Allergic Disorders and Systemic Lupus Erythematosus: Common Pathogenesis and Caveats in Management
Abstract
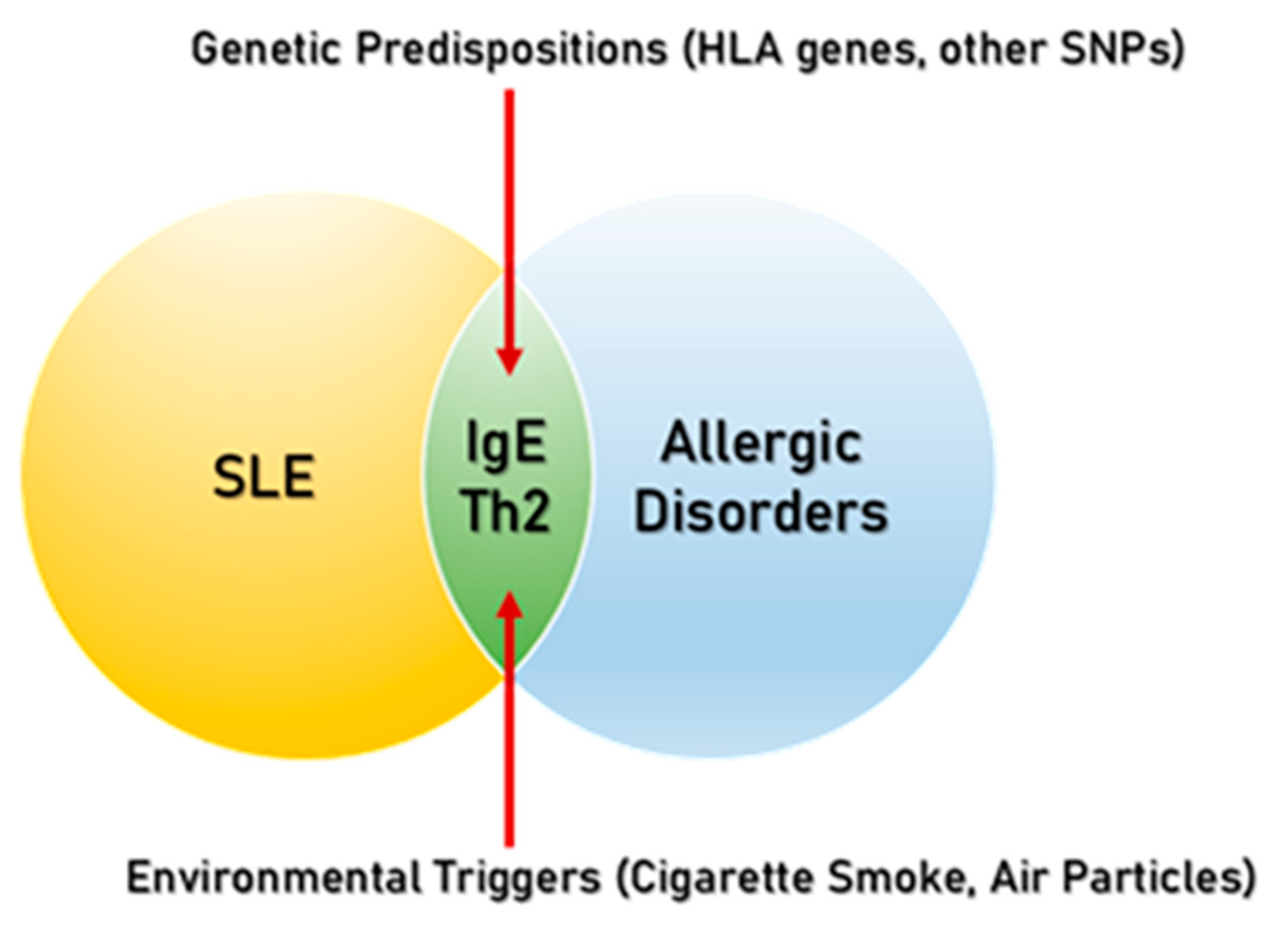
1. Introduction
2. Associations Between Allergic Disorders and SLE
2.1. Allergic Rhinitis (AR) and SLE
2.2. Asthma and SLE
3. Immunoglobulin E in Allergic Disorders and SLE
4. Common Risk Factors of Allergic Disorders and SLE
5. Treatment Options in Allergic Disorders and SLE
5.1. Allergy Therapy and SLE
5.1.1. Allergen Immunotherapy
5.1.2. Omalizumab
5.1.3. Baricitinib
5.2. SLE Treatments and Allergic Disorders
5.2.1. Hydroxychloroquine
5.2.2. Methotrexate
5.2.3. Azathioprine
5.2.4. Mycophenolate Mofetil
5.2.5. Anifrolumab
5.2.6. Belimumab
5.2.7. Rituximab
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bach, J.-F. The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Duarte-García, A.; Hocaoglu, M.; Valenzuela-Almada, M.; Osei-Onomah, S.-A.; Dabit, J.Y.; Sanchez-Rodriguez, A.; Duong, S.Q.; Giblon, R.E.; Langenfeld, H.E.; Alarcón, G.S.; et al. Rising Incidence and Prevalence of Systemic Lupus Erythematosus: A Population-Based Study over Four Decades. Ann. Rheum. Dis. 2022, 81, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Zheng, M.; Lou, H.F.; Wang, C.S.; Zhang, Y.; Bo, M.Y.; Ge, S.Q.; Zhang, N.; Zhang, L.; Bachert, C. An Increased Prevalence of Self-Reported Allergic Rhinitis in Major Chinese Cities from 2005 to 2011. Allergy 2016, 71, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.E.; Barr, S.G.; Clarke, A.E. The Global Burden of SLE: Prevalence, Health Disparities and Socioeconomic Impact. Nat. Rev. Rheumatol. 2016, 12, 605–620. [Google Scholar] [CrossRef]
- Romagnani, S. Immunologic Influences on Allergy and the TH1/TH2 Balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef]
- Krishna, M.T.; Subramanian, A.; Adderley, N.J.; Zemedikun, D.T.; Gkoutos, G.V.; Nirantharakumar, K. Allergic Diseases and Long-Term Risk of Autoimmune Disorders: Longitudinal Cohort Study and Cluster Analysis. Eur. Respir. J. 2019, 54, 1900476. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The Immunology of the Allergy Epidemic and the Hygiene Hypothesis. Nat. Immunol. 2017, 18, 1076–1083. [Google Scholar] [CrossRef]
- Ga, R.; Mgl, W.; Chris, W. The Role of Immunometabolism in the Pathogenesis of Systemic Lupus Erythematosus. Front. Immunol. 2022, 12, 806560. [Google Scholar] [CrossRef]
- Rekvig, O.P. Autoimmunity and SLE: Factual and Semantic Evidence-Based Critical Analyses of Definitions, Etiology, and Pathogenesis. Front. Immunol. 2020, 11, 569234. [Google Scholar] [CrossRef]
- Accapezzato, D.; Caccavale, R.; Paroli, M.P.; Gioia, C.; Nguyen, B.L.; Spadea, L.; Paroli, M. Advances in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 6578. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of Allergen-Specific Immunotherapy: Multiple Suppressor Factors at Work in Immune Tolerance to Allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, J.F.; Cesic, D.; Keser, G.; Bukelica, M.; Karanagnostis, S.; Khamashta, M.A.; Hughes, G.R. Allergic Disorders in Systemic Lupus Erythematosus. Lupus 1993, 2, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Lorber, M. Allergy and SLE: Common and Variable. Isr. J. Med. Sci. 1997, 33, 147–149. [Google Scholar]
- Elkayam, O.; Tamir, R.; Pick, A.I.; Wysenbeek, A. Serum IgE Concentrations, Disease Activity, and Atopic Disorders in Systemic Lupus Erythematosus. Allergy 1995, 50, 94–96. [Google Scholar]
- Morton, S.; Palmer, B.; Muir, K.; Powell, R.J. IgE and Non-IgE Mediated Allergic Disorders in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 1998, 57, 660–663. [Google Scholar] [CrossRef]
- Sekigawa, I.; Yoshiike, T.; Iida, N.; Hashimoto, H.; Ogawa, H. Allergic Disorders in Systemic Lupus Erythematosus: Prevalence and Family History. Lupus 2002, 11, 426–429. [Google Scholar] [CrossRef]
- Ponvilawan, B.; Charoenngam, N.; Wongtrakul, W.; Ungprasert, P. Association of Atopic Dermatitis with an Increased Risk of Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. J. Postgrad. Med. 2021, 67, 139–145. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Muo, C.-H.; Sung, F.-C. Associations of Chronic Urticaria with Atopic and Autoimmune Comorbidities: A Nationwide Population-Based Study. Int. J. Dermatol. 2018, 57, 822–829. [Google Scholar] [CrossRef]
- Wongtrakul, W.; Charoenngam, N.; Ponvilawan, B.; Ungprasert, P. Allergic Rhinitis and Risk of Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Int. J. Rheum. Dis. 2020, 23, 1460–1467. [Google Scholar] [CrossRef]
- Charoenngam, N.; Ponvilawan, B.; Wongtrakul, W.; Ungprasert, P. Patients with Asthma Have a Higher Risk of Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2021, 40, 529–536. [Google Scholar] [CrossRef]
- Chan, S.C.W.; Yeung, W.W.Y.; Wong, J.C.Y.; Chui, E.S.H.; Lee, M.S.H.; Chung, H.Y.; Cheung, T.T.; Lau, C.S.; Li, P.H. Prevalence and Impact of Reported Drug Allergies among Rheumatology Patients. Diagnostics 2020, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-P.; Tsai, J.-D.; Muo, C.-H.; Tsai, C.-H.; Sung, F.-C.; Liao, Y.-T.; Chang, Y.-J.; Yang, J.-H. Atopic Diseases and Systemic Lupus Erythematosus: An Epidemiological Study of the Risks and Correlations. Int. J. Environ. Res. Public Health 2014, 11, 8112–8122. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hung, P.-H.; Hu, H.-Y.; Chung, C.-J.; Chen, T.-H.; Hung, K.-Y. Clinically Diagnosed Urticaria and Risk of Systemic Lupus Erythematosus in Children: A Nationwide Population-Based Case-Control Study. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2018, 29, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.B.; Nelsen, L.; Hawes, J.C.L.; Holick, C.N.; Maloney, J.; Mehta, V.; Mines, D.; Pianka, G.; Lanes, S.F. Autoimmune Disorders in Allergen Immunotherapy, Allergic Rhinitis, and Non-Allergic Patients. Eur. Respir. J. 2014, 44, 3472. [Google Scholar]
- Suebsarakam, P.; Kaweeyanont, K.; Srisutthikamol, S.; Mairiang, D. Coexistence of Allergic Diseases in Pediatric Systemic Lupus Erythematosus Patients: Prevalence, Clinical Manifestation and Severity. Glob. Pediatr. Health 2024, 11, 2333794X241251615. [Google Scholar] [CrossRef]
- Shen, T.-C.; Tu, C.-Y.; Lin, C.-L.; Wei, C.-C.; Li, Y.-F. Increased Risk of Asthma in Patients with Systemic Lupus Erythematosus. Am. J. Respir. Crit. Care Med. 2014, 189, 496–499. [Google Scholar] [CrossRef]
- Rossides, M.; Nguyen, C.; Arkema, E.V.; Simard, J.F. Asthma in Children of Mothers with Systemic Lupus Erythematosus and the Role of Preterm Birth. Arthritis Care Res. 2018, 70, 1269–1274. [Google Scholar] [CrossRef]
- Lindau, D.; Mussard, J.; Rabsteyn, A.; Ribon, M.; Kötter, I.; Igney, A.; Adema, G.J.; Boissier, M.-C.; Rammensee, H.-G.; Decker, P. TLR9 Independent Interferon α Production by Neutrophils on NETosis in Response to Circulating Chromatin, a Key Lupus Autoantigen. Ann. Rheum. Dis. 2014, 73, 2199–2207. [Google Scholar] [CrossRef]
- Hjorton, K.; Hagberg, N.; Israelsson, E.; Jinton, L.; Berggren, O.; Sandling, J.K.; Thörn, K.; Mo, J.; DISSECT consortium; Eloranta, M.-L.; et al. Cytokine Production by Activated Plasmacytoid Dendritic Cells and Natural Killer Cells Is Suppressed by an IRAK4 Inhibitor. Arthritis Res. Ther. 2018, 20, 238. [Google Scholar] [CrossRef]
- Corzo, C.A.; Varfolomeev, E.; Setiadi, A.F.; Francis, R.; Klabunde, S.; Senger, K.; Sujatha-Bhaskar, S.; Drobnick, J.; Do, S.; Suto, E.; et al. The Kinase IRAK4 Promotes Endosomal TLR and Immune Complex Signaling in B Cells and Plasmacytoid Dendritic Cells. Sci. Signal. 2020, 13, eaaz1053. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting Neutrophils Are Major Inducers of Type I IFN Production in Pediatric Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel; Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; et al. Atopic Dermatitis (Eczema) Guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-Based Recommendations. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Shamji, M.H. Allergen Immunotherapy: Past, Present and Future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Valenta, R.; Mittermann, I.; Werfel, T.; Garn, H.; Renz, H. Linking Allergy to Autoimmune Disease. Trends Immunol. 2009, 30, 109–116. [Google Scholar] [CrossRef]
- Sin, E.; Anand, P.; Frieri, M. A Link: Allergic Rhinitis, Asthma & Systemic Lupus Erythematosus. Autoimmun. Rev. 2016, 15, 487–491. [Google Scholar] [CrossRef]
- Gruber, B.L.; Kaufman, L.D.; Marchese, M.J.; Roth, W.; Kaplan, A.P. Anti-Ige Autoantibodies in Systemic Lupus Erythematosus. Arthritis Rheum. 1988, 31, 1000–1006. [Google Scholar] [CrossRef]
- Wozniacka, A.; Sysa-Jedrzejowska, A.; Robak, E.; Samochocki, Z.; Zak-Prelich, M. Allergic Diseases, Drug Adverse Reactions and Total Immunoglobulin E Levels in Lupus Erythematosus Patients. Mediators Inflamm. 2003, 12, 95–99. [Google Scholar] [CrossRef]
- Brilland, B.; Scherlinger, M.; Khoryati, L.; Goret, J.; Duffau, P.; Estibaliz, L.; Manon, C.; Vivien, G.; Christophe, R.; Patrick, B. Platelets and IgE: Shaping the Innate Immune Response in Systemic Lupus Erythematosus. Clin. Rev. Allergy Immunol. 2020, 58, 194–212. [Google Scholar] [CrossRef]
- Charles, N.; Hardwick, D.; Daugas, E.; Illei, G.G.; Rivera, J. Basophils and the T Helper 2 Environment Can Promote the Development of Lupus Nephritis. Nat. Med. 2010, 16, 701–707. [Google Scholar] [CrossRef]
- Dema, B.; Pellefigues, C.; Hasni, S.; Gault, N.; Jiang, C.; Ricks, T.K.; Bonelli, M.M.; Scheffel, J.; Sacré, K.; Jablonski, M.; et al. Autoreactive IgE Is Prevalent in Systemic Lupus Erythematosus and Is Associated with Increased Disease Activity and Nephritis. PLoS ONE 2014, 9, e90424. [Google Scholar] [CrossRef] [PubMed]
- Henault, J.; Riggs, J.M.; Karnell, J.L.; Liarski, V.M.; Li, J.; Shirinian, L.; Xu, L.; Casey, K.A.; Smith, M.A.; Khatry, D.B.; et al. Self-Reactive IgE Exacerbates Interferon Responses Associated with Autoimmunity. Nat. Immunol. 2016, 17, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Brown, E.E.; Kimberly, R.P.; Langefeld, C.D. Genetic Factors Predisposing to Systemic Lupus Erythematosus and Lupus Nephritis. Semin. Nephrol. 2010, 30, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, B.M.; Al-Hadithi, A.T.R.; Raouf, H.; Zalzala, H.H.; Abid, L.A.; Nehad, Z. Effect of HLA on Development of Asthma. Ann. Med. Surg. 2018, 36, 118–121. [Google Scholar] [CrossRef]
- Kreiner, E.; Waage, J.; Standl, M.; Brix, S.; Pers, T.H.; Couto Alves, A.; Warrington, N.M.; Tiesler, C.M.T.; Fuertes, E.; Franke, L.; et al. Shared Genetic Variants Suggest Common Pathways in Allergy and Autoimmune Diseases. J. Allergy Clin. Immunol. 2017, 140, 771–781. [Google Scholar] [CrossRef]
- Chen, M.; Meng, Y.; Shi, X.; Zhu, C.; Zhu, M.; Tang, H.; Zheng, H. Identification of ENTPD1 as a Novel Biomarker Linking Allergic Rhinitis and Systemic Lupus Erythematosus. Sci. Rep. 2024, 14, 18266. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Li, Z. Association Between Immune-Related Disease and Allergic Rhinitis: A Two-Sample Mendelian Randomization Study. Am. J. Rhinol. Allergy 2024, 38, 31–37. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Kim, D.J.; Peerzada, J.; Lockman, S.; Nobles-Knight, D.; Petri, M.; Karlson, E.W. Cigarette Smoking and the Risk of Systemic Lupus Erythematosus: A Meta-Analysis. Arthritis Rheum. 2004, 50, 849–857. [Google Scholar] [CrossRef]
- Speyer, C.B.; Costenbader, K.H. Cigarette Smoking and the Pathogenesis of Systemic Lupus Erythematosus. Expert Rev. Clin. Immunol. 2018, 14, 481–487. [Google Scholar] [CrossRef]
- Stapleton, M.; Howard-Thompson, A.; George, C.; Hoover, R.M.; Self, T.H. Smoking and Asthma. J. Am. Board Fam. Med. 2011, 24, 313–322. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Li, J.; Deng, X.; Dai, T.; Ji, Q.; Xiong, D.; Xie, H. Environmental Risk Factors, Protective Factors, and Biomarkers for Allergic Rhinitis: A Systematic Umbrella Review of the Evidence. Clin. Rev. Allergy Immunol. 2023, 65, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Brunner, H.I. Environmental Triggers in Systemic Lupus Erythematosus. Semin. Arthritis Rheum. 2018, 47, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. The Epithelial Barrier Hypothesis Proposes a Comprehensive Understanding of the Origins of Allergic and Other Chronic Noncommunicable Diseases. J. Allergy Clin. Immunol. 2022, 149, 41–44. [Google Scholar] [CrossRef]
- Quaglia, M.; Merlotti, G.; De Andrea, M.; Borgogna, C.; Cantaluppi, V. Viral Infections and Systemic Lupus Erythematosus: New Players in an Old Story. Viruses 2021, 13, 277. [Google Scholar] [CrossRef]
- Klimek, L.; Kündig, T.; Kramer, M.F.; Guethoff, S.; Jensen-Jarolim, E.; Schmidt-Weber, C.B.; Palomares, O.; Mohsen, M.O.; Jakob, T.; Bachmann, M. Virus-like Particles (VLP) in Prophylaxis and Immunotherapy of Allergic Diseases. Allergo J. Int. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Edwards, M.R.; Strong, K.; Cameron, A.; Walton, R.P.; Jackson, D.J.; Johnston, S.L. Viral Infections in Allergy and Immunology: How Allergic Inflammation Influences Viral Infections and Illness. J. Allergy Clin. Immunol. 2017, 140, 909–920. [Google Scholar] [CrossRef]
- Nelson, P.; Rylance, P.; Roden, D.; Trela, M.; Tugnet, N. Viruses as Potential Pathogenic Agents in Systemic Lupus Erythematosus. Lupus 2014, 23, 596–605. [Google Scholar] [CrossRef]
- Pan, Q.; Chen, J.; Guo, L.; Lu, X.; Liao, S.; Zhao, C.; Wang, S.; Liu, H. Mechanistic Insights into Environmental and Genetic Risk Factors for Systemic Lupus Erythematosus. Am. J. Transl. Res. 2019, 11, 1241–1254. [Google Scholar]
- Zimmer, A.; Bouley, J.; Le Mignon, M.; Pliquet, E.; Horiot, S.; Turfkruyer, M.; Baron-Bodo, V.; Horak, F.; Nony, E.; Louise, A.; et al. A Regulatory Dendritic Cell Signature Correlates with the Clinical Efficacy of Allergen-Specific Sublingual Immunotherapy. J. Allergy Clin. Immunol. 2012, 129, 1020–1030. [Google Scholar] [CrossRef]
- Gueguen, C.; Bouley, J.; Moussu, H.; Luce, S.; Duchateau, M.; Chamot-Rooke, J.; Pallardy, M.; Lombardi, V.; Nony, E.; Baron-Bodo, V.; et al. Changes in Markers Associated with Dendritic Cells Driving the Differentiation of Either TH2 Cells or Regulatory T Cells Correlate with Clinical Benefit during Allergen Immunotherapy. J. Allergy Clin. Immunol. 2016, 137, 545–558. [Google Scholar] [CrossRef]
- Ling, E.M.; Smith, T.; Nguyen, X.D.; Pridgeon, C.; Dallman, M.; Arbery, J.; Carr, V.A.; Robinson, D.S. Relation of CD4+CD25+ Regulatory T-Cell Suppression of Allergen-Driven T-Cell Activation to Atopic Status and Expression of Allergic Disease. Lancet 2004, 363, 608–615. [Google Scholar] [CrossRef]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; Gerth van Wijk, R.; Halken, S.; Larenas-Linnemann, D.; Pawankar, R.; Pitsios, C.; et al. EAACI Guidelines on Allergen Immunotherapy: Allergic Rhinoconjunctivitis. Allergy 2018, 73, 765–798. [Google Scholar] [CrossRef]
- Fujioka, K.; Kasahara, A.; Kida, T.; Fujii, W.; Seno, T.; Wada, M.; Kohno, M.; Kawahito, Y. Effectiveness and Safety of Allergen Immunotherapy in Patients with Allergic Rhinitis Complicated by Rheumatic Autoimmune Diseases: A Case Series Study. Allergy Asthma Clin. Immunol. 2022, 18, 63. [Google Scholar] [CrossRef]
- Bozek, A.; Mućka, S.; Miodonska, M.; Zlik, A.; Mroz-Dybowska, M. Effect of Sublingual Immunotherapy on Clinical and Laboratory Autoimmunity. Immunotherapy 2024, 16, 235–241. [Google Scholar] [CrossRef]
- Robinson, S.; Thomas, R. Potential for Antigen-Specific Tolerizing Immunotherapy in Systematic Lupus Erythematosus. Front. Immunol. 2021, 12, 654701. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Pioggia, G.; Navarra, M.; Gangemi, S. Omalizumab for the Treatment of Chronic Idiopathic Urticaria: Systematic Review of the Literature. Pharmacotherapy 2017, 37, 464–480. [Google Scholar] [CrossRef]
- D’Amato, G.; Salzillo, A.; Piccolo, A.; D’Amato, M.; Liccardi, G. A Review of Anti-IgE Monoclonal Antibody (Omalizumab) as Add on Therapy for Severe Allergic (IgE-Mediated) Asthma. Ther. Clin. Risk Manag. 2007, 3, 613–619. [Google Scholar] [CrossRef]
- Hasni, S.; Gupta, S.; Davis, M.; Poncio, E.; Temesgen-Oyelakin, Y.; Joyal, E.; Fike, A.; Manna, Z.; Auh, S.; Shi, Y.; et al. Safety and Tolerability of Omalizumab: A Randomized Clinical Trial of Humanized Anti-IgE Monoclonal Antibody in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1135–1140. [Google Scholar] [CrossRef]
- Petri, M.; Bruce, I.N.; Dörner, T.; Tanaka, Y.; Morand, E.F.; Kalunian, K.C.; Cardiel, M.H.; Silk, M.E.; Dickson, C.L.; Meszaros, G.; et al. Baricitinib for Systemic Lupus Erythematosus: A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial (SLE-BRAVE-II). Lancet Lond. Engl. 2023, 401, 1011–1019. [Google Scholar] [CrossRef]
- Morand, E.F.; Vital, E.M.; Petri, M.; van Vollenhoven, R.; Wallace, D.J.; Mosca, M.; Furie, R.A.; Silk, M.E.; Dickson, C.L.; Meszaros, G.; et al. Baricitinib for Systemic Lupus Erythematosus: A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial (SLE-BRAVE-I). Lancet Lond. Engl. 2023, 401, 1001–1010. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.-C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR Recommendations for the Management of Systemic Lupus Erythematosus: 2023 Update. Ann. Rheum. Dis. 2024, 83, 15–29. [Google Scholar] [CrossRef]
- Fox, R.I. Mechanism of Action of Hydroxychloroquine as an Antirheumatic Drug. Semin. Arthritis Rheum. 1993, 23, 82–91. [Google Scholar] [CrossRef]
- Lu, S.; Sung, T.; Lin, N.; Abraham, R.T.; Jessen, B.A. Lysosomal Adaptation: How Cells Respond to Lysosomotropic Compounds. PLoS ONE 2017, 12, e0173771. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine Inhibits Autophagic Flux by Decreasing Autophagosome-Lysosome Fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Goldstein, J.A. Hydroxychloroquine for Asthma. Am. Rev. Respir. Dis. 1983, 128, 1100–1101. [Google Scholar] [CrossRef]
- Roberts, J.A.; Gunneberg, A.; Elliott, J.A.; Thomson, N.C. Hydroxychloroquine in Steroid Dependent Asthma. Pulm. Pharmacol. 1988, 1, 59–61. [Google Scholar] [CrossRef]
- Charous, B.L.; Halpern, E.F.; Steven, G.C. Hydroxychloroquine Improves Airflow and Lowers Circulating IgE Levels in Subjects with Moderate Symptomatic Asthma. J. Allergy Clin. Immunol. 1998, 102, 198–203. [Google Scholar] [CrossRef]
- Bonzano, L.; Cassone, G.; Tarallo, L.; Pellacani, G. The Rediscovery of Hydroxychloroquine in Allergic Diseases in the COVID-19 Era. J. Investig. Allergol. Clin. Immunol. 2021, 31, 85–86. [Google Scholar] [CrossRef]
- Ban, G.-Y.; Pham, D.L.; Trinh, T.H.K.; Lee, S.-I.; Suh, D.-H.; Yang, E.-M.; Ye, Y.-M.; Shin, Y.S.; Chwae, Y.-J.; Park, H.-S. Autophagy Mechanisms in Sputum and Peripheral Blood Cells of Patients with Severe Asthma: A New Therapeutic Target. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2016, 46, 48–59. [Google Scholar] [CrossRef]
- Adam, D.N.; Gooderham, M.J.; Beecker, J.R.; Hong, C.H.; Jack, C.S.; Jain, V.; Lansang, P.; Lynde, C.W.; Papp, K.A.; Prajapati, V.H.; et al. Expert Consensus on the Systemic Treatment of Atopic Dermatitis in Special Populations. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1135–1148. [Google Scholar] [CrossRef]
- Caron, A.G.M.; Bloem, M.; El Khattabi, H.; de Waal, A.C.; van Huizen, A.M.; Denswil, N.P.; Gerbens, L.A.A.; Spuls, P.I. The Wide Variety of Methotrexate Dosing Regimens for the Treatment of Atopic Dermatitis: A Systematic Review. J. Dermatol. Treat. 2024, 35, 2292962. [Google Scholar] [CrossRef]
- Davies, H.; Olson, L.; Gibson, P. Methotrexate as a Steroid Sparing Agent for Asthma in Adults. Cochrane Database Syst. Rev. 2000, 1998, CD000391. [Google Scholar] [CrossRef]
- Comet, R.; Domingo, C.; Larrosa, M.; Morón, A.; Rué, M.; Amengual, M.-J.; Marín, A. Benefits of Low Weekly Doses of Methotrexate in Steroid-Dependent Asthmatic Patients. A Double-Blind, Randomized, Placebo-Controlled Study. Respir. Med. 2006, 100, 411–419. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS Guidelines on Definition, Evaluation and Treatment of Severe Asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Dean, T.P.; Dewey, A.; Bara, A.; Lasserson, T.J.; Walters, E.H. Azathioprine as an Oral Corticosteroid Sparing Agent for Asthma. Cochrane Database Syst. Rev. 2003, 4, CD003270. [Google Scholar] [CrossRef]
- Chu, A.W.L.; Wong, M.M.; Rayner, D.G.; Guyatt, G.H.; Díaz Martinez, J.P.; Ceccacci, R.; Zhao, I.X.; McMullen, E.; Srivastava, A.; Wang, J.; et al. Systemic Treatments for Atopic Dermatitis (Eczema): Systematic Review and Network Meta-Analysis of Randomized Trials. J. Allergy Clin. Immunol. 2023, 152, 1470–1492. [Google Scholar] [CrossRef]
- Kravčenia, B.; Maślanka, T. Mycophenolate Mofetil, an Inhibitor of Inosine Monophosphate Dehydrogenase, and Tofacitinib, a Janus Kinase Inhibitor, Attenuate Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma. Molecules 2024, 29, 5293. [Google Scholar] [CrossRef]
- Frémond, M.-L.; David, C.; Richez, C. Anifrolumab: The New Frontier in the Treatment of Genetic Interferonopathies. RMD Open 2024, 10, e004780. [Google Scholar] [CrossRef]
- Gonzales-van Horn, S.R.; Farrar, J.D. Interferon at the Crossroads of Allergy and Viral Infections. J. Leukoc. Biol. 2015, 98, 185–194. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S.; et al. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cancro, M.P.; D’Cruz, D.P.; Khamashta, M.A. The Role of B Lymphocyte Stimulator (BLyS) in Systemic Lupus Erythematosus. J. Clin. Investig. 2009, 119, 1066–1073. [Google Scholar] [CrossRef]
- Levy, R.A.; Gonzalez-Rivera, T.; Khamashta, M.; Fox, N.L.; Jones-Leone, A.; Rubin, B.; Burriss, S.W.; Gairy, K.; van Maurik, A.; Roth, D.A. 10 Years of Belimumab Experience: What Have We Learnt? Lupus 2021, 30, 1705–1721. [Google Scholar] [CrossRef]
- Alturaiki, W. The Roles of B Cell Activation Factor (BAFF) and a Proliferation-Inducing Ligand (APRIL) in Allergic Asthma. Immunol. Lett. 2020, 225, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Casal Moura, M.; Berti, A.; Keogh, K.A.; Volcheck, G.W.; Specks, U.; Baqir, M. Asthma Control in Eosinophilic Granulomatosis with Polyangiitis Treated with Rituximab. Clin. Rheumatol. 2020, 39, 1581–1590. [Google Scholar] [CrossRef]
- Asproudis, I.; Kanari, M.; Ntountas, I.; Ragos, V.; Goussia, A.; Batistatou, A.; Voulgari, P.V. Successful Treatment with Rituximab of IgG4-Related Disease Coexisting with Adult-Onset Asthma and Periocular Xanthogranuloma. Rheumatol. Int. 2020, 40, 671–677. [Google Scholar] [CrossRef]
- Baqir, M.; Garrity, J.A.; Vassallo, R.; Witzig, T.E.; Ryu, J.H. Asthma and Orbital Immunoglobulin G4-Related Disease. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2016, 116, 313–316. [Google Scholar] [CrossRef]
| Allergic Disorder | Incident Risk |
|---|---|
| Allergic rhinitis | 1.4 |
| Asthma | 1.37~2.54 |
| Allergic conjunctivitis | 1.43 |
| Atopic dermatitis | 1.46~2.13 |
| Immune Cells | Allergic Disorders | SLE |
|---|---|---|
| B-cells | IgE production | Autoantibody production Long-lived plasma cells |
| Th1 cells | - | Promotion of oxidative stress Interferon-γ (IFNγ) production |
| Th2 cells | Stimulate B-cells via IL-4, IL-13 to stimulate B-cell class switching to IgE Eosinophil recruitment | Decrease in IL-4-producing cells |
| Th17 cells | - | Increased IL-17 production |
| Regulatory T-cells | - | Unclear |
| T-follicular cells | - | Involved in autoreactive B-cells |
| CD8+ T-cells | - | Impaired cytolytic function |
| γδ-T-cells | - | High levels in SLE |
| Neutrophils | - | Reduced phagocytosis Reduced removal of apoptotic cells Type 1 interferon (IFN-1) production |
| Dendritic cells | Allergen detection and stimulation of Th2 cells | Plasmacytoid dendritic cells produce high levels of IFN-1 |
| Mast cells | Activated by IgE Release of cytokines, prostaglandins, leukotrienes and histamines | - |
| Medication Used in SLE | Allergic Rhinitis | Asthma | Atopic Dermatitis |
|---|---|---|---|
| Hydroxychloroquine | Not studied * | Possible benefit | Not studied |
| Methotrexate | Not studied | Steroid-sparing, high risk profile | Off-label use |
| Azathioprine | Not studied | No steroid-sparing effect | May have benefit High risk profile |
| Mycophenolate mofetil | Not studied | Possible benefit | Steroid-sparing, high risk profile |
| Anifrolumab | Not studied | Theoretical harm | Not studied |
| Belimumab | Not studied | Theoretical benefit | Not studied |
| Rituximab | Not studied | Possible benefit | Not studied |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.-J.; Mustafa Ali, S.; Khianey, R.; Mikdashi, J. Allergic Disorders and Systemic Lupus Erythematosus: Common Pathogenesis and Caveats in Management. Allergies 2025, 5, 10. https://doi.org/10.3390/allergies5020010
Jung H-J, Mustafa Ali S, Khianey R, Mikdashi J. Allergic Disorders and Systemic Lupus Erythematosus: Common Pathogenesis and Caveats in Management. Allergies. 2025; 5(2):10. https://doi.org/10.3390/allergies5020010
Chicago/Turabian StyleJung, Hee-Jae, Saja Mustafa Ali, Reena Khianey, and Jamal Mikdashi. 2025. "Allergic Disorders and Systemic Lupus Erythematosus: Common Pathogenesis and Caveats in Management" Allergies 5, no. 2: 10. https://doi.org/10.3390/allergies5020010
APA StyleJung, H.-J., Mustafa Ali, S., Khianey, R., & Mikdashi, J. (2025). Allergic Disorders and Systemic Lupus Erythematosus: Common Pathogenesis and Caveats in Management. Allergies, 5(2), 10. https://doi.org/10.3390/allergies5020010





