5 × 106) in 100 µL EMEM media without FBS were seeded into a 96-well plate with or without sensitization using 0.25 µg/mL of anti-crude-peanut-extract (CPE) IgE monoclonal antibody or left unsensitized (control) and incubated at 37 °C for 1 h. Following the sensitization incubation, RBL-2H3 cells were washed twice with EMEM media without FBS and subsequently stimulated with pre-incubated CPE with neutralizer spray formulated with or without AYA22AR321 at different concentrations or with control unrelated aptamer for 30 min at room temperature. The treated CPE samples were then added to the RBL-2H3 cells and incubated for 1 h at 37 °C in 200 µL of media. Degranulation was quantified by measuring the release of -hexosaminidase, a granule-stored enzyme, into the supernatant. The enzymatic activity was determined spectroscopically using the p-nitrophenyl-N-acetyl--d-glucosamine substrate. Percent (%) degranulation was calculated using the following formula: (Experimental -hexosaminidase release − vehicle control -hexosaminidase release)/(Triton-X-100 -hexosaminidase release − vehicle control -hexosaminidase release) × 100. All data are representative of at least two independent experiments. Error bars represent mean ± standard deviation (SD). Statistical significance was determined using an unpaired Student’s t test. **** denotes p < 0.0001. The dashed line indicates the results of the control condition. Results above the line indicate degranulation, while results below it indicate a lack of degranulation.
3.6. Formulation and Efficacy of FISTOQ: A Stable, Eco-Friendly Peanut Allergen Neutralizer
To finalize an effective peanut-allergen-neutralizing formulation, we developed a preparation named “FISTOQ” with a specific combination of components: 0.01% Tween-20 as a mild surfactant, 0.01% PS as a preservative, 1 mM MgCl2 to stabilize the oligonucleotide, 1X PBS (pH 7.2–7.4) as a buffered medium, and 1 µM AYA22AR321 as the active ingredient for allergen neutralization. Our data collectively demonstrate that FISTOQ effectively mitigates peanut allergen activity, prompting further validation of its efficacy on surfaces contaminated by peanut food products.
To assess its performance under practical conditions, we tested FISTOQ on peanut-butter-contaminated surfaces (polyethylene plastic, polystyrene petri dishes, and cell culture 6-well plate). After contaminating a hard surface with peanut butter and removing the excess, we applied FISTOQ in three experimental conditions: single (Figure 6A), double (Figure 6B), and triple (Figure 6C) spray applications, compared against a control solution (FISTOQ Control, with a non-targeting aptamer). The results indicated that FISTOQ substantially reduced allergen-induced degranulation in RBL-2H3 cells across all conditions compared to allergen (peanut butter containing insoluble peanut allergens) alone or allergen+control aptamer groups, demonstrating robust allergen neutralization (Figure 6). Reapplication of FISTOQ after water-wiping steps further enhanced allergen mitigation, indicating that the AYA22AR321 aptamer effectively rebinds residual peanut allergens on the surface, suggesting that three FISTOQ applications yield optimal protection.
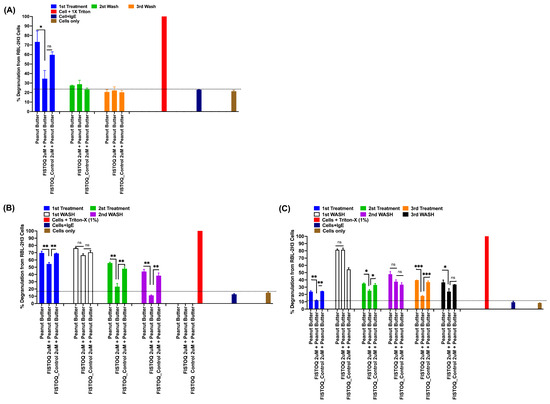
Figure 6.
FISTOQ (AYA22AR321 neutralizer spray) effectively neutralizes peanut allergen contamination on surfaces with single, double, or triple applications. RBL-2H3 cells (0.5 × 106) in 100 µL EMEM media without FBS were seeded into a 96-well plate with or without sensitization using 0.25 µg/mL of anti-crude peanut extract (CPE) IgE monoclonal antibody or left unsensitized (control) and incubated at 37 °C for 1 h. Following the sensitization incubation, RBL-2H3 cells were washed twice with EMEM media without FBS and subsequently stimulated with pre-incubated peanut-butter-contaminated surface, which was treated with or without FISTOQ 2µM and FISTOQ control (control unrelated aptamer, 2 µM solution) for 30 min incubation at room temperature at each treatment, and then treated flow-through was collected as indicated in (A) single spray treatment, (B) double spray treatment, and (C) triple spray treatment. The collected flow-through was then added to the RBL-2H3 cells and incubated for 1 h at 37 °C in 200 µL of media. Degranulation was quantified by measuring the release of -hexosaminidase, a granule-stored enzyme, into the supernatant. The enzymatic activity was determined spectroscopically using the p-nitrophenyl-N-acetyl--d-glucosamine substrate. Percent (%) degranulation was calculated using the following formula: (Experimental -hexosaminidase release − vehicle control -hexosaminidase release)/(Triton-X-100 -hexosaminidase release − vehicle control -hexosaminidase release) × 100. All data are representative of at least two independent experiments. Error bars represent mean ± standard deviation (SD). Statistical significance was determined using an unpaired Student’s t test. * denotes p < 0.05, ** p < 0.01, *** p < 0.001, and “ns”denotes non-significant. The dashed line indicates the results of the control condition. Results above the line indicate degranulation, while results below it indicate a lack of degranulation.
To refine the interaction dynamics, we next evaluated the time course of AYA22AR321 binding to CPE (Figure 7A) or contaminated surfaces (Figure 7B) by incubating either CPE or contaminated surfaces with FISTOQ or FISTOQ control for varied intervals (0–30 min). The results showed that FISTOQ rapidly and effectively neutralizes peanut allergens over a range of incubation times, reducing allergen-induced cell degranulation compared to allergen alone or allergen+control aptamer groups. These findings affirm that AYA22AR321 specifically binds peanut allergens and shields them, reducing the allergenic response.
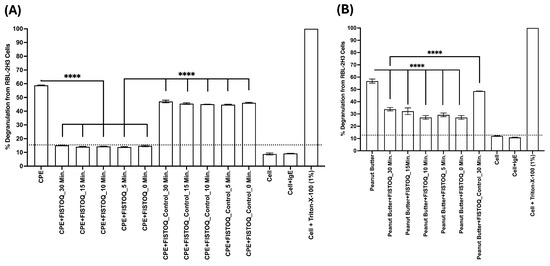
Figure 7.
Consistent neutralizing efficiency of FISTOQ against CPE- and peanut-butter-induced RBL-2H3 cell degranulation across different incubation times. RBL-2H3 cells (0.5 × 106 cells/well) were seeded into 96-well plates in EMEM medium without FBS. Cells were sensitized with 0.25 µg/mL anti-CPE IgE monoclonal antibodies at 37 °C for 1 h, with unsensitized wells serving as controls. Following two washes with FBS-free EMEM medium, cells were exposed to 10 µg/mL CPE (A) or extracts from peanut-butter-contaminated surfaces (B) in the presence or absence of FISTOQ (AYA22AR321, 1 µM) or a control unrelated aptamer (1 µM). Treatments were performed at various incubation times (30, 15, 10, 5, and 0 min) at room temperature. Degranulation was quantified by measuring the release of -hexosaminidase, a granule-stored enzyme, into the supernatant. The enzymatic activity was determined spectroscopically using the p-nitrophenyl-N-acetyl--d-glucosamine substrate. Percent (%) degranulation was calculated using the following formula: (Experimental -hexosaminidase release − vehicle control -hexosaminidase release)/(Triton-X-100 -hexosaminidase release − vehicle control -hexosaminidase release) × 100. All data are representative of at least two independent experiments. Error bars represent mean ± standard deviation (SD). Statistical significance was determined using an unpaired Student’s t test. **** denotes p < 0.0001. The dashed line indicates the results of the control condition. Results above the line indicate degranulation, while results below it indicate a lack of degranulation.
To rigorously evaluate the efficacy of FISTOQ in neutralizing insoluble peanut allergens under practical conditions, additional experiments were conducted on a diverse range of hard surfaces, including black stone, glass, ceramic, stainless steel, granite, marble, and wood (Supplementary Figure S8A,B). Each surface was systematically contaminated with peanut butter, followed by the removal of excess residue to simulate real-world exposure scenarios. A single spray application of FISTOQ was then applied, and its performance was compared against a control solution (FISTOQ control, containing a non-targeting aptamer). A quantitative analysis of residual allergenicity demonstrated that FISTOQ treatment significantly reduced allergen-induced responses across all tested surfaces compared to both untreated peanut butter residues and the allergen + control aptamer groups. These findings provide strong evidence supporting the efficacy of FISTOQ in mitigating peanut allergen contamination on various hard surfaces, reinforcing its potential application in food processing and allergen-sensitive settings.
Finally, we evaluated the stability of FISTOQ by assessing the functionality of AYA22AR321 over time at room temperature. Remarkably, AYA22AR321 retained its allergen-neutralizing efficacy, as evidenced by reduced degranulation responses in RBL-2H3 cells at both 3-day and 28-day and 90-day intervals (Supplementary Figure S9A,B); additional long-term stability studies extending to two years are planned to ensure its suitability for extended storage and application in diverse environments. These findings provide a strong foundation for the continued development of FISTOQ as a stable and effective allergen neutralizer. Together, these findings position FISTOQ as a stable, eco-friendly peanut allergen neutralizer capable of providing sustained allergen protection on surfaces, suitable for application in diverse environments. These promising results warrant further long-term stability studies.
4. Discussion
Peanut allergy is a major public health concern due to the risk of severe IgE-mediated reactions triggered by peanut-allergenic proteins. Although strategies like early peanut introduction in high-risk infants, as demonstrated in the LEAP (Learning Early About Peanut Allergy) and ITN (Immune Tolerance Network) studies have shown success in reducing peanut allergy prevalence, these methods do not benefit individuals who develop peanut allergies later in life. For such individuals, minimizing accidental exposure to peanut allergens becomes crucial, as cross-contamination in environments like schools, restaurants, grocery stores, and food processing facilities remains a substantial risk. Effective allergen sanitation is therefore essential in these shared spaces. Currently, no commercial products are specifically designed to target peanut allergens effectively and safely. This underscores a critical need for a product that complements the existing peanut allergy treatments and interventions by addressing allergen decontamination to reduce accidental exposure risks. Building on our previous work, where we developed aptamers that bind specifically to peanut allergens Ara h1 and Ara h2 and demonstrated effective neutralization of allergen-induced degranulation [20], we aimed to develop an aptamer-based allergen-neutralizing cleaner specifically targeting peanut proteins.
In this study, we formulated an aptamer-based cleaning solution capable of neutralizing peanut allergens on hard surfaces. Our findings indicate that this solution effectively neutralizes peanut proteins across all tested surfaces. Comparisons with common household products, including Lysol, Clorox, Germ-X sanitizer, and Line-A-Way cleaner, Microban, Allersearch ADMS, ALPET D2 Surface Sanitizing Wipes, and Nozin revealed that our aptamer-based solution was significantly more effective at neutralizing peanut allergens on contaminated surfaces. Additionally, safety assessments demonstrated that the active ingredient AYA22AR321 is non-immunogenic, non-cytotoxic in human PBMCs, and non-mutagenic, suggesting it is safe for both human use and the environment.
Peanut allergens are easily spread within homes, schools, and restaurants, creating challenges for individuals with peanut sensitivities. The study by Brough et al. found that peanut protein persisted on hands and in saliva for up to 3 h after consumption and remained on surfaces like laminate, wood, pillows, and sofa covers even after cleaning with standard detergents [28]. Most commercial wipes, including those used in healthcare settings, have primarily demonstrated efficacy against Ara h1, with limited data on their effectiveness against other allergens. In contrast, our aptamer-based peanut allergy neutralizing solution effectively targets multiple peanut allergens, including Ara h1, Ara h2, Ara h3, and peanut crude extract, offering a more comprehensive solution for allergen decontamination.
Previous studies have examined cleaning techniques for removing peanut residues. For instance, Kim et al. demonstrated that washing with hot water (above 50 °C) and using specific cleaning tools could effectively reduce peanut butter residue on certain surfaces, though removal on wooden surfaces remained challenging [29]. Our product, however, does not require elevated temperatures or specialized tools and effectively neutralize peanut allergenic protein in food residue from wood and other surfaces, as shown in our results.
While various strategies exist for mitigating allergenicity, managing allergic reactions, and preventing peanut allergy, no single approach offers complete protection. Comprehensive allergen management requires a multi-faceted approach, including employee training, allergen labeling, color-coding utensils, segregating allergenic and non-allergenic products, and routine food allergen testing [30]. Given the severe reactions and economic impact associated with peanut exposure, this study aimed to evaluate whether an aptamer-based cleaning product could effectively neutralize peanut allergens (Ara h1, Ara h2, Ara h3) on different surfaces. Our findings indicate that this aptamer-based peanut-allergen-neutralizing solution provides an effective and safe approach to reducing the risk of accidental allergen exposure in high-sensitivity settings, such as schools, homes, and restaurants.
5. Conclusions
This study presents a novel aptamer-based cleaning solution designed specifically for neutralizing peanut allergens on contaminated surfaces. Our solution demonstrates superior effectiveness compared to commercial cleaners in neutralizing multiple peanut allergens, including Ara h1, Ara h2, Ara h3, and crude peanut extract proteins. Unlike traditional cleaning methods that may require specific conditions, such as elevated temperatures, our solution works effectively at room temperature across hard surface types. Safety studies confirm that the cleaning solution is non-immunogenic, non-cytotoxic, and non-mutagenic, making it suitable for use in environments where accidental peanut exposure is a concern. This aptamer-based cleaner represents a promising new tool in comprehensive allergen management strategies for schools, restaurants, and homes, addressing the critical need for effective peanut allergen decontamination in shared spaces.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/allergies5020011/s1, Figure S1: Characterization of AYA22AR321 aptamer binding to peanut allergens, Ara h1, Ara h2, Ara h3, and crude peanut extract (CPE); Figure S2: Assessment of peanut-allergen-induced degranulation in RBL-2H3 cells and the neutralizing effect of AYA22AR321 aptamer; Figure S3: AYA22AR321 combined with Commercial solution Spray Effectively Neutralizes Peanut Product-Contaminated Surfaces, Preventing Degranulation; Figure S4: Cytotoxicity assessment of AYA22AR321 aptamer; Figure S5: Microbial mutagenicity test for AYA22AR321; Figure S6: Comparative Functionality of AYA22AR321 Aptamer with Commercial Eco-Friendly Detergents and detergents containing Tween-20; Figure S7: Comparative functionality of AYA22AR321 Aptamer in buffer containing Tween-20 with or without preservatives; Figure S8: Stability of FISTOQ by assessing AYA22AR321 functionality over time at room temperature (18–25 °C); Figure S9: Stability of FISTOQ by assessing AYA22AR321 functionality over time at room temperature (18–25 °C); Table S1: Materials and key resources used in the methods.
Author Contributions
M.A.A. participated in the conceptualization and design of the research project, reviewed the data, and supervised in conducting the research and reporting the results. T.T. and L.A.-M. designed the experiments. T.T., R.R.N., T.O., V.P. and N.G. conducted the experiments and data analyses. J.Z. and T.T. conducted the literature review and manuscript writing. L.A.-M., T.T., K.Z. and J.Z. reviewed the data. T.T. and N.G. oversaw the research operation. L.A.-M. supervised the research. All authors contributed to manuscript editing and discussion of the results in the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Ayass Bioscience, LLC, a privately-held Texas limited liability company.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This study did not involve humans.
Data Availability Statement
This article has accompanying Supplementary Files. All data generated or analyzed during this study are included in this published article (and its Supplementary Material Files).
Acknowledgments
We thank Larisa Marder for all illustrative work.
Conflicts of Interest
Ayass Bioscience, LLC is a privately-held Texas limited liability company (“Ayass Bioscience”). The research project was funded by Ayass Bioscience. The principal investigator, Ayass is the founder of Ayass Bioscience. All staff who worked on this research project were employees of Ayass Bioscience. The value of compensation paid to such employees did not influence the results. Moreover, the participation of employees of Ayass Bioscience did not affect the design, conduct, reporting, or outcome of the research. The employees of Ayass Bioscience who performed the research related to the study do not have a proprietary interest in any product or services, such as patent rights, trademark, copyright, or licensing agreement related to the research or any outcome. Any and all work product derived from the research project and proprietary rights related to such work product belong exclusively to Ayass Bioscience.
References
- Turner, P.; Bognanni, A.; Arasi, S.; Ansotegui, I.; Schnadt, S.; La Vieille, S.; Hourihane, J.; Zuberbier, T.; Eigenmann, P.; Ebisawa, M. Others Time to ACT-UP: Update on precautionary allergen labelling (PAL). World Allergy Organ. J. 2024, 17, 100972. [Google Scholar]
- Schaible, A.; Kabourek, J.; Elverson, W.; Venter, C.; Cox, A.; Groetch, M. Precautionary Allergen Labeling: Avoidance for All? Curr. Allergy Asthma Rep. 2024, 24, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Pele, M.; Brohée, M.; Anklam, E.; Hengel, A. Peanut and hazelnut traces in cookies and chocolates: Relationship between analytical results and declaration of food allergens on product labels. Food Addit. Contam. 2007, 24, 1334–1344. [Google Scholar] [PubMed]
- Manny, E.; La Vieille, S.; Barrere, V.; Théolier, J.; Godefroy, S. Peanut and hazelnut occurrence as allergens in foodstuffs with precautionary allergen labeling in Canada. NPJ Sci. Food 2021, 5, 11. [Google Scholar] [PubMed]
- Watson, W.; Woodrow, A.; Stadnyk, A. Persistence of peanut allergen on a table surface. Allergy Asthma Clin. Immunol. 2013, 9, 7. [Google Scholar]
- Cherkaoui, S.; Ben-Shoshan, M.; Alizadehfar, R.; Asai, Y.; Chan, E.; Cheuk, S.; Shand, G.; St-Pierre, Y.; Harada, L.; Allen, M. Others Accidental exposures to peanut in a large cohort of Canadian children with peanut allergy. Clin. Transl. Allergy 2015, 5, 16. [Google Scholar]
- Yang, W.; Mwakatage, N.; Goodrich-Schneider, R.; Krishnamurthy, K.; Rababah, T. Mitigation of major peanut allergens by pulsed ultraviolet light. Food Bioprocess Technol. 2012, 5, 2728–2738. [Google Scholar] [CrossRef]
- Szymkiewicz, A.; Jedrychowski, L. Effect of acylation and enzymatic modification on pea proteins allergenicity. Pol. J. Food Nutr. Sci. 2008, 58, 344–350. [Google Scholar]
- Apostolovic, D.; Luykx, D.; Warmenhoven, H.; Verbart, D.; Stanic-Vucinic, D.; Jong, G.; Velickovic, T.; Koppelman, S. Reduction and alkylation of peanut allergen isoforms Ara h 2 and Ara h 6; characterization of intermediate-and end products. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 2832–2842. [Google Scholar]
- Huang, H.; Yang, B.; Wang, C. Effects of high pressure processing on immunoreactivity and microbiological safety of crushed peanuts. Food Control 2014, 42, 290–295. [Google Scholar]
- Cabanillas, B.; Maleki, S.; Rodríguez, J.; Burbano, C.; Muzquiz, M.; Jiménez, M.; Pedrosa, M.; Cuadrado, C.; Crespo, J. Heat and pressure treatments effects on peanut allergenicity. Food Chem. 2012, 132, 360–366. [Google Scholar] [CrossRef]
- Hsu, F.; Lin, W.; Hsieh, K.; Cheng, K.; Wu, J.; Ting, Y. Mitigating the allergenicity of peanut allergen Ara h 1 by cold atmospheric pressure argon plasma jet. J. Sci. Food Agric. 2023, 103, 3017–3027. [Google Scholar] [CrossRef]
- Romero, A.; Sartori, A.; Caetano-Silva, M.; Alencar, S.; Calori, M.; Augusto, P. Ozone processing of peanut “milk”: Degradation of aflatoxins, impact on quality attributes and the potential effect on peanut allergens. J. Clean. Prod. 2023, 405, 136950. [Google Scholar] [CrossRef]
- Pi, X.; Yang, Y.; Sun, Y.; Wang, X.; Wan, Y.; Fu, G.; Li, X.; Cheng, J. Food irradiation: A promising technology to produce hypoallergenic food with high quality. Crit. Rev. Food Sci. Nutr. 2021, 62, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, J.; Chen, K.; Li, Q.; Wang, T.; Luo, T.; Jiang, S. The effects of ultrasound-assisted glycation on the allergenicity and functional properties of peanut proteins. Int. J. Biol. Macromol. 2024, 282, 136664. [Google Scholar] [CrossRef] [PubMed]
- Bedford, B.; Liggans, G.; Williams, L.; Jackson, L. Allergen removal and transfer with wiping and cleaning methods used in retail and food service establishments. J. Food Prot. 2020, 83, 1248–1260. [Google Scholar] [CrossRef]
- Watson, W.; Woodrow, A.; Stadnyk, A. Removal of peanut allergen Ara h 1 from common hospital surfaces, toys and books using standard cleaning methods. Allergy Asthma Clin. Immunol. 2015, 11, 4. [Google Scholar] [CrossRef]
- Perry, T.; Conover-Walker, M.; Pomés, A.; Chapman, M.; Wood, R. Distribution of peanut allergen in the environment. J. Allergy Clin. Immunol. 2004, 113, 973–976. [Google Scholar] [CrossRef]
- Da Silva, E.; Varraso, R.; Lenzotti, A.; Fezeu, L.; Sit, G.; Galan, P.; Hercberg, S.; Touvier, M.; Paris, C.; Dumas, O. Others Household Use of Green Cleaning Products, Disinfecting Wipes, and Asthma Control Among Adults. J. Allergy Clin. Immunol. Pract. 2024, 12, 919–926. [Google Scholar] [CrossRef]
- Ayass, M.; Tripathi, T.; Griko, N.; Ramankutty Nair, R.; Okyay, T.; Zhang, J.; Zhu, K.; Melendez, K.; Pashkov, V.; Abi-Mosleh, L. AYA22A Aptamers Mitigate Peanut Allergenicity: Insights from Degranulation Assays and Modulating Immune Responses. Allergies 2024, 4, 94–123. [Google Scholar] [CrossRef]
- Morita, Y.; Siraganian, R. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J. Immunol. 1981, 127, 1339–1344. [Google Scholar] [PubMed]
- Ayass, M.; Tripathi, T.; Griko, N.; Pashkov, V.; Dai, J.; Zhang, J.; Herbert, F.; Nair, R.R.; Okyay, T.; Zhu, K.; et al. Highly efficacious and safe neutralizing DNA aptamer of SARS-CoV-2 as an emerging therapy for COVID-19 disease. Virol. J. 2022, 19, 227. [Google Scholar] [PubMed]
- Thiel, W.; Esposito, C.; Dickey, D.; Dassie, J.; Long, M.; Adam, J.; Streeter, J.; Schickling, B.; Takapoo, M.; Flenker, K. Others Smooth muscle cell–targeted RNA aptamer inhibits neointimal formation. Mol. Ther. 2016, 24, 779–787. [Google Scholar] [PubMed]
- Kummrow, A.; Frankowski, M.; Bock, N.; Werner, C.; Dziekan, T.; Neukammer, J. Quantitative assessment of cell viability based on flow cytometry and microscopy. Cytom. Part A 2013, 83, 197–204. [Google Scholar]
- Rieger, A.; Nelson, K.; Konowalchuk, J.; Barreda, D. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. JoVE 2011, 50, 2597. [Google Scholar]
- Ayass, M.; Griko, N.; Pashkov, V.; Tripathi, T.; Zhang, J.; Nair, R.R.; Okyay, T.; Zhu, K.; Abi-Mosleh, L. New high-affinity thrombin aptamers for advancing coagulation therapy: Balancing thrombin inhibition for clot prevention and effective bleeding management with antidote. Cells 2023, 12, 2230. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, X.; Tang, B.; Chang, C.; Liu, G.; Feng, L.; Yu, Y.; Zhang, D.; Hou, J. Tween-20 transiently changes the surface morphology of PK-15 cells and improves PCV2 infection. BMC Vet. Res. 2018, 14, 138. [Google Scholar] [CrossRef]
- Brough, H.; Makinson, K.; Penagos, M.; Maleki, S.; Cheng, H.; Douiri, A.; Stephens, A.; Turcanu, V.; Lack, G. Distribution of peanut protein in the home environment. J. Allergy Clin. Immunol. 2013, 132, 623–629. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Shin, J.; Shim, W. Cleaning Methods to Effectively Remove Peanut Allergens from Food Facilities or Utensil Surfaces. J. Food Hyg. Saf. 2023, 38, 228–235. [Google Scholar]
- Marriott, N.; Schilling, M.; Gravani, R.; Marriott, N.; Schilling, M.; Gravani, R. The Relationship of Allergens to Sanitation. In Principles of Food Sanitation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 73–81. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).