Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI
Abstract
1. Introduction
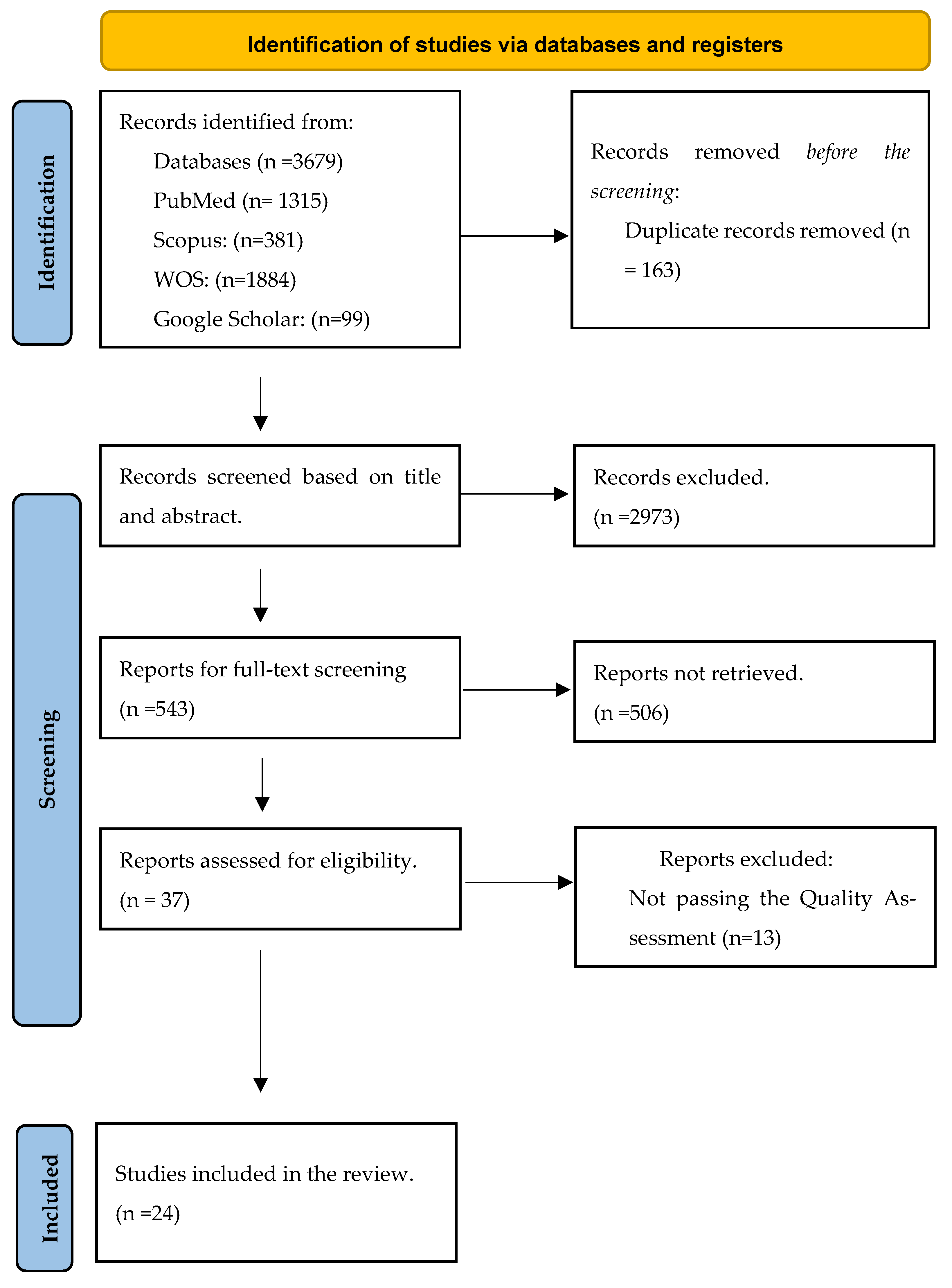
2. Method
2.1. Inclusion and Exclusion Criteria
- (1)
- To mitigate the possible confounding effect of any intervention, observation methods are used.
- (2)
- Included for consideration were original studies, all types of cardiac imaging modules, and studies from all countries, which were in English.
- (3)
- Excluded from consideration were studies that were review papers, animal studies, populations without a heart condition, case reports, case series, other languages that were not English, articles that were only abstract and lacked full text, and grey literature.
2.2. Data Extraction and Study Quality Assessment
3. Result
4. Discussion
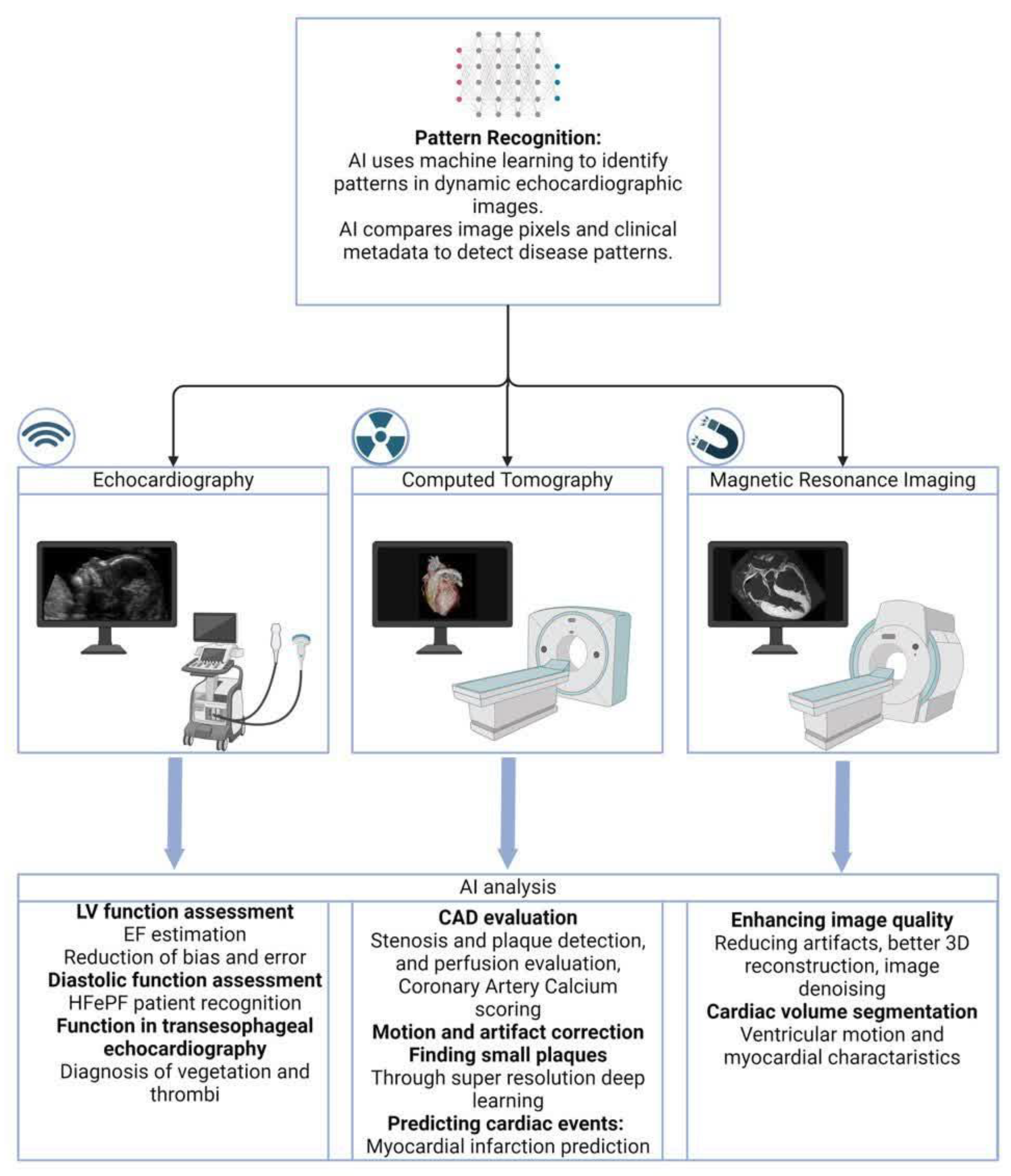
4.1. AI Patterns in Cardiac Imaging
4.2. AI Applications in Echocardiography
4.3. AI and CT Imaging in Cardiology
4.4. AI and Cardiac MRI
4.5. Limitation of Using AI in Cardiac Imaging
4.6. Current Challenges and Gaps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kronenberg, M.W.; Price, R.R.; Smith, C.W.; Robertson, R.M.; Perry, J.M.; Pickens, D.R.; Domanski, M.J.; Partain, C.L.; Friesinger, G.C. Evaluation of left ventricular performance using digital subtraction angiography. Am. J. Cardiol. 1983, 51, 837–842. [Google Scholar] [CrossRef] [PubMed]
- New Directions in Cardiac Imaging. Ann. Intern. Med. 1985, 102, 795–799. [CrossRef] [PubMed]
- Reeves, R.A.; Halpern, E.J.; Rao, V.M. Cardiac Imaging Trends from 2010 to 2019 in the Medicare Population. Radiol. Cardiothorac. Imaging 2021, 3, e210156. [Google Scholar] [CrossRef] [PubMed]
- van Assen, M.; Lee, S.J.; De Cecco, C.N. Artificial intelligence from A to Z: From neural network to legal framework. Eur. J. Radiol. 2020, 129, 109083. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.T.; Meyersohn, N.M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Foldyna, B.; Mueller, M.E.; Hearne, S.; Yang, C.; et al. Central Core Laboratory versus Site Interpretation of Coronary CT Angiography: Agreement and Association with Cardiovascular Events in the PROMISE Trial. Radiology 2018, 287, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Rushlow, D.R.; Inselman, J.W.; McCoy, R.G.; Thacher, T.D.; Behnken, E.M.; Bernard, M.E.; Rosas, S.L.; Akfaly, A.; Misra, A.; et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: A pragmatic, randomized clinical trial. Nat. Med. 2021, 27, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yin, Y.; Yang, Q.; Huo, T. Artificial intelligence in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. J. Med. Res. 2023, 28, 242. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.; Farina, J.M.; Chao, C.-J.; Ayoub, C.; Jeong, J.; Patel, B.N.; Banerjee, I.; Arsanjani, R. The Role of Artificial Intelligence in Echocardiography. J. Imaging 2023, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kusunose, K.; Abe, T.; Haga, A.; Fukuda, D.; Yamada, H.; Harada, M.; Sata, M. A deep learning approach for assessment of regional wall motion abnormality from echocardiographic images. Cardiovasc. Imaging 2020, 13, 374–381. [Google Scholar] [CrossRef]
- Pandey, A.; Kagiyama, N.; Yanamala, N.; Segar, M.W.; Cho, J.S.; Tokodi, M.; Sengupta, P.P. Deep-learning models for the echocardiographic assessment of diastolic dysfunction. Cardiovasc. Imaging 2021, 14, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Salte, I.M.; Østvik, A.; Smistad, E.; Melichova, D.; Nguyen, T.M.; Karlsen, S.; Brunvand, H.; Haugaa, K.H.; Edvardsen, T.; Lovstakken, L. Artificial intelligence for automatic measurement of left ventricular strain in echocardiography. Cardiovasc. Imaging 2021, 14, 1918–1928. [Google Scholar] [CrossRef]
- Upton, R.; Mumith, A.; Beqiri, A.; Parker, A.; Hawkes, W.; Gao, S.; Porumb, M.; Sarwar, R.; Marques, P.; Markham, D. Automated echocardiographic detection of severe coronary artery disease using artificial intelligence. Cardiovasc. Imaging 2022, 15, 715–727. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.J.; Ahn, H.G.; Cha, K.-C.; Yang, S. Left ventricle segmentation in transesophageal echocardiography images using a deep neural network. PLoS ONE 2023, 18, e0280485. [Google Scholar] [CrossRef]
- Krishna, H.; Desai, K.; Slostad, B.; Bhayani, S.; Arnold, J.H.; Ouwerkerk, W.; Hummel, Y.; Lam, C.S.; Ezekowitz, J.; Frost, M. Fully automated artificial intelligence assessment of aortic stenosis by echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yuki, H.; Niida, T.; Suzuki, K.; Kinoshita, D.; McNulty, I.; Broersen, A.; Dijkstra, J.; Lee, H.; Kakuta, T. A novel deep learning model for a computed tomography diagnosis of coronary plaque erosion. Sci. Rep. 2023, 13, 22992. [Google Scholar] [CrossRef]
- Salte, I.M.; Østvik, A.; Olaisen, S.H.; Karlsen, S.; Dahlslett, T.; Smistad, E.; Eriksen-Volnes, T.K.; Brunvand, H.; Haugaa, K.H.; Edvardsen, T. Deep learning for improved precision and reproducibility of left ventricular strain in echocardiography: A test-retest study. J. Am. Soc. Echocardiogr. 2023, 36, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Zamzmi, G.; Hsu, L.-Y.; Rajaraman, S.; Li, W.; Sachdev, V.; Antani, S. Evaluation of an artificial intelligence-based system for echocardiographic estimation of right atrial pressure. Int. J. Cardiovasc. Imaging 2023, 39, 2437–2450. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.F.; Rohnean, A.; Giroussens, H.; Pressat-Laffouilhere, T.; Wong, T. Evaluation of a deep learning model on coronary CT angiography for automatic stenosis detection. Diagn. Interv. Imaging 2022, 103, 316–323. [Google Scholar] [CrossRef]
- Andre, F.; Fortner, P.; Aurich, M.; Seitz, S.; Jatsch, A.K.; Schöbinger, M.; Wels, M.; Kraus, M.; Gülsün, M.A.; Frey, N.; et al. Human AI Teaming for Coronary CT Angiography Assessment: Impact on Imaging Workflow and Diagnostic Accuracy. Diagn. 2023, 13, 3574. [Google Scholar] [CrossRef]
- Cobo, M.; Pérez-Rojas, F.; Gutiérrez-Rodríguez, C.; Heredia, I.; Maragaño-Lizama, P.; Yung-Manriquez, F.; Lloret Iglesias, L.; Vega, J.A. Novel deep learning method for coronary artery tortuosity detection through coronary angiography. Sci. Rep. 2023, 13, 11137. [Google Scholar]
- Dey, D.; Gaur, S.; Ovrehus, K.A.; Slomka, P.J.; Betancur, J.; Goeller, M.; Hell, M.M.; Gransar, H.; Berman, D.S.; Achenbach, S.; et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: A multicentre study. Eur. Radiol. 2018, 28, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Dundas, J.; Leipsic, J.A.; Sellers, S.; Blanke, P.; Miranda, P.; Ng, N.; Mullen, S.; Meier, D.; Akodad, M.; Sathananthan, J.; et al. Artificial Intelligence–based Coronary Stenosis Quantification at Coronary CT Angiography versus Quantitative Coronary Angiography. Radiol. Cardiothorac. Imaging 2023, 5, e230124. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.F.; Choi, A.D.; Riess, J.S.; Marques, H.; Chang, H.J.; Choi, J.H.; Doh, J.H.; Her, A.Y.; Koo, B.K.; Nam, C.W.; et al. AI Evaluation of Stenosis on Coronary CTA, Comparison With Quantitative Coronary Angiography and Fractional Flow Reserve: A CREDENCE Trial Substudy. JACC Cardiovasc. Imaging 2023, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Luo, N.; Xu, L.; Cao, J.; Guo, N.; He, Y.; Hong, M.; Jia, X.; Wang, Z.; Yang, Z. Artificial intelligence stenosis diagnosis in coronary CTA: Effect on the performance and consistency of readers with less cardiovascular experience. BMC Med. Imaging 2022, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Ihdayhid, A.R.; Lan, N.S.R.; Williams, M.; Newby, D.; Flack, J.; Kwok, S.; Joyner, J.; Gera, S.; Dembo, L.; Adler, B.; et al. Evaluation of an artificial intelligence coronary artery calcium scoring model from computed tomography. Eur. Radiol. 2023, 33, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Sun, J.; Zhang, L.; Ding, Z.; Wang, L.; Zhao, K.; Pan, Z.; Li, Q.; Guo, N.; et al. Fully automated artificial intelligence-based coronary CT angiography image processing: Efficiency, diagnostic capability, and risk stratification. Eur. Radiol. 2024, 34, 4909–4919. [Google Scholar] [CrossRef] [PubMed]
- van Assen, M.; Martin, S.S.; Varga-Szemes, A.; Rapaka, S.; Cimen, S.; Sharma, P.; Sahbaee, P.; De Cecco, C.N.; Vliegenthart, R.; Leonard, T.J.; et al. Automatic coronary calcium scoring in chest CT using a deep neural network in direct comparison with non-contrast cardiac CT: A validation study. Eur. J. Radiol. 2021, 134, 109428. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Nakajima, K.; Taki, J.; Wakabayashi, H.; Matsuo, S.; Konishi, T.; Okuda, K.; Shibutani, T.; Onoguchi, M.; Kinuya, S. Ability of artificial intelligence to diagnose coronary artery stenosis using hybrid images of coronary computed tomography angiography and myocardial perfusion SPECT. Eur. J. Hybrid Imaging 2019, 3, 4. [Google Scholar] [CrossRef]
- Åkesson, J.; Ostenfeld, E.; Carlsson, M.; Arheden, H.; Heiberg, E. Deep learning can yield clinically useful right ventricular segmentations faster than fully manual analysis. Sci. Rep. 2023, 13, 1216. [Google Scholar] [CrossRef]
- Cau, R.; Pisu, F.; Pintus, A.; Palmisano, V.; Montisci, R.; Suri, J.S.; Salgado, R.; Saba, L. Cine-cardiac magnetic resonance to distinguish between ischemic and non-ischemic cardiomyopathies: A machine learning approach. Eur. Radiol. 2024, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.H.; Augusto, J.B.; Bhuva, A.; Xue, H.; Treibel, T.A.; Ye, Y.; Hughes, R.K.; Bai, W.; Lau, C.; Shiwani, H.; et al. Precision measurement of cardiac structure and function in cardiovascular magnetic resonance using machine learning. J. Cardiovasc. Magn. Reson. 2022, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, G.; Gao, Z.; Xu, C.; Zhang, Y.; Shi, R.; Keegan, J.; Xu, L.; Zhang, H.; Fan, Z.; et al. Deep Learning for Diagnosis of Chronic Myocardial Infarction on Nonenhanced Cardiac Cine MRI. Radiology 2019, 291, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Shah, S.M.I.; Naeem, A.; Shujauddin, S.M.; Jabeen, A.; Kazmi, S.; Siddiqui, S.A.; Kumar, P.; Salman, S.; Hassan, S.A.; et al. Artificial intelligence in the diagnosis and detection of heart failure: The past, present, and future. RCM 2021, 22, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Valueva, M.V.; Nagornov, N.; Lyakhov, P.A.; Valuev, G.V.; Chervyakov, N.I. Application of the residue number system to reduce hardware costs of the convolutional neural network implementation. Math. Comput. Simul. 2020, 177, 232–243. [Google Scholar] [CrossRef]
- Lee, J.-G.; Jun, S.; Cho, Y.-W.; Lee, H.; Kim, G.B.; Seo, J.B.; Kim, N. Deep learning in medical imaging: General overview. Korean J. Radiol. 2017, 18, 570. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Ghesu, F.-C.; Georgescu, B.; Zheng, Y.; Grbic, S.; Maier, A.; Hornegger, J.; Comaniciu, D. Multi-scale deep reinforcement learning for real-time 3D-landmark detection in CT scans. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 41, 176–189. [Google Scholar] [CrossRef]
- Zheng, Y.; Barbu, A.; Georgescu, B.; Scheuering, M.; Comaniciu, D. Four-chamber heart modeling and automatic segmentation for 3-D cardiac CT volumes using marginal space learning and steerable features. IEEE Trans. Med. Imaging 2008, 27, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.A.; Lu, Z.; Carneiro, G. Combining deep learning and level set for the automated segmentation of the left ventricle of the heart from cardiac cine magnetic resonance. Med. Image Anal. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Davis, A.; Billick, K.; Horton, K.; Jankowski, M.; Knoll, P.; Marshall, J.E.; Paloma, A.; Palma, R.; Adams, D.B. Artificial intelligence and echocardiography: A primer for cardiac sonographers. J. Am. Soc. Echocardiogr. 2020, 33, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Sehly, A.; Jaltotage, B.; He, A.; Maiorana, A.; Ihdayhid, A.R.; Rajwani, A.; Dwivedi, G. Artificial Intelligence in echocardiography: The time is now. Rev. Cardiovasc. Med. 2022, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, C.; Bekkers, S.C.; Schummers, G.; Schreckenberg, M.; Muraru, D.; Badano, L.P.; Franke, A.; Bavishi, C.; Omar, A.M.S.; Sengupta, P.P. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: The FAST-EFs multicenter study. J. Am. Coll. Cardiol. 2015, 66, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.J.; Lapidaire, W.; Leeson, P. Machine learning augmented echocardiography for diastolic function assessment. Front. Cardiovasc. Med. 2021, 8, 711611. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chang, H.; Yang, D.; Yang, F.; Wang, Q.; Deng, Y.; Li, L.; Lv, W.; Zhang, B.; Yu, L. A deep learning framework assisted echocardiography with diagnosis, lesion localization, phenogrouping heterogeneous disease, and anomaly detection. Sci. Rep. 2023, 13, 3. [Google Scholar] [CrossRef]
- Yusuf, S.; Reddy, S.; Ôunpuu, S.; Anand, S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001, 104, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, L.R.M.; Bucolo, G.M.; Muscogiuri, G.; Sironi, S.; Gaeta, M.; Ascenti, G.; Booz, C.; Vogl, T.J.; Blandino, A.; Mazziotti, S.; et al. Artificial Intelligence in Cardiovascular CT and MR Imaging. Life 2023, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Nakaura, T.; Yanagawa, M.; Fujita, S.; Kamagata, K.; Ito, R.; Kawamura, M.; Fushimi, Y.; Ueda, D.; Matsui, Y.; et al. Recent advances in artificial intelligence for cardiac CT: Enhancing diagnosis and prognosis prediction. Diagn. Interv. Imaging 2023, 104, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, S.; Cheezum, M.K.; Hulten, E.A.; Bittencourt, M.S.; Silverman, M.G.; Nasir, K.; Blankstein, R. Use of cardiac CT and calcium scoring for detecting coronary plaque: Implications on prognosis and patient management. Br J Radiol 2015, 88, 20140594. [Google Scholar] [CrossRef]
- Išgum, I.; Rutten, A.; Prokop, M.; van Ginneken, B. Detection of coronary calcifications from computed tomography scans for automated risk assessment of coronary artery disease. Med. Phys. 2007, 34, 1450–1461. [Google Scholar] [CrossRef]
- Takx, R.A.P.; de Jong, P.A.; Leiner, T.; Oudkerk, M.; de Koning, H.J.; Mol, C.P.; Viergever, M.A.; Išgum, I. Automated Coronary Artery Calcification Scoring in Non-Gated Chest CT: Agreement and Reliability. PLoS ONE 2014, 9, e91239. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, P.; Su, Z.; Yao, X.; Wang, Y.; Wang, C.; Du, X.; Li, K. Effect of a novel motion correction algorithm (SSF) on the image quality of coronary CTA with intermediate heart rates: Segment-based and vessel-based analyses. Eur. J. Radiol. 2014, 83, 2024–2032. [Google Scholar] [CrossRef]
- Wang, M.; Fan, J.; Shi, X.; Qin, L.; Yan, F.; Yang, W. A deep-learning reconstruction algorithm that improves the image quality of low-tube-voltage coronary CT angiography. Eur. J. Radiol. 2022, 146, 110070. [Google Scholar] [CrossRef] [PubMed]
- Otgonbaatar, C.; Ryu, J.-K.; Shin, J.; Woo, J.Y.; Seo, J.W.; Shim, H.; Hwang, D.H. Improvement in image quality and visibility of coronary arteries, stents, and valve structures on CT angiography by deep learning reconstruction. Korean J. Radiol. 2022, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Higaki, T.; Kawashita, I.; Fukumoto, W.; Nakamura, Y.; Matsuura, M.; Lee, T.-C.; Zhou, J.; Cai, L.; Kitagawa, T. Improvement of spatial resolution on coronary CT angiography by using Super-resolution Deep Learning Reconstruction. Acad. Radiol. 2023, 30, 2497–2504. [Google Scholar] [CrossRef]
- Lin, A.; Manral, N.; McElhinney, P.; Killekar, A.; Matsumoto, H.; Kwiecinski, J.; Pieszko, K.; Razipour, A.; Grodecki, K.; Park, C.; et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: An international multicentre study. Lancet Digit. Health 2022, 4, e256–e265. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Slomka, P.J.; Rios, R.; Betancur, J.; Blaha, M.J.; Nasir, K.; Miedema, M.D.; Rumberger, J.A.; Gransar, H.; Shaw, L.J.; et al. Machine Learning Adds to Clinical and CAC Assessments in Predicting 10-Year CHD and CVD Deaths. JACC Cardiovasc. Imaging 2021, 14, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the future—Big data, machine learning, and clinical medicine. N. Engl. J. Med. 2016, 375, 1216. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.A.; Manning, W.J.; Nezafat, R. Present and Future Innovations in AI and Cardiac MRI. Radiology 2024, 310, e231269. [Google Scholar] [CrossRef]
- Hauptmann, A.; Arridge, S.; Lucka, F.; Muthurangu, V.; Steeden, J.A. Real-time cardiovascular MR with spatio-temporal artifact suppression using deep learning-proof of concept in congenital heart disease. Magn. Reson. Med. 2019, 81, 1143–1156. [Google Scholar] [CrossRef]
- Tian, C.; Fei, L.; Zheng, W.; Xu, Y.; Zuo, W.; Lin, C.-W. Deep learning on image denoising: An overview. Neural Netw. 2020, 131, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szemes, A.; Muscogiuri, G.; Schoepf, U.J.; Wichmann, J.L.; Suranyi, P.; De Cecco, C.N.; Cannaò, P.M.; Renker, M.; Mangold, S.; Fox, M.A. Clinical feasibility of a myocardial signal intensity threshold-based semi-automated cardiac magnetic resonance segmentation method. Eur. Radiol. 2016, 26, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, C.; Dacher, J.N. A review of segmentation methods in short axis cardiac MR images. Med. Image Anal. 2011, 15, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Han, K.; Lee, S.; Yang, Y.J.; Kim, P.K.; Choi, B.W.; Suh, Y.J. Automated measurement of native T1 and extracellular volume fraction in cardiac magnetic resonance imaging using a commercially available deep learning algorithm. Korean J. Radiol. 2022, 23, 1251. [Google Scholar] [CrossRef] [PubMed]
- Cordero, D. The downsides of artificial intelligence in healthcare. Korean J. Pain 2024, 37, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Bazoukis, G.; Hall, J.; Loscalzo, J.; Antman, E.M.; Fuster, V.; Armoundas, A.A. The inclusion of augmented intelligence in medicine: A framework for successful implementation. Cell Rep. Med. 2022, 3, 100485. [Google Scholar] [CrossRef]
- Griffin, F. Artificial intelligence and liability in health care. Health Matrix 2021, 31, 65. [Google Scholar]
- Chung, C.T.; Lee, S.; King, E.; Liu, T.; Armoundas, A.A.; Bazoukis, G.; Tse, G. Clinical significance, challenges and limitations in using artificial intelligence for electrocardiography-based diagnosis. Int. J. Arrhythmia 2022, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Tamang, S.; Yazdany, J.; Schmajuk, G. Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern. Med. 2018, 178, 1544–1547. [Google Scholar] [CrossRef]
- Norori, N.; Hu, Q.; Aellen, F.M.; Faraci, F.D.; Tzovara, A. Addressing bias in big data and AI for health care: A call for open science. Patterns 2021, 2, 100347. [Google Scholar] [CrossRef]
- Brady, A.P.; Allen, B.; Chong, J.; Kotter, E.; Kottler, N.; Mongan, J.; Oakden-Rayner, L.; Dos Santos, D.P.; Tang, A.; Wald, C.; et al. Developing, purchasing, implementing and monitoring AI tools in radiology: Practical considerations. A multi-society statement from the ACR, CAR, ESR, RANZCR & RSNA. Insights Imaging 2024, 15, 16. [Google Scholar] [PubMed]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Singh, S.; Pathania, M.; Gosavi, S.; Abhishek, S.; Parchani, A.; Dhar, M. Artificial intelligence in clinical medicine: Catalyzing a sustainable global healthcare paradigm. Front. Artif. Intell. 2023, 6, 1227091. [Google Scholar] [CrossRef]
| Databases | Mesh Term | Result | Date |
|---|---|---|---|
| PubMed | ((((Artificial intelligence[MeSH Terms]) OR (machine intelligence[MeSH Terms])) OR (AI[Title/Abstract])) OR (cognitive computing[Title/Abstract])) OR (robotic intelligence[Title/Abstract]) AND ((((cardiac imaging[MeSH Terms]) OR (cardiac ultrasound[MeSH Terms])) OR (cardiac MRI[MeSH Terms])) OR (angiography[MeSH Terms])) OR (cardiac US[MeSH Terms]) | 1315 | 16 April 2024 |
| SCOPUS | (artificial AND intelligence OR machine AND intelligence OR ai OR cognitive AND computing OR robotic AND intelligence) AND (cardiac AND imaging OR cardiac AND MRI OR cardiac AND ultrasound OR angiography OR cardiac AND sonography OR heart AND sonography) | 381 | 16 April 2024 |
| Web of Science | (ALL = (Artificial intelligence OR machine intelligence OR AI)) AND ALL = (cardiac imaging OR cardiac MRI OR Cardiac US OR cardiac ultrasound) and Article (Document Types) | 1884 | 16 April 2024 |
| Google Scholar | (Artificial intelligence OR machine intelligence OR AI OR cognitive computing OR robotic intelligence) AND (cardiac imaging OR cardiac MRI OR Cardiac US OR cardiac ultrasound OR cardiac sonography) | 99 | 16 April 2024 |
| Author, Year. | Number of Participants | Cardiac Abnormality | Type of DLM | Result | Conclusion |
|---|---|---|---|---|---|
| Kusunose et al., 2021 [10]. | 400 | Regional wall motion abnormalities | Deep convolutional neural network | DLM algorithm showed comparable performance to cardiologists in detecting WMAs: AUC of 0.99 vs. 0.98 (p = 0.15). | Deep convolutional neural network can be Use to diagnose myocardial ischemia with echocardiography. |
| Pandey et al., 2021 [11]. | 1242 | Heart failure with preserved EF | 1. unsupervised clustering approach (topological data analysis network) 2. cloud-based automated ML | The AUC for predicting elevated left ventricular filling pressure is higher compared to the 2016 ASE guidance grades (0.88 vs. 0.67; p = 0.01). | The neural network. classifier in this study provides a practical solution to overcome the limitations of current clinical standards in accurately characterizing the burden of LVDD in HFpEF. |
| Salte et al., 2021 [12]. | 200 | Various cardiac pathologies (measuring LV function) | Different type of artificial neural networks (ANN) 1. recurrent ANN architecture 2. U-net architecture | -The AI method accurately classified all apical views and timed cardiac events in 89% of patients. -Mean GLS was −12.1% ± 5.0% (AI) and −13.5% ± 5.3% (conventional). | DL networks eliminate manual tracing, increasing efficiency and reproducibility. Fully automated AI measurements facilitate the implementation of GLS in clinical practice. |
| Upton et al., 2022 [13]. | 578 | Severe Coronary artery disease | convolutional neural network. | -Using AI to identify severe coronary artery disease achieved 92.7% specificity and 84.4% sensitivity with cross-fold validation. -The AI tool increased inter-reader agreement, confidence, and disease detection sensitivity by 10%, with an AUC of 0.93. | Automated analysis of stress echocardiograms is possible using AI. Providing automated classifications to clinicians could improve accuracy, inter-reader agreement, and reader confidence when reading stress echocardiograms. |
| Kang et al., 2023 [14]. | 9 | Systolic function abnormality | U-Net and new AI model based on U-Net | U-Net had an AUC of 0.982, 0.996, 0.983, 0.996, and 0.992 in each fold, while the new model achieved AUCs of 0.994, 0.998, 0.997, 0.997, and 0.994. | Compared to U-Net, the proposed model demonstrated better performance across all metrics, enabling more accurate segmentation of the LV for detailed evaluation of the heart’s systolic function during CPR. |
| Krishna et al., 2023 [15]. | 256 | Aortic stenosis | ANN (Us2.ai) | AI demonstrated strong correlation with human measurements in: -Aortic valve peak velocity (r = 0.97, p < 0.001) -Mean pressure gradient (r = 0.94, p < 0.001) -Stroke volume index (r = 0.79, p < 0.001) | This AI technology could reduce interscan variability, enhance AS interpretation and diagnosis, and enable accurate and reproducible management of AS patients. |
| Park et al., 2023. [16]. | 395 | Acute coronary syndromes | -MD-CTA model. -standard convolution neural network | The novel DL model showed significantly improved AUC, sensitivity, and specificity compared to the convolution neural network for patient-level prediction: AUC (0.899 vs. 0.724), sensitivity (87.1% vs. 71.0%), and specificity (85.3% vs. 68.0%). | This new DL model shows promise in identifying atherosclerotic plaque erosion using non-invasive coronary CTA images and significantly outperforms experienced cardiologists |
| Salte et al., 2023 [17]. |
| LV GLS | ANN | -The AI method correctly classified the view in 96% (231/240) of recordings in dataset I and 97% (187/192) in dataset II. -AI accurately classified cardiac events (end diastole, systole, and end systole) in 99% (238/240) of recordings in dataset I and 97% (187/192) in dataset II. | This AI method, with automated LV GLS measurements, reduced test-retest variability, eliminated reader bias, and can facilitate LV GLS implementation, improving workflow in clinical echocardiography. |
| Zamzmi et al., 2023 [18]. | 255 | Estimation of RAP by IVC measurement during echocardiography | Conventional ML models | Strong agreement (r = 0.96) between automatically calculated and manually measured IVC values. | -Fully automated and cost-effective tool for analyzing dIVC and cIVC. -Enhances clinical decision-making through quantitative data insights. -Potential to improve patient outcomes through informed medical interventions. |
| Author, Year | Number of Patients | Cardiac Abnormality | Type of Deep Learning | Result | Conclusion |
|---|---|---|---|---|---|
| Paul et al., 2022 [19]. | 53 | Coronary artery disease | neural network | Accuracy of the DLM was 96%. | Accuracy was excellent for differentiating patients with vs. without stenoses ≥50%. |
| Andre et al., 2023 [20]. | 120 | Coronary artery disease | Siemens Automatic Coronary Analysis | The time for the coronary CTA assessment was reduced in the human AI group vs. standard group by approximately 27%. | AI-based analysis significantly improves clinical by reducing CTA analysis reporting time without compromising diagnostic accuracy. |
| Cobo et al., 2023 [21]. | 658 | Coronary artery tortuosity (CAT) | convolutional neural network | DLM Vs expert radiological visual examination for detecting CAT, with a sensitivity of 87 ± 10% versus 84 ± 2% and a specificity of 88 ± 10% versus 86 ± 4% | DLM could have a beneficial impact on preventing cardiac complication, shortening coronary angiography examination times. |
| Dey et al., 2018 [22]. | 254 | Coronary artery disease | supervised learning model usable in predicting revascularization after ischemia. | Results suggest that ML approach may outperform conventional statistical integration of the same data | The Integrated ML ischemia risk score improved the prediction of lesion-specific ischemia. |
| Dundas et al., 2023 [23]. | 120 | Coronary artery disease | AI-based coronary stenosis quantification (AI-CSQ) software V1. | The AI-CSQ tool demonstrated a sensitivity of 95%, specificity of 57%, and accuracy of 76%. On a per-vessel basis, sensitivity was 90%, specificity 76%, and accuracy 80%. | AI-based coronary stenosis quantification at coronary CT angiography shows high diagnostic performance and sensitivity for stenosis detection compared to quantitative coronary angiography, both per-patient and per-vessel. |
| Griffin et al., 2023 [24]. | 303 | Coronary artery disease | convolutional neural network models. | Per-patient for detecting ≥50% stenosis sensitivity (94%,), specificity (68%), positive predictive value (81%), negative predictive value (90%), and accuracy (84%) and for detecting ≥70% stenosis were 94%, 82%, 69%, 97%, and 86%, respectively. | AI-based evaluation demonstrated high diagnostic performance for the identification, exclusion, discrimination, and correlation to a quantitative coronary angiography reference standard. |
| Han et al., 2022 [25]. | 196 | Coronary Artery Disease | Automated AI algorithms. | The AI system demonstrated 94% sensitivity at the patient level and 78% sensitivity at the vessel level. These sensitivity rates were higher than those of non-AI assisted readings by two cardiovascular radiologists. | using AI increased the sensitivity of inexperienced readers and improved the consistency of coronary stenosis diagnosis via coronary CT angiography. |
| Ihdayhid et al., 2022 [26]. | 1849 | Coronary artery calcium score | three-dimensional (3D) fully convolutional neural network. | The AI-based fully automated coronary artery calcium scoring model accurately detected coronary artery calcium, closely aligning with human readings, and demonstrated comparably lower analysis times. | fully automated coronary artery calcium (CAC) scoring model shows high accuracy and low analysis times. |
| Zhang et al., 2024 [27]. | 1801 | Coronary artery disease. | Convolutional Neural Networks | Observed greatly improved efficiency, and maintains high diagnostic accuracy and the effectiveness in stratifying patients for cardiovascular events. | Fully automated AI-based coronary CT angiography significantly improves workflow efficiency compared to the semi-automated mode. |
| Assen et al., 2020 [28]. | Cohort one: 95 Cohort two: 168 | Coronary artery calcium score | Deep-learning convolution neural and a fully connected neural network. | Deep-learning-based automated calcium quantification on chest CT shows excellent correlation with manual calcium volume quantification and Agatston scores from cardiac CTs. | Automated analysis can increase workflow efficiency and help manage the growing number of acquisition requests, assisting radiologists. |
| Yoneyama et al., 2019 [29]. | 59 | Coronary artery disease | artificial neural network. | 1. Observer A detected CAD with 83.6% accuracy in the RCA, 89.3% in the LAD, and 94.4% in the LCX. 2. Observer B achieved 72.9% accuracy in the RCA, 84.2% in the LAD, and 89.3% in the LCX. 3. The artificial neural network (ANN) had 79.1% accuracy in the RCA, 89.8% in the LAD, and 89.3% in the LCX. | AI diagnoses are comparable to those by nuclear medicine physicians. |
| Author, Year | Number of Patients | Cardiac Abnormality | Type of Deep Learning | Result | Conclusion |
|---|---|---|---|---|---|
| Åkesson et al. 2023 [30]. | 1434 | Right ventricular. abnormalities | Convolutional Neural Networks | The average time reduction using DLM was 5 min and 17 s. | A DL-based model can sufficiently make the right ventricular assessments faster. |
| Cau et al. 2024 [31]. | 107 | Ischemic and non-ischemic cardiomyopathy | gradient boosting generalized additive model (GB-GAM) | GB-GAM had The Sensitivity of 0.72 and Specificity of 0.68 (AUC = 0.82) in discriminating between ICM and NICM. | DLM can discriminate between CM and NICM with high accuracy reducing cost and time of the examination. |
| Davies et al. 2022 [32]. | 109 | Cardiac structure and function abnormalities | Convolutional neuronal network | Measuring left ventricular metrics had precision of 0.94–0.95 | DLM was faster and more precision. Compare to human performance. |
| Zhang et al. 2019 [33]. | 212 | Coronary artery disease and MI | 1. convolutional neural network 2. recurrent neural network | Using DLM in nonenhanced cardiac MRI has per-segment Sensitivity of 89.8% And Specificity of 99.1% in detecting chronic MI. | Using DLM in nonenhanced cardiac cine MRI can find the likely location of chronic MI |
| Author | Selection | Comparability | Outcome | Overall |
|---|---|---|---|---|
| Kusunose et al. [10] | 3 | 1 | 3 | good |
| Pandey et al. [11] | 3 | 2 | 3 | good |
| Salte et al. [12] | 3 | 1 | 3 | good |
| Upton et al. [13] | 3 | 1 | 3 | good |
| Kang et al. [14] | 4 | 1 | 3 | good |
| Krishna et al. [15] | 3 | 1 | 3 | good |
| Park et al. [16] | 3 | 2 | 3 | good |
| Salte et al. [17] | 3 | 1 | 3 | good |
| Zamzmi et al. [18] | 3 | 1 | 3 | good |
| Paul et al. [19] | 3 | 2 | 3 | good |
| Andre et al. [20] | 3 | 1 | 3 | good |
| Cobo et al. [21] | 3 | 1 | 3 | good |
| Dey et al. [22] | 3 | 1 | 3 | good |
| Dundas et al. [23] | 3 | 1 | 3 | good |
| Griffin et al. [24] | 3 | 1 | 3 | good |
| Han et al. [25] | 3 | 1 | 3 | good |
| Ihdayhid et al. [26] | 3 | 1 | 3 | good |
| Zhang et al. [27] | 3 | 1 | 3 | good |
| Assen et al. [28]. | 3 | 1 | 3 | good |
| Yoneyama et al. [29] | 3 | 1 | 3 | good |
| Åkesson et al. [30] | 3 | 1 | 3 | good |
| Cau et al. [31] | 3 | 1 | 3 | good |
| Davies et al. [32] | 3 | 1 | 3 | good |
| Zhang et al. [33] | 3 | 1 | 3 | good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, A.; Olanisa, O.O.; Nzeako, T.; Shahrokhi, M.; Esfahani, E.; Fakher, N.; Khazeei Tabari, M.A. Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI. J. Imaging 2024, 10, 193. https://doi.org/10.3390/jimaging10080193
Moradi A, Olanisa OO, Nzeako T, Shahrokhi M, Esfahani E, Fakher N, Khazeei Tabari MA. Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI. Journal of Imaging. 2024; 10(8):193. https://doi.org/10.3390/jimaging10080193
Chicago/Turabian StyleMoradi, Ali, Olawale O. Olanisa, Tochukwu Nzeako, Mehregan Shahrokhi, Eman Esfahani, Nastaran Fakher, and Mohamad Amin Khazeei Tabari. 2024. "Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI" Journal of Imaging 10, no. 8: 193. https://doi.org/10.3390/jimaging10080193
APA StyleMoradi, A., Olanisa, O. O., Nzeako, T., Shahrokhi, M., Esfahani, E., Fakher, N., & Khazeei Tabari, M. A. (2024). Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI. Journal of Imaging, 10(8), 193. https://doi.org/10.3390/jimaging10080193






