Real-World Outcomes and Healthcare Utilization of Lanadelumab in Spain: Insights from First Cohort of Difficult-to-Treat Hereditary Angioedema Cases
Abstract
1. Introduction
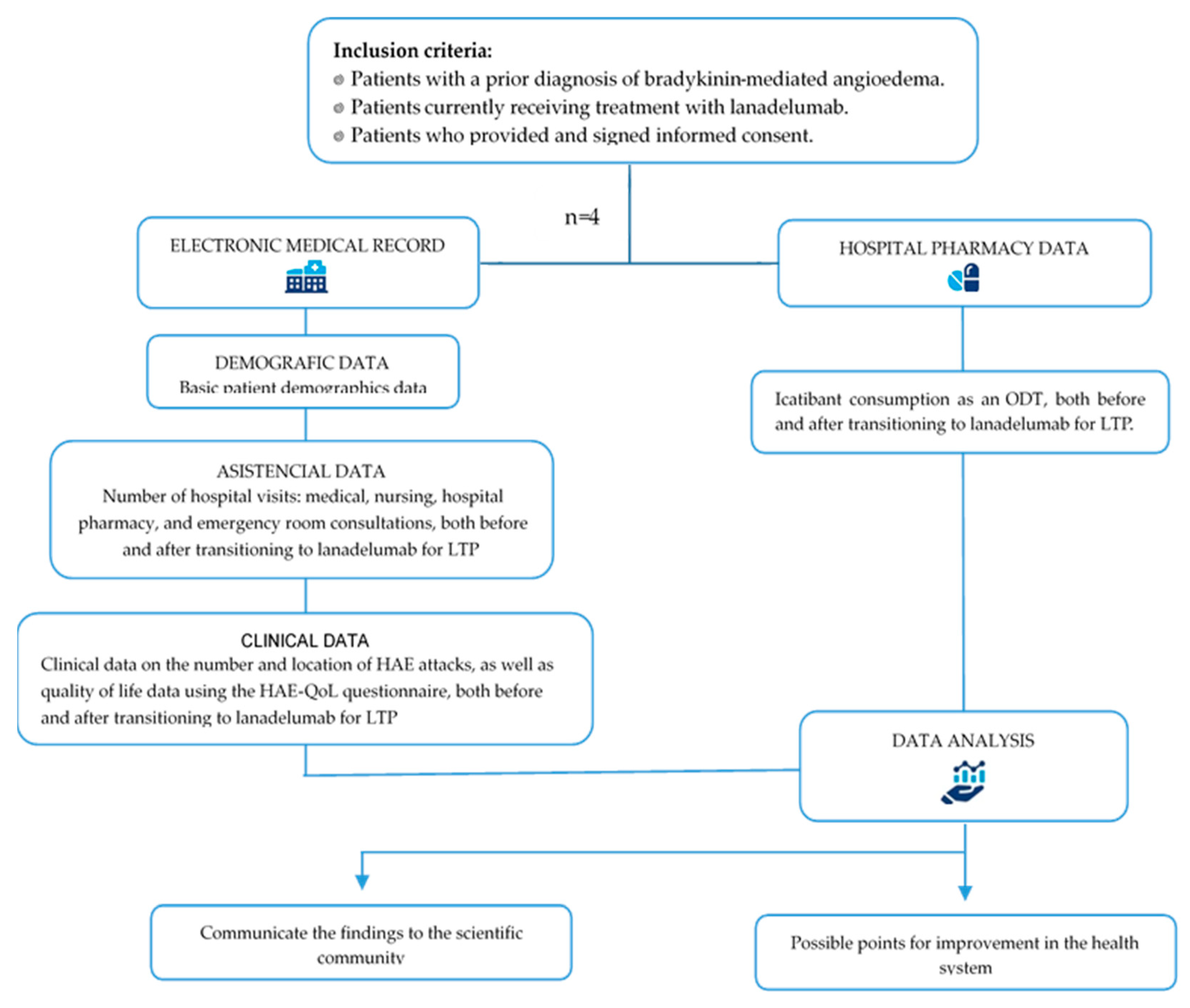
2. Materials and Methods
2.1. Subjects and Study Variables
2.2. Statistical Analysis
3. Results
3.1. Characteristics of Investigated Patients
- HAE type I: 10 patients (47.6%) with a fully confirmed diagnosis;
- HAE type II: eight patients (38%);
- HAE-FXII: two patients (9.5%);
- Acquired angioedema (AEA): one patient with C1q deficiency.
- In total, 14 (66.6%) did not require LTP;
- Two males (9.5%) with HAE type I and type II underwent LTP with danazol;
- Four females (19%) were receiving LTP with lanadelumab.
3.2. Roles of Medical, Nursing, and Hospital Pharmacy Teams in the Management and Control of HAE in Patients Receiving Biological Therapies
3.3. Healthcare Resource Utilization
3.3.1. Allergy Department (Allergist and Nursing) Consultations
3.3.2. Hospital Pharmacy Visits
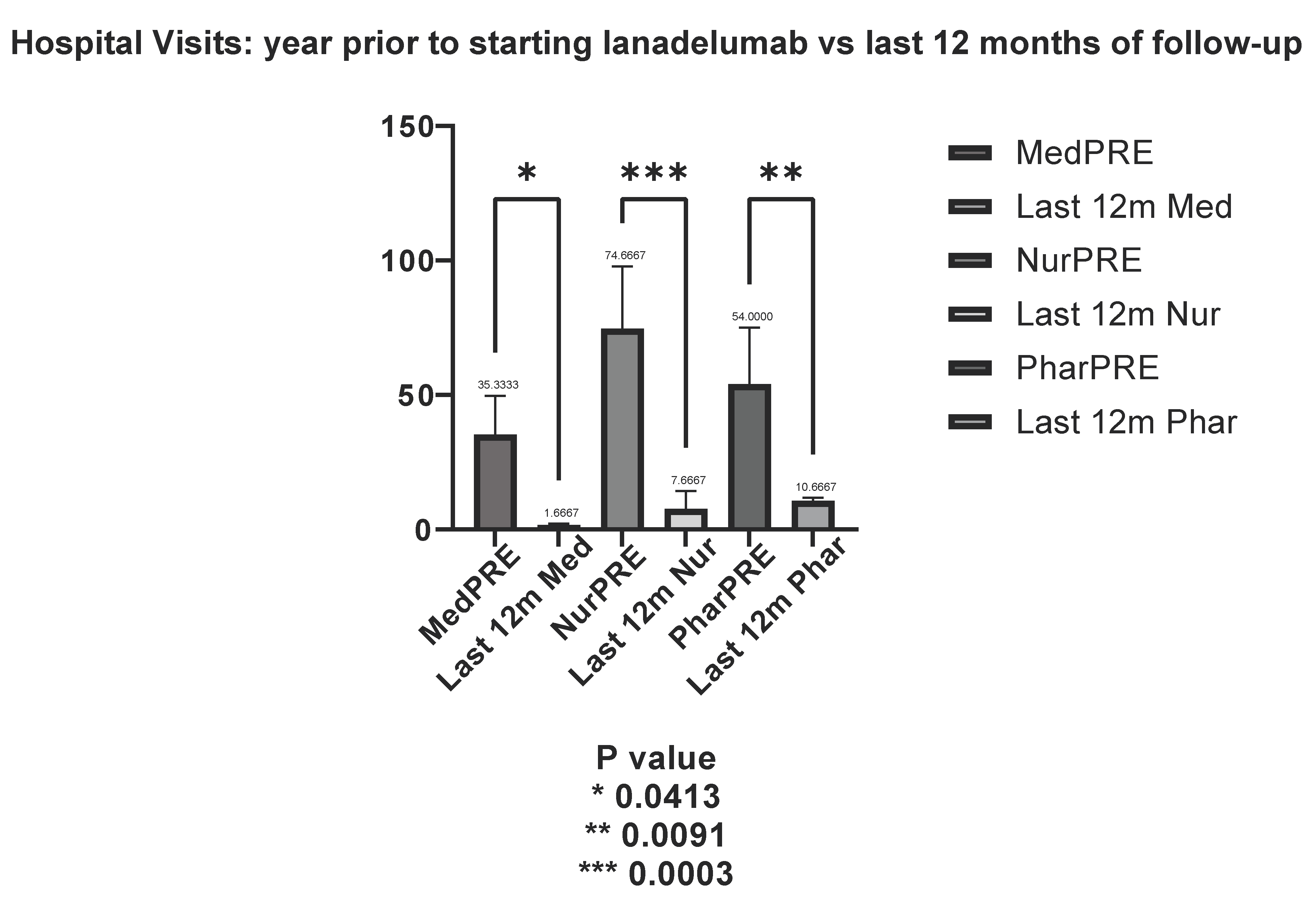
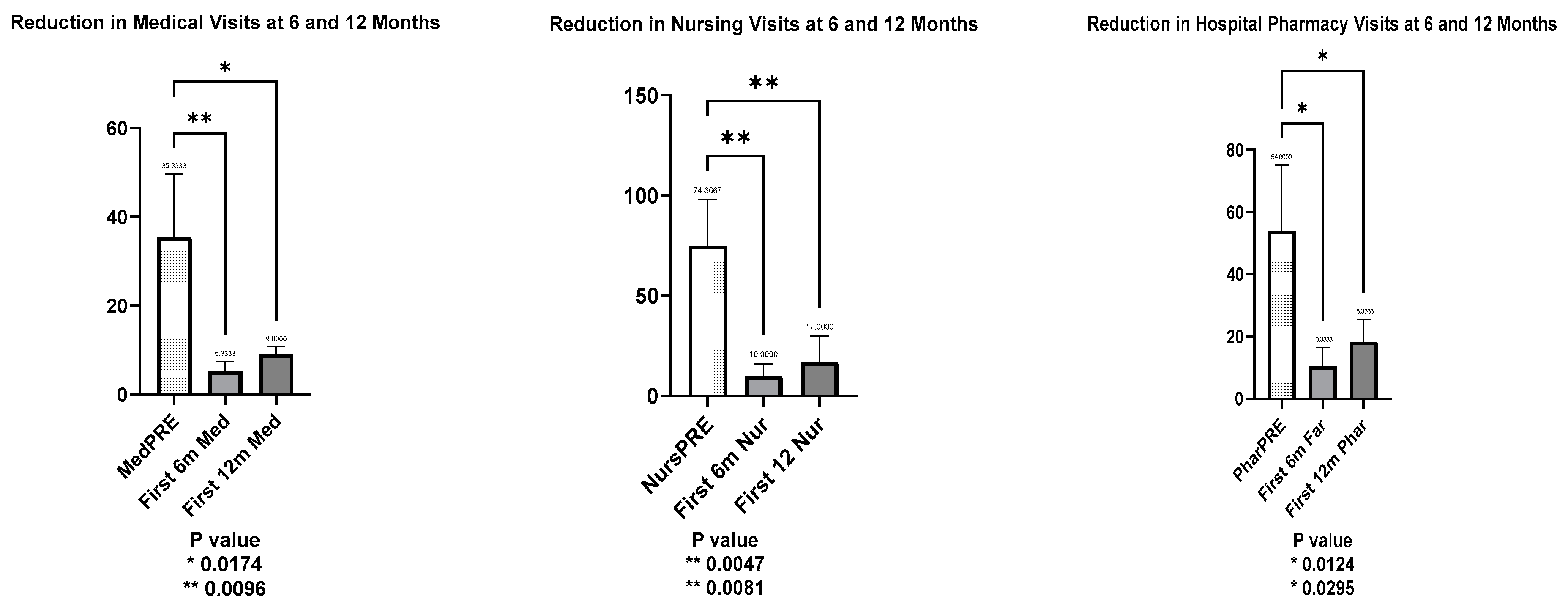
3.3.3. Trends in Hospital Visits After LTP with Lanadelumab
- Medical visits decreased significantly, with a mean reduction to 30.00 visits at 6 months (t = 4.354, p = 0.0096) and 26.33 visits at 12 months (t = 3.822, p = 0.017).
- Nursing visits followed a similar pattern, with a mean reduction of 64.67 visits at 6 months (t = 5.052, p = 0.0047) and 57.67 visits at 12 months (t = 4.5054, p = 0.0081).
- Hospital pharmacy visits also declined, with a mean reduction of 43.67 visits at 6 months (t = 4.017, p = 0.0124) and 35.67 visits at 12 months (t = 3.281, p = 0.0295).
3.3.4. Emergency Department Visits
3.3.5. WhatsApp Consultations
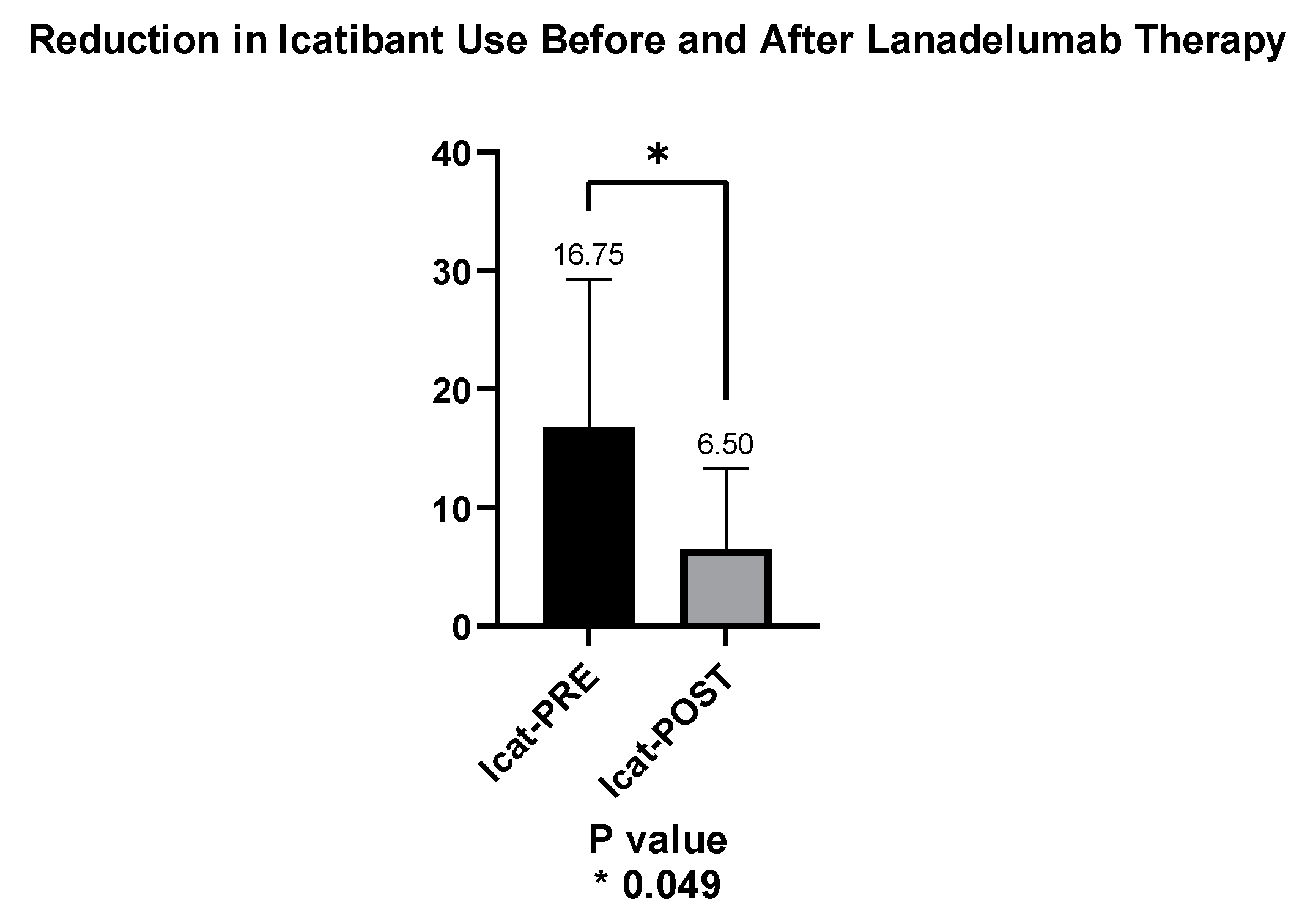
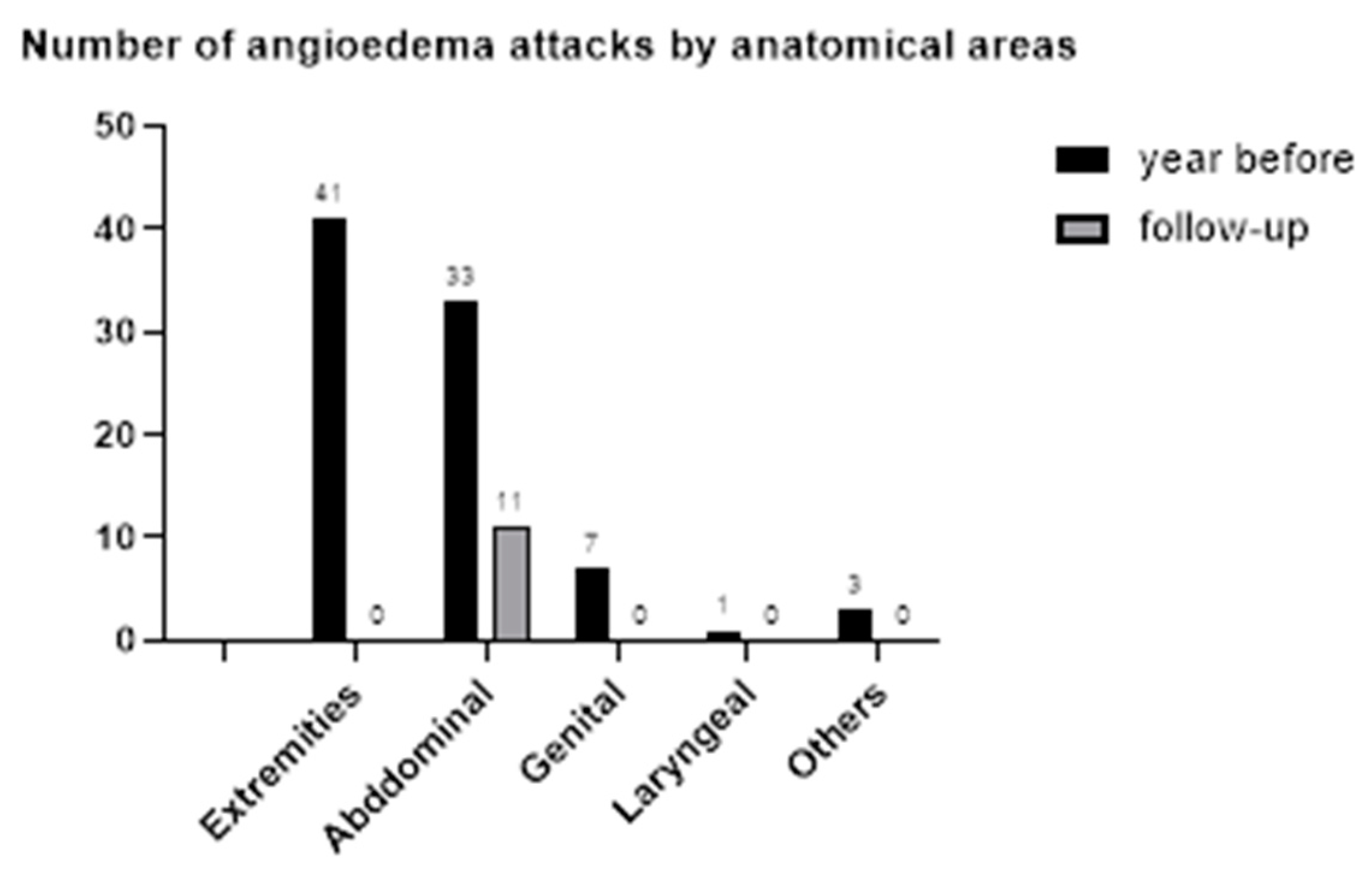
3.4. Number of Angioedema Episodes and Use of ODT with Icatibant
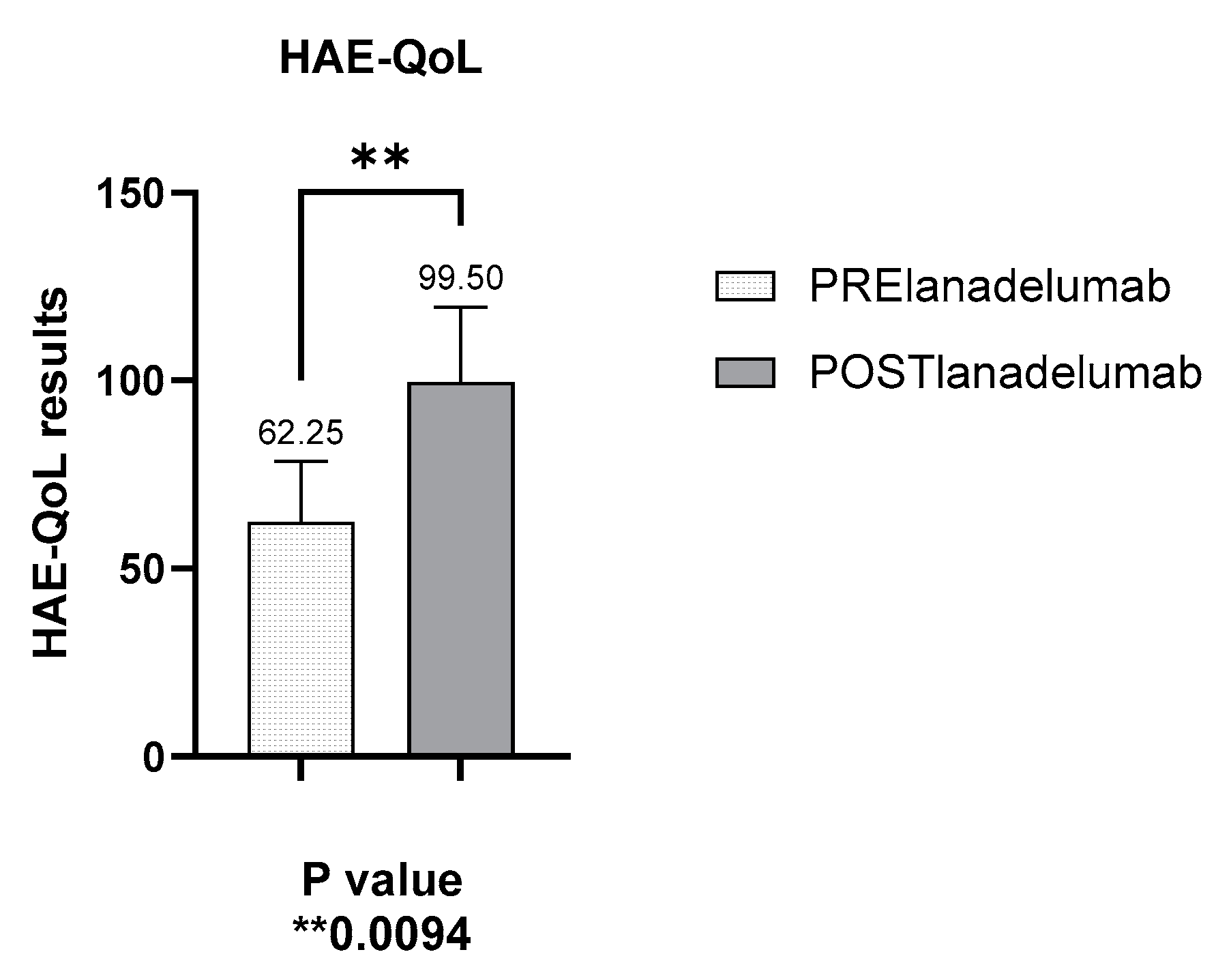
3.5. Improved Disease Control as Measured by the HAE-QoL (Hereditary Angioedema Quality of Life) Questionnaire
3.6. Adverse Reactions Related to the Use of Lanadelumab
3.7. Use of Short-Term Prophylaxis After Initiation of Lanadelumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aygören-Pürsün, E.; Magerl, M.; Maetzel, A.; Maurer, M. Epidemiology of Bradykinin-mediated angioedema: A systematic investigation of epidemiological studies. Orphanet J. Rare Dis. 2018, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zuraw, B.L.; Christiansen, S.C. HAE pathophysiology and underlying mechanisms. Clin. Rev. Allergy Immunol. 2016, 51, 216–229. [Google Scholar] [CrossRef]
- Reshef, A.; Buttgereit, T.; Betschel, S.D.; Caballero, T.; Farkas, H.; Grumach, A.S.; Maurer, M. Definition, acronyms, nomenclature, and classification of angioedema (DANCE): AAAAI, ACAAI, ACARE, and APAAACI DANCE consensus. J. Allergy Clin. Immunol. 2024, 154, 398–411.e1. [Google Scholar] [CrossRef] [PubMed]
- Rosen, F.S.; Pensky, J.; Donaldson, V.; Charache, P. Hereditary angioneurotic edema: Two genetic variants. Science 1965, 148, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Caballero, T. Treatment of Hereditary Angioedema. J. Investig. Allergol. Clin. Immunol. 2021, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Maat, S.; Hofman, Z.L.M.; Maas, C. Hereditary angioedema: The plasma contact system out of control: Reply. J. Thromb. Haemost. 2018, 16, 2349–2351. [Google Scholar] [CrossRef]
- Available online: https://www.aemps.gob.es/informa/informes-de-posicionamiento-terapeutico/informe-de-posicionamiento-terapeutico-de-lanadelumab-takhzyro-en-prevencion-rutinaria-de-las-crisis-recurrentes-de-angioedema-hereditario-aeh-en-pacientes-a-partir-de-los-12-anos-de-edad/ (accessed on 2 May 2024).
- Caballero, T.; Lleonart-Bellfill, R.; Pedrosa, M.; Ferrer, L.; Guilarte, M. Expert Review and Consensus on the treat-to-Target Management of Hereditary Angioedema: From scientific evidence to clinical practice. J. Investig. Allergy Clin. Immunol. 2023, 33, 238–249. [Google Scholar] [CrossRef]
- Población de la Comunidad Autónoma de Canarias en 2023 y 2024, Por Isla. Available online: https://es.statista.com/estadisticas/474029/poblacion-de-canarias-por-isla/#:~:text=Esta%20estad%C3%ADstica%20muestra%20la%20poblaci%C3%B3n,unas%20869.000%20personas%20en%202024 (accessed on 2 June 2024).
- Buttgereit, T.; Vera Ayala, C.; Aykanat, S.; Weller, K.; Gutsche, A.; Maurer, M.; Magerl, M. The real life experience goes on: Update after 4 years on the first cohort treated with lanadelumab at our center. Front. Immunol. 2024, 15, 1405317. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saguer, I.; Knop, J.; Flemming, A.; Thomann, M.; Maurer, M. Real World treatment patterns of hereditary angioedema with lanadelumab in Germany: A prescription data analysis. J. Dtsch. Dermatol. Ges. 2022, 20, 1127–1129. [Google Scholar] [CrossRef]
- Abuzakouk, M.; Ghorab, O.; Al-Hameli, H.; Salvo, F.; Grandon, D.; Maurer, M. Using an extended treatment regimen of lanadelumab in the prophylaxis of hereditary angioedema: A single-centre experience. World Allergy Organ. J. 2022, 15, 100664. [Google Scholar] [CrossRef] [PubMed]
- Dorr, A.D.; Chopra, C.; Coulter, T.I.; Dempster, J.; Dziadzio, M.; El-Shanawany, T.; Garcez, T.; Gompels, M.; Herriot, R.; Jain, R.; et al. Lanadelumab for the prevention of hereditary angioedema attacks: A real-world UK audit. Allergy 2023, 78, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.H.; Princic, N.; Evans, K.A.; Schultz, B.G. Real-world changes in costs over time among patients in the United States with hereditary angioedema on long-term prophylaxis with lanadelumab. J. Med. Econ. 2023, 26, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad. Available online: https://www.sanidad.gob.es/areas/farmacia/precios/comisionInteministerial/acuerdosNotasInformativas/docs/ACUERDOS_CIPM_208_de_17_de_DICIEMBRE_web.pdf (accessed on 5 October 2024).
- Hahn, J.; Trainotti, S.; Wigand, M.; Schuler, P.; Hoffmann, T.; Greve, J. Prospective analysis in patients with HAE under prophylaxis with lanadelumab a real-life experience. J. Drugs Dermatol. 2020, 19, 978–983. [Google Scholar] [CrossRef]
- Bernardino, A.G.; Ferreira, M.B.; Costa, C.; Caiado, J.; Pedro, E.; Santos, A.S. Experience of lanadelumab administration in hereditary angioedema: A case series of 4 patients in Portugal. Asia Pac. Allergy 2023, 13, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, A.; Porębski, G.; Rząd, M.; Grzela, K.; Juchacz, A.; Kurowski, M.; Kuziemski, K.; Łukaszyk, M.; Matuszewski, T.; Pawlukiewicz, M.; et al. Lanadelumab Demonstrates High Efficacy in Reducing the Frequency of Angioedema Attacks in Patients with Severe HAE in Real-Life Settings. Available online: https://pimr.pl/index.php/issues/2023-vol-19-no-4/lanadelumab-demonstrates-high-efficacy-in-reducing-the-frequency-of-angioedema-attacks-in-patients-with-severe-hae-in-real-life-settings#overlay (accessed on 30 October 2024).
- Latysheva, E.A.; Manto, I.A.; Aleshina, L.V.; Bobrikova, E.N.; Viktorova, E.A.; Gracheva, E.M.; Demina, D.V.; Fomina, D.S.; Shcherbina, A.Y.; Latysheva, T.V. Preliminary results of a non-interventional single-center study evaluating the efficacy of long-term use of lanadelumab in routine clinical practice in the Russian Federation. Russ. J. Allergy 2023, 20, 164–176. [Google Scholar] [CrossRef]
- Banerji, A.; Riedl, M.; Bernstein, J.; Cicardi, M.; Longhurst, H.; Zuraw, B.; Busse, P.J.; Anderson, J.; Magerl, M.; Martinez-Saguer, I.; et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: A randomized clinical trial. JAMA 2018, 320, 2108–2121. [Google Scholar] [CrossRef]
- Iaboni, A.; Kanani, A.; Lacuesta, G.; Song, C.; Kan, M.; Betschel, S.D. Impact of lanadelumab in hereditary angioedema: A case series of 12 patients in Canada. Allergy Asthma Clin. Immunol. 2021, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Banerji, A.; Nurse, C.; Lu, P.; Aygören-Pürsün, E.; Kiani-Alikhan, S.; Soteres, D.; Lugar, P.; Zuraw, B.; Lockey, R.; et al. Long-term safety of lanadelumab in hereditary angioedema (HAE): Interim results from the HELP OLE Study. Ann. Allergy Asthma Immunol. 2019, 123, S30. [Google Scholar] [CrossRef]
- Magerl, M.; Schiffhorst, G.; Fanter, L.; Müller, G.; Hirche, C.; Berkemeier, F.; Aygören, E. Patient-level indirect treatment comparison of lanadelumab versus pdC1-INH iv in hereditary angioedema patients: PATCH study. Allergy 2024, 79, 215–224. [Google Scholar] [CrossRef]
- Zozaya, N.; Caballero, T.; González-Quevedo, T.; Setien, P.G.; González, M.Á.; Jódar, R.; Hidalgo-Vega, Á. A multicriteria decision analysis (MCDA) applied to three long-term prophylactic treatments for hereditary angioedema in Spain. Glob. Reg. Health Technol. Assess. 2022, 9, 14. [Google Scholar] [CrossRef]
- Watt, M.; Malmenäs, M.; Romanus, D.; Haeussler, K. Network meta-analysis for indirect comparison of lanadelumab and berotralstat for the treatment of hereditary angioedema. J. Comp. Eff. Res. 2023, 12, e220188. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Gender/age (y) | ♀/78 | ♀/53 | ♀/39 | ♀/48 | ||||
| HAE type | 1 | 1 | 1 | 1 | ||||
| Comorbidities | Non-Hodgkin lymphoma HBP Nodular thyroid hyperplasia AMI Depression | Androgenic alopecia secondary to danazol Depression | Premature birth | HBP | ||||
| Age at diagnosis of HAE | 27 y | 2 y | 27 y | 18 y | ||||
| LTP prior to switching to lanadeluma | Pd1-inh i.v. 2x/week (Since 2017) | Pd1-inh i.v. 2x/week (Since 2019) | Pd1-inh i.v. 2x/week (Since 2022) | Danazol (Since 2002) + Berotralstat (since Oct 2023) | ||||
| Lanadelumab started | March 2021 | March 2022 | January 2023 | February 2024 | ||||
| Number and location of acute attacks in previous year and months after switch to lanadelumab | ||||||||
| Before | After | Before | After | Before | After | Before | After | |
| Extremities | 7 | 0 | 3 | 0 | 27 | 4 | 0 | |
| Abdominal | 5 | Two attacks in first 6 months after switch | 8 | Four attacks in first 12 months after switch | 17 | Five attacks in first 3 months after switch | 3 | |
| Genital | 0 | 0 | 0 | 0 | 5 | 3 | 0 | |
| Laryngeal | 0 | 0 | 0 | 0 | 1 | |||
| Others | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| Attack-free months since last HAE attack, post lanadelumab therapy | 33 months | 16 months | 14 months | 5 months | ||||
| Pedigrees of Families with Various Types of Angioedema | |
|---|---|
| Family 1: One patient with HAE type II on danazol for LTP. | 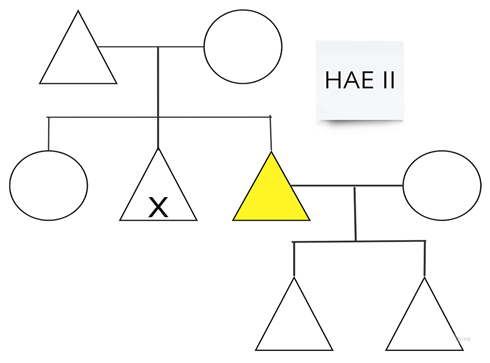 |
| Family 2: In this family, a baby is awaiting diagnosis. | 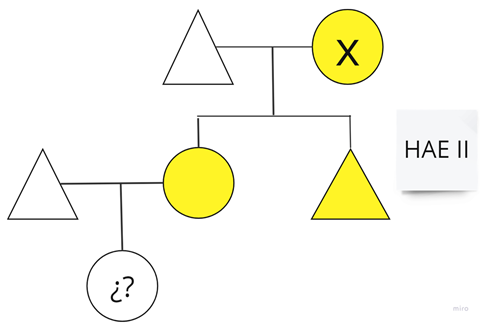 |
| Family 3: This family is from the United Kingdom and has minimal symptoms. | 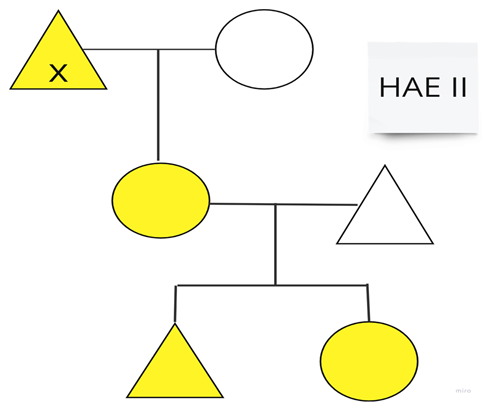 |
| Family 4 | 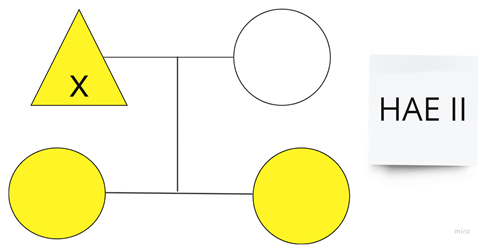 |
| Family 5 | 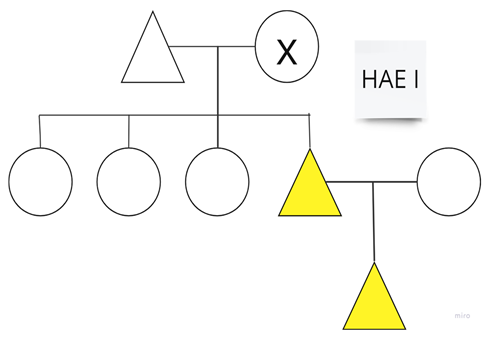 |
| Family 6: One of the sisters overcame leukemia, during which she required C1-INH therapy; currently, she does not require LTP. | 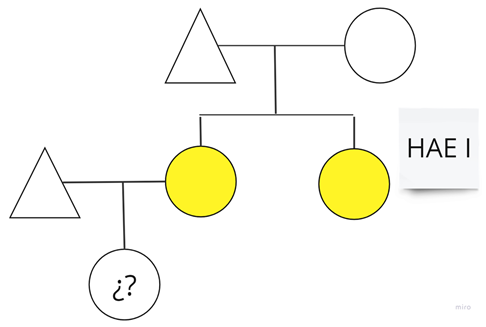 |
| Family 7: This family includes patients number 1 and 2 from Table 1. | 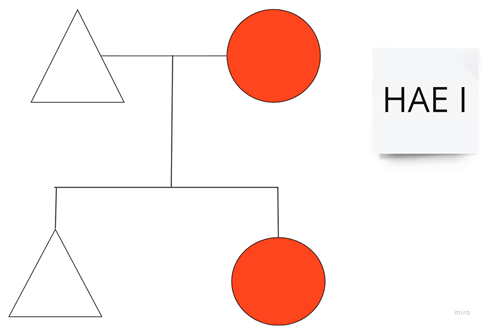 |
| Family 8: In this family, the patient marked in red is patient number 3 from Table 1, and her brother is on LTP with danazol. | 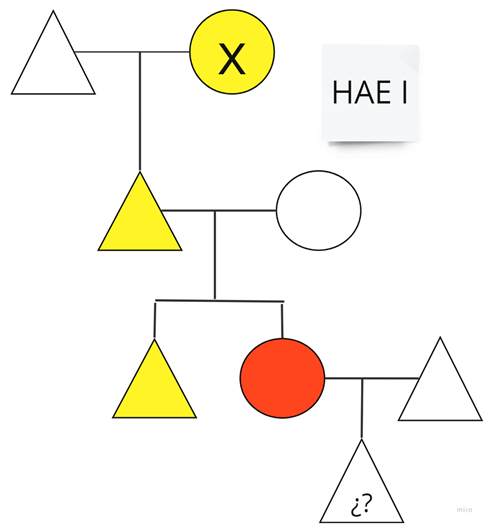 |
| Family 9: This family includes patient number 4 from Table 1. | 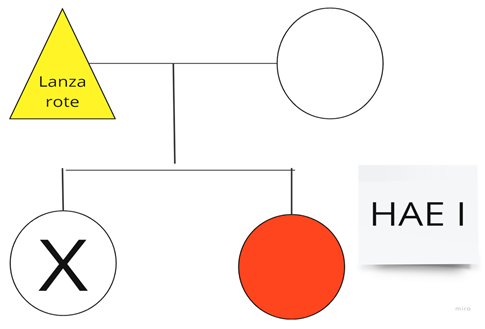 |
| Family 10: Two members of this family live in Galicia, Spain; only one sister lives in our Autonomous Community (Canary Islands, Spain). | 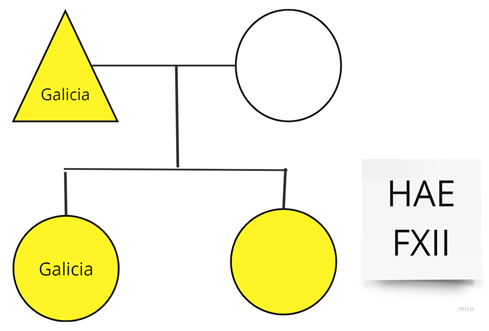 |
| Family 11: The patient’s two daughters have not been studied; they currently live in Germany. | 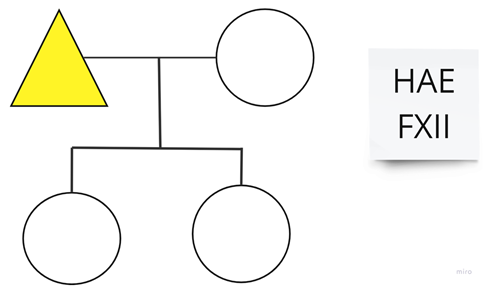 |
| Family 12: We have known this patient since 2000. She has never associated any medical process associated with C1q consumption. She is maintained on amchafibrin for LTP. | 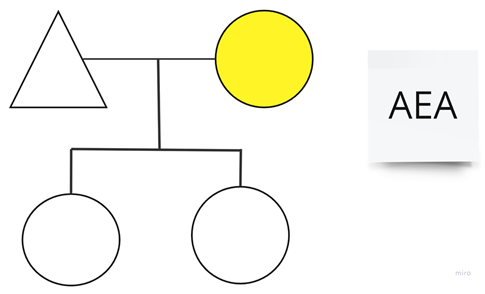 |
| Disciplines | Roles |
|---|---|
| Medical Care | |
| Monitoring of Effectiveness and Safety | Conduct regular assessments to measure treatment response, adjusting doses or changing therapies if necessary; monitor side effects or adverse reactions, and manage urgent episodes. |
| Coordination with Multidisciplinary Teams | Collaborate with pharmacy, nursing, and other healthcare professionals to implement administration, monitoring, and comprehensive patient management protocols. |
| Patient Education and Guidance | Inform patients of the benefits, risks, and possible side effects of biological therapy, ensuring they fully understand their treatment. |
| Management of Adverse Effects and Complications | Develop intervention strategies to address adverse events or complications related to therapy, including treatment adjustments and emergency care. Report adverse effects to pharmacovigilance. |
| Ongoing Research and Continuing Education | Participate in clinical studies, review the scientific literature, and update knowledge on new therapies and advancements in biological treatments. |
| Cost-effectiveness Evaluation | Assess the cost–benefit relationship of biological therapies, especially in complex diseases, aiding in decision-making about treatment sustainability. |
| Clinical Documentation and Reporting | Maintain a detailed record of patient progress, treatment response, and any relevant incidents, ensuring a complete electronic medical record is kept. |
| Nursing Care | |
| Patient and Family Education | Explain the purpose of biological therapy, administration methods, and possible side effects, promoting self-care and addressing questions. |
| Safe Medication Administration | Prepare and administer biological therapies according to strict protocols, ensuring proper administration techniques (subcutaneous, intravenous) and minimizing errors. |
| Monitoring of Adverse Effects | Observe and record signs of adverse reactions or undesirable events after administration, responding rapidly in emergencies and notifying the healthcare team. |
| Adherence Tracking and Support | Ensure that patients follow the prescribed treatment, identifying barriers to adherence and providing support to ensure continuity of treatment. |
| Self-care and Self-administration Support | Train patients who must self-administer medication at home, teaching safe techniques and supervising their ability to perform the administration correctly. |
| Appointment Coordination and Scheduled Administration | Manage dosing schedules according to the treatment plan, ensuring patients receive therapies at the appropriate time. |
| Documentation and Reporting | Record all relevant information on administration, treatment response, and any incidents, maintaining open communication with the healthcare team. Nursing maintains its own electronic nursing record. |
| Emotional and Psychological Support | Provide emotional support, helping patients manage anxiety or concerns related to their treatment, promoting better adaptation to therapy. |
| Hospital Pharmacy Care | |
| Personalized Dispensing and Patient Education | Provide detailed information on the use and handling of biological therapies, instructing patients on administration and potential side effects. |
| Pharmacotherapeutic Monitoring | Monitor treatment response, side effects, and make adjustments according to the patient’s progress and clinical outcomes. |
| Pharmacovigilance | Identify and record adverse reactions and potential interactions, collaborating on adverse event reporting to pharmacovigilance systems. |
| Resource Optimization | Manage inventory and ensure availability of biological medications, considering their high cost and the need for special storage conditions. |
| Treatment Adherence Support | Supervise patient compliance with the therapeutic regimen, implementing strategies to improve adherence in long-term treatments. |
| Participation in Committees and Decision-making | Collaborate in multidisciplinary committees to evaluate the inclusion of new biological therapies and establish evidence-based usage protocols. |
| Cost and Efficiency Consulting | Analyze the cost–benefit relationship of biological therapies, supporting decisions on the funding and prioritization of treatments for patients with specific clinical needs. |
| Use of ODT | Before (n) | After (n) | Change (%) |
|---|---|---|---|
| Patient #1 | 17 units in 12 mo. | 4 units in 40 mo. | −76 |
| Patient #2 | 11 units in 12 mo. | 6 units in 28 mo. | −45 |
| Patient #3 | 34 units in 12 mo. | 16 units in 18 mo. | −53 |
| Patient #4 | 5 units in 12 mo. | 0 units in 5 mo. | −100 |
| TOTAL | 67 | 26 | −61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Machín, I.; González-Pérez, R.; Mederos-Luis, E.; García-Gil, S.; Poza-Guedes, P. Real-World Outcomes and Healthcare Utilization of Lanadelumab in Spain: Insights from First Cohort of Difficult-to-Treat Hereditary Angioedema Cases. Allergies 2025, 5, 3. https://doi.org/10.3390/allergies5010003
Sánchez-Machín I, González-Pérez R, Mederos-Luis E, García-Gil S, Poza-Guedes P. Real-World Outcomes and Healthcare Utilization of Lanadelumab in Spain: Insights from First Cohort of Difficult-to-Treat Hereditary Angioedema Cases. Allergies. 2025; 5(1):3. https://doi.org/10.3390/allergies5010003
Chicago/Turabian StyleSánchez-Machín, Inmaculada, Ruperto González-Pérez, Elena Mederos-Luis, Sara García-Gil, and Paloma Poza-Guedes. 2025. "Real-World Outcomes and Healthcare Utilization of Lanadelumab in Spain: Insights from First Cohort of Difficult-to-Treat Hereditary Angioedema Cases" Allergies 5, no. 1: 3. https://doi.org/10.3390/allergies5010003
APA StyleSánchez-Machín, I., González-Pérez, R., Mederos-Luis, E., García-Gil, S., & Poza-Guedes, P. (2025). Real-World Outcomes and Healthcare Utilization of Lanadelumab in Spain: Insights from First Cohort of Difficult-to-Treat Hereditary Angioedema Cases. Allergies, 5(1), 3. https://doi.org/10.3390/allergies5010003







