Copper and Trace Elements in Gallbladder form Patients with Wilson’s Disease Imaged and Determined by Synchrotron X-ray Fluorescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Control Gallbladder (C-GB)
2.2. Wilson’s Disease Gallbladder (WD-GB)
2.3. Tissue Preparation for µ-SRXRF Investigations
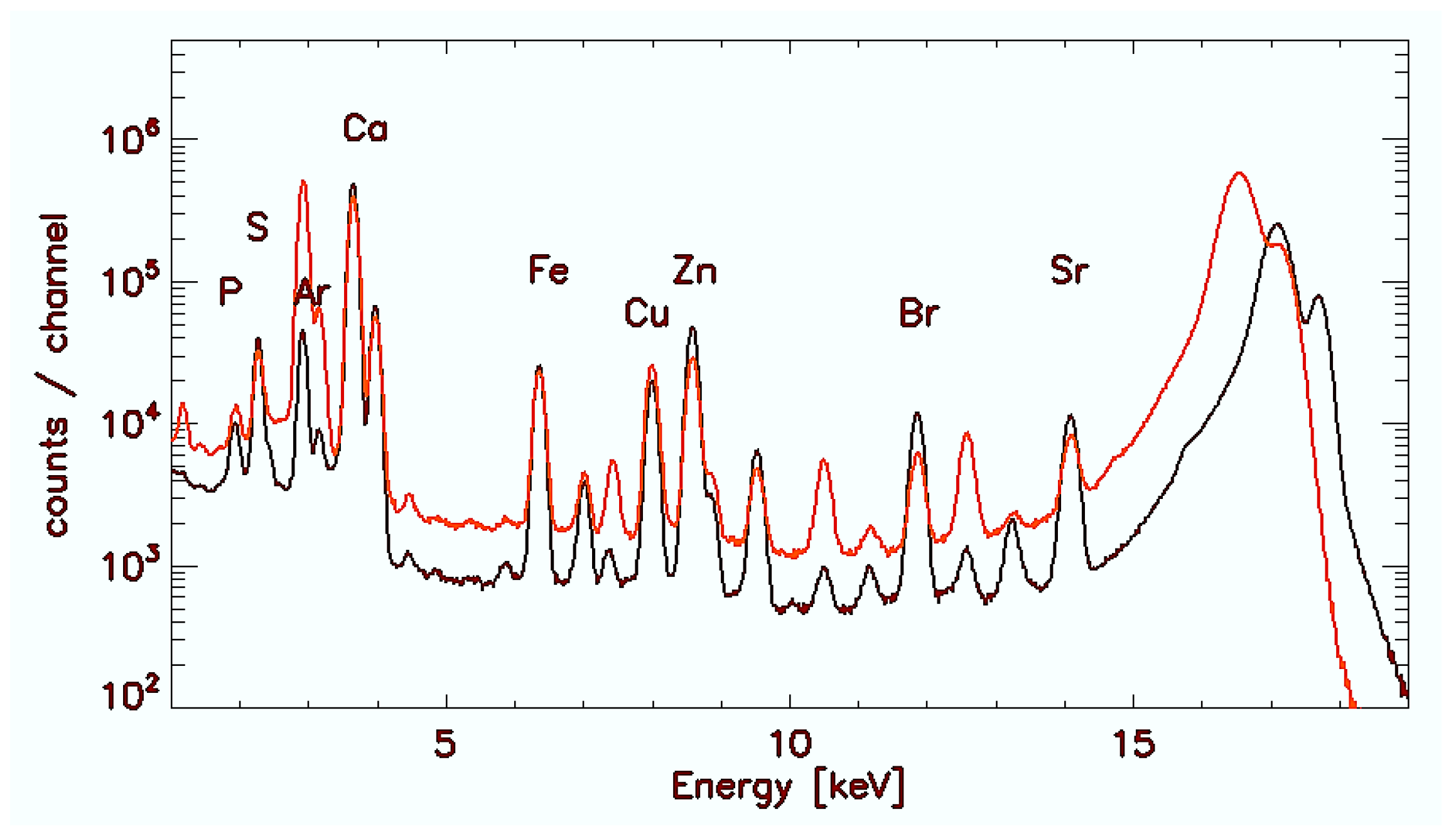
2.4. Microscopic Synchrotron Radiation X-ray Fluorescence Analysis
2.5. Quantification of Elements
2.6. Statistical Analysis
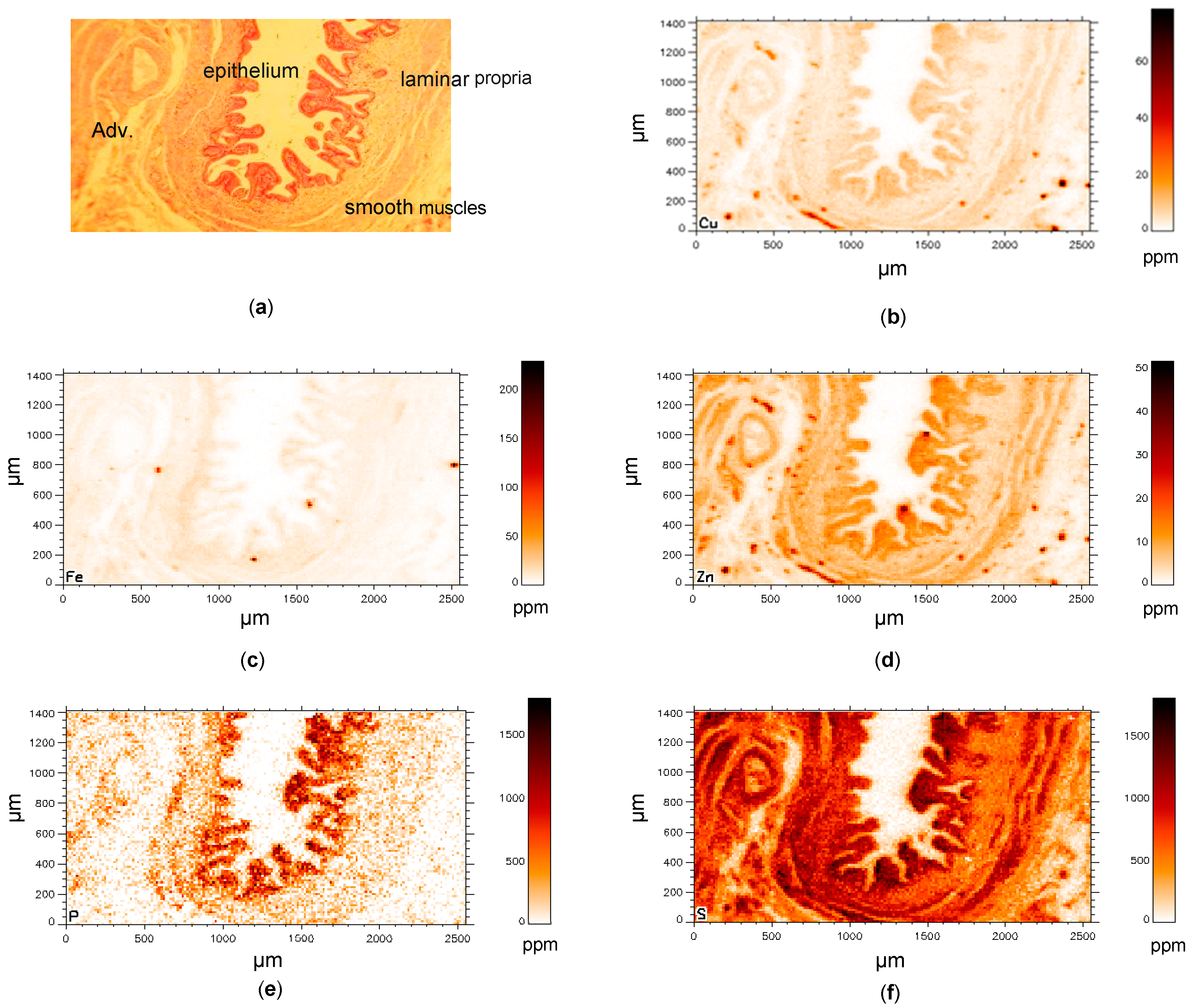
3. Results
3.1. Control Gallbladder (C-GB)
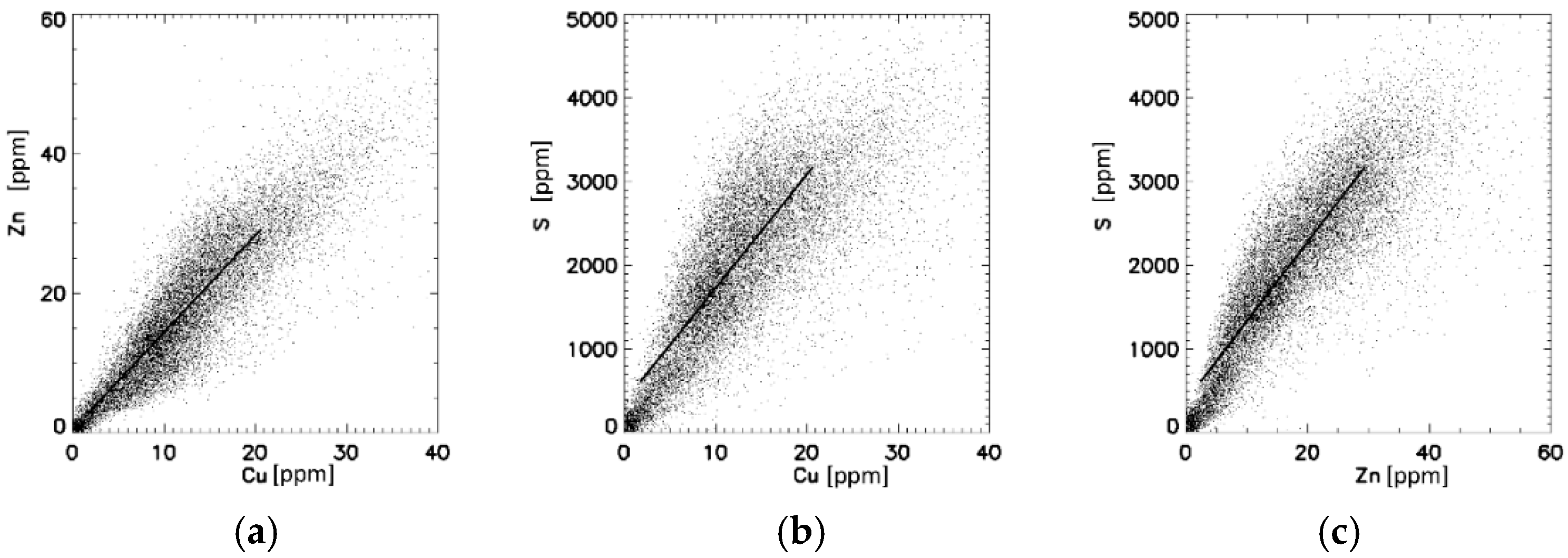
3.2. Wilson’s Disease Gallbladder (WD-GB)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WD | Wilson’s disease |
| GB | Gallbladder |
| SRXRF | Synchrotron X-ray fluorescence |
| MT | Metallothionein |
References
- Gitlin, J.D. Wilson disease. Gastroenterology 2003, 125, 1868–1877. [Google Scholar] [CrossRef]
- Ferenci, P. Wilson’s disease. Clin. Gastroenterol. Hepatol. 2005, 3, 726–733. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver, EASL. Clinical Practice Guidelines: Wilson´s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef]
- Evans, G.W. Copper homeostasis in the mammalian system. Physiol. Rev. 1973, 53, 535–570. [Google Scholar] [CrossRef]
- Richards Mark, P. Recent Developments in Trace Element Metabolism and Function: Role of Metallothionein in Copper and Zinc Metabolism. J. Nutr. 1989, 119, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef]
- Frommer, D.J. Defective biliary excretion of copper in Wilson’s disease. Gut 1974, 15, 125–129. [Google Scholar] [CrossRef]
- Gupta, M.S.; Singh, S.P.; Shukla, V.K. Copper, zinc, and Cu/Zn ratio in carcinoma of the gallbladder. J. Surgical. Oncol. 2005, 91, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Lowe, N.M.; Araya, M.; Brown, K.H. Assessment of the trace element status of individuals and populations: The example of zinc and copper. J Nutr. 2003, 133 (Suppl. 1), 1563S–1568S. [Google Scholar] [CrossRef]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, S3, 0495. [Google Scholar] [CrossRef]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and its complexes in medicine: A biochemical approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef]
- Davis, W.; Chowrimootoo, G.F.; Seymour, C.A. Defective biliary copper excretion in Wilson’s disease: The role of caeruloplasmin. Eur. J. Clin. Investig. 1996, 26, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Gucev, Z.S.; Pop-Jordanova, N.; Calovska, V.; Tasic, V.; Slavevska, N.; Laban, N.; Noli, M.C.; Lepori, M.B.; Loudianos, G. Acute Gallbladder Hydrops and Arthritis: Unusual initial manifestations of Wilson’s Disease (WD): Case Report. Prilozi 2011, 32, 307–315. [Google Scholar]
- Basu, S.; Singh, M.K.; Singh, T.B.; Bhartiya, S.K.; Singh, S.P.; Shukla, V.K. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg. 2013, 37, 2641–2646. [Google Scholar] [CrossRef]
- Rosenfield, N.; Grand, R.J.; Watkins, J.B.; Ballantine, T.V.; Levey, R.H. Cholelithiasis and Wilson disease. J. Pediatr. 1978, 92, 210–213. [Google Scholar] [CrossRef]
- Debray, D.; Franchi-Abella, S.; Irtan, S.; Girard, M. Lithiase biliaire du nourrisson, de l’enfant et de l’adolescent [Cholelithiasis in infants, children and adolescents]. Presse Med. 2012, 41, 466–473. [Google Scholar] [CrossRef]
- Kindness, A.; Sekaran, C.N.; Feldmann, J. Two-dimensional mapping of copper and zinc in liver sections by laser ablation-inductively coupled plasma mass spectrometry. Clin. Chem. 2003, 49, 1916–1923. [Google Scholar] [CrossRef]
- Watanabe, K.; Miyakawa, O.; Kobayashi, M. New method for quantitative mapping of metallic elements in tissue sections by electron probe microanalyser with wavelength dispersive spectrometers. J. Electron. Microsc. (Tokyo) 2001, 50, 77–82. [Google Scholar] [CrossRef]
- Osterode, W.; Falkenberg, G.; Höftberger, R.; Wrba, F. Iron, copper, zinc and bromine mapping in cirrhotic liver slices from patients with hemochromatosis studied by μSRXRF in continuous scanning mode, Spectrochim. Acta Part B Atom. Spectrosc. 2007, 62, 682–688. [Google Scholar] [CrossRef]
- Osterode, W.; Falkenberg, G.; Ferenci, P.; Wrba, F. Quantitative trace element mapping in liver tissue from patients with Wilson’s disease determined by micro X-ray fluorescence. J. Trace Elem. Med. Biol. 2019, 51, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Osterode, W.; Falkenberg, G.; Regele, H. Gadolinium distribution in kidney tissue determined and quantified by micro synchrotron X-ray fluorescence. Biometals 2021, 34, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hachmöller, O.; Buzanich, A.G.; Aichler, M.; Radtke, M.; Dietrich, D.; Schwamborn, K.; Lutz, L.; Werner, M.; Sperling, M.; Walch, A.; et al. Elemental bioimaging and speciation analysis for the investigation of Wilson’s disease using μXRF and XANES. Metallomics 2016, 8, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, W. Osterode, Räumliche Abbildung der Verteilung von Spurenelementen auf mikorskopischem Niveau: Wo sitzt das Blei in menschlichen Knochen? Mikroskopische Röntgenfluoreszenzanalyse. In Forschung mit Synchrotronstrahlung, Eine Einführung in die Grundlagen und Anwendungen; Falta, J., Möller, T., Eds.; Vieweg + Teubner: Wiesbaden, Germany, 2010. [Google Scholar]
- Vekemans, B.; Janssens, K.; Vince, L.; Adams, F.; Van Espen, P. Analysis of X-ray spectra by iterative least squares (AXIL). New Dev. 1994, 23, 278–285. [Google Scholar]
- Feuerborn, J.; Knöchel, A.; Meyer, A.K.; Lechtenberg, F.; Falkenberg, G.; Rickers, K. Sputtered Germanium Films as an Internal Standard for Quantitative X-ray Fluorescence Analysis of Thin Film Samples, in Hasylab Annual Report. 2002. Available online: https://hasyweb.desy.de/science/annual_reports/2002_report/index.html (accessed on 30 November 2021).
- Arredondo, M.; Cambiazo, V.; Tapia, L.; González-Agüero, M.; Núñez, M.T.; Uauy, R.; González, M. Copper overload affects copper and iron metabolism in Hep-G2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G27–G32. [Google Scholar] [CrossRef]
- Harris, E.D.; Qian, Y.; Tiffany-Castiglioni, E.; Lacy, A.R.; Reddy, M.C. Functional analysis of copper homeostasis in cell culture models: A new perspective on internal copper transport. Am. J. Clin. Nutr. 1998, 67 (Suppl. 5), 988S–995S. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Verkman, A.S. Water Transport in the Gastrointestinal Tract. In Physiology of the Gastrointestinal Tract, 6th ed.; Said, H.M., Ed.; Academic Press: San Diego, CA, USA, 2018; pp. 1249–1272. [Google Scholar] [CrossRef]
- Santon, A.; Giannetto, S.; Sturniolo, G.C.; Medici, V.; D’Incà, R.; Irato, P.; Albergoni, V. Interactions between Zn and Cu in LEC rats, an animal model of Wilson’s disease. Histochem. Cell Biol. 2002, 117, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Albergoni, V. Interaction between copper and zinc in metal accumulation in rats with particular reference to the synthesis of induced-metallothionein. Chem. Biol. Interact. 2005, 155, 155–164. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Brewer, G.J. Zinc acetate for the treatment of Wilson’s disease. Expert Opin Pharmacother. 2001, 2, 1473–1477. [Google Scholar] [CrossRef]
- Wu, L.M.; Ekladious, A.; Wheeler, L.; Mohamad, A.A. Wilson disease: Copper deficiency and iatrogenic neurological complications with zinc therapy. Intern. Med. J. 2020, 50, 121–123. [Google Scholar] [CrossRef]
- Dijkstra, M.; Vonk, R.J.; Kuipers, F. How does copper get into bile? New insights into the mechanism(s) of hepatobiliary copper transport. J. Hepatol. 1996, 24 (Suppl. 1), 109–120. [Google Scholar]
- Hidalgo, J.; Dingman, A.; Garvey, J.S. Role of extracellular zinc and copper on metallothionein regulation in cultured rat hepatocytes. Hepatology 1991, 14, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T.P.; Janssens, A.R.; Verspaget, H.W.; van Hattum, J.; Lamers, C.B. Metallothionein concentration in the liver of patients with Wilson’s disease, primary biliary cirrhosis, and liver metastasis of colorectal cancer. J. Hepatol. 1992, 16, 346–350. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Mestriner, C.; Irato, P.; Albergoni, V.; Longo, G.; D’Incà, R. Zinc therapy increases duodenal concentrations of metallothionein and iron in Wilson’s disease patients. Am. J. Gastroenterol. 1999, 94, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Garrick, M.D.; Núñez, M.T.; Olivares, M.; Harris, E.D. Parallels and contrasts between iron and copper metabolism. Biometals 2003, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.L. The copper-iron chronicles: The story of an intimate relationship. Biometals 2003, 16, 9–40. [Google Scholar] [CrossRef] [PubMed]
- Lalioti, V.; Muruais, G.; Tsuchiya, Y.; Pulido, D.; Sandoval, I.V. Molecular mechanisms of copper homeostasis. Front. Biosci. (Landmark Ed.) 2009, 14, 4878–4903. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Cozzi, A.; Santambrogio, P. Iron Pathophysiology in Neurodegeneration with Brain Iron Accumulation. Adv. Exp. Med. Biol. 2019, 1173, 153–177. [Google Scholar] [CrossRef]
- Tello, C.; Darling, A.; Lupo, V.; Pérez-Dueñas, B.; Espinós, C. On the complexity of clinical and molecular bases of neurodegeneration with brain iron accumulation. Clin. Genet. 2018, 93, 731–740. [Google Scholar] [CrossRef]
- Faa, G.; Lisci, M.; Caria, M.P.; Ambu, R.; Sciot, R.; Nurchi, V.M.; Silvagni, R.; Diaz, A.; Crisponi, G. Brain copper, iron, magnesium, zinc, calcium, sulfur and phosphorus storage in Wilson’s disease. J. Trace Elem. Med. Biol. 2001, 15, 155–160. [Google Scholar] [CrossRef]
- Litwin, T.; Gromadzka, G.; Szpak, G.M.; Jablonka-Salach, K.; Bulska, E.; Czlonkowska, A. Brain metal accumulation in Wilson’s disease. J. Neurol. Sci. 2013, 329, 55–58. [Google Scholar] [CrossRef]
- Dusek, P.; Bahn, E.; Litwin, T.; Jabłonka-Salach, K.; Łuciuk, A.; Huelnhagen, T.; Madai, V.I.; Dieringer, M.A.; Bulska, E.; Knauth, M.; et al. Brain iron accumulation in Wilson disease: A post mortem 7 Tesla MRI—Histopathological study. Neuropathol. Appl. Neurobiol. 2017, 43, 514–532. [Google Scholar] [CrossRef]
- Chwiej, J.; Szczerbowska-Boruchowska, M.; Lankosz, M.; Wojcik, S.; Falkenberg, G.; Setkowicz, Z.; Stegowski, Z. Preparation of tissue samples for X-ray fluorescence microscopy. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 1531–1537. [Google Scholar] [CrossRef]
- Schrag, M.; Dickson, A.; Jiffry, A.; Kirsch, D.; Vinters, H.V.; Kirsch, W. The effect of formalin fixation on the levels of brain transition metals in archived samples. Biometals 2010, 23, 1123–1127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perrin, L.; Carmona, A.; Roudeau, S.; Ortega, R. Evaluation of sample preparation methods for single cell quantitative elemental imaging using proton or synchrotron radiation focused beams. J. Anal. At. Spectrom. 2015, 30, 2525–2532. [Google Scholar] [CrossRef]
- Surowka, A.D.; Gianoncelli, A.; Birarda, G.; Sala, S.; Cefarin, N.; Matruglio, A.; Szczerbowska-Boruchowska, M.; Ziomber-Lisiak, A.; Vaccari, L. Soft X-ray induced radiation damage in thin freeze-dried brain samples studied by FTIR microscopy. J. Synchrotron Radiat. 2020, 27, 1218–1226. [Google Scholar] [CrossRef]
- Jin, Q.; Paudesku, T.; Lai, B.; Gleber, S.C.; Chen, S.; Finney, L.; Vine, D.; Vogt, S.; Woloschak, G.; Jacobsen, C. Preserving elemental content in adherent mammalian cells for analysis by synchrotron-based X-ray fluorescence microscopy. J. Microscopy 2017, 265, 81–93. [Google Scholar] [CrossRef]


| No. | Age [Years] | Gender | Tissue | Histology | ||
|---|---|---|---|---|---|---|
| FDG | SC | Sol | ||||
| 1 | 51 | m | Block | no | ± | no |
| 2 | 40 | m | Block | no | no | no |
| 3 | 36 | f | Block | no | no | ? |
| 4 | 48 | f | Block | ± | no | no |
| 5 | 53 | m | Block | no | no | no |
| 6 | 46 | f | Block | no | no | ± |
| No. | Age [Years] | Gender | Tissue | Histology | ||
|---|---|---|---|---|---|---|
| FDG | SC | Sol | ||||
| 1 | 29 | f | Block | no | ± | ± |
| 2 | 40 | m | Block | + | no | ? |
| 3 | 18 | f | Block | no | no | ? |
| 4 | 32 | f | Block | ± | no | no |
| 5 | 48 | m | Block | no | no | no |
| 6 | 25 | f | Block | ± | + | ± |
| N = 6 | P | S | Cu | Fe | Zn | |
|---|---|---|---|---|---|---|
| C-GB | 849 | 1250 | 10 | 8 | 12 | [ppm] |
| SD | 261 | 148 | 4 | 4 | 3 | [ppm] |
| WD-GB | 1796 * | 2350 ** | 23 ** | 67 * | 30 ** | [ppm] |
| SD | 912 | 651 | 9 | 28 | 11 | [ppm] |
| N = 6 | P | S | Cu | Fe | Zn | |
|---|---|---|---|---|---|---|
| C-L | 1818 | 2699 | 20 | 299 | 64 | |
| SD | 497 | 457 | 5 | 34 | 19 | |
| WD-L | Fibr. | 1269 * | 1687 ** | 83 ** | 293 | 55 |
| SD | 319 | 275 | 14 | 99 | 12 | |
| Hep. | 1301 | 2121 * | 434 ** | 381 | 71 | |
| SD | 401 | 369 | 107 | 151 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osterode, W.; Falkenberg, G.; Wrba, F. Copper and Trace Elements in Gallbladder form Patients with Wilson’s Disease Imaged and Determined by Synchrotron X-ray Fluorescence. J. Imaging 2021, 7, 261. https://doi.org/10.3390/jimaging7120261
Osterode W, Falkenberg G, Wrba F. Copper and Trace Elements in Gallbladder form Patients with Wilson’s Disease Imaged and Determined by Synchrotron X-ray Fluorescence. Journal of Imaging. 2021; 7(12):261. https://doi.org/10.3390/jimaging7120261
Chicago/Turabian StyleOsterode, Wolf, Gerald Falkenberg, and Fritz Wrba. 2021. "Copper and Trace Elements in Gallbladder form Patients with Wilson’s Disease Imaged and Determined by Synchrotron X-ray Fluorescence" Journal of Imaging 7, no. 12: 261. https://doi.org/10.3390/jimaging7120261
APA StyleOsterode, W., Falkenberg, G., & Wrba, F. (2021). Copper and Trace Elements in Gallbladder form Patients with Wilson’s Disease Imaged and Determined by Synchrotron X-ray Fluorescence. Journal of Imaging, 7(12), 261. https://doi.org/10.3390/jimaging7120261






