Nut Allergenicity: Effect of Food Processing
Highlights
Abstract
:1. Introduction
2. Most Prevalent Allergenic Nuts and Their Predominant Allergens
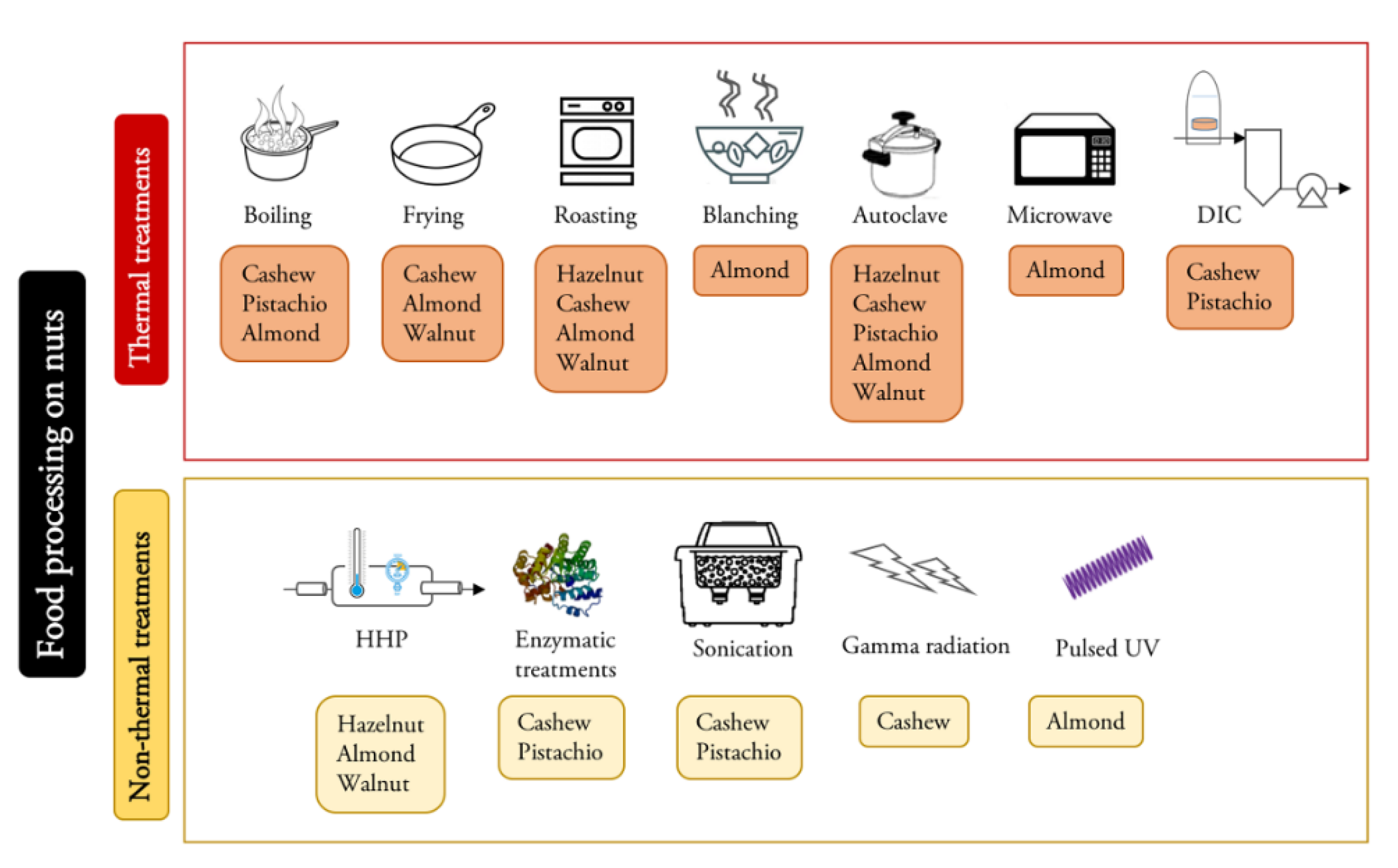
3. Food Processing
4. Effect of Thermal Processing on Food Allergens
5. Effect of Non-Thermal Processing on Food Allergens
6. Effect of Processing on Nut Allergens
6.1. Hazelnut
6.2. Cashew
6.3. Pistachio
6.4. Almond
6.5. Walnut
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jerschow, E.; Lin, R.Y.; Scaperotti, M.M.; McGinn, A.P. Fatal anaphylaxis in the United States, 1999–2010: Temporal patterns and demographic associations. J. Allergy Clin. Immunol. 2014, 134, 1318–1328.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraro, A.; Agache, I.; Clark, A.; Sheikh, A.; Roberts, G.; Akdis, C.A.; Borrego, L.M.; Higgs, J.; Hourihane, J.O.; Jorgensen, P.; et al. EAACI food allergy and anaphylaxis guidelines: Managing patients with food allergy in the community. Allergy 2014, 69, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 1169/2011EC of the European Parliament and of the Council of 25 October 2011on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council; Official Journal of the European Union: Bruxelles, Belgium, 2011; pp. 18–43.
- Fernandez Rivas, M. Food allergy in Alergologica-2005. J. Investig. Allergol. Clin. Immunol. 2009, 2, 37–44. [Google Scholar]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [Green Version]
- Crespo, J.F.; James, J.M.; Fernandez-Rodriguez, C.; Rodriguez, J. Food allergy: Nuts and tree nuts. Br. J. Nutr. 2006, 96, S95–S102. [Google Scholar] [CrossRef] [Green Version]
- Roux, K.H.; Teuber, S.S.; Sathe, S.K. Tree nut allergens. Int. Arch. Allergy Immunol. 2003, 131, 234–244. [Google Scholar] [CrossRef]
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007, 120, 518. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Effects of daily food processing on allergenicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.; Johnson, P.E.; Zuidmeer-Jongejan, L.; Critenden, R.; Wal, J.M.; Asero, R. Effect of Processing on the Allergenicity of Foods. In Risk Management for Food Allergy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 227–251. [Google Scholar]
- Wal, J.M. Thermal processing and allergenicity of foods. Allergy 2003, 58, 727–729. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, J.; Guillamón, E.; Crespo, J.F.; Cuadrado, C.; Burbano, C.; Rodríguez, J.; Fernández, C.; Muzquiz, M. Effects of extrusion, boiling, autoclaving, and microwave heating on lupine allergenicity. J. Agric. Food Chem. 2005, 53, 1294–1298. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.M.; Varela, A.; Guillamon, E.; Muzquiz, M.; Crespo, J.F.; Rodriguez, J.; Burbano, C. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol. Nutr. Food Res. 2009, 53, 1462–1468. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cheng, H.; Sanchiz, A.; Ballesteros, I.; Easson, M.; Grimm, C.C.; Dieguez, M.C.; Linacero, R.; Burbano, C.; Maleki, S.J. Influence of enzymatic hydrolysis on the allergenic reactivity of processed cashew and pistachio. Food Chem. 2018, 241, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Sanchiz, A.; Cuadrado, C.; Dieguez, M.C.; Ballesteros, I.; Rodriguez, J.; Crespo, J.F.; Cuevas, N.L.; Rueda, J.; Linacero, R.; Cabanillas, B.; et al. Thermal processing effects on the IgE-reactivity of cashew and pistachio. Food Chem. 2018, 245, 595–602. [Google Scholar] [CrossRef]
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Burbano, C.; Muzquiz, M.; Aranzazu Jimenez, M.; Pedrosa, M.M.; Cuadrado, C.; Crespo, J.F. Heat and pressure treatments effects on peanut allergenicity. Food Chem. 2012, 132, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Cheng, H.; Teuber, S.S.; Wallowitz, M.L.; Muzquiz, M.; Pedrosa, M.M.; Linacero, R.; Burbano, C.; et al. Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem. 2014, 157, 141–147. [Google Scholar] [CrossRef]
- Cabanillas, B.; Pedrosa, M.M.; Rodriguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of Enzymatic Hydrolysis on the Allergenicity of Roasted Peanut Protein Extract. Int. Arch. Allergy Immunol. 2012, 157, 41–50. [Google Scholar] [CrossRef]
- Ortolani, C.; Ballmer-Weber, B.K.; Hansen, K.S.; Ispano, M.; Wüthrich, B.; Bindslev-Jensen, C. Hazelnut allergy: A double- blind, placebo-controlled food challenge multicenter study. J. Allergy Clin. Immunol. 2000, 105, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B.P.P. Hazelnut Allergens: Molecular Characterization, Detection, and Clinical Relevance. Crit. Rev. Food Sci. Nutr. 2016, 56, 2579–2605. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Tawde, P.; Sathe, S.K.; Roux, K.H. Ana o 1, a cashew (Anacardium occidentale L.) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002, 110, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Ana o 2, a major cashew (Anacardium occidentale L.) nut allergen of the legumin family. Int. Arch. Allergy Immunol. 2003, 132, 27–39. [Google Scholar] [CrossRef]
- Robotham, J.M.; Wang, F.; Seamon, V.; Teuber, S.S.; Sathe, S.K.; Sampson, H.A.; Beyer, K.; Seavy, M.; Roux, K.H. Ana o 3, an important cashew nut (Anacardium occidentale L.) allergenof the 2S albumin family. J. Allergy Clin. Immunol. 2005, 115, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Tehrani, M.; Azad, F.J.; Behmanesh, F.; Varasteh, A. Pistachio allergy-prevalence and in vitro cross-reactivity with other nuts. Allergol. Int. 2011, 60, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, K.; Bardina, L.; Grishina, G.; Beyer, K.; Sampson, H.A. Identification of two pistachio allergens, Pis v 1 and Pis v 2, belonging to the 2S albumin and 11S globulin family. Clin. Exp. Allergy 2009, 39, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, R.; Grishina, G.; Ahn, K. Identification of a MnSOD-like protein as a new major pistachio allergen. J. Allergy Clin. Immunol. 2007, 119, s115. [Google Scholar] [CrossRef]
- Willison, L.N.; Tawde, P.; Robotham, J.M.; Penney, R.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Pistachio vicilin, Pis v 3, is immunoglobulin E-reactive and cross-reacts with the homologous cashew allergen, Ana o 1. Clin. Exp. Allergy 2008, 38, 1229–1238. [Google Scholar] [CrossRef]
- Barre, A.; Nguyen, C.; Granier, C.; Benoist, H.; Rougé, P. IgE-Binding Epitopes of Pis v 1, Pis v 2 and Pis v 3, the Pistachio (Pistacia vera) Seed Allergens. Allergies 2021, 1, 63–91. [Google Scholar] [CrossRef]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B. Almond allergens: Molecular characterization, detection, and clinical relevance. J. Agric. Food Chem. 2012, 60, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Carrapatoso, I.; Oliveira, M.B.; Mafra, I. Walnut allergens: Molecular characterization, detection and clinical relevance. Clin. Exp. Allergy 2014, 44, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.; Mackie, A.; Burney, P.; Beyer, K.; Frewer, L.; Madsen, C.; Botjes, E.; Crevel, R.; van Ree, R. The prevalence, cost and basis of food allergy across Europe. Allergy 2007, 62, 717–722. [Google Scholar] [CrossRef]
- Lyons, S.; Clausen, M.; Knulst, A.; Ballmer-Weber, B.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Pérez, C.; Jędrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746. [Google Scholar] [CrossRef]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218.e4. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Munoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2012, 125, 1322–1326. [Google Scholar] [CrossRef]
- Ribeiro, M.; Costa, J.; Mafra, I.; Cabo, S.; Silva, A.P.; Gonçalves, B.; Hillion, M.; Hébraud, M.; Igrejas, G. Natural Variation of Hazelnut Allergenicity: Is There Any Potential for Selecting Hypoallergenic Varieties? Nutrients 2020, 12, 2100. [Google Scholar] [CrossRef]
- Clark, A.T.; Anagnostou, K.; Ewan, P.W. Cashew nut causes more severe reactions than peanut: Case matched comparison in 141 children. Allergy 2007, 62, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Costa, J.; Vicente, A.A.; Oliveira, M.B.; Mafra, I. Cashew Nut Allergy: Clinical Relevance and Allergen Characterisation. Clin. Rev. Allergy Immunol. 2019, 57, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rance, F.; Bidat, E.; Bourrier, T.; Sabouraud, D. Cashew allergy: Observations of 42 children without associated peanut allergy. Allergy 2003, 58, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.P.; Dubois, A.E.; Gerth van Wijk, R.; Wichers, H.J.; de Jong, N.W. Systematic review on cashew nut allergy. Allergy 2014, 69, 692–698. [Google Scholar] [CrossRef]
- Costa, J.; Silva, I.; Vicente, A.A.; Oliveira, M.B.P.P.; Mafra, I. Pistachio nut allergy: An updated overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 546–562. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, C.; Pedrosa, M. Legume Allergenicity: The effect of food processing In Legumes for Global Food Security; Jimenez-Lopez, J.C., Clemente, A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 223–248. [Google Scholar]
- Costa, J.; Bavaro, S.; Benedé, S.; Diaz-Perales, A.; Bueno-Diaz, C.; Gelencser, E.; Klüber, J.; Larre, C.; Lozano-Ojalvo, D.; Lupi, R.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Plant Allergens? Clin. Rev. Allergy Immunol. 2020, 1–36. [Google Scholar] [CrossRef]
- Masthoff, L.J.; Hoff, R.; Verhoeckx, K.C.; van Os-Medendorp, H.; Michelsen-Huisman, A.; Baumert, J.L.; Pasmans, S.G.; Meijer, Y.; Knulst, A.C. A systematic review of the effect of thermal processing on the allergenicity of tree nuts. Allergy 2013, 68, 983–993. [Google Scholar] [CrossRef] [Green Version]
- Maleki, S.J. Food processing: Effects on allergenicity. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 241–245. [Google Scholar] [CrossRef]
- Schmitt, D.A.; Nesbit, J.B.; Hurlburt, B.K.; Cheng, H.; Maleki, S.J. Processing can alter the properties of peanut extract preparations. J. Agric. Food Chem. 2010, 58, 1138–1143. [Google Scholar] [CrossRef]
- Davis, P.; Williams, S. Protein modification by thermal processing. Allergy 1998, 53, 102–105. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of Processing Technologies on the Allergenicity of Food Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917. [Google Scholar] [CrossRef] [Green Version]
- Vanga, S.K.; Raghavan, V. Processing Effects On Tree Nut Allergens: A Review. Crit. Rev. Food Sci. Nutr. 2016, 57, 2077–2094. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Burbano, C.; Iniesto, E.; Rodriguez, J.; Cabanillas, B.; Crespo, J.; Pedrosa, M.; Muzquiz, M.; del Pozo, J.; Linacero, R.; et al. A Novel Proteomic Analysis of the Modifications Induced by High Hydrostatic Pressure on Hazelnut Water-Soluble Proteins. Foods 2014, 3, 279–289. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of roasting, blanching, autoclaving, and microwave heating on antigenicity of almond (Prunus dulcis L.) proteins. J. Agric. Food Chem. 2002, 50, 3544–3548. [Google Scholar] [CrossRef]
- Downs, M.L.; Simpson, A.; Custovic, A.; Semic-Jusufagic, A.; Bartra, J.; Fernandez-Rivas, M.; Taylor, S.L.; Baumert, J.L.; Mills, E.N.C. Insoluble and soluble roasted walnut proteins retain antibody reactivity. Food Chem. 2016, 194, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Sathe, S.K.; Sharma, G.M. Effects of food processing on food allergens. Mol. Nutr. Food Res. 2009, 53, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.S.; Ballmer-Weber, B.K.; Leuttkopf, D.; Skov, P.S.; Weuthrich, B.; Bindslev-Jensen, C.; Vieths, S.; Poulsen, L.K. Roasted hazelnuts—Allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy Eur. J. Allergy Clin. Immunol. 2003, 58, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Hompes, S.; Fiedler, E.M.; Illner, A.K.; Zuberbier, T.; Vieths, S. Impact of native, heat-processed and encapsulated hazelnuts on the allergic response in hazelnut-allergic patients. Clin. Exp. Allergy 2009, 39, 159–166. [Google Scholar] [CrossRef]
- Lopez, E.; Cuadrado, C.; Burbano, C.; Jimenez, M.A.; Rodriguez, J.; Crespo, J.F. Effects of autoclaving and high pressure on allergenicity of hazelnut proteins. J. Clin. Bioinform. 2012, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, C.; Sanchiz, A.; Vicente, F.; Ballesteros, I.; Linacero, R. Changes Induced by Pressure Processing on Immunoreactive Proteins of Tree Nuts. Molecules 2020, 25, 954. [Google Scholar] [CrossRef] [Green Version]
- Mattison, C.P.; Desormeaux, W.A.; Wasserman, R.L.; Yoshioka-Tarver, M.; Condon, B.; Grimm, C.C. Decreased immunoglobulin E (IgE) binding to cashew allergens following sodium sulfite treatment and heating. J. Agric. Food Chem. 2014, 62, 6746–6755. [Google Scholar] [CrossRef]
- Su, M.; Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Impact of γ-irradiation and thermal processing on the antigenicity of almond, cashew nut and walnut proteins. J. Sci. Food Agric. 2004, 84, 1119–1125. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Monaghan, E.K.; Kshirsagar, H.H.; Robotham, J.M.; O’Donnell, S.E.; Gerber, M.S.; Roux, K.H.; Sathe, S.K. Effects of processing on immunoreactivity of cashew nut (Anacardium occidentale L.) seed flour proteins. J. Agric. Food Chem. 2008, 56, 8998–9005. [Google Scholar] [CrossRef]
- Vicente, F.; Sanchiz, A.; Rodriguez-Perez, R.; Pedrosa, M.; Quirce, S.; Haddad, J.; Besombes, C.; Linacero, R.; Allaf, K.; Cuadrado, C. Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts. Molecules 2020, 25, 1742. [Google Scholar] [CrossRef] [Green Version]
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Maleki, S.J.; Nasiraii, L.R.; Falak, R.; Sima, H.R.; Varasteh, A. Influence of processing on the allergenic properties of pistachio nut assessed in vitro. J. Agric. Food Chem. 2010, 58, 10231–10235. [Google Scholar] [CrossRef]
- Lucas, J.S.; Atkinson, R.G. What is a food allergen? Clin.Exp. Allergy 2008, 38, 1095–1099. [Google Scholar] [CrossRef]
- Moreno, F.J. Gastrointestinal digestion of food allergens: Effect on their allergenicity. Biomed. Pharmacother. 2007, 61, 50–60. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Untersmayr, E.; Klems, M.; Jensen-Jarolim, E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients 2018, 10, 1129. [Google Scholar] [CrossRef] [Green Version]
- FAO. Evaluation of Allergenicity of Genetically Modified Foods. In Report of a Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology; FAO: Rome, Italy, 2001. [Google Scholar]
- Somkuti, J.; Smeller, L. High pressure effects on allergen food proteins. Biophys. Chem. 2013, 4622, 19–29. [Google Scholar] [CrossRef]
- Wigotzki, M.; Steinhart, H.; Paschke, A. Influence of varieties, storage and heat treatment on IgE-binding proteins in hazelnuts (Corylus avellana). Food Agric. Immunol. 2000, 12, 217–229. [Google Scholar] [CrossRef]
- Cucu, T.; Platteau, C.; Taverniers, I.; Devreese, B.; de Loose, M.; de Meulenaer, B. ELISA detection of hazelnut proteins: Effect of protein glycation in the presence or absence of wheat proteins. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2011, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; Grimm, C.C.; Wasserman, R.L. In vitro digestion of soluble cashew proteins and characterization of surviving IgE-reactive peptides. Mol. Nutr. Food Res. 2014, 58, 884–893. [Google Scholar] [CrossRef]
- Mattison, C.P.; Bren-Mattison, Y.; Vant-Hull, B.; Vargas, A.M.; Wasserman, R.L.; Grimm, C.C. Heat-induced alterations in cashew allergen solubility and IgE binding. Toxicol. Rep. 2016, 3, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.Y.; Mattison, C.P.; Reed, S.; Wasserman, R.L.; Desormeaux, W.A. Treatment with oleic acid reduces IgE binding to peanut and cashew allergens. Food Chem. 2015, 180, 295–300. [Google Scholar] [CrossRef]
- Shi, X.; Guo, R.; White, B.L.; Yancey, A.; Sanders, T.H.; Davis, J.P.; Burks, A.W.; Kulis, M. Allergenic properties of enzymatically hydrolyzed peanut flour extracts. Int. Arch. Allergy Immunol. 2013, 162, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.H.; Teuber, S.S.; Robotham, J.M.; Sathe, S.K. Detection and stability of the major almond allergen in foods. J. Agric. Food Chem. 2001, 49, 2131–2136. [Google Scholar] [CrossRef]
- Su, M.; Liu, C.; Roux, K.H.; Gradziel, T.M.; Sathe, S.K. Effects of processing and storage on almond (Prunus dulcis L.) amandin immunoreactivity. Food Res. Int. 2017, 100, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Chung, S.-Y.; Chen, H.; Ye, M.; Teixeira, A.; Gregory, J.; Welt, B.; Shriver, S. Effect of Pulsed Ultraviolet Light and High Hydrostatic Pressure on the Antigenicity of Almond Protein Extracts. Food Bioprocess Technol. 2011, 6, 431–440. [Google Scholar] [CrossRef]
- Doi, H.; Touhata, Y.; Shibata, H.; Sakai, S.; Urisu, A.; Akiyama, H.; Teshima, R. Reliable enzyme-linked immunosorbent assay for the determination of walnut proteins in processed foods. J. Agric. Food Chem. 2008, 56, 7625–7630. [Google Scholar] [CrossRef]
- Barre, A.; Sordet, C.; Culerrier, R.; Rance, F.; Didier, A.; Rouge, P. Vicilin allergens of peanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol. Immunol. 2008, 45, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
| Source | Allergen | Protein Family | MW* (kDa) |
|---|---|---|---|
| Hazelnut (Corylus avellana) | Cor a 1 Cor a 2 Cor a 8 Cor a 9 Cor a 11 Cor a 12 Cor a 13 Cor a 14 Cor a 15 | Pathogen-related protein (PR10) Profilin Non-specific lipid transfer protein (LTP) 11S globulin/legumin 7S globulin/vicilin Oleosin Oleosin 2S albumin Oleosin | 17 14 9 40 47 17 14–16 16 17 |
| Cashew (Anacardium occidentale) | Ana o 1 Ana o 2 Ana o 3 | Vicilin-like protein Legumin-like protein 2S albumin | 50 55 14 |
| Pistachio (Pistachia vera) | Pis v 1 Pis v 2 Pis v 3 Pis v 4 Pis v 5 | 2S albumin 11S globulin/legumin 7S globulin/vicilin Manganese Superoxide dismutase 11S globulin/legumin | 7 32 55 25.7 36 |
| Almond (Prunus dulcis) | Pru du 3 Pru du 4 Pru du 5 Pru du 6 Pru du 8 Pru du 10 | Non-specific lipid transfer protein 1(LTP) Profilin 60S acidic ribosomal protein P2 11S globulin/legumin Antimicrobial seed storage protein Mandelonitrile lyase 2 | 9 14 10 60 31 60 |
| Walnut (Juglans regia) | Jug r 1 Jug r 2 Jug r 3 Jug r 4 Jug r 5 | 2S albumin 7S globulin/vicilinNon-specific lipid transfer protein (LTP) 11S globulin/legumin Profilin | 15 44 9 36 20 |
| Brazil nut (Bertholletia excelsa) | Ber e 1 Ber e 2 | 2S sulfur-rich albumin 11S globulin | 9 29 |
| Chestnut (Castanea sativa) | Cas s 5 Cas s 8 Cas s 9 | Chitinase Non-specific lipid transfer protein 1 Cytosolic class I small heat shock protein | 12–13 17 |
| Source | Processing Conditions | IgE Reactivity * | Reference |
|---|---|---|---|
| Hazelnut | Roasting 140 °C, 40 min | ↓ | Hansen et al. [55] |
| Roasting 144 °C, time not indicated | ↓ | Worm et al. [56] | |
| Autoclaving 138 °C, 15 and 30 min | ↓↓ | Lopez et al. [57]; Cuadrado et al. [58] | |
| HHP 300–600 MPa, 15 min | = | Prieto et al. [50]; Cuadrado et al. [58] | |
| Cashew | Boiling, 100 °C, 30 and 60 min | = (~↓) | Cuadrado et al. [14]; Sanchiz et al. [15] |
| Boiling, 100 °C, 15 min + sodium sulfite | ~↓ | Mattison et al. [59] | |
| Frying, 191 °C, 1 min | = (~↓) | Su et al. [60] | |
| Roasting 200 °C, 15 min | ~↑ | Venkatachalam et al. [61] | |
| Autoclaving 138 °C, 15 and 30 min | ↓ | Cuadrado et al. [14]; Sanchiz et al. [15] | |
| Autoclaving 138 °C, 30 min + Amano 3DS 120 min | ↓↓ | Cuadrado et al. [14] | |
| DIC 7 bar, 2 min | ↓↓ | Vicente et al. [62] | |
| Pistachio | Boiling, 100 °C, 30 and 60 min | = (~↓) | Cuadrado et al. [14]; Sanchiz et al. [15] |
| Steaming | ~↓ | Noorbakhsh et al. [63]; | |
| Autoclaving 138 °C, 15 and 30 min | ↓ | Cuadrado et al. [14]; Sanchiz et al. [15] | |
| Autoclaving 138 °C, 30 min + Amano SD 60 min | ↓↓ | Cuadrado et al. [14] | |
| DIC 7 bar, 2 min | ↓↓ | Vicente et al. [62] | |
| Almond | Boiling, 100 °C, 5 and 10 min | = | Su et al. [60] |
| Frying, 191 °C, 1 min | = (~↓) | Su et al. [60] | |
| Roasting 180 °C, 15 min | = | Su et al. [60] | |
| Autoclaving 121 °C, 30 min | = | Venkatachalam et al. [51] | |
| Autoclaving 138 °C, 15 and 30 min | ↓↓ | Cuadrado et al. [58] | |
| HHP 300–600 MPa, 15 min | = | Cuadrado et al. [58] | |
| Walnut | Frying, 191°C, 1 min | = (~↓) | Su et al. [60] |
| Roasting 160 °C, 30 min; 177 °C, 12 min | = | Su et al. [60] | |
| Autoclaving 138 °C, 15 and 30 min | ↓↓ | Cabanillas et al. [17]. | |
| HHP 300–600 MPa, 15 min | = | Cabanillas et al. [17]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadrado, C.; Sanchiz, Á.; Linacero, R. Nut Allergenicity: Effect of Food Processing. Allergies 2021, 1, 150-162. https://doi.org/10.3390/allergies1030014
Cuadrado C, Sanchiz Á, Linacero R. Nut Allergenicity: Effect of Food Processing. Allergies. 2021; 1(3):150-162. https://doi.org/10.3390/allergies1030014
Chicago/Turabian StyleCuadrado, Carmen, África Sanchiz, and Rosario Linacero. 2021. "Nut Allergenicity: Effect of Food Processing" Allergies 1, no. 3: 150-162. https://doi.org/10.3390/allergies1030014
APA StyleCuadrado, C., Sanchiz, Á., & Linacero, R. (2021). Nut Allergenicity: Effect of Food Processing. Allergies, 1(3), 150-162. https://doi.org/10.3390/allergies1030014








