Seed Storage Protein, Functional Diversity and Association with Allergy
Abstract
:1. Introduction
2. Classification of Seed Storage Proteins
3. Structural Studies on Seed Storage Proteins
3.1. Structural Features of Prolamin Superfamily
3.2. Structural Features of Cupin Superfamily
3.3. Structural Features of Bet V1 (Pathogenesis-Related) Superfamily
4. Physiological Function of Seed Storage Proteins
4.1. Germination
4.2. Nutrient Accumulation
4.3. Thiamine Storage
4.4. Plant Defense Proteins
4.5. Sugar-Binding Proteins
4.6. Antimicrobial Role
4.7. Ribosome-Inactivating Proteins (RIPs)
4.8. Stress Tolerance
4.9. Antioxidative Properties
4.10. Antihyperglycaemic and Antitumor Activity
5. Seed Storage Proteins and Association with Allergy
5.1. Ligand or Metabolite Binding
5.2. Lipids or Lipid–Membrane Interactions
5.3. Protein Stability and Mobility
5.4. Glycosylation
5.5. Repeated Structures, Aggregates and Glycation
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Agarwal, D.K.; Kumar, S.; Reddy, Y.M.; Chintagunta, A.D.; Saritha, K.; Pal, G. Nutraceuticals derived from seed storage proteins: Implications for health wellness. Biocatal. Agric. Biotechnol. 2019, 17, 710–719. [Google Scholar] [CrossRef]
- Gołda, A.; Szyniarowski, P.; Ostrowska, K.; Kozik, A.; Rąpała-Kozik, M. Thiamine binding and metabolism in germinating seeds of selected cereals and legumes. Plant Physiol. Biochem. 2004, 42, 187–195. [Google Scholar] [CrossRef]
- Agrawal, L.; Narula, K.; Basu, S.; Shekhar, S.; Ghosh, S.; Datta, A.; Chakraborty, N.; Chakraborty, S. Comparative proteomics reveals a role for seed storage protein AmA1 in cellular growth, development, and nutrient accumulation. J. Proteome Res. 2013, 12, 4904–4930. [Google Scholar] [CrossRef]
- Delatorre, P.; Silva-Filho, J.C.; Rocha, B.A.M.; Santi-Gadelha, T.; da Nóbrega, R.B.; Gadelha, C.A.A.; Nascimento, K.S.D.; Nagano, C.S.; Sampaio, A.H.; Cavada, B.S. Interactions between indole-3-acetic acid (IAA) with a lectin from Canavalia maritima seeds reveal a new function for lectins in plant physiology. Biochimie 2013, 95, 1697–1703. [Google Scholar] [CrossRef] [Green Version]
- Businge, E.; Bygdell, J.; Wingsle, G.; Moritz, T.; Egertsdotter, U. The effect of carbohydrates and osmoticum on storage reserve accumulation and germination of Norway spruce somatic embryos. Physiol. Plant. 2013, 149, 273–285. [Google Scholar] [CrossRef]
- de Souza Cândido, E.; Pinto, M.F.S.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-De-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef]
- Ribeiro, S.F.; Taveira, G.B.; Carvalho, A.O.; Dias, G.B.; Da Cunha, M.; Santa-Catarina, C.; Rodrigues, R.; Gomes, V.M. Antifungal and other biological activities of two 2S albumin-homologous proteins against pathogenic fungi. Protein J. 2012, 31, 59–67. [Google Scholar] [CrossRef]
- Nair, D.N.; Singh, V.; Yamaguchi, Y.; Singh, D.D. Jatropha curcas hemagglutinin is similar to a 2S albumin allergen from the same source and has unique sugar affinities. Planta 2012, 236, 1499–1505. [Google Scholar] [CrossRef]
- Borad, V.; Sriram, S. Pathogenesis-related proteins for the plant protection. Asian J. Exp. Sci. 2008, 22, 189–196. [Google Scholar]
- Peumans, W.J.; Hao, Q.; van Damme, E. Ribosome-inactivating proteins from plants: More than RNA N-glycosidases? FASEB J. 2001, 15, 1493–1506. [Google Scholar] [CrossRef]
- Shikhi, M.; Jain, A.; Salunke, D.M. Comparative study of 7S globulin from Corylus avellana and Solanum lycopersicum revealed importance of salicylic acid and Cu-binding loop in modulating their function. Biochem. Biophys. Res. Commun. 2019, 522, 127–132. [Google Scholar] [CrossRef]
- Jain, A.; Salunke, D.M. Purification, identification and preliminary crystallographic studies of an allergenic protein from Solanum melongena. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Rose, T.L.; Conceição, A.D.S.; Xavier-Filho, J.; Okorokov, L.A.; Fernandes, K.V.S.; Marty, F.; Marty-Mazars, D.; Carvalho, A.O.; Gomes, V.M. Defense proteins from Vigna unguiculata seed exudates: Characterization and inhibitory activity against Fusarium oxysporum. Plant Soil 2006, 286, 181–191. [Google Scholar] [CrossRef]
- Viernes, L.; Garcia, R.; Torio, M.; Angelia, M. Antihypertensive Peptides from Vicilin, the Major Storage Protein of Mung Bean (Vigna radiata (L.) R. Wilczek). J. Biol. Sci. 2012, 12, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Salunke, D.M. Crystal structure of nonspecific lipid transfer protein from Solanum melongena. Proteins Struct. Funct. Bioinform. 2017, 85, 1820–1830. [Google Scholar] [CrossRef]
- Jain, A.; Kumar, A.; Shikhi, M.; Nair, D.T.; Salunke, D.M. The structure of MP-4 from Mucuna pruriens at 2.22 Å resolution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2020, 76, 47–57. [Google Scholar] [CrossRef]
- Fang, E.F.; Wong, J.H.; Ng, T.B. Thermostable Kunitz trypsin inhibitor with cytokine inducing, antitumor and HIV-1 reverse transcriptase inhibitory activities from Korean large black soybeans. J. Biosci. Bioeng. 2010, 109, 211–217. [Google Scholar] [CrossRef]
- Ye, X.J.; Ng, T.B. Antitumor and HIV-1 Reverse Transcriptase Inhibitory Activities of a Hemagglutinin and a Protease Inhibitor from Mini-Black Soybean. Evid.-Based Complement. Altern. Med. 2011, 2011, 851396. [Google Scholar] [CrossRef] [Green Version]
- Edstam, M.M.; Viitanen, L.; Salminen, T.A.; Edqvist, J. Evolutionary history of the non-specific lipid transfer proteins. Mol. Plant 2011, 4, 947–964. [Google Scholar] [CrossRef] [Green Version]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Osborne, T.B. The Vegetable Proteins (Monographs on Biochemistry), 2nd ed.; Longmans, Green and Co.: London, UK, 1924; p. 154. [Google Scholar]
- Kreis, M.; Shewry, P.R. Unusual features of cereal seed protein structure and evolution. Bioessays 1989, 10, 201–207. [Google Scholar] [CrossRef]
- Breiteneder, H.; Mills, E.C. Molecular properties of food allergens. J. Allergy Clin. Immunol. 2005, 115, 14–23. [Google Scholar] [CrossRef]
- Templeman, T.S.; DeMaggio, A.E.; Stetler, D.A. Biochemistry of fern spore germination: Globulin storage proteins in Matteuccia struthiopteris L. Plant Physiol. 1987, 85, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Kumar, A.; Salunke, D.M. Crystal structure of the vicilin from Solanum melongena reveals existence of different anionic ligands in structurally similar pockets. Sci. Rep. 2016, 6, 23600. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J.; Boulter, D. The structure of legumin, a storage protein of broad bean (Vicia faba) seed. Eur. J. Biochem. 1970, 17, 460–466. [Google Scholar] [CrossRef]
- Konopska, L. Legumin and albumin of pea cotyledons during seed germination. Biol. Plant. 1983, 25, 15–20. [Google Scholar] [CrossRef]
- Lawrence, M.C.; Izard, T.; Beuchat, M.; Blagrove, R.; Colman, P. Structure of Phaseolin at 2·2 Å Resolution: Implications for a Common Vicilin/Legumin Structure and the Genetic Engineering of Seed Storage Proteins. J. Mol. Biol. 1994, 238, 748–776. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H.; Jain, A.; Nair, D.T.; Salunke, D.M. Docking, thermodynamics and molecular dynamics (MD) studies of a non-canonical protease inhibitor, MP-4, from Mucuna pruriens. Sci. Rep. 2018, 8, 689. [Google Scholar] [CrossRef] [Green Version]
- Radauer, C.; Lackner, P.; Breiteneder, H. The Bet v 1 fold: An ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol. Biol. 2008, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Hauser, M.; Ferreira, F.; Egger, M.; Wallner, M.; Wopfner, N.; Schmidt, G. Molecular properties of plant food allergens: A current classification into protein families. Open Immunol. J. 2008, 1, 1–12. [Google Scholar]
- Lee, J.Y.; Min, K.; Cha, H.; Shin, D.H.; Hwang, K.Y.; Suh, S.W. Rice non-specific lipid transfer protein: The 1.6 A crystal structure in the unliganded state reveals a small hydrophobic cavity. J. Mol. Biol. 1998, 276, 437–448. [Google Scholar] [CrossRef] [PubMed]
- van Ree, R.; Cabanes-Macheteau, M.; Akkerdaas, J.; Milazzo, J.P.; Loutelier-Bourhis, C.; Rayon, C.; Villalba, M.; Koppelman, S.; Aalberse, R.; Rodriguez, R.; et al. β (1, 2)-xylose and α (1, 3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 2000, 275, 11451–11458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douliez, J.P.; Michon, T.; Marion, D. Steady-state tyrosine fluorescence to study the lipid-binding properties of a wheat non-specific lipid-transfer protein (nsLTP1). Biochim. Biophys. Acta 2000, 1467, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoh, F.; Pons, J.-L.; Gautier, M.-F.; de Lamotte, F.; Dumas, C. Structure of a liganded type 2 non-specific lipid-transfer protein from wheat and the molecular basis of lipid binding. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 397–406. [Google Scholar] [CrossRef]
- Rueckert, D.G.; Schmidt, K. Lipid transfer proteins. Chem. Phys. Lipids 1990, 56, 1–20. [Google Scholar] [CrossRef]
- Thoma, S.; Kaneko, Y.; Somerville, C. A non-specific lipid transfer protein from Arabidopsis is a cell wall protein. Plant J. 1993, 3, 427–436. [Google Scholar] [CrossRef] [PubMed]
- García-Olmedo, F.; Molina, A.; Segura, A.; Moreno, M. The defensive role of nonspecific lipid-transfer proteins in plants. Trends Microbiol. 1995, 3, 72–74. [Google Scholar] [CrossRef] [Green Version]
- Marion, D.; Bakan, B.; Elmorjani, K. Plant lipid binding proteins: Properties and applications. Biotechnol. Adv. 2007, 25, 195–197. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.W.; Kim, W.; Hwang, B. Three pathogen-inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant Cell Environ. 2003, 26, 915–928. [Google Scholar] [CrossRef]
- Park, C.-J.; Shin, R.; Park, J.M.; Lee, G.-J.; You, J.-S.; Paek, K.-H. Induction of pepper cDNA encoding a lipid transfer protein during the resistance response to tobacco mosaic virus. Plant Mol. Biol. 2002, 48, 243–254. [Google Scholar] [CrossRef]
- Nielsen, K.K.; Nielsen, J.E.; Madrid, S.M. New antifungal proteins from sugar beet (Beta vulgaris L.) showing homology to non-specific lipid transfer proteins. Plant Mol. Biol. 1996, 31, 539–552. [Google Scholar] [CrossRef]
- Gonorazky, A.G.; Regente, M.; de la Canal, L. Stress induction and antimicrobial properties of a lipid transfer protein in germinating sunflower seeds. J. Plant Physiol. 2005, 162, 618–624. [Google Scholar] [CrossRef]
- Molina, A.; García-Olmedo, F. Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2. Plant J. 1997, 12, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, A.M.; Doerner, P.; Dixon, R.A.; Lamb, C.J.; Cameron, R.K. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 2002, 419, 399–403. [Google Scholar] [CrossRef]
- Lleonart, R.; Cisteró, A.; Carreira, J.; Batista, A.; Del Prado, J.M. Food allergy: Identification of the major IgE-binding component of peach (Prunus persica). Ann. Allergy 1992, 69, 128–130. [Google Scholar]
- Wijesinha-Bettoni, R.; Alexeev, Y.; Johnson, P.; Marsh, J.; Sancho, A.I.; Abdullah, S.U.; Mackie, A.R.; Shewry, P.R.; Smith, L.J.; Mills, E.N.C. The structural characteristics of nonspecific lipid transfer proteins explain their resistance to gastroduodenal proteolysis. Biochemistry 2010, 49, 2130–2139. [Google Scholar] [CrossRef]
- Diaz-Perales, A.; Garcia-Casado, G.; Sanchez-Monge, R.; Garcia-Selles, F.J.; Barber, D.; Salcedo, G. cDNA cloning and heterologous expression of the major allergens from peach and apple belonging to the lipid-transfer protein family. Clin. Exp. Allergy 2002, 32, 87–92. [Google Scholar] [CrossRef]
- Salcedo, G.; Sanchez-Monge, R.; Díaz-Perales, A.; García-Casado, G.; Barber, D. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin. Exp. Allergy 2004, 34, 1336–1341. [Google Scholar] [CrossRef]
- Asero, R. Detection and clinical characterization of patients with oral allergy syndrome caused by stable allergens in Rosaceae and nuts. Ann. Allergy Asthma Immunol. 1999, 83, 377–383. [Google Scholar] [CrossRef]
- Ballmer-Weber, B. Lipid transfer protein as a potential panallergen? Allergy 2002, 57, 873–875. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Robino, A.M. Clinical role of lipid transfer proteins in food allergy. Mol. Nutr. Food Res. 2004, 48, 356–362. [Google Scholar] [CrossRef]
- Pantoja-Uceda, D.; Bruix, M.; Giménez-Gallego, G.; Rico, M.; Santoro, J. Solution structure of RicC3, a 2S albumin storage protein from Ricinus communis. Biochemistry 2003, 42, 13839–13847. [Google Scholar] [CrossRef]
- Pantoja-Uceda, D.; Palomares, O.; Bruix, M.; Villalba, M.; Rodríguez, R.; Rico, M.; Santoro, J. Solution structure and stability against digestion of rproBnIb, a recombinant 2S albumin from rapeseed: Relationship to its allergenic properties. Biochemistry 2004, 43, 16036–16045. [Google Scholar] [CrossRef]
- Pantoja-Uceda, D.; Shewry, P.R.; Bruix, M.; Tatham, A.S.; Santoro, J.; Rico, M. Solution structure of a methionine-rich 2S albumin from sunflower seeds: Relationship to its allergenic and emulsifying properties. Biochemistry 2004, 43, 6976–6986. [Google Scholar] [CrossRef]
- Hennig, M.; Pfeffer-Hennig, S.A.; Dauter, Z.B.; Wilson, K.S.; Schlesier, B.; Nong, V.H. Crystal structure of narbonin at 1.8 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 1995, 51, 177–189. [Google Scholar] [CrossRef]
- Gaur, V.; Qureshi, I.A.; Singh, A.; Chanana, V.; Salunke, D.M. Crystal structure and functional insights of hemopexin fold protein from grass pea. Plant Physiol. 2010, 152, 1842–1850. [Google Scholar] [CrossRef] [Green Version]
- Gaur, V.; Chanana, V.; Jain, A.; Salunke, D.M. The structure of a haemopexin-fold protein from cow pea (Vigna unguiculata) suggests functional diversity of haemopexins in plants. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Mariutti, R.B.; Masood, R.; Caruso, I.P.; Costa, G.H.G.; de Freita, C.M.; Santos, C.R.; Zanphorlin, L.M.; Mutton, M.J.R.; Murakami, M.T.; et al. Crystal structure of mature 2S albumin from Moringa oleifera seeds. Biochem. Biophys. Res. Commun. 2015, 468, 365–371. [Google Scholar] [CrossRef]
- Shewry, P.R.; Napier, J.; Tatham, A. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945. [Google Scholar]
- Gane, P.J.; Dunwell, J.; Warwickr, J. Modeling based on the structure of vicilins predicts a histidine cluster in the active site of oxalate oxidase. J. Mol. Evol. 1998, 46, 488–493. [Google Scholar] [CrossRef]
- Maleki, S.J.; Kopper, R.A.; Shin, D.S.; Park, C.-W.; Compadre, C.M.; Sampson, H.; Burks, A.W.; Bannon, G.A. Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J. Immunol. 2000, 164, 5844–5849. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-Q.; Møller, I.; Song, S.-Q. Proteomic analysis of embryonic axis of Pisum sativum seeds during germination and identification of proteins associated with loss of desiccation tolerance. J. Proteom. 2012, 77, 68–86. [Google Scholar] [CrossRef]
- Rose, T.; Gomes, V.; Da Cunha, M.; Fernandes, K.; Xavier-Filho, J. Effect of sugars on the association between cowpea vicilin (7S storage proteins) and fungal cells. Biocell 2003, 27, 173–179. [Google Scholar] [CrossRef]
- Dooper, M.; Plassen, C.; Holden, L.; Lindvik, H.; Faeste, C.K. Immunoglobulin E cross-reactivity between lupine conglutins and peanut allergens in serum of lupine-allergic individuals. J. Investig. Allergol. Clin. Immunol. 2009, 19, 283–291. [Google Scholar]
- Ko, T.-P.; Day, J.; McPherson, A. The refined structure of canavalin from jack bean in two crystal forms at 2.1 and 2.0 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, M.C.; Suzuki, E.; Varghese, J.; Davis, P.; Van Donkelaar, A.; Tulloch, P.; Colman, P. The three-dimensional structure of the seed storage protein phaseolin at 3 A resolution. EMBO J. 1990, 9, 9–15. [Google Scholar] [CrossRef]
- Maruyama, N.; Maruyama, Y.; Tsuruki, T.; Okuda, E.; Yoshikawa, M.; Utsumi, S. Creation of soybean β-conglycinin β with strong phagocytosis-stimulating activity. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2003, 1648, 99–104. [Google Scholar] [CrossRef]
- Adachi, M.; Kanamori, J.; Masuda, T.; Yagasaki, K.; Kitamura, K.; Mikami, B.; Utsumi, S. Crystal structure of soybean 11S globulin: Glycinin A3B4 homohexamer. Proc. Natl. Acad. Sci. USA 2003, 100, 7395–7400. [Google Scholar] [CrossRef] [Green Version]
- Adachi, M.; Takenaka, Y.; Gidamis, A.B.; Mikami, B.; Utsumi, S. Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol. 2001, 305, 291–305. [Google Scholar] [CrossRef]
- Koiwa, H.; Kato, H.; Nakatsu, T.; Oda, J.I.; Yamada, Y.; Sato, F. Crystal structure of tobacco PR-5d protein at 1.8 Å resolution reveals a conserved acidic cleft structure in antifungal thaumatin-like proteins. J. Mol. Biol. 1999, 286, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Batalia, M.A.; Monzingo, A.F.; Ernst, S.; Roberts, W.; Robertus, J.D. The crystal structure of the antifungal protein zeamatin, a member of the thaumatin-like, PR-5 protein family. Nat. Genet. 1996, 3, 19–22. [Google Scholar] [CrossRef]
- Ko, T.-P.; Day, J.; Greenwood, A.; McPherson, A. Structures of three crystal forms of the sweet protein thaumatin. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 813–825. [Google Scholar] [CrossRef]
- Shih, C.-Y.T.; Wu, J.; Jia, S.; Khan, A.A.; Ting, K.-L.H.; Shih, D.S. Purification of an osmotin-like protein from the seeds of Benincasa hispida and cloning of the gene encoding this protein. Plant Sci. 2001, 160, 817–826. [Google Scholar] [CrossRef]
- Marković-Housley, Z.; Degano, M.; Lamba, D.; von Roepenack-Lahaye, E.; Clemens, S.; Susani, M.; Ferreira, F.; Scheiner, O.; Breiteneder, H. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. J. Mol. Biol. 2003, 325, 123–133. [Google Scholar] [CrossRef]
- Biesiadka, J.; Bujacz, G.; Sikorski, M.M.; Jaskolski, M. Crystal structures of two homologous pathogenesis-related proteins from yellow lupine. J. Mol. Biol. 2002, 319, 1223–1234. [Google Scholar] [CrossRef]
- Śliwiak, J.; Dolot, R.; Michalska, K.; Szpotkowski, K.; Bujacz, G.; Sikorski, M.; Jaskolski, M. Crystallographic and CD probing of ligand-induced conformational changes in a plant PR-10 protein. J. Struct. Biol. 2016, 193, 55–66. [Google Scholar] [CrossRef]
- Ruszkowski, M.; Sliwiak, J.; Ciesielska, A.; Barciszewski, J.; Sikorski, M.; Jaskolski, M. Specific binding of gibberellic acid by Cytokinin-Specific Binding Proteins: A new aspect of plant hormone-binding proteins with the PR-10 fold. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 2032–2041. [Google Scholar] [CrossRef]
- Sliwiak, J.; Jaskolski, M.; Dauter, Z.; McCoy, A.J.; Read, R.J. Likelihood-based molecular-replacement solution for a highly pathological crystal with tetartohedral twinning and sevenfold translational noncrystallographic symmetry. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Ruszkowski, M.; Szpotkowski, K.; Sikorski, M.; Jaskolski, M. The landscape of cytokinin binding by a plant nodulin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 2365–2380. [Google Scholar] [CrossRef] [Green Version]
- Huet, J.; Mbosso, E.J.T.; Soror, S.; Meyer, F.; Looze, Y.; Wintjens, R.; Wohlkönig, A. High-resolution structure of a papaya plant-defence barwin-like protein solved by in-house sulfur-SAD phasing. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 2017–2026. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Cellular events during germination and seedling growth. In Seeds; Springer: Boston, MA, USA, 1994; pp. 147–197. [Google Scholar]
- Oriowo, M.A.; Bevan, R.; Bevan, J. Variation in the interaction of some phenylethylamine and imidazoline derivatives with alpha-1 adrenoceptors in rabbit arteries: Further evidence for the variable receptor affinity hypothesis. J. Pharmacol. Exp. Ther. 1991, 257, 651–656. [Google Scholar]
- Boulter, D. Biochemistry of storage protein synthesis and deposition in the developing legume seed. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 1984; pp. 1–31. [Google Scholar]
- Below, F.; Cazetta, J.; Seebauer, J. Carbon/nitrogen interactions during ear and kernel development of maize. Physiol. Model. Kernel Set Maize 2000, 29, 15–24. [Google Scholar]
- Finkelstein, R.R.; Rock, C.D. Abscisic acid biosynthesis and response. In The Arabidopsis Book/American Society of Plant Biologists; Somerville, C., Meyerowitz, E., Eds.; NIH: Rockville, MD, USA, 2002; pp. 1–52. [Google Scholar]
- Leung, J.; Giraudat, J. Abscisic Acid Signal Transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard Cell Signal Transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef] [Green Version]
- Hunter, T. The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: Its role in cell growth and disease. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 583–605. [Google Scholar] [CrossRef]
- Ashton, F.M. Mobilization of storage proteins of seeds. Annu. Rev. Plant Physiol. 1976, 27, 95–117. [Google Scholar] [CrossRef]
- Sangwan, R.S.; Singh, N.; Plaxton, W. Phosphoenolpyruvate carboxylase activity and concentration in the endosperm of developing and germinating castor oil seeds. Plant Physiol. 1992, 99, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Mitsunaga, T.; Inaba, K.; Yoshida, T.; Iwashima, A. Accumulation of thiamine and thiamine-binding protein during development of rice seed. J. Plant Physiol. 1990, 137, 123–124. [Google Scholar] [CrossRef]
- Molin, W.T.; Wilkerson, C.; Fites, R. Thiamin phosphorylation by thiamin pyrophosphotransferase during seed germination. Plant Physiol. 1980, 66, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Howle, P.K.; Fites, R. GTP-specific pyrophosphorylation of thiamin in dark-grown soybean (Glycine max) seedling axes. Physiol. Plant. 1991, 81, 24–30. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Shimizu, M.; Iwashima, A. A possible role for thiamine-binding protein in the germination of rice seed. J. Plant Physiol. 1987, 130, 279–284. [Google Scholar] [CrossRef]
- Sexton, A.C.; Howlett, B. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Peumans, W.J.; Van Damme, E. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef]
- Brinda, K.; Mitra, N.; Surolia, A.; Vishveshwara, S. Determinants of quaternary association in legume lectins. Protein Sci. 2004, 13, 1735–1749. [Google Scholar] [CrossRef]
- Loris, R.; Hamelryck, T.; Bouckaert, J.; Wyns, L. Legume lectin structure. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1998, 1383, 9–36. [Google Scholar] [CrossRef]
- Farahnak, A.; Golmohamadi, T.; Ra, M.M. Carbohydrate Detection and Lectin Isolation from Tegumental Tissue of Fasciola hepatica. Iran. J. Parasitol. 2010, 5, 20–24. [Google Scholar]
- Komath, S.S.; Kavitha, M.; Swamy, M. Beyond carbohydrate binding: New directions in plant lectin research. Org. Biomol. Chem. 2006, 4, 973–988. [Google Scholar] [CrossRef]
- Jean-François, F.; Elezgaray, J.; Berson, P.; Vacher, P.; Dufourc, E.J. Pore formation induced by an antimicrobial peptide: Electrostatic effects. Biophys. J. 2008, 95, 5748–5756. [Google Scholar] [CrossRef] [Green Version]
- Araujo, A.P.; Hansen, D.; Vieira, D.F.; de Oliveira, C.; Santana, L.A.; Beltramini, L.M.; Sampaio, C.A.; Sampaio, M.U.; Oliva, M.L.V. Kunitz-type Bauhinia bauhinioides inhibitors devoid of disulfide bridges: Isolation of the cDNAs, heterologous expression and structural studies. Biol. Chem. 2005, 386, 561–568. [Google Scholar] [CrossRef]
- Yount, N.Y.; Yeaman, M. Peptide antimicrobials: Cell wall as a bacterial target. Ann. N. Y. Acad. Sci. 2013, 1277, 127–138. [Google Scholar] [CrossRef]
- Nawrot, R.; Zauber, H.; Schulze, W.X. Global proteomic analysis of Chelidonium majus and Corydalis cava (Papaveraceae) extracts revealed similar defense-related protein compositions. Fitoterapia 2014, 94, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiao, Y.; Wang, X.; Pei, Y. Expression of a novel small antimicrobial protein from the seeds of motherwort (Leonurus japonicus) confers disease resistance in tobacco. Appl. Environ. Microbiol. 2007, 73, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987, 262, 8128–8130. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Yeung, H.W.; Pan, L.P.; Chan, S.I. Trichosanthin, a potent HIV-1 inhibitor, can cleave supercoiled DNA in vitro. Nucleic Acids Res. 1991, 19, 6309–6312. [Google Scholar] [CrossRef]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide: Adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly (A). Nucleic Acids Res. 1997, 25, 518–522. [Google Scholar] [CrossRef]
- Hudak, K.A.; Wang, P.; Tumer, N. A novel mechanism for inhibition of translation by pokeweed antiviral protein: Depurination of the capped RNA template. Rna 2000, 6, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, L.; Gorini, P.; Valbonesi, P.; Castiglioni, P.; Stirpe, F. Unexpected activity of saporins. Nature 1994, 372, 624. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O. Intracellular glasses and seed survival in the dry state. C. R. Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, E.D.; Regnier, F.E.; Bressan, R.A. Characterization of osmotin A thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Goyal, K.; Walton, L.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Grelet, J.; Benamar, A.; Teyssier, E.; Avelange-Macherel, M.-H.; Grunwald, D.; Macherel, D. Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. 2005, 137, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Houston, N.L.; Hajduch, M.; Thelen, J. Quantitative proteomics of seed filling in castor: Comparison with soybean and rapeseed reveals differences between photosynthetic and nonphotosynthetic seed metabolism. Plant Physiol. 2009, 151, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Marsoni, M.; Bracale, M.; Espen, L.; Prinsi, B.; Negri, A.S.; Vannini, C. Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Rep. 2008, 27, 347–356. [Google Scholar] [CrossRef]
- Cooper, B.; Clarke, J.D.; Budworth, P.; Kreps, J.; Hutchison, D.; Park, S.; Guimil, S.; Dunn, M.; Luginbühl, P.; Ellero, C.; et al. A network of rice genes associated with stress response and seed development. Proc. Natl. Acad. Sci. USA 2003, 100, 4945–4950. [Google Scholar] [CrossRef]
- Subbaiah, C.C.; Kollipara, K.; Sachs, M.M. A Ca2+-dependent cysteine protease is associated with anoxia-induced root tip death in maize. J. Exp. Bot. 2000, 51, 721–730. [Google Scholar] [CrossRef]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Contento, A.L.; Nguyen, P.Q.; Bassham, D.C. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 2007, 143, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Graf, E.; Eaton, J. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Akond, A.; Khandaker, L.; Berthold, J.; Gates, L.; Peters, K.; Delong, H.; Hossain, K. Anthocyanin, total polyphenols and antioxidant activity of common bean. Am. J. Food Technol. 2011, 6, 385–394. [Google Scholar]
- Empson, K.L.; Labuza, T.; Graf, E. Phytic acid as a food antioxidant. J. Food Sci. 1991, 56, 560–563. [Google Scholar] [CrossRef]
- Graf, E.; Empson, K.L.; Eaton, J.W. Phytic acid. A natural antioxidant. J. Biol. Chem. 1987, 262, 11647–11650. [Google Scholar] [CrossRef]
- Sedláková, K.; Straková, E.; Suchý, P.; Krejcarová, J.; Herzig, I. Lupin as a perspective protein plant for animal and human nutrition—A review. Acta Vet. Brno 2016, 85, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Teugwa, C.M.; Boudjeko, T.; Tchinda, B.T.; Mejiato, P.C.; Zofou, D. Anti-hyperglycaemic globulins from selected Cucurbitaceae seeds used as antidiabetic medicinal plants in Africa. BMC Complement. Altern. Med. 2013, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- De Mejia, E.; Ben, O. Soybean bioactive peptides: A new horizon in preventing chronic diseases. Sex. Reprod. Menopause 2006, 4, 91–95. [Google Scholar] [CrossRef]
- Johansson, S.; Hourihane, J.B.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; Van Cauwenberge, P.; et al. A revised nomenclature for allergy: An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef]
- Sampson, H.A. Food allergy. Part 1: Immunopathogenesis and clinical disorders. J. Allergy Clin. Immunol. 1999, 103, 717–728. [Google Scholar] [CrossRef]
- Taylor, S.L.; Hefle, S.L. Food allergen labeling in the USA and Europe. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 186–190. [Google Scholar] [CrossRef]
- Sokol, K.; Rasooly, M.; Dempsey, C.; Lassiter, S.; Gu, W.; Lumbard, K.; Frischmeyer-Guerrerio, P.A. Prevalence and diagnosis of sesame allergy in children with IgE-mediated food allergy. Pediatr. Allergy Immunol. 2020, 31, 214. [Google Scholar] [CrossRef]
- Sampson, H.A. Update on food allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819, quiz 820. [Google Scholar] [CrossRef]
- Aalberse, R.C. Structural biology of allergens. J. Allergy Clin. Immunol. 2000, 106, 228–238. [Google Scholar] [CrossRef]
- Bugajska-Schretter, A.; Elfman, L.; Fuchs, T.; Kapiotis, S.; Rumpold, H.; Valenta, R.; Spitzauer, S. Parvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletion. J. Allergy Clin. Immunol. 1998, 101, 67–74. [Google Scholar] [CrossRef]
- Tassin, S.; Broekaert, W.F.; Marion, D.; Acland, D.P.; Ptak, M.; Vovelle, F.; Sodano, P. Solution structure of Ace-AMP1, a potent antimicrobial protein extracted from onion seeds. Structural analogies with plant nonspecific lipid transfer proteins. Biochemistry 1998, 37, 3623–3637. [Google Scholar] [CrossRef]
- Selitrennikoff, C.P. Antifungal proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.; Bavaro, S.L.; Benedé, S.; Diaz-Perales, A.; Bueno-Diaz, C.; Gelencser, E.; Klueber, J.; Larré, C.; Lozano-Ojalvo, D.; Lupi, R.; et al. Are physicochemical properties shaping the allergenic potency of plant allergens? Clin. Rev. Allergy Immunol. 2022, 62, 37–63. [Google Scholar] [CrossRef]
- Dominguez, J.; Cuevas, M.; Ureña, V.; Muñoz, T.; Moneo, I. Purification and characterization of an allergen of mustard seed. Ann. Allergy 1990, 64, 352–357. [Google Scholar]
- Murtagh, G.J.; Archer, D.B.; Dumoulin, M.; Ridout, S.; Matthews, S.; Arshad, S.H.; Alcocer, M.J.C. In vitro stability and immunoreactivity of the native and recombinant plant food 2S albumins Ber e 1 and SFA-8. Clin. Exp. Allergy 2003, 33, 1147–1152. [Google Scholar] [CrossRef]
- Bublin, M.; Radauer, C.; Wilson, I.B.H.; Kraft, D.; Scheiner, O.; Breiteneder, H.; Hoffmann-Sommergruber, K. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003, 17, 1697–1699. [Google Scholar] [CrossRef]
- Pedrosa, C.; De Felice, F.G.; Trisciuzzi, C.; Ferreira, S.T. Selective neoglycosylation increases the structural stability of vicilin, the 7S storage globulin from pea seeds. Arch. Biochem. Biophys. 2000, 382, 203–210. [Google Scholar] [CrossRef]
- Koppelman, S.J.; Bruijnzeel-Koomen, C.A.F.M.; Hessing, M.; de Jongh, H.H.J. Heat-induced conformational changes of Ara h 1, a major peanut allergen, do not affect its allergenic properties. J. Biol. Chem. 1999, 274, 4770–4777. [Google Scholar] [CrossRef]
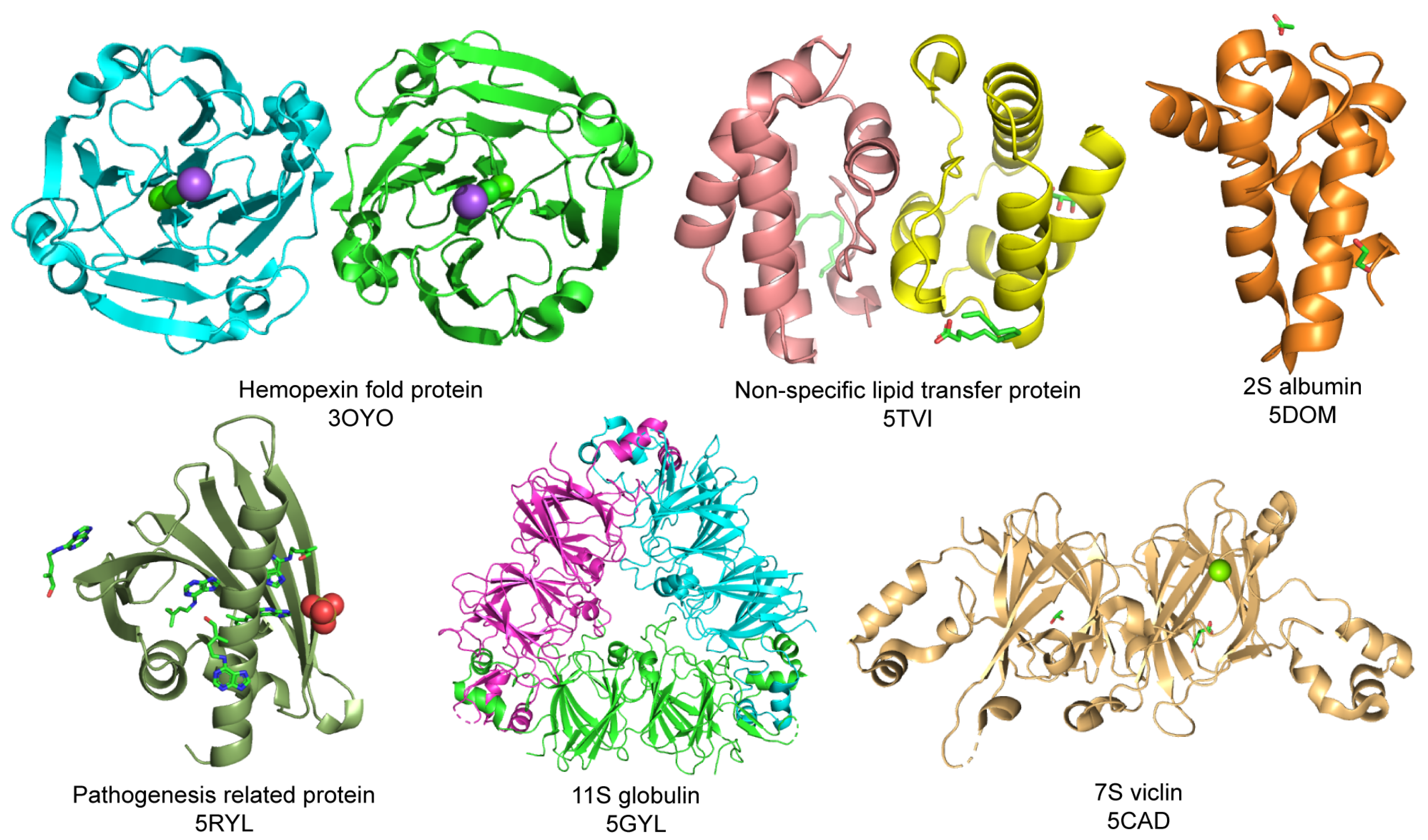
| Family | Characteristics | Members | Example of Structures from Protein Data Bank (PDB) |
|---|---|---|---|
| Prolamin | Conserved skeleton of cysteine Rich in proline and glutamine Rich in α-helix | 2S albumin nsLTPs cereal prolamins α-amylase inhibitors | Vigna unguiculata (3OYO) Moringa oleifera (5DOM) Solanum melongena (5TVI) |
| Cupin | Have one or more β-barrel cores | 7S vicilin 11S vicilin | Solanum melongena (5VF5) Cicer arietinum (5GYL) |
| BetV1 or pathogenesis-related class | Cytoplasmic disease resistance-related proteins | PRs Proteases Kunitz type of protease inhibitor and more | Betula pendula (1BV1) Fragaria x ananassa (4C94) |
| S.No | Type of Reactions | Symptoms |
|---|---|---|
| 1 | Cutaneous | Rash, scratching, urticarial plaques, papules, urticaria and angioedema. |
| 2 | Respiratory | Asthma, laryngeal edema, rhinitis and nasal congestion. |
| 3 | Gastrointestinal | Colic, acute nausea, vomiting, emesis, abdominal pain, weight loss and failure to thrive and/or diarrhea. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A. Seed Storage Protein, Functional Diversity and Association with Allergy. Allergies 2023, 3, 25-38. https://doi.org/10.3390/allergies3010003
Jain A. Seed Storage Protein, Functional Diversity and Association with Allergy. Allergies. 2023; 3(1):25-38. https://doi.org/10.3390/allergies3010003
Chicago/Turabian StyleJain, Abha. 2023. "Seed Storage Protein, Functional Diversity and Association with Allergy" Allergies 3, no. 1: 25-38. https://doi.org/10.3390/allergies3010003
APA StyleJain, A. (2023). Seed Storage Protein, Functional Diversity and Association with Allergy. Allergies, 3(1), 25-38. https://doi.org/10.3390/allergies3010003






