Abstract
Seafood is a crucial source of nutrients, with global consumption steadily increasing. Among seafood-related allergies, shellfish are a significant cause of food allergy and anaphylaxis worldwide, affecting approximately 0.5–2.5% of the general population. While the majority of existing research has focused on crustaceans, allergic reactions to mollusks, including their clinical characteristics, remain poorly understood. In the Canary Islands, limpets (a type of marine gastropod) are widely consumed as part of the traditional cuisine. Despite isolated reports of limpet allergy, no large-scale studies or comprehensive clinical analyses have been published on this topic. A cohort of patients sensitized to limpets was analyzed: 66 patients were monosensitized to limpets (Group A), while 64 patients demonstrated additional sensitization to other shellfish (Group B). Limpet ingestion was associated with delayed and severe symptoms, including anaphylaxis and severe asthma. Notably, only 11.5% of patients in Group A tested positive for shellfish allergens using ALEX testing compared to 67.9% in Group B. The identification of protein bands in the 25–40 and 50–200 kDa molecular weight ranges in monosensitized patients provides a novel finding that differentiates this study from prior research. Our study represents the largest reported series of patients with documented limpet allergy to date.
1. Introduction
Seafood, encompassing both fish and shellfish, serves as a rich source of essential nutrients, including high-quality proteins and antioxidants, and plays a critical role in human nutrition. Its importance is particularly pronounced in regions where seafood constitutes a dietary staple due to cultural and dietary practices [1,2]. In recent years, the global consumption of seafood has risen substantially, accompanied by a corresponding increase in reported allergic reactions [1,2].
The term “shellfish” generally refers to both crustaceans and mollusks, which are recognized for their nutritional importance. Shellfish belong to the invertebrate group within the Animal Kingdom Eumatozoa, which is divided into four phyla: Mollusca, Arthropoda, Nematoda, and Echinodermata. The phylum Arthropoda includes the subphylum Crustacea, comprising a wide range of crustacean species consumed as food. It also encompasses other classes, such as Arachnida and Insecta, which include arthropods of known allergic relevance (e.g., mites and parasites). Mollusca represents the largest marine phylum, with approximately 85,000 described species [3]. These species are classified into eight distinct classes, although only three are significant for human consumption: Cephalopoda (e.g., cuttlefish, squid, and octopus), Bivalvia (e.g., clams, cockles, mussels, blue mussels, scallops, and oysters), and Gastropoda (e.g., limpets, conchs, periwinkles, sea slugs, whelks, snails, and abalone) [4].
Food allergy (FA) is defined as an adverse immune response triggered by specific foods [5]. The precise prevalence of FA remains challenging to determine; however, systematic reviews estimate that it affects approximately 3.5–4% of the population worldwide [3,4]. Among the major triggers, shellfish ranks as one of the leading causes of FA and anaphylaxis globally, with an estimated prevalence of 0.5–2.5% of the general population. This prevalence varies significantly depending on geographical location and dietary habits [3].
In Spain, shellfish ranks as the third most common cause of FA in adults, with a prevalence of approximately 14.8%; in the Canary Islands, it is specifically 8.1% [6]. Notably, cases of shellfish allergy are increasingly reported in younger individuals, following allergies relative to milk, egg, fruit, and fish [6]. Furthermore, in many Asian countries, including Thailand, Taiwan, Hong Kong, Vietnam, and Singapore, where shellfish consumption is considerably higher, shellfish allergy is among the most prevalent causes of FA across all age groups [7,8].
The coastal regions of Asia are major consumers of crustaceans and mollusks, while Southern Europe, particularly Spain, exhibits a preference for cephalopods, such as octopus and squid, in addition to other types of shellfish. In Japan, diets are characterized by a higher consumption of squid, whereas countries such as Italy, France, Portugal, and Spain include significant quantities of terrestrial gastropods (snails) in their cuisine [3,7]. Despite the growing recognition of mollusk allergies, their prevalence remains poorly defined [9]. This is primarily due to the research emphasis on crustacean allergies, resulting in a notable scarcity of studies focused on mollusk allergies, particularly those involving gastropods [7].
The probability of cross-reactivity between different shellfish and mollusk classes remains poorly understood. Only a limited number of proteins, such as tropomyosin—long regarded as the major allergen implicated in cross-reactivity between shellfish and mites—appear to be shared by crustaceans and mollusks [10]. The reported homology in the protein sequence of tropomyosin within various crustacean families (e.g., prawn, shrimp, lobster) is high; however, the degree of homology between tropomyosins from mollusks is comparatively lower [4,9,10,11,12]. Emerging evidence suggests that additional proteins may play a role in the immunological cross-reactivity of shellfish; however, these proteins have yet to be definitively identified [10].
In the Canary Islands, regional gastronomy prominently features limpets, a type of sea gastropod, as a traditional local dish. Two species are primarily consumed in this region: Patella crenata (black limpet) and Patella aspera (white limpet). While isolated cases of limpet allergy have been reported, no published studies have examined a large cohort of patients with this specific food allergy. Furthermore, there is a lack of in-depth clinical analysis or investigation into the allergenic composition of these two species.
The aim of this study is to analyze the clinical and immunological characteristics of a cohort of patients with local limpet allergy, thereby enhancing the understanding of this emerging condition.
2. Materials and Methods
2.1. Subjects
Patients, both children and adults, presenting with symptoms suggestive of limpet allergy following ingestion were recruited from the Outpatient Allergy Clinic at Hospital Universitario de Canarias (Tenerife, Spain) between February 2022 and February 2023. This open, longitudinal, prospective study was previously approved by the Institutional Ethical Committee of Hospital Universitario de Canarias (Tenerife, Spain; CHUC_2022_10, ECCIAALT). Written informed consent was obtained from and signed by all adult participants, as well as from parents or legal guardians for participants under the age of 18, prior to their inclusion in the current investigation.
2.2. Clinical History
The following clinical data were collected: sociodemographic information (gender, age, and allergic comorbidities) and details of food reactions, including the clinical presentation and severity of symptoms (e.g., anaphylaxis, severe asthma, urticaria, angioedema, oral allergy syndrome, etc.), latency following consumption, emergency room visits, and prescribed treatments. Additionally, patients were evaluated for tolerance or allergy relative to other shellfish, with clinical symptoms recorded following the ingestion of crustaceans, cephalopods, bivalves, or other gastropods. The severity of allergic reactions was assessed by trained allergists based on established guidelines [13]. Moreover, we recorded whether urgent medical intervention was required.
The inclusion criteria involved a clinical history suggestive of allergic symptoms, accompanied by confirmed allergic sensitization, as evidenced by positive skin tests and/or elevated serum-specific IgE (sIgE) levels against gastropods (including limpets and/or terrestrial snails).
Patients were excluded if they lacked a suggestive history of symptoms following limpet ingestion, did not demonstrate positive sIgE results against gastropods, were pregnant, or were undergoing treatment with immunomodulatory agents, including biological therapies or immunosuppressants.
2.3. Skin Tests
The skin prick test (SPT) was carried out in accordance with European standards [14] using two common commercial allergen batteries (Laboratorios Inmunotek®, Madrid, Spain): local aeroallergens (Dermatophagoides pteronyssinnus, Dermatophagoides farinae, Blomia tropicalis, Lepidoglyphus destructor, Tyrophagus putrescentiae, Alternaria alternata, cat and dog epithelium, grass mix—Poa pratensis, Dactilis glomerata, Lolium perenne, Phleum pratense, and Festuca pratensis—Parietaria judaica, and Artemisia vulgaris) and shellfish extracts (shrimp, mussel, clam, squid, and oyster). A skin test was considered positive if the resulting wheal diameter was equal to or greater than 3 mm, with a negative control (saline solution) and a positive control (histamine 10 mg/mL) [14]. The wheal diameters were measured after 20 min.
In the absence of commercial limpet extract, a prick-by-prick skin test was performed using natural food samples on the volar side of each subject’s forearm. Both raw and cooked limpet samples were used for testing [5].
2.4. Limpet Extracts
Four limpet extracts were prepared based on the most frequently consumed species of limpet in our region: raw and cooked black limpet (Patella crenata) and raw and cooked white limpet (Patella aspera). The extracts were prepared by extracting this material in 1/10 wt/vol 0.01 M phosphate-buffered saline buffers (PBS, pH 7.2) for 16 h at 5 ± 3 °C under magnetic stirring. Then, the extracts were centrifuged at 15,000× g for 10 min at 4 °C. Afterwards, the supernatant was recollected, clarified via filtration, and dialyzed. Finally, the native extracts were frozen and lyophilized. The protein content was measured via the Bradford method.
2.5. Serological Analysis
The RAST technique (Radio Allergo Sorbent Test, Pharmacia®, Stockholm, Sweden) was used to determine the presence of seric sIgE (range 0.1 kUA/L–100 kUA/L) against common aeroallergens and shellfish allergens and against terrestrial snails, the only gastropod sIgE available at this moment.
The levels of total seric IgE and sIgE were also measured via a multiplex array (ALEX® MacroArray Diagnostics, Vienna, Austria) according to the manufacturer’s instructions in all included subjects [15]. The ALEX test performed contained 295 reagents—178 molecules and 117 extracts of airborne allergens and cross-reactive food allergens—with the ability to simultaneously measure the concentration of seric sIgE (test range of 0.3–50 kUA/L) and total IgE (test range of 1–2500 kU/L). The different allergens and components are coupled onto polystyrene nanobeads; then, the allergen beads are deposited onto a nitrocellulose membrane, as formerly published [16]. A total of 5 shellfish molecular allergens were included in the ALEX test: Pen m 1, Pen m 2, Pen m 3, Pen m 4, and Cra c 6.
2.6. SDS-PAGE and IgE Western Blot
Proteins from limpet extracts were separated via mini-PROTEAN TGX Stain-Free precast gels (Bio-Rad, Hercules, CA, USA) with sodium dodecylsulfate (SDS-PAGE) under reducing conditions according to Laemmli’s method [17]. Proteins were visualized using the GelCode Blue stain reagent (Life Technologies, Carlsbad, CA, USA).
For Western Blot, proteins from gel electrophoresis were electrotransferred to nitrocellulose membranes of 0.45 µm (Bio-Rad Laboratories, Hercules, CA, USA). Ponceau Red 5% was added to previsualize the bands, and after washing with distilled water, the membrane was blocked with PBS 0.25%–Tween 20 0.5% (blocking solution) for 1 h at room temperature. Then, the membrane was incubated with the corresponding serum overnight at 4 °C.
We performed a two-step assay via Western blot with the aim of analyzing the specific binding of IgE antibodies to allergens. This was the first time Western Blot was carried out using pooled sera from randomly selected patients (16 from Group A and another 16 from Group B) relative to the raw and cooked extracts of both types of limpets: black limpet (Patella crenata) and white limpet (Patella aspera). This was followed by the individual testing of N patients with the same types of limpets, and shrimp extracts were also tested.
2.7. Use of AI-Assisted Tools
OpenAI’s ChatGPT (GPT-4, OpenAI, San Francisco, CA, USA) was exclusively used to assist with text refinement and improving clarity. All content was reviewed and edited by the authors to ensure accuracy, and the final manuscript reflects the author’s original research and conclusions.
3. Results
3.1. Classification of the Study Population
Over a 12-month period from February 2022 to February 2023, we enrolled a total of 130 patients with a suggestive clinical history of allergy symptoms and confirmed allergic sensitization to limpets. Subsequently, individuals were categorized into two groups based on food (shellfish) sensitization: Group A (n = 66), comprising exclusively limpet-sensitized individuals, and Group B (n = 64), consisting of those sensitized to limpets, as well as other shellfish (cephalopods, bivalves, and/or crustaceans).
3.2. Demographic and Clinical Characteristics of Investigated Patients
After categorizing the patients into two groups, the analysis of clinical presentations and demographic data was conducted separately for each group. No statistically significant differences (p > 0.05) were found in demographic data between the groups (Table 1). The majority of participants were young adults, with a median age of <30 years old (p-value = 0.4433), although the range was broad (4–62 years old). Additionally, no significant gender differences were observed (p-value = 0.3003).

Table 1.
Clinical and demographic characteristics between the 2 groups: monosensitized limpet patients (Group A) and patients with limpet and other shellfish allergies (Group B).
3.2.1. Personal History: Respiratory Disease
Additionally, all patients reported a personal history of allergic rhino-conjunctivitis (92.5% Group A and 84.4% Group B) and/or asthma (39.4% Group A and 42.2% Group B). Notably, 100% of our sample was sensitized to mites, with animal epithelia being the second most common aeroallergen. The completed results are summarized in Table 2, as previously described in our region [18].

Table 2.
Results of sensitization relative to local aeroallergens from the skin prick test (SPT) (n = 130).
3.2.2. Food Allergy
Clinical symptoms following limpet ingestion manifested later than anticipated, with a mean onset time of 120 min (range: 5 to 360 min in both groups), and they were typically severe. The incidence of anaphylaxis was significantly (p = 0.0027) higher in Group A (66.7%) compared to Group B (41.2%). This is also reflected in the need for urgent medical assistance, which is required in more than 50% of cases in both groups (Table 1).
In our cohort of patients with limpet allergy, severe bronchospasm occurred following limpet ingestion regardless of a prior asthma diagnosis. In Group A, 40 out of 66 patients (60.6%) had no pre-existing asthma diagnosis, while in Group B, 37 out of 64 patients (57.8%) similarly lacked such a diagnosis. Despite this, respiratory symptoms were observed in both groups. In fact, in Group A, 29 out of 40 patients (72.5%) without previous asthma diagnoses experienced respiratory symptoms after ingesting limpets. This included 7 cases of isolated severe bronchospasm and 22 cases of anaphylaxis (which included bronchospasm). In Group B, 14 out of 37 patients (37.8%) without prior asthma diagnoses also presented with respiratory symptoms, consisting of five cases of isolated severe asthma and nine cases of anaphylaxis (Figure 1).
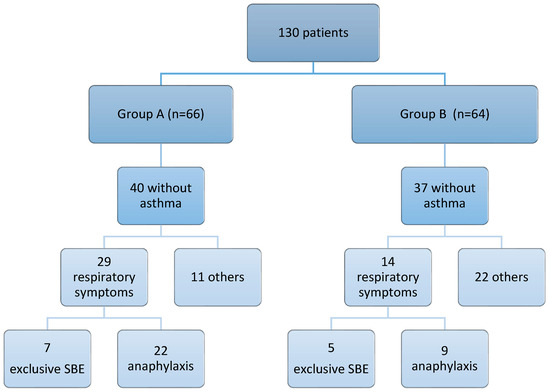
Figure 1.
Summary of respiratory symptoms following ingestion of limpets in patients without previous diagnoses of asthma. SBE: severe bronchospasm; others: urticaria, angioedema, rinoconjuncthivitis, etc.
Regarding the tolerance to other shellfish groups, all patients in Group A strictly avoided limpets following their allergic reaction. Among these patients, 48 out of 66 individuals (72.7%) demonstrated confirmed clinical tolerance to cephalopods, bivalves, and crustaceans. In contrast, 18 out of 66 individuals (27.3%) refrained from consuming any shellfish after their reaction, primarily due to fear of re-experiencing the sensation of impending doom.
In Group B, 34 out of 64 patients (53%) developed symptoms after ingesting limpets. The remaining 30 patients in Group B (47%) exhibited symptoms after consuming other types of shellfish and were incidentally found to be sensitized to limpets despite having never consumed them, as they had intentionally avoided limpet consumption. Furthermore, 14 out of the 34 patients (41.2%) who experienced symptoms following limpet ingestion were able to tolerate cephalopods without further complications after their reaction.
3.3. Skin Tests, Total IgE, and sIgE Reactivity
Regarding the prick-by-prick test with natural limpets, no statistically significant differences were found between the results for raw or cooked limpets in both groups (p = 0.9644 in Group A; p = 0.5894 in Group B) or between the results of Group A and Group B (Table 3). The sensitivity of this cutaneous test as a diagnostic method ranged from 61% to 71%.

Table 3.
Immunological characteristics: total IgE levels, skin test results, and sIgE measurements were compared between Group A and Group B. The prick-by-prick test (P-P) and sIgE levels were assessed, with “+” indicating a positive result.
We found that the levels of total IgE were higher in Group B than in Group A; however, the difference was not statistically significant (p = 0.0525). Additionally, there was a greater recognition of specific IgE (sIgE) relative to terrestrial gastropods (snails) in Group B (91%) compared to Group A (51%) (Table 3).
3.4. Molecular Profile According to Clinical Phenotypes
We then conducted a specific molecular analysis using the multiplex platform ALEX® technique on a random sample of 105 patients (52 from Group A and 53 from Group B). In both groups, we observed distinct patterns of positive results for at least one of the six shellfish allergens included in this technique, with tropomyosin, arginine kinase, and troponin C being the most commonly identified allergens (Table 4).

Table 4.
Comparison of allergen recognition between the monosensitized limpet group (Group A) and the group with allergies to limpets and other shellfish (Group B).
In Group A, only 6 out of 52 patients (11.5%) exhibited a positive detection of shellfish allergens via the ALEX® technique (Table 5). Notably, the most frequently recognized allergen was Cra c 6 (troponin C), and it was identified in 5 out of 52 patients (9.6%) (Table 5). Interestingly, one of these individuals exhibited sensitization for four allergens simultaneously: Pen m 1, Pen m 3, Pen m 4, and Cra c 6 (Table 5).

Table 5.
(1) Selected patients (6 out of 52) with positive results of shellfish allergens sensitization analysis via ALEX® (KU/L) were the group monosensitized to limpets (Group A). (2) Specific IgE profiles aggregated into selected shellfish allergens (6 out of 52 subjects) tested via microarray ALEX (KU/L). Profiles are ordered by the number of recognized molecules. (*) indicates sIgE sensitization to a single shellfish molecular allergen.
In Group B, 36 out of 53 patients (67.9%) exhibit a positive detection of shellfish allergens via the ALEX® technique (Table 6). The most commonly identified allergen was Pen m 1 (tropomyosin), recognized by 25 out of 53 patients, accounting for 47.17% (Table 6). Cra c 6 (troponin C) was identified in 17 out of 53 patients (32.08%), while Pen m 2 (arginine kinase) was recognized in 11 out of 53 patients, constituting 20.75% (Table 6). The majority of patients identified one or two shellfish allergens. Only 4 out of 53 patients recognized three allergens simultaneously: one patient recognized Pen m 1, Pen m 2, and Pen m 3, while three patients recognized Pen m 1, Pen m 2, and Cra c 6 (Table 6).

Table 6.
(1) Selected patients (36 out of 53) with positive results of shellfish allergen sensitization via ALEX® (KU/L) in allergies relative to limpets and other shellfish groups (Group B). (2) Specific IgE profiles aggregated into selected shellfish allergens (6 out of 53 subjects) tested via microarray ALEX® (KU/L). Profiles are ordered by the number of recognized molecules. (*) indicates positive sIgE relative to a single shellfish molecular allergen.
3.5. SDS PAGE and IgE Western Blot
Subsequently, Western Blot was carried out in a first assay using pooled sera from both groups, followed by individual testing using sera from randomly selected patients: 16 monosensitized to limpets (Group A) and another 16 sensitized to limpets and other shellfish (Group B) (Figure 2). The first Western blot analysis revealed a distinctive band recognition pattern among monosensitized patients (Group A) for each analyzed sample.
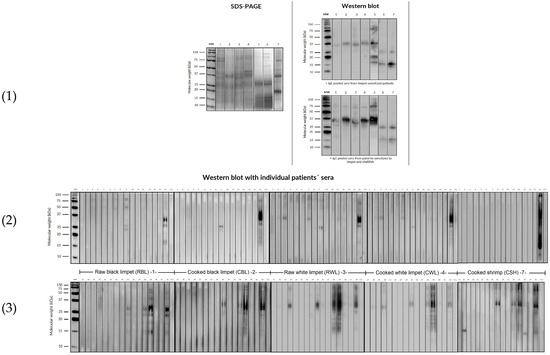
Figure 2.
SDS-PAGE (1) and IgE Western blot (2) under reducing conditions with the sera pool of all patients from Group A and Group B relative to limpet extracts and with the individual sera (3) of 16 random patients from Group A and Group B relative to allergens of raw and cooked black limpets (Patella crenata), raw and cooked white limpets (Patella aspera), and cooked shrimp extract.
Specifically, a band between 36 and 40 kDa was consistently recognized in both raw and cooked limpet extracts, along with weaker bands between 50 and 75 kDa. On the other hand, in the individual assay of the selected six patients, Western blot displayed several band recognitions between 50 and 200 kDa, and the pattern band was more pronounced in raw extracts (contrasting with previous observations in shrimp extracts, where band recognition is typically more prominent in cooked extracts).
In cooked shrimp extracts, while several bands were recognized with pooled sera, upon individual analysis, bands were only observed in one patient. Notably, there was a patient who exhibited a mixture of bands across all extracts, a phenomenon that is challenging to interpret due to clinical tolerance (Figure 2).
Additionally, the Western blot analysis conducted with pooled sera from Group B (patients sensitized to limpets and other shellfish) revealed a band recognition pattern similar to Group A, albeit with greater intensity in Group B. Furthermore, individually, the allergenic profile of the 16 patients sensitized to limpets and other shellfish exhibited recognition of approximately four bands between 15 and 40 kDa in both raw and cooked limpet extracts. Additionally, only five patients recognized bands between 50 and 200 kDa, with this recognition being more pronounced in the raw limpet extracts.
In the case of cooked shrimp extract, it was observed that seven patients recognized some bands between 50 and 200 kDa, although quantification proved challenging (Figure 2).
4. Limitations
Currently, the absence of a specific molecular diagnosis and commercial extract for gastropods, such as limpets, limits the diagnostic process, particularly in geographic regions where limpet consumption is more prevalent. Additionally, in mild cases, many patients may choose not to undergo the oral food challenge, even in instances where they have experienced multiple episodes following isolated consumption of limpets. It is essential to note that our study, conducted in a single center with a limited patient population, primarily reflects the restricted scope of our sample and results.
Additionally, in mild cases, many patients may choose not to undergo the oral food challenge.
5. Discussion
Allergy to gastropods is underreported in the scientific literature, with only a small number of documented cases. This limited documentation may be attributed to the regional nature of limpet consumption [7], as only a few studies have published concise case series specifically addressing patients who are monosensitized to limpets [9,19,20,21,22,23].
The first two reported cases of limpet allergy were documented in 1991 by Carrillo et al. in the Canary Islands [19]. This initial study described two patients who were monosensitized to limpets, and both experienced anaphylaxis following isolated limpet ingestion. In 1994, Carrillo et al. expanded their investigation to include six additional patients, concluding that limpets could be potentially serious allergens for individuals who are already sensitized to mites [20]. Conversely, in 2003, Azofra and coworkers presented a brief series of five patients with a history of limpet allergy, and they identified a 75 kDa protein that may be related to Der p 4 amylase (60 kDa) [21]. In 2017, Azofra further identified actin (45 kDa) in limpets, suggesting its potential role in the cross-reactivity between dust mites and gastropods [9]. However, the exact mechanisms underlying this cross-reactivity remain unclear. In populations such as ours, it is plausible that shared proteins between dust mites and gastropods may exist, suggesting the potential for co-sensitization. Notably, our study represents the most extensive series of patients with limpet allergy reported to date.
In our current study, we enrolled 130 outpatients with a clinically documented history of limpet allergy. The patient cohort was predominantly composed of young adults, although there was a wide age range, with patients as young as four years old and as elderly as older adults. There were no significant differences in gender distribution. Notably, patients frequently reported experiencing severe reactions following limpet ingestion, ranging from severe bronchospasm to anaphylaxis, with a substantial proportion requiring urgent medical intervention. As a result, strict avoidance of limpet consumption was strongly recommended for all patients.
Unlike other shellfish allergies, where symptoms typically manifest quickly [22], the clinical symptoms in our study appeared later than expected, with a mean onset of up to 120 min post-ingestion. However, the timing of reactions varied, ranging from immediate responses (within 30 min) to delayed reactions occurring up to 6–8 h after ingestion. This contrasts with findings by Azofra et al. [9,21], who reported that symptoms in a series of five patients occurred within the first hour after ingestion in all cases. In line with other studies, a significant percentage of our patients exhibited severe respiratory symptoms, including anaphylaxis [19,24,25]. These symptoms were particularly prevalent in patients monosensitized to limpets (Group A), and even patients without a prior asthma diagnosis experienced severe bronchospasm. Further research is needed to explore whether mechanisms such as the O-glycosylation of proteins contribute to this delayed response, akin to the IgE-mediated reactions observed in alpha-gal syndrome in meat-allergic patients, where symptoms typically appear with a delayed onset [26,27,28]. Alternatively, the delayed nature of symptoms could be explained by the antigen’s route through the digestive tract. The onset of the allergic response is delayed because, following ingestion, the proteins within the food must undergo enzymatic modifications to activate the responsible allergen. This process may involve the cleavage of binding links between subunits in high-molecular-weight proteins or alterations in the three-dimensional structure that expose specific epitopes. These modifications often require the action of gastric or intestinal enzymes, thereby accounting for the delayed onset of symptoms.
While the gold standard for diagnosing food allergies remains the oral food challenge, in many cases, due to the severity of symptoms and the heightened concern among patients, our diagnostic approach—similarly to that in other published series—relied on a suggestive clinical history in combination with the detection of sIgE, either through serum or cutaneous methods. This approach may provide sufficient evidence to confirm the diagnosis [5].
Despite previous studies suggesting that patients exhibited positive results solely for prick-by-prick skin tests with cooked limpet extracts [19], our study observed variations in sensitivity between both raw and cooked limpet extracts, ranging from 61 to 71%. These discrepancies between our findings and those of other studies may be attributed to the limited statistical power inherent in smaller series.
The unavailability of serum sIgE laboratory tests for limpets led us to conduct tests using the only available gastropod extract, that of the terrestrial snail. Unfortunately, this approach did not consistently contribute to confirming sensitization, as indicated by our results. This may be due to the unknown cross-reactivity between limpets and terrestrial snails. The high percentage of negative results relative to terrestrial snail sIgE in the monosensitized group (49%) may be attributed to this unknown cross-reactivity, which is further complicated by the limited consumption of terrestrial snails in our region, which hinders the study of cross-reactivity between these gastropods.
Additionally, we utilized the ALEX® technique, which includes a panel of five well-described shellfish allergens (Pen m 1, Pen m 2, Pen m 3, Pen m 4, and Cra c 6). In Group A, despite being monosensitized to limpets, up to six patients exhibited positive detections of several shellfish allergens. Notably, the recognition of the allergens in this group was low, with Cra c 6 (troponin C) being the most frequently recognized, detected in five out of six patients. Intriguingly, only one patient recognized up to three allergens simultaneously: Pen m 1, Pen m 3, and Pen m 4.
In contrast, Group B displayed a higher percentage of patients recognizing Pen m 1, Pen m 2, and Cra c 6. Further analysis revealed that 8 out of 53 patients in Group B recognized multiple allergens simultaneously: 4 recognized Pen m 1, Pen m 2, and Cra c 6; 2 recognized Pen m 1 and Pen m 2; and 1 recognized Pen m 1, Pen m 2, and Pen m 3. This difference between both groups may be attributed to the higher prevalence of associated allergies to crustaceans and cephalopods in Group B. These proteins may play a role in the potential cross-reactivity between limpets and other mollusks or crustaceans, suggesting that polysensitization may be a contributing factor. Additionally, cross-reactivity between various seafood allergens could explain the varying patterns of sensitization observed in this group.
A noteworthy distinction in our study compared to others is the observation that a substantial number of our patients were not only monosensitized to limpets but also exhibited allergies to other classes of mollusks and crustaceans. This phenomenon may be attributed to either co-sensitization or cross-reactivity. Published studies investigating protein sequence homology have reported ranges of 68% to 88% similarity between different mollusk species and 56% to 68% between mollusks and crustaceans. Notably, these figures are lower than the protein sequence homology observed within different classes of crustaceans, which is approximately 98% [4,9,10,11,12]. These findings highlight the complexity of allergic responses both within and across different marine species, underscoring the need for further investigation to delineate the specific mechanisms underlying these sensitivities. However, comprehensive studies are required to elucidate and clarify these relationships.
Conversely, there is a prevailing hypothesis suggesting shared allergens between gastropods and dust mites, as evidenced by the allergic symptoms triggered by gastropods in patients who have been sensitized to dust mites prior to the onset of food allergies [3,9,12,24,25,29,30,31,32]. In our cohort, all patients presented with mite allergic rhinoconjunctivitis and/or asthma, indicating a very close relationship between both allergies, consistent with previous observations [3,9,12,24,25,29,30,31,32]. The cross-reactivity between house dust mites and terrestrial snails has been linked to several mite allergens, including Der p 4, Der p 5, Der p 7, and hemocyanin [33]. Unlike crustaceans, tropomyosin does not appear to play a significant role in gastropod allergy [21]. In a minority of our patients (3.8% in Group A and 47.17% in Group B), we observed the recognition of tropomyosin (Pen m 1) as an allergenic protein. However, it is noteworthy that mollusk allergy has also been reported in patients who tolerate crustaceans, where tropomyosin was not identified as a relevant allergen [9].
Nevertheless, comprehensive series and molecular studies are required to fully elucidate the complexities surrounding this issue and provide a better understanding of the mechanisms underlying cross-reactivity between dust mites and gastropods. Cross-reactivity among different gastropods, or between gastropods and other shellfish, remains understudied. Additionally, recent research suggests that O-glycosylation may play a role in patients experiencing anaphylaxis due to snails and allergy to Artemisia vulgaris [34]. This finding highlights the complexity of allergenic mechanisms and underscores the importance of further investigation into the role of glycosylation and its implications for shellfish allergy management and diagnosis [7,26,27,28].
The molecular-level identification of allergens in mollusks is relatively limited, with myosin (100 kDa) being the only allergen extensively characterized, particularly as the major allergen in abalone [4,35,36]. In addition, Azofra et al., in northern Spain, identified three novel allergens in mollusks: actin (45 kDa), recognized as the major allergen in razor fish and limpets; enolase (50 kDa) in razorfish; and a putative C1q-domain-containing protein (42 kDa) in mussels [9]. These findings underscore the complexity of allergenic proteins in mollusks and highlight the urgent need for further molecular research in order to better understand the underlying mechanisms of allergic reactions to these marine species.
Nevertheless, the majority of our patients exhibited recognition of protein bands in the 25–40 kDa and 50–200 kDa ranges, a phenomenon that has not been extensively described in other studies of limpet allergy. Bands exceeding 200 kDa suggest the potential involvement of protein domains related to the three-dimensional structure of the myosin heavy chain [9]. Interestingly, similar high molecular weight bands have been previously identified as major allergens in Helix aspersa [24]. While Lourenço Martins et al. ruled out the role of this high-molecular-weight protein in the cross-reactivity between gastropods and meat, they acknowledged its potential implication in cross-reactivity among mollusks, crustaceans, and arachnids [24].
Intriguingly, 1 of the 16 patients recognized protein bands across all extracts, a finding that aligns with the observations of Misnan R et al. [31]. However, the underlying cause of this pattern remains unclear in our study. Further investigation is required to elucidate the significance of this recognition and its potential implications for limpet and related allergen sensitivities. This observation highlights the heterogeneous nature of allergic profiles in patients sensitized to limpets.
6. Unmet Needs and Future Directions
Currently, our diagnostic capabilities for gastropod allergies are limited. We primarily rely on commercially available snail extracts for conducting skin prick tests and sIgE testing against snail allergens. Unfortunately, there are no commercial extracts or specific serum IgE tests available for limpets, which necessitates the use of fresh raw and cooked limpet samples for skin prick tests to confirm allergic sensitization to this gastropod [7].
Currently, the lack of both specific molecular diagnosis tools and commercial extracts for limpets restricts the diagnostic process, particularly in regions where the consumption of this gastropod is more prevalent. There is an urgent need to optimize diagnostic procedures for limpet allergy to improve the quality of allergy studies and enhance the accuracy of precision diagnoses. By achieving this, we aim to reduce the unnecessary avoidance of limpets and related mollusks, thereby providing better and safer management options for patients [7].
Additionally, efforts should be directed toward identifying allergenic proteins from various consumable gastropods to incorporate them into diagnostic tools. This approach aims to determine whether the coexistence of dust mite allergy and gastropod shellfish allergy, along with allergies to other shellfish groups, is due to shared proteins (cross-reactivity) or simply co-sensitization. Such research would provide valuable insight into the likelihood of cross-reactivity between these groups. Given that allergic reactions to gastropods are often severe and can be potentially life-threatening, it is essential to provide comprehensive health education, prescribe epinephrine auto-injectors, and offer guidance on the appropriate use of necessary medications. However, addressing these challenges requires significant time and resources.
7. Conclusions
In conclusion, limpets, which are widely consumed sea mollusks in the Canary Islands, have contributed to a notable local prevalence of limpet allergies. To the best of our knowledge, our study presents the largest series of patients with limpet allergies reported to date. The identification of protein bands within the 25–40 and 50–200 kDa ranges in monosensitized patients differentiates our findings from those of other studies on limpet allergy. However, further research is crucial to identify the specific allergens recognized by our patients and to better understand their functions. Such advancements will not only facilitate the development of accurate diagnostic methods but will also contribute to a deeper understanding of this condition, ultimately improving knowledge, management, and therapeutic interventions for limpet allergies in the future.
Author Contributions
Conceptualization and methodology, I.S.-M., P.P.-G. and E.M.-L.; software, E.M.-L.; validation and formal analysis, R.G.-P., I.S.-M. and P.P.-G.; investigation and resources, T.G., M.J.M., F.P. and E.M.-L.; data curation, E.M.-L.; writing—original draft preparation, I.S.-M. and E.M.-L.; writing—review and editing, F.P., R.G.-P. and P.P.-G.; visualization and supervision, I.S.-M. and R.G.-P.; project administration, E.M.-L. and P.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hospital Universitario de Canarias (CHUC_2022_10 (ECCIAALT), February 2022) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The corresponding authors have data obtained from SCS.
Acknowledgments
We thank the AAVV “Por la defensa del Caletón” (La Matanza, Tenerife, Spain) for their invaluable collaboration in the development of the project and all nurses of the Allergy Department of HUC for their contributions and dedication. The authors acknowledge OpenAI’s ChatGPT (GPT-4, OpenAI, San Francisco, CA, USA) for assistance in refining the manuscript’s text. However, all interpretations, analyses, and conclusions were carried out by the authors.
Conflicts of Interest
The authors declare no conflicts of interest. T.G., M.J.M. and F.P. are full-time employees of Inmunotek SL Laboratories with no competing conflicts of interest that could influence the outcomes of this research.
References
- Giovannini, M.; Beken, B.; Buyuktiryaki, B.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Pontone, M.; Bartha, I.; Mori, F.; et al. IgE-Mediated Shellfish Allergy in Children. Nutrients 2023, 15, 2714. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cai, J.; Gao, T.; Ma, A. Shellfish consumption and health: A comprehensive review of human studies and recommendations for enhanced public policy. Crit. Rev. Food Sci. Nutr. 2022, 62, 4656–4668. [Google Scholar] [CrossRef] [PubMed]
- Khora, S.S. Seafood-Associated Shellfish Allergy: A Comprehensive Review. Immunol. Invest. 2016, 45, 504–530. [Google Scholar] [CrossRef]
- Taylor, S.L. Molluscan Shellfish Allergy. Adv. Food Nutr. Res. 2008, 54, 139–177. [Google Scholar] [CrossRef]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Álvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K.; et al. EAACI guidelines on the diagnosis of IgEmediated food allergy. Allergy 2023, 78, 3057–3076. [Google Scholar] [CrossRef]
- Alergológica 2015, SEAIC. Available online: https://www.seaic.org/inicio/noticias-general/alergologica-2015.html (accessed on 15 June 2017).
- Mederos-Luis, E.; Poza-Guedes, P.; Pineda, F.; Sánchez-Machín, I.; González-Pérez, R. Gastropod Allergy: A Comprehensive Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 5950–5964. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.; Leung, N.Y.; Leung, A.S.; Wong, G.W.; Leung, T.F. Seafood allergy in Asia: Geographical specificity and beyond. Front. Allergy 2021, 2, 676903. [Google Scholar] [CrossRef] [PubMed]
- Azofra, J.; Echechipia, S.; Irazábal, B.; Muñoz, D.; Bernedo, N.; Gacía, B.E.; Gastaminza, G.; Goikoetxea, M.J.; Joral, A.; Lasa, E.; et al. Heterogenecity in allergy to mollusks: A clinicalimmunological study in a population from the north of Spain. J. Investig. Allergol. Clin. Immunol. 2017, 27, 252–260. [Google Scholar] [CrossRef]
- Gelis, S.; Rueda, M.; Valero, A.; Fernández, E.A.; Moran, M.; Fernández-Caldas, E. Shellfish allergy: Unmet needs in diagnosis and treatment. J. Investig. Allergol. Clin. Immunol. 2020, 30, 409–420. [Google Scholar] [CrossRef]
- Ayuso, R. Update on diagnosis and treatment of shellfish allergy. Curr. Allergy Asthma Rep. 2011, 11, 309–316. [Google Scholar] [CrossRef]
- Pedrosa, M.; Boyano-Martínez, T.; García-Ara, C.; Quirce, S. Shellfish Allergy: A comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Arasi, S.; Nurmatov, U.; Dunn-Galvin, A.; Daher, S.; Roberts, G.; Turner, P.J.; Shinder, S.B.; Gupta, R.; Eigenmann, P.; Nowak-Wegrzyn, A.; et al. Consensus on Definition of food allergy severity (DEFASE) an integrated mixed methods systematic review. World Allergy Organ. J. 2021, 14, 100503. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test-European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Bojcukova, J.; Vlas, T.; Forstenlechner, P.; Panzner, P. Comparison of two multiplex arrays in the diagnostics of allergy. Clin. Transl. Allergy 2019, 9, 31. [Google Scholar] [CrossRef]
- Lis, K.; Bartuzi, Z. Selected Technical Aspects of Molecular Allergy Diagnostics. Curr. Issues Mol. Biol. 2023, 45, 5481–5493. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the Assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- González-Pérez, R.; Poza-Guedes, P.; Pineda, F.; Galán, T.; Mederos-Luis, E.; Abel-Fernández, E.; Martínez, M.J.; Sánchez-Machín, I. Molecular Mapping of allergen exposome among different atopic phenotypes. Int. J. Mol. Sci. 2023, 24, 10467. [Google Scholar] [CrossRef]
- Carrillo, T.; De Castro, F.R.; Cuevas, M.; Caminero, J.; Cabrera, P. Allergy to limpet. Allergy 1991, 46, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, T.; Rodríguez de Castro, F.; Blanco, C.; Castillo, R.; Quiralte, J.; Cuevas, M. Anaphylaxis due to limpet ingestión. Ann. Allergy 1994, 73, 504–508. [Google Scholar]
- Azofra, J.; Lombardero, M. Limpet Anaphylaxis: Cross-reactivity between limpet and house-dust mite Dermatophagoides pteronyssinus. Allergy 2003, 58, 146–149. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, D.; Fuentes-Vallejo, M.S.; Bartolomé-Zavala, B.; Foncubierta-Fernández, A.; Lucas-Velarde, J.; León-Jiménez, A. Urticaria-angioedema due to limpet ingestión. J. Allergol. Clin. Immunol. 2009, 19, 64–79. [Google Scholar]
- Mederos-Luis, E.; Poza-Guedes, P.; Martínez, M.J.; González-Pérez, R.; Galán, T.; Sánchez-Machín, I. Limpet molecular profile: Tropomyosin or not tropomyosin, that is the question. Thematic poster session (TPS). Allergy 2023, 78, 283–682. [Google Scholar] [CrossRef]
- Lourenço-Martins, L.M.; Peltre, G.; Fialho da Costa-Faro, C.J.; Vieira-Pires, E.M.; Da Cruz-Inacio, F.F. The Helix aspersa (brown garden snail) allergens repertoire. Int. Arch. Allergy Immunol. 2005, 136, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lopata, A.L.; Zinn, C.; Potter, P.C. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J. Allergy Clin. Immunol. 1997, 100, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Mirakhur, B.; Chan, E.; Le, Q.-T.; Berlin, J.; Morse, M.; Murphy, B.A.; Satinover, S.M.; Hosen, J.; Mauro, D.; et al. Cetuximab induced anaphylaxis and IgE specific for galactose-a-1,3-galactose. N. Engl. J. Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef]
- Commins, S.P.; Satinover, S.M.; Hosen, J.; Mozena, J.; Borish, L.; Lewis, B.; Woodfolk, J.A.; Platts-Mills, T.A. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-a-1,3-galactose. J. Allergy Clin. Immunol. 2009, 123, 426–433. [Google Scholar] [CrossRef]
- Homann, A.; Schramm, G.; Jappe, U. Glycans andglycan-specific IgE in clinical and molecular allergology: Sensitization, diagnostics and clinical symptoms. J. Allergy Clin. Immunol. 2017, 140, 356–368. [Google Scholar] [CrossRef]
- Van Ree, R.; Antonicelli, L.; Akkerdaas, J.H.; Pajno, G.B.; Barberio, G.; Corbetta, L.; Ferro, G.; Zambito, M.; Garritani, M.S.; Aalberse, R.C.; et al. Asthma after consumption of snails in house-dust-mite-allergic patients: A case of IgE cross-reactivity. Allergy 1996, 51, 387–393. [Google Scholar]
- Vidal, C.; Bartolome, B.; Rodríguez, V.; Armisén, M.; Linneberg, A.; González-Quintela, A. Sensitization pattern of crustacea-allergic individuals can indicate allergy to mollusks. Allergy 2015, 70, 1493–1496. [Google Scholar] [CrossRef]
- Misnan, R.; Abd Aziz, N.S.; Yadzir, Z.H.M.; Bakhtiar, F.; Abdullah, N.; Murad, S. Impact of thermal treatments on major and minor allergens of sea snail, Cerithidea obtuse (obtuse horn shell). Ira J. Allergy Asthma Immunol. 2016, 15, 309–316. [Google Scholar]
- Klaewsongkram, J. High prevalence of shellfish and house dust mite allergies in Asia-Pacific: Probably not just a coincidence. Asian Pac. J. Allergy Immunol. 2012, 30, 247–248. [Google Scholar]
- Guilloux, L.; Vuitton, D.A.; Delbourg, M.; Lagier, A.; Adessi, B.; Marchand, C.R.; Ville, G. Cross-reactivity between terrestial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). II. In vitro study. Allergy 1998, 53, 151–158. [Google Scholar]
- Prados-Castaño, M.; Cimbollek, S.; Bartolomé, B.; Castillo, M.; Quiralte, J. Snailinduced anaphylaxis in patients with underlying Artemisia vulgaris pollinosis: The role of carbohydrates. Allergol. Immunopathol. 2024, 52, 60–64. [Google Scholar] [CrossRef] [PubMed]
- López-Matas, M.A.; de Larramendi, C.H.; Moya, R.; Sánchez-Guerrero, I.; Ferrer, A.; Huertas, A.J.; Flores, I.; Navarro, L.A.; García-Abujeta, J.L.; Vicario, S.; et al. In vivo diagnosis with purified tropomyosin in mite and shellfish allergic patients. Ann. Allergy Asthma Immunol. 2016, 116, 538–543. [Google Scholar] [CrossRef]
- Suzuki, M.; Kobayashi, Y.; Hiraki, Y.; Nakata, H.; Shiomi, K. Paramyosin of the disc abalone Haliotis discus discos: Identification as a new allergen and cross-reactivity with tropomyosin. Food Chemestry 2011, 124, 921–926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).