Abstract
Three-dimensional soft tissue simulation has become a popular tool in the process of virtual orthognathic surgery planning and patient–surgeon communication. To apply 3D soft tissue simulation software in routine clinical practice, both qualitative and quantitative validation of its accuracy are required. The objective of this study was to systematically review the literature on the accuracy of 3D soft tissue simulation in orthognathic surgery. The Web of Science, PubMed, Cochrane, and Embase databases were consulted for the literature search. The systematic review (SR) was conducted according to the PRISMA statement, and 40 articles fulfilled the inclusion and exclusion criteria. The Quadas-2 tool was used for the risk of bias assessment for selected studies. A mean error varying from 0.27 mm to 2.9 mm for 3D soft tissue simulations for the whole face was reported. In the studies evaluating 3D soft tissue simulation accuracy after a Le Fort I osteotomy only, the upper lip and paranasal regions were reported to have the largest error, while after an isolated bilateral sagittal split osteotomy, the largest error was reported for the lower lip and chin regions. In the studies evaluating simulation after bimaxillary osteotomy with or without genioplasty, the highest inaccuracy was reported at the level of the lips, predominantly the lower lip, chin, and, sometimes, the paranasal regions. Due to the variability in the study designs and analysis methods, a direct comparison was not possible. Therefore, based on the results of this SR, guidelines to systematize the workflow for evaluating the accuracy of 3D soft tissue simulations in orthognathic surgery in future studies are proposed.
1. Introduction
In orthognathic surgery, two-dimensional (2D) planning programs based on lateral cephalograms and clinical profile photographs have been used for decades in clinical practice. However, the use of 2D lateral cephalograms is prone to analysis bias due to the superimposition of three-dimensional (3D) anatomical structures [1]. The main limitations of 2D planning programs are the simplifications of the algorithms in the simulation of soft tissue changes, because they use fixed hard-to-soft-tissue ratios for the prediction of soft tissue results, and they are unable to predict changes in the transverse plane [2].
The introduction of 3D planning software based on cone beam computed tomography (CBCT) and patients’ high aesthetic demands have led to a paradigm shift in the philosophy of 3D surgical planning [3], where “the bite indicates a problem—the face indicates how to treat the ‘bite’” [4]. During the surgical procedure, surgeons do not directly operate on facial soft tissues but rely on their passive change after the repositioning of the bony segments [5]. While virtual treatment planning (VTP) of bony movements is predictable [6,7], currently, a reliable algorithm for predicting the postoperative facial soft tissue appearance does not exist [8,9].
The application of 3D soft tissue simulation extends beyond mere visualization, offering valuable insights into the aesthetic implications of orthognathic surgery and facilitating effective communication between the surgeon, the orthodontist, and the patient [10]. This collaborative approach fosters informed decision making and clearer understanding of the proposed treatment plan and realistic expectations for the surgical outcome.
Before applying 3D simulation software in routine clinical practice, both qualitative and quantitative validation are required [11] to evaluate whether the simulations are accurate representations of the expected soft tissue changes [10]. Hence, a series of studies have been published evaluating the accuracy of 3D soft tissue simulation by comparing it to the actual postsurgical soft tissue outcome. However, whereas the superimposition and measurement techniques of planned and postoperative images in the 2D environment were well established many years ago, the 3D environment is much more complex, with significant inconsistency, and there is no consensus regarding the ideal assessment method [7].
The objective of this study was to systematically review the literature on the accuracy of 3D soft tissue simulation in orthognathic surgery. Based on the insights gained from this review, we propose standardizing the methodology for evaluating the accuracy of 3D soft tissue simulation in orthognathic surgery. This standardized approach aims to minimize the risk of errors and analysis bias in future studies.
2. Materials and Methods
This study was planned based on the Population Intervention Comparison Outcome Study design (PICOS) format, as presented in Table 1.

Table 1.
PICOS format.
On 13 January 2023, the Web of Science, PubMed, Cochrane, and Embase databases were used for the literature search. Specific search strategies using the search terms “soft tissue” and “orthognathic surgery” were performed in each database in collaboration with a professional librarian. The full search string for each database is included in Supplementary Materials Table S1. There were no restrictions in the search strategy regarding the year of publication. No additional search of the gray literature was performed. On 20 April 2024, before finishing the manuscript, the search was repeated to detect any new studies that could also be included. The inclusion and exclusion criteria are listed in Table 2.

Table 2.
Inclusion and exclusion criteria.
This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020130214). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was used for selecting studies (http://www.prisma-statement.org/ accessed on 20 April 2024) [12]. The PRISMA flow diagram can be found in Figure 1.
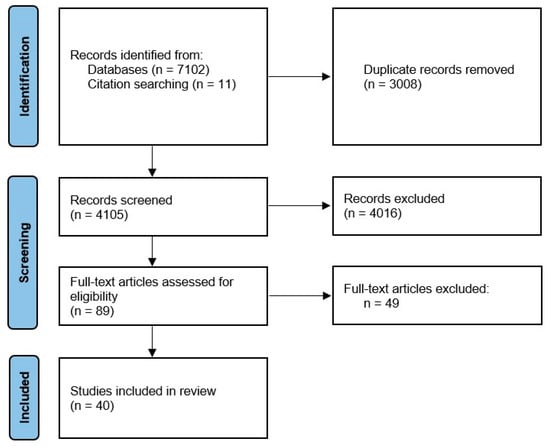
Figure 1.
PRISMA 2020 flow diagram.
The Covidence (Veritas Health Innovation, Melbourne, Australia) tool was used for the screening phase and full text review. The eligibility of the studies was checked independently by two junior authors. In case of disagreement, the study was discussed with the senior author. Studies that did not meet the eligibility criteria were excluded from further analysis.
Qualitative and quantitative data were independently extracted from the studies using a standardized form. The following data were registered: year of publication, first author, study design, sample size, mean age (years), gender, type of facial deformity, type of surgery, medical imaging technique (CBCT/MSCT, 3D photographs), image acquisition protocol, software package and/or algorithm, type of rigid registration method for soft tissue evaluation, method of analysis, fixed point of accuracy, and results.
Study Quality and Risk of Bias Assessment
The revised Quadas2 tool for assessing risk of bias and applicability in systematic reviews for diagnostic accuracy-related studies was used in this study. This tool comprises five domains: patient selection, index test, reference standard, flow, and timing. It allows for a transparent rating of the bias and applicability of included studies. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed for applicability concerns [13]. The assessment was carried out independently by two junior authors. In case of disagreement, the issue was discussed with the senior author.
3. Results
The initial search yielded 7113 articles, which were processed through abstract screening, from which 89 articles were selected for full text reading. Finally, 40 articles fulfilled the inclusion and exclusion criteria after the full text review.
The studies included in this review assessed the accuracy of 3D soft tissue simulation by comparing the actual postoperative soft tissue outcome to the 3D soft tissue simulation based on the VTP. For the VTP and 3D soft tissue simulation, various commercially available programs were used, as well as advanced software platforms that are limited in use to engineers only (Table 3).

Table 3.
Reported software packages used for VTP and 3D soft tissue simulation.
Descriptive data on the included studies are presented in Table 4. While this paper presents an abridged table, an equivalent but complete table is included in Supplementary Materials Table S2. The papers included in this SR were assessed in terms of risk of bias, as described above, and these assessments are presented in Supplementary Materials Table S3.
In the 40 included studies, the sample size varied from 3 to 100 patients. A total of 1021 simulations were evaluated. Among the studies, there was variability in the types of facial deformities that were included in the study sample: 10 studies [10,15,16,18,19,29,30,31,36,37] included patients with skeletal class III malocclusion, 3 studies [22,32,33] included patients with skeletal class II malocclusion, 12 studies [3,9,17,24,25,26,34,35,38,39,40,41] included heterogenous groups, and 1 study [20] only included facial asymmetry. In 14 studies [8,11,14,21,23,27,28,42,43,44,45,46,47,48], information about the type of deformity was missing or unclear.
Three-dimensional soft tissue simulations of different orthognathic surgical procedures were described. Six studies [15,16,19,30,37,45] evaluated the simulation of Le Fort I osteotomy, two studies [22,33] evaluated that of mandibular osteotomy, fifteen studies [9,10,14,17,21,23,27,28,29,31,32,34,36,47,48] evaluated that of bimaxillary osteotomy (with or without genioplasty), and in thirteen studies [3,11,18,20,24,25,26,38,39,40,41,44,46], different types of procedures were considered. In four studies [8,35,42,43], information about the surgical procedure was not reported or unclear.
Multi-slice computed tomography (MSCT) records were used in 12 studies [3,8,9,27,29,32,35,41,42,43,46,48], whereas in 23 studies [10,11,14,15,16,17,19,20,21,22,23,24,25,28,30,31,33,34,37,38,39,40,45,47] CBCT records were utilized. Three other studies [18,26,44] used both MSCT and CBCT records. One study combined CBCT and MRI records [47]. In one study [36], 3D photographs and 2D lateral cephalograms were combined, and in thirteen studies [3,9,10,11,15,31,33,34,38,39,40,41,44], both 3D photographs and MSCT/CBCT records were taken.
The time interval for postoperative image acquisition varied from 3 to 6 months in seven studies [3,11,24,25,26,34,46] to at least 4 months in one study [18], exactly 6 months in seven studies [27,28,29,35,38,44,47], at least 6 months in ten studies [9,10,15,21,22,23,31,32,41,45], 6–12 months in five studies [14,17,19,20,37], 12 months in two studies [16,33], and 11–15 months in one study [30]. In seven studies [8,36,39,40,42,43,48], information about the postoperative image acquisition time interval was not reported.

Table 4.
Descriptive data on the studies included in the review.
Table 4.
Descriptive data on the studies included in the review.
| Year, First Author | Sample Size | CBCT/MSCT a | Three-Dimensional Photo a | Software Package and/or Algorithm | Type of Surgery | Results b |
|---|---|---|---|---|---|---|
| 2004, Chabanas [42] | 3 | MSCT ** | No | FEM | NR | ME range: 1–1.5 mm, MaxE: 3–6 mm |
| 2007, Mollemans [3] | 10 | MSCT ** | Yes | (1) Linear FEM; (2) non-linear FEM; (3) MSM; (4) MTM | TRIMAX, BIMAX, BSSO, BSSO + Ch, LFI + Ch | Average median distance for MTM: 0.60 mm, FEM: 0.60 mm, MSM: 0.64 mm, NFEM: 0.63 mm; average 90th percentile distance for MTM: 1.48 mm, FEM: 1.51 mm, MSM: 1.67 mm, NFEM: 1.71 mm; highest accuracy: FEM and MTM |
| 2007, Marchetti [46] | 25 | MSCT | No | VISU system | LFI, BSSO, LFI + Ch, BSSO + Ch, BIMAX, TRIMAX | Error < 2 mm in 80% (20 of 25) of the patients |
| 2010, Bianchi [28] | 10 | CBCT | No | SurgiCase CMF Pro v.1.2 | BIMAX/TRIMAX | ME: 0.94 mm; error < 2 mm in 86.8% of the simulations; 90th percentile: 2.24 mm; 95th percentile: 2.81 mm |
| 2010, Ulusoy [43] | 6 | MSCT ** | No | Dynamic volume spline | BIMAX * | ME: 1.8 mm |
| 2011, Centenero [26] | 16 | MSCT/CBCT | No | SimPlant ProOMS v.10.1 | BIMAX, TRIMAX, BSSO + Ch | 5 of 8 ST measurements: high degree of correlation; 3 measurements: medium degree of correlation |
| 2011, Marchetti [29] | 10 | MSCT | No | SurgiCase CMF Pro v.1.2 | BIMAX, TRIMAX | ME: 0.75 +/− 0.78 mm; error < 2 mm in 91% of the simulations; 90th percentile: 1.94 mm; 95th percentile: 2.47 mm |
| 2013, Schendel [38] | 23 | CBCT | Yes c | 3dMDVultus—MSM | LFI, BSSO, Ch | Entire face ME: 0.27 mm, ComR: 1.10 mm, ComL: 0.99 mm, Pog: 0.79 mm |
| 2013, Shafi [19] | 13 | CBCT | No | Maxilim v.2.2.0—MTM | LFI | ME: 0.97 mm; all anatomical regions with error significantly < 3.0 mm, exception UL error: 2.73 +/− 1.72; overprediction of UL |
| 2013, Nadjmi [11] | 13 | CBCT ** lat ceph | Yes ** | (1) 2D Dolphin v.10—fixed hard-tissue-to-soft-tissue ratios; (2) Maxilim—MTM | LFI, LFI+Ch, BIMAX, TRIMAX | Dolphin range of error in horizontal position: −1.41 to 1.20 mm, in vertical position: −1.85 to 1.55 mm; Maxilim range of error in horizontal position: −1.60 to 1.50 mm, in vertical position: −4.25 to 2.42 mm. No statistical differences between software, exception SA in Maxilim |
| 2014, Terzic [44] | 13 | MSCT/CBCT | Yes c | 3dMDvultus v.2.2.0.8—MSM | BSSO, BSSO+Ch, BIMAX, TRIMAX | ME for the upper part: +0.27 mm, the lower part: –0.64 mm; in the lower part, error < +/− 1 mm 26.9%, >+/− 1 mm 73.1%, >+/− 2 mm 49.5%, and >+/− 3 mm 29.8% |
| 2014, Nadjmi [24] | 20 | CBCT | No | Maxilim—MTM | BSSO, BIMAX, TRIMAX | ME: 1.18 mm; 84% of errors between −2 mm and +2 mm |
| 2015, Ullah [37] | 13 | CBCT | No | 3dMDVultus v.2.2.0—MSM | LFI | ME: 0.92 mm (0.3–2.4 mm); 90th percentile from 0.65 mm (chin) to 1.17 mm (UL); ME significantly < 3 mm; the 95% CI in all regions < 2 mm |
| 2015, Khambay [45] | 10 | CBCT ** | No | 3dMDvultus v.2.2.0—MSM | LFI | ME for 95th percentile: 0.98–0.56 mm, for 90th percentile: 0.91–0.50 mm; error < 2 mm: 94.4%—85.2% points; RMS error: 2.49–0.94 mm; RMS difference for all measurements: 1.3 mm |
| 2015, Nam [27] | 29 | MSCT | No | Simplant Pro | BIMAX, TRIMAX | ME in all landmarks: 2.03 mm; error < 2 mm: 52.8%; absolute error values in the x-axis: 0.73 mm, y-axis: 1.39 mm, z-axis 0.85 mm; error significantly > 2 mm: ChR, ChL, LL, Pog; MaxE: 2.38 mm in ChL, MinE: 0.84 mm in pronasale |
| 2015a, Liebregts [23] | 60 | CBCT | No | Maxilim—MTM | BIMAX | Landmarks: MaxE at LI: 3.1 +/− 1.4 mm, MinE at SN: 1.5 +/− 0.6 mm; surfaces: entire face ME: 0.81 +/− 0.22 mm, for UL: 1.2 +/− 0.6 mm, LL: 1.4 +/− 0.5 mm, chin: 1.1 +/− 0.6 mm; error equal to or < 1 mm: 83.3%, < 2 mm: 100%; ME among patients who had a V-Y closure was significantly smaller than those without a V-Y closure |
| 2015b, Liebregts [22] | 100 | CBCT | No | Maxilim v.2.2.2.1—MTM | BSSO | Landmarks: ME at SN: 1.1 +/− 0.5 mm, at LS: 1.5 +/− 0.7 mm, at LI: 2.0 +/− 1.0 mm, at sublabial: 1.7 +/− 1.1 mm, at Pog: 1.5 +/− 0.9 mm; surface: entire face ME: 0.9 +/− 0.3 mm; error equal to or <2 mm: 100%, <1 mm: 78%; ME for UL: 0.9+/− 0.5, LL: 1.2+/− 0.5, and chin: 0.8 +/− 0.5 mm; average absolute error less or equal to 2 mm for UL: 98%, for LL: 94%, and for the chin: 97% |
| 2015, Van Hemelen [25] | 31 | CBCT | No | Maxilim—MTM | BSSO, BSSO + Ch, LFI, LFI + Ch, BIMAX, TRIMAX | ME in the horizontal direction: 1.48 mm, in the vertical direction: 1.46 mm |
| 2016, Liebregts [21] | 60 | CBCT | No | Maxilim—MTM | BIMAX | ME: 1.0 +/− 0.9 mm in alar width |
| 2016, Resnick [15] | 7 | CBCT | Yes c | Dolphin 3D v.11.8—sparse landmark-based algorithm | LFI | ME: 2.91 +/− 2.16 mm, for midline points: 1.66 +/− 1.82 mm, for lateral points: 3.84 +/− 1.92 mm; 2 (33%) midline points with error > 2 mm (SN, SA), 6 (75%) lateral points > 2 mm; ME at NLA: 8.1 +/− 5.6 degrees |
| 2017, Kim [9] | 40 | MSCT ** | Yes c | FEM with the mucosa sliding effect | BIMAX, TRIMAX | Quantitative: entire face ME: 1.1 +/− 0.3 mm, UL: 1.2 +/− 0.7 mm, LL: 1.5 +/− 0.7 mm, chin: 1.3 +/− 0.7; qualitative: 80% (32/40) clinically acceptable |
| 2021, Kim [41] | 35 | MSCT ** | Yes c | FEM with the sliding effect of the lip and the mucosa | BSSO, BIMAX, TRIMAX | Quantitative: entire face ME: 1.03 +/− 0.30 mm, UL: 0.86 +/− 0.36 mm, LL: 1.10 +/− 0.41 mm, chin: 1.08 +/− 0.51 mm; qualitative: improvement in lips compared with previous FEM methods |
| 2017, Mundluru [20] | 13 | CBCT | No | Maxilim—MTM | BIMAX, BSSO, BSSO+Ch | Underprediction of ST changes; signed ME from −0.55 to 0.43 mm; absolute ME from 0.6 to 1.3 mm |
| 2018, Holzinger [35] | 16 | MSCT | No | SOTIRIOS | NR—surgery first | ME: 1.46 +/− 1.53 mm; 50% < 1.03 mm, 80% < 2.20 mm, and 95% up to 4.34 mm |
| 2019, Knoops [16] | 7 | CBCT | No | (1) Dolphin 3D v.11.95—sparse landmark-based algorithm; (2) ProPlan CMF v.3.0.1—FDM; (3) PFEM | LFI | RMSDolphin = 1.8 +/− 0.8 mm, RMSPro-Plan = 1.2+/− 0.4 mm, and RMSPFEM = 1.3+/− 0.4 mm; average percentage of points < 2 mm: PDolphin = 83+/− 12%, PProPlan = 91+/− 9%, and PPFEM = 88+/− 10%; better results for ProPlan and PFEM compared to Dolphin |
| 2019, Elshebiny [14] | 20 | CBCT | No | Dolphin 3D v.11.9—sparse landmark-based algorithm | BIMAX/TRIMAX | Statistically significant differences in 2 angular measurements (FNA and NLA) and in 3 linear measurements (SA, UL length, and subalar width) |
| 2021, Cunha [32] | 16 | MSCT ** | No | OrtogOnBlender-OOB—MSM | BIMAX/TRIMAX | ME for all landmarks < 2 mm, entire face ME: 1.07 mm; MaxE: ChR, ChL, and SB |
| 2021, Willinger [18] | 19 | MSCT/CBCT ** | No | (1) IPS Case Designer—MTM; (2) Dolphin 3D v.11.95—sparse landmark-based algorithm | Modified IQLFIIO +/− BSSO | IR level: Dolphin ME: 2.90 +/− 2.1 mm, IPS ME: 1.70 +/− 1.3 mm; SF level: Dolphin ME: 3.57 +/− 2.0 mm, IPS ME: 1.34 +/− 0.9 mm; Li level: Dolphin ME: 2.48 +/− 1.9 mm, IPS ME: 2.25 +/− 1.6 mm; MaxE for Dolphin at SF level |
| 2021, Tanikawa [36] | 72 | No—lat ceph | Yes | Geometric morphometric methods (GMMs), DL | BIMAX | System error: 0.89 ± 0.30 mm; MaxE of 0.8–1.2 mm in the nasal ala, chin, corner of the mouth; total success rate at <1 mm: 54%, and at <2 mm: 100% |
| 2021, ter Horst [33] | 14 | CBCT | Yes c | DL; IPS CaseDesigner—MTM | BSSO | DL-based: lower face ME: 1.0 +/− 0.6 mm, simulations with MaxE of 1 mm: 64.3% and of 2 mm: 92.9%; RMS: 1.2 +/− 0.6 mm; ME: for LL 1.1 +/− 0.9 mm; for the chin: 1.4 +/− 0.9 mm. MTM-based: lower face ME: 1.5 +/− 0.5 mm, simulations with MaxE of 1 mm: 21.4% and of 2 mm: 85.7%; RMS: 2.0 +/− 0.7 mm; ME for LL: 1.7 +/− 0.9 mm; chin: 2.0 +/− 1.0 mm; DL model had higher accuracy |
| 2021, Alcañiz [39] | 10 | CBCT ** | Yes ** | FEM | LFI, LFII, BSSO, USSO, Ch, BIMAX | Surface with error < 3 mm with coarse meshes: 92%, with fine meshes: 95% |
| 2022, Lee [10] | 10 | CBCT ** | Yes **c | ProPlan CMF—FDM | BIMAX | Entire face ME: 0.73 +/− 0.21 mm, for LL: 1.42 +/− 0.77 mm, for UL: 1.14 +/− 0.80 mm, for chin: 0.95 +/− 0.58 mm; error < 2 mm: 90.9% |
| 2022, Gutiérrez [40] | 10 | CBCT ** | Yes ** | FEM | LFI, LFII, BSSO, USSO, Ch, BIMAX | All distances for both meshes and their mean distances significantly < 2 mm, except LL, RGo, and LGo; distances for all landmarks significantly < 3 mm, except for LL of the fine mesh |
| 2022, Yamashita [17] | 88 | CBCT | No | Dolphin 3D v.11.95—sparse landmark-based algorithm | BIMAX, TRIMAX | C II: underprediction with downward direction in S-Y, S-Z, LI-Y, SB-Y, Pog-Y, Pog-Z, Gn-Y, Gn-Z, Me-Y, Me-Z, values > 2 mm: LI-Y, SB-Y, Pog-Y, Gn-Y, Gn-Z, Me-Y; MaxE LI-Y: 2.73 mm. C III: overprediction and downward direction in Pog-Z, Gn-Y, Gn-Z, Me-Y, and Me-Z, all discrepancies < 2 mm |
| 2022, Ma [8] | 40 | MSCT ** | No | FSC-Net, point cloud DL | NR | Qualitative: FSC-Net comparable with FEM-RLSE; quantitative: landmarks entire face ME: 2.95 +/− 0.61 mm; surface entire face ME: 1.55 +/− 0.30 mm, lips: 1.58 +/− 0.26 mm, chin: 2.11 +/− 0.77 mm; FSC-Net comparable with FEM-RLSE |
| 2022, Awad [34] | 20 | CBCT | Yes | IPS CaseDesigner v.2.1.4.4—MTM | BIMAX | Entire face ME: −1.5 to 1.4 mm, UL: −2.5 to 1.3 mm, LL: −2.1 to 2.5 mm, chin: −1.8 to 2.6 mm |
| 2022, Hou [31] | 58 | CBCT | Yes c | ProPlan CMF—FDM | BIMAX | Entire face ME: 1.43 +/− 0.40 mm; error of UL, LL, chin, right external buccal, and left external buccal > 2.0 mm; LL the least predictable: 2.69 ± 1.25 mm |
| 2023, Şenyürek [30] | 16 | CBCT ** | No | ProPlan CMF v.3.0—FDM | LFI | Error in UL and LL: 1.49 +/− 0.77 mm, in cheeks: 0.98 +/− 0.34 mm, nose: 0.86 +/− 0.23 mm, and eyes: 0.76 +/− 0.32 mm |
| 2023, Ruggiero [47] | 5 | CBCT + MRI | No | FEM with patient-specific model generated from CBCT and MRI | BIMAX | Midface ME: 0.55 +/− 2.29 mm |
| 2024, Fang [48] | 40 | MSCT ** | No | DL, ACMT-Net with the CPSA module | BIMAX | Quantitative: surface entire face ME: 1.06 +/− 0.43 mm, UL: 1.13 +/− 0.71 mm, LL: 1.23 +/− 0.48 mm, chin: 1.13 +/− 0.62 mm; landmarks entire face: ME 2.44 +/− 0.45 mm, upper face: 1.23 +/− 0.47 mm, lower face: 3.25 +/− 0.66 mm Qualitative: 77.5% (31/40) of the simulations clinically acceptable |
* Not clear; ** device not specified; a details in Table S4; b most relevant results; c 3D photograph fused with the MSCT/CBCT skin surface; BIMAX, bimaxillary osteotomy; BSSO, bilateral mandibular sagittal split osteotomy; Ch, genioplasty; ChL, cheilion left; ChR, cheilion right; ComL, left commissure; ComR, right commissure; DL, deep learning; FDM, finite difference method; FEM, finite element model; FNA, frontonasal angle; Gn, soft tissue gnathion; IR, infraorbital rim; IQLFIIO, intraoral quadrangular Le Fort II osteotomy; lat ceph, lateral cephalograms; LFI, Le Fort I maxillary osteotomy; LGo, soft tissue left gonion; Li, crown of the lateral incisor; LI, labrale inferior; LL, lower lip; LS, labrale superior; MaxE, maximal error; Me, soft tissue menton; ME, mean error; MinE, minimal error; MSM, mass spring model; MTM, mass tensor model; NLA, nasolabial angle; NR, not reported; PFEM, probabilistic FEM; Pog, soft tissue pogonion; RGo, soft tissue right gonion; RMS, root mean square; S, stomion; SA, soft tissue A point; SB, soft tissue B point; SF, sinus floor; SN, subnasale; ST, soft tissue; TRIMAX, bimaxillary osteotomy and genioplasty; UL, upper lip; USSO, unilateral mandibular sagittal split osteotomy.
In the majority of studies, real bony movements were used for the generation of 3D soft tissue simulations; however, in nine studies [8,17,25,26,27,32,34,43,46], measurements were only based on the initial virtual treatment plan.
In the studies reviewed in this SR, the following two quantitative analysis methods were used: (1) 3D landmark-based evaluation was performed in 18 studies [8,11,14,15,17,18,21,22,23,25,26,27,31,32,38,40,45,48], and (2) surface mesh-based evaluation was performed in 28 studies [3,8,9,10,16,19,20,22,23,24,28,29,30,31,33,34,35,36,37,39,41,42,43,44,45,46,47,48]. In six studies [8,22,23,31,45,48], both methods were combined.
Furthermore, the definition of accuracy, referring to the comparison of the actual postoperative soft tissue outcome to the 3D soft tissue simulation, varied between the studies reviewed in this SR. Some studies defined accuracy as a clinically insignificant error of less than 0.5 mm, while others used thresholds of 1 mm, 2 mm, or even up to 3 mm.
Regarding the mean error of the 3D soft tissue simulations of the whole face, fluctuations from 0.27 mm [38] to 2.9 mm [8,15] were reported. Due to variability in analysis methods, however, direct comparison is not possible. In the studies evaluating 3D soft tissue simulation accuracy after a Le Fort I osteotomy only, the upper lip and paranasal regions were reported to have the largest error [15,19,30,37,45], while after an isolated bilateral sagittal split osteotomy (BSSO), the largest error was reported for the lower lip and chin regions [22,33]. In the studies evaluating simulation after bimaxillary osteotomy with or without genioplasty, the highest inaccuracy was reported at the level of the lips, predominantly the lower lip, chin, and, sometimes, the paranasal regions [9,10,14,17,23,27,29,31,32,34,36,43].
The overall inconsistency in methodology encouraged the authors of this SR to summarize the various methodologies (shown in Table 5), since such inconsistency could be considered an additional risk of bias.

Table 5.
Methodological data on the studies included in this review.
4. Discussion
Three-dimensional VTP has become the state of the art for surgical planning for patients needing orthognathic surgery. Nonetheless, there is still a significant lack of evidence-based data regarding the accuracy of its associated 3D soft tissue simulation. In order to discuss the findings of this SR in a structured manner, a framework was set up, which resulted in a proposal of guidelines (Table 6) to systematize the workflow for evaluating the accuracy of 3D soft tissue simulations in orthognathic surgery in future studies.

Table 6.
Guidelines to systematize the workflow for evaluating the accuracy of 3D soft tissue simulation in orthognathic surgery in future studies, based on this SR.
4.1. Image Acquisition (Pre- and Postoperative)
In the studies reviewed in this SR, different medical image acquisition techniques were reported: MSCT, CBCT, and 3D photographs and MRI. These were used separately or in combination. Only a few studies fully reported their pre- and postoperative image acquisition protocol details: imaging device, patient’s head position, lip morphology and posture, mandible positioning (centric relation, centric occlusion, the use of wax bite), and time interval between the surgery and postoperative image acquisition [15,16,17,19,22,24,32,33,34,35,37,38,44]. Most of the studies only considered these details partially. Regarding the imaging device and patient’s head position, 14 studies reported an MSCT scanning protocol performed in a horizontal position. Only one study presented a scanning protocol with a CBCT apparatus that scanned the patient in a supine position [28]. This is crucial for clinicians, since scanning the patient in a horizontal position inherently modifies and falsifies the 3D facial soft tissue mask due to the effects of gravity [49]. A study by Iblher et al. [50] showed that gravitational facial soft tissue deformation could range from 4 to 6 mm when comparing horizontal and vertical image acquisition. Therefore, it is of paramount importance to scan the patient in a vertical position, avoiding gravitational distortion of the facial soft tissues. An additional advantage of CBCT scanning compared to MSCT is that patients are exposed to a much lower radiation dose [51]. A limiting factor of CBCT scanning, however, is a potentially smaller field of view (FOV) in some CBCT devices, which can result in incomplete capture of the facial soft tissue mask, e.g., the tip of the nose [32]. Currently, the majority of CBCT scanners are equipped with specific algorithms to partly solve this issue. Unfortunately, the nose tip still cannot always be visualized with the correct 3D geometry. Stratemann et al. [52] observed statistical differences in measurements between different CBCT devices (NewTom and CB MercuRay). Therefore, to superimpose and compare two sets of CBCT data, it is important that the image acquisition is performed with the same CBCT device.
The limitations of 3D soft tissue simulation that were identified in the studies included in this SR also relate to lip morphology and posture, which indicates the importance of a standardized scanning protocol. It has repeatedly been suggested [22,23,33,38,53] that it is important for patients to relax their lips to avoid muscle hyperfunction during scanning.
In eight studies [10,15,16,21,22,23,28,31], the generated 3D soft tissue simulations were obtained from preoperative images with fixed orthodontic appliances in place, while the postoperative image acquisition was performed after these appliances had been removed. Resnick et al. [15] and Liebregts et al. [23] indicated that this could probably have influenced the final lip position and morphology. Eidson et al. [53] and Kim et al. [54] used 3D stereophotogrammetry and reported statistically significant differences in the right and left commissures, as well as the lower lip, after the orthodontic appliances were removed.
In the presented studies, the time interval for postoperative CBCT image acquisition varies from 3 months [26,46] to 72 months [32]. In a prospective study by van der Vlis et al. [55], which quantified changes in postoperative swelling, it was reported that 50% of facial swelling resolves within the first three weeks post operation, 20% persists after three months, and 11.2% of the initial swelling volume remains at six months. Moreover, facial soft tissue swelling continues to decline at a statistically significant rate from six to twelve months postoperatively.
4.2. Virtual Osteotomies and VTP
The accuracy of 3D soft tissue simulation depends on two main factors: the computation model itself and the mismatch between surgical planning and the actual surgical movements [16]. Khambay and Ullah [45] and Baan et al. [56] stated that surgeons are generally unable to transfer the virtually predicted surgical plan perioperatively in an accurate way, and significant errors are introduced. Knoops et al. [16] compared the accuracy of 3D soft tissue simulation based on planned skeletal movements and actual postoperative bony movements. An increase in root mean square distance between the simulation and postoperative soft tissue outcome was observed when using the initially planned segments positions. Therefore, the analysis method should rely on determining the exact skeletal changes that occurred after the surgery (and any potential relapse). The use of the initial bony virtual surgical plan as a template to evaluate the accuracy of the 3D soft tissue simulation may cause a discrepancy between 3D soft tissue simulation and postoperative facial outcome and bias the accuracy of the results.
4.3. Considerations Regarding Additional Surgical Procedures
According to Holzinger et al. [35], the higher rate of error in predicting the 3D outcome of the soft tissues in the paranasal region and upper lip could be explained by additional intraoperative surgical maneuvers, such as septoplasty, reshaping of the anterior nasal spine (ANS) or nasal base, or soft tissue closure methods. Current 3D virtual planning software programs cannot reproduce the effect of different suturing techniques, resulting in non-linear soft-to-hard-tissue ratios, especially in the lip regions [32]. Thus, additional surgical techniques are, in fact, an uncontrolled factor that jeopardizes the 3D soft tissue simulation algorithm to a potentially clinically relevant extent. Nevertheless, Liebregts et al. [23] found a statistically significant favorable result for 3D simulation of the facial soft tissue mask when performing V-Y closure compared to surgeries without V-Y closure. With regard to the alar cinch suture, their findings were not significant. Moreover, additional surgical procedures were only briefly reported in 12 studies [15,16,17,18,19,20,21,23,27,32,37,38]. To improve the accuracy of 3D soft tissue simulation, additional surgical procedures, such as septoplasty, rhinoplasty, bony reshaping (ANS, nasal base, lateral nasal walls, chin, gonial angles, and zygomas), bone augmentation (grafts and PSIs), soft tissue closure methods after Le Fort I osteotomy (V-Y closure, alar base cinch suture, and paranasal cross sutures), lipofilling, or liposuction, should be reported in the methodology of the study and, ideally, in the future, they should be incorporated into the 3D soft tissue simulation model. Ter Horst et al. [33] therefore suggested a deep-learning-based algorithm as a suitable model for incorporating all these factors.
4.4. Soft Tissue Simulation Algorithms
The simulation of a patient’s new facial outlook requires a mathematical model that can process the deformation of the facial tissues due to underlying bony movements [3]. The algorithms of computational modeling that have been applied to 3D facial soft tissue morphing can be divided into five broad categories: (1) mass spring models (MSMs), (2) finite element models (FEMs), (3) mass tensor models (MTMs), (4) sparse landmark-based algorithms, and (5) methods that use artificial intelligence (AI). Each of these have their particular advantages and drawbacks [57], which are presented in Table 7. Mollemans et al. [3] compared four different computational strategies: a linear FEM, a non-linear FEM, an MSM, and an MTM. They found that the most accurate results were obtained with the FEM and the MTM.

Table 7.
Characteristics of 3D soft tissue simulation algorithms.
No mathematical model, however, has been generally accepted as the gold standard [15].
Recently, AI applications have spread rapidly in various fields of medicine and maxillofacial surgery [60]. The rationale for incorporating AI in soft tissue simulation methods is that it improves accuracy. AI includes machine learning (ML), which comprises both deep learning (DL) and artificial neural networks (ANNs) [61]. In this SR, only four studies presented models based on DL [8,33,36,48], and the results showed that the 3D soft tissue simulation accuracy is comparable to [8] or surpasses [33] the accuracy of the mathematical biomechanical algorithms. AI models require a huge database of MSCT, CBCT, or MR images, including data on additional surgical procedures, which are currently not available [59]. Hence, Ter Horst et al. [33] suggested that a web-based data sharing platform, to which multiple centers can upload standardized preoperative, planned, and postoperative 3D data, is the most likely way forward.
4.5. Rigid Registration of Preoperative/Simulated and Postoperative Data
Superimposition of 3D data, also called image rigid registration or image fusion, involves the spatial alignment of similar structures (e.g., a CBCT soft tissue mask and a 3D photograph or a 3D virtual treatment plan and post-treatment imaging data) [49]. There are different types of rigid registration: landmark-based, surface-based, and voxel-based rigid registration [7]. In order to evaluate 3D soft tissue simulation and enable measurements, 4 studies used landmark-based [15,30,34,35], 17 studies [9,10,16,18,19,28,29,31,32,33,37,38,41,43,44,45,47] applied surface-based, and 10 studies [3,14,17,20,21,22,23,24,25,29] used voxel-based rigid registration of the simulation and actual outcome. Voxel-based registration processes the raw information of a DICOM image by using the gray scale intensity of the voxels for superimposition. In contrast, surface-based registration requires an additional step of 3D model rendering to generate a 3D surface mesh model, which leads to a potential source of error [62]. Point-based rigid registration only uses corresponding points to compute the rotation and translation between datasets [49] and is prone to human error due to manual tracing of 3D cephalometric landmarks. Moreover, it has been shown to be inferior to surface- and voxel-based registration [63], which was confirmed by Andriola et al. [64], who showed in an SR that voxel-based superimposition protocols presented the highest accuracy and reproducibility. Voxel-based registration should, however, ideally be performed using only one type of user-independent software and based on a stable volume of interest (VOI) (e.g., the anterior cranial base, the total cranial base, or both zygomatic arches).
On the other hand, 3D photographs, as a non-ionizing imaging method, have relevant clinical potential for diagnosis and longitudinal non-radiation virtual treatment outcome analysis [49]. A total of eight studies in this review used a fusion of MSCT or CBCT data with 3D photogrammetry images to replace the MSCT or CBCT 3D soft tissue mask. Resnick et al. [15] stated that errors are created with each additional step in the imaging registration process, and thus, inferior accuracy can be expected with subsequent registration of a 3D photograph. Image registration errors were mainly located in the cheek and orbital regions and were reported to be larger than 1.5 mm [65].
4.6. Postprocessing and Analysis
The evaluation of the accuracy of 3D soft tissue simulation may be either quantitative or qualitative.
Quantitative validation measures the error between the virtually simulated 3D orthognathic treatment plan and its actual postoperative results. Qualitative validation uses questionnaires that are answered by surgeons or shape analysis [39]. In the reviewed studies, two main quantitative analysis methods have been used: (1) 3D landmark-based evaluation, where linear and angular differences between reference points placed on superimposed predicted and postoperative 3D models are measured, and (2) surface mesh-based evaluations, where surface-to-surface distances are measured [10]. This SR showed that 3D landmark-based evaluation has important shortcomings. One of them is the variability in the identification of 3D cephalometric landmarks [40], which is prone to human error, ranging from 0.3 to 2.8 mm, particularly when the landmarks have to be placed manually [7,66]. This source of error, however, could be minimized by the automatic identification of landmarks, for example, by means of AI algorithms [40]. In this SR, 7 [8,17,21,22,25,27,32] out of the 18 studies using landmark evaluation methods reported employing corresponding points, while others did not report correspondences or used the distance between one point on one surface and the closest point on the second surface. This might not be the corresponding anatomical point and would result in an underestimation of the error [45]. In the surface mesh-based method, there is no need to define 3D cephalometric landmarks, which eliminates the potential errors associated with this process. However, in the majority of the studies in this SR, measurements were taken using the minimal Euclidean distance between the two nearest points of the two surface meshes (the shortest distance between the triangle vertices of the adjacent meshes), with no actual anatomical correspondence [67]. This may explain the resulting underestimations of the error. Therefore, in a few studies [3,20,35,36], the authors used a generic mesh to overcome this problem. The generic mesh is a 3D virtual mask that resembles the human face, with a predefined number of equally sized triangles and a set of indexed vertices. It has the potential to mimic the morphology of a specific face by creating a deformation through a process known as “conformation”. Conformation enables the vertices that have been displaced by morphological changes (e.g., simulation or surgery) to be tracked. This provides an anatomical correspondence of vertices in two surface meshes [68,69]. Many studies have assessed the error of 3D soft tissue simulation in regard to the entire face, which includes large areas that are not affected at all by the performed surgery and could decrease the actual error [10]. Furthermore, when reporting based on the entire face, the site of the error remains unknown and is not clinically meaningful. In the studies that divided the face, the anatomical regions involved in orthognathic surgery were defined by the authors themselves [31].
Kim et al. [9], however, suggested surface deviation error alone to be an intuitive notion rather than a representation of the true anatomical correspondence. The unnatural shape and position of the lips, the labio-mental fold, the chin, and the soft tissue mesh distortion in the cheek regions next to the inferior border of the mandible could only be recognized by qualitative analysis using the “clinical human eye”. Therefore, in addition to quantitative analysis, they introduced a qualitative evaluation and confirmed that the quantitative error does not necessarily correspond to the clinicians’ qualitative evaluation. This was their rationale for introducing a lip shape analysis [41], evaluating the geometrical difference in the 2D lip profiles between the soft tissue simulation and the postoperative outcome.
Shafi et al. [19] reported that the overpredicted displacement of the soft tissue in the 3D simulation (positive values) and the underpredicted displacement (negative values) should be treated equally, i.e., as absolute values, to measure the mean difference. If some parts of the simulated surface lie behind and some in front of the postoperative surface, distance measurements for this region would comprise signed distances, i.e., positive and negative values. Any positive values would cancel out any negative values, thus underestimating the mean error and thereby biasing the results [45]. Therefore, the absolute mean values or Euclidean distances and the root mean square error should be measured.
4.7. Accuracy Cut-Off
Finally, in order to compare results reported in the literature regarding the accuracy of 3D soft tissue simulation in orthognathic surgery, it is important to define what accuracy means. In the studies reviewed in this SR, accuracy, referring to the comparison of the actual postoperative soft tissue outcome to the 3D soft tissue simulation, was defined in different ways [40], as an error of less than 0.5 mm, 1 mm, 2 mm, or up to 3 mm. Lee et al. [10] suggested setting the value of clinical insignificance at 2 mm, as this has been proposed in conventional 2D lateral cephalometric analysis. Kim et al. [9] reported a clinically acceptable error between the simulated and the actual soft tissue result below 2 mm (mean error) or 3 mm (maximum error). However, while there have been different attempts to set a fixed value of error that is clinically acceptable (i.e., unnoticeable by a lay person’s eye), there is no consensus in the current literature. Further studies with a proper study design are necessary in the future in order to gain evidence-based data.
5. Conclusions
This systematic review aimed to evaluate the accuracy of 3D soft tissue simulations in orthognathic surgery that have been reported in the literature. The findings underscore the diverse methodologies and approaches used in assessing simulation accuracy, emphasizing the critical need for standardization in this domain.
The current software packages and algorithms used in 3D soft tissue simulations have inherent limitations. A mean error varying from 0.27 mm to 2.9 mm for 3D soft tissue simulations for the whole face was reported. In the studies evaluating 3D soft tissue simulation accuracy after a Le Fort I osteotomy only, the upper lip and paranasal regions were reported to have the largest error, while after an isolated bilateral sagittal split osteotomy, the largest error was reported for the lower lip and chin regions. In the studies evaluating simulation after bimaxillary osteotomy with or without genioplasty, the highest inaccuracy was reported at the level of the lips, predominantly the lower lip, chin, and, sometimes, the paranasal regions.
The integration of artificial intelligence (AI) algorithms represents a promising advancement in enhancing the accuracy and efficiency of 3D soft tissue simulation. However, further research and a huge database of MSCT, CBCT, or MR images are needed to fully leverage the potential of AI in this context.
Due to significant variability in methodology and study design, meta-analysis was not feasible. Therefore, based on the results of this SR, guidelines to systematize the workflow for evaluating the accuracy of 3D soft tissue simulations in orthognathic surgery are proposed. These guidelines aim to streamline future research efforts and enhance comparability across studies.
In conclusion, while 3D soft tissue simulation holds promise for improving surgical outcomes in orthognathic procedures, ongoing efforts to address methodological challenges and advance technology are essential to realize its full potential in clinical practice.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jimaging10050119/s1: Table S1: The full search string for each database; Table S2: Methodological data on the studies included in this review; Table S3: The revised Quadas2 tool for risk of bias and applicability assessment; Table S4: Image acquisition technique and device.
Author Contributions
Conceptualization, G.R.J.S. and C.P.; methodology, A.O. and L.V.; validation, A.O., L.V. and T.-M.C.; formal analysis, A.O., T.-M.C. and G.R.J.S.; resources, A.O. and L.V.; writing—original draft preparation, A.O.; writing—review and editing, L.V., T.-M.C. and G.R.J.S.; supervision, G.R.J.S.; project administration, G.R.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Reinhilde Jacobs and Eman Shaheen from KU Leuven for their support for this research.
Conflicts of Interest
The authors declare that there are no competing interests.
References
- Swennen, G.R.J.; Schutyser, F.; Barth, E.-L.; De Groeve, P.; De Mey, A. A New Method of 3-D Cephalometry Part I. J. Craniofacial Surg. 2006, 17, 314–325. [Google Scholar] [CrossRef]
- Swennen, G.R.J.; Schutyser, F.; Hausamen, J.-E. Three-Dimensional Cephalometry; Springer: Berlin/Heidelberg, Germany, 2006; Chapter 8; p. 291. [Google Scholar]
- Mollemans, W.; Schutyser, F.; Nadjmi, N.; Maes, F.; Suetens, P. Predicting soft tissue deformations for a maxillofacial surgery planning system: From computational strategies to a complete clinical validation. Med. Image Anal. 2007, 11, 282–301. [Google Scholar] [CrossRef]
- Arnett, G.W.; Gunson, M.J. Facial planning for orthodontists and Oral surgeons. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kuang, T.; Rodrigues, Y.L.; Gateno, J.; Shen, S.G.F.; Wang, X.; Deng, H.; Yuan, P.; Alfi, D.M.; Liebschner, M.A.K.; et al. A New Approach of Predicting Facial Changes Following Orthognathic Surgery Using Realistic Lip Sliding Effect; Springer: Berlin/Heidelberg, Germany, 2019; pp. 336–344. [Google Scholar]
- Tondin, G.M.; de Oliveira Coelho Dutra Leal, M.; Costa, S.T.; Grillo, R.; Jodas, C.R.P.; Teixeira, R.G. Evaluation of the accuracy of virtual planning in bimaxillary orthognathic surgery: A systematic review. Br. J. Oral Maxillofac. Surg. 2022, 60, 412–421. [Google Scholar] [CrossRef]
- Gaber, R.M.; Shaheen, E.; Falter, B.; Araya, S.; Politis, C.; Swennen, G.R.J.; Jacobs, R. A Systematic Review to Uncover a Universal Protocol for Accuracy Assessment of 3-Dimensional Virtually Planned Orthognathic Surgery. J. Oral Maxillofac. Surg. 2017, 75, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xiao, D.; Kim, D.; Lian, C.; Kuang, T.; Liu, Q.; Deng, H.; Yang, E.; Liebschner, M.A.K.; Gateno, J.; et al. Simulation of Postoperative Facial Appearances via Geometric Deep Learning for Efficient Orthognathic Surgical Planning. IEEE Trans. Med. Imaging 2022, 42, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ho, D.C.; Mai, H.; Zhang, X.; Shen, S.G.F.; Shen, S.; Yuan, P.; Liu, S.; Zhang, G.; Zhou, X.; et al. A clinically validated prediction method for facial soft-tissue changes following double-jaw surgery. Med. Phys. 2017, 44, 4252–4261. [Google Scholar] [CrossRef]
- Lee, K.J.C.; Tan, S.L.; Low, H.Y.A.; Chen, L.J.; Yong, C.W.; Chew, M.T. Accuracy of 3-dimensional soft tissue prediction for orthognathic surgery in a Chinese population. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Nadjmi, N.; Tehranchi, A.; Azami, N.; Saedi, B.; Mollemans, W. Comparison of soft-tissue profiles in Le Fort I osteotomy patients with Dolphin and Maxilim softwares. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 654–662. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Elshebiny, T.; Morcos, S.; Mohammad, A.; Quereshy, F.; Valiathan, M. Accuracy of Three-Dimensional Soft Tissue Prediction in Orthognathic Cases Using Dolphin Three-Dimensional Software. J. Craniofacial. Surg. 2019, 30, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Resnick, C.M.; Dang, R.R.; Glick, S.J.; Padwa, B.L. Accuracy of three-dimensional soft tissue prediction for Le Fort I osteotomy using Dolphin 3D software: A pilot study. Int. J. Oral Maxillofac. Surg. 2017, 46, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Knoops, P.G.M.; Borghi, A.; Breakey, R.W.F.; Ong, J.; Jeelani, N.U.O.; Bruun, R.; Schievano, S.; Dunaway, D.J.; Padwa, B.L. Three-dimensional soft tissue prediction in orthognathic surgery: A clinical comparison of Dolphin, ProPlan CMF, and probabilistic finite element modelling. Int. J. Oral Maxillofac. Surg. 2019, 48, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.L.; Iwaki Filho, L.; Ferraz, F.W.d.S.; Ramos, A.L.; Previdelli, I.T.d.S.; Pereira, O.C.N.; Tolentino, E.d.S.; Chicarelli, M.; Iwaki, L.C.V. Accuracy of three-dimensional soft tissue profile prediction in orthognathic surgery. Oral Maxillofac. Surg. 2022, 26, 271–279. [Google Scholar] [CrossRef]
- Willinger, K.; Guevara-Rojas, G.; Cede, J.; Schicho, K.; Stamm, T.; Klug, C. Accuracy of Soft Tissue Prediction of 2 Virtual Planning Systems in Patients Undergoing Intraoral Quadrangular Le Fort II Osteotomy. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3326. [Google Scholar] [CrossRef]
- Shafi, M.I.; Ayoub, A.; Ju, X.; Khambay, B. The accuracy of three-dimensional prediction planning for the surgical correction of facial deformities using Maxilim. Int. J. Oral Maxillofac. Surg. 2013, 42, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Mundluru, T.; Almukhtar, A.; Ju, X.; Ayoub, A. The accuracy of three-dimensional prediction of soft tissue changes following the surgical correction of facial asymmetry: An innovative concept. Int. J. Oral Maxillofac. Surg. 2017, 46, 1517–1524. [Google Scholar] [CrossRef]
- Liebregts, J.; Xi, T.; Schreurs, R.; van Loon, B.; Bergé, S.; Maal, T. Three-dimensional virtual simulation of alar width changes following bimaxillary osteotomies. Int. J. Oral Maxillofac. Surg. 2016, 45, 1315–1321. [Google Scholar] [CrossRef]
- Liebregts, J.H.F.; Timmermans, M.; de Koning, M.J.J.; Bergé, S.J.; Maal, T.J.J. Three-Dimensional Facial Simulation in Bilateral Sagittal Split Osteotomy: A Validation Study of 100 Patients. J. Oral Maxillofac. Surg. 2015, 73, 961–970. [Google Scholar] [CrossRef]
- Liebregts, J.; Xi, T.; Timmermans, M.; de Koning, M.; Bergé, S.; Hoppenreijs, T.; Maal, T. Accuracy of three-dimensional soft tissue simulation in bimaxillary osteotomies. J. Cranio-Maxillofac. Surg. 2015, 43, 329–335. [Google Scholar] [CrossRef]
- Nadjmi, N.; Defrancq, E.; Mollemans, W.; Hemelen, G.; Bergé, S. Quantitative validation of a computer-aided maxillofacial planning system, focusing on soft tissue deformations. Ann. Maxillofac. Surg. 2014, 4, 171. [Google Scholar] [CrossRef]
- van Hemelen, G.; van Genechten, M.; Renier, L.; Desmedt, M.; Verbruggen, E.; Nadjmi, N. Three-dimensional virtual planning in orthognathic surgery enhances the accuracy of soft tissue prediction. J. Cranio-Maxillofac. Surg. 2015, 43, 918–925. [Google Scholar] [CrossRef]
- Aboul-Hosn Centenero, S.; Hernández-Alfaro, F. 3D planning in orthognathic surgery: CAD/CAM surgical splints and prediction of the soft and hard tissues results—Our experience in 16 cases. J. Cranio-Maxillofac. Surg. 2012, 40, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-U.; Hong, J. Is Three-Dimensional Soft Tissue Prediction by Software Accurate? J. Craniofacial. Surg. 2015, 26, e729–e733. [Google Scholar] [CrossRef]
- Bianchi, A.; Muyldermans, L.; di Martino, M.; Lancellotti, L.; Amadori, S.; Sarti, A.; Marchetti, C. Facial Soft Tissue Esthetic Predictions: Validation in Craniomaxillofacial Surgery with Cone Beam Computed Tomography Data. J. Oral Maxillofac. Surg. 2010, 68, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Bianchi, A.; Muyldermans, L.; di Martino, M.; Lancellotti, L.; Sarti, A. Validation of new soft tissue software in orthognathic surgery planning. Int. J. Oral Maxillofac. Surg. 2011, 40, 26–32. [Google Scholar] [CrossRef]
- Şenyürek, S.A.; Ajami, S.; Ruggiero, F.; van de Lande, L.; Caron, C.J.J.M.; Schievano, S.; Dunaway, D.J.; Padwa, B.; Koudstaal, M.J.; Borghi, A. The Accuracy of Computer-Assisted Surgical Planning in Predicting Soft Tissue Responses After Le Fort I Osteotomy: Retrospective Analysis. J. Craniofacial. Surg. 2023, 34, 131–138. [Google Scholar] [CrossRef]
- Hou, L.; He, Y.; Yi, B.; Wang, X.; Liu, X.; Zhang, Y.; Li, Z. Evaluation of soft tissue prediction accuracy for orthognathic surgery with skeletal class III malocclusion using maxillofacial regional aesthetic units. Clin. Oral Investig. 2022, 27, 173–182. [Google Scholar] [CrossRef]
- Cunha, H.S.; da Costa Moraes, C.A.; de Faria Valle Dornelles, R.; da Rosa, E.L.S. Accuracy of three-dimensional virtual simulation of the soft tissues of the face in OrtogOnBlender for correction of class II dentofacial deformities: An uncontrolled experimental case-series study. Oral Maxillofac. Surg. 2021, 25, 319–335. [Google Scholar] [CrossRef]
- ter Horst, R.; van Weert, H.; Loonen, T.; Bergé, S.; Vinayahalingam, S.; Baan, F.; Maal, T.; de Jong, G.; Xi, T. Three-dimensional virtual planning in mandibular advancement surgery: Soft tissue prediction based on deep learning. J. Cranio-Maxillofac. Surg. 2021, 49, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.; Reinert, S.; Kluba, S. Accuracy of Three-Dimensional Soft-Tissue Prediction Considering the Facial Aesthetic Units Using a Virtual Planning System in Orthognathic Surgery. J. Pers. Med. 2022, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Juergens, P.; Shahim, K.; Reyes, M.; Schicho, K.; Millesi, G.; Perisanidis, C.; Zeilhofer, H.-F.; Seemann, R. Accuracy of soft tissue prediction in surgery-first treatment concept in orthognathic surgery: A prospective study. J. Cranio-Maxillofac. Surg. 2018, 46, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, C.; Yamashiro, T. Development of novel artificial intelligence systems to predict facial morphology after orthognathic surgery and orthodontic treatment in Japanese patients. Sci. Rep. 2021, 11, 15853. [Google Scholar] [CrossRef]
- Ullah, R.; Turner, P.J.; Khambay, B.S. Accuracy of three-dimensional soft tissue predictions in orthognathic surgery after Le Fort I advancement osteotomies. Br. J. Oral Maxillofac. Surg. 2015, 53, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Schendel, S.A.; Jacobson, R.; Khalessi, S. 3-Dimensional Facial Simulation in Orthognathic Surgery: Is It Accurate? J. Oral Maxillofac. Surg. 2013, 71, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz, P.; Pérez, J.; Gutiérrez, A.; Barreiro, H.; Villalobos, Á.; Miraut, D.; Illana, C.; Guiñales, J.; Otaduy, M.A. Soft-Tissue Simulation for Computational Planning of Orthognathic Surgery. J. Pers. Med. 2021, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Venturini, A.; Guiñales Díaz de Cevallos, J.; del Castillo Pardo de Vera, J.L.; Alcañiz Aladrén, P.; Illana Alejandro, C.; Cebrián Carretero, J.L. A Quantitative and Qualitative Clinical Validation of Soft Tissue Simulation for Orthognathic Surgery Planning. J. Pers. Med. 2022, 12, 1460. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kuang, T.; Rodrigues, Y.L.; Gateno, J.; Shen, S.G.F.; Wang, X.; Stein, K.; Deng, H.H.; Liebschner, M.A.K.; Xia, J.J. A novel incremental simulation of facial changes following orthognathic surgery using FEM with realistic lip sliding effect. Med. Image Anal. 2021, 72, 102095. [Google Scholar] [CrossRef]
- Chabanas, M.; Marécaux, C.; Chouly, F.; Boutault, F.; Payan, Y. Evaluating soft tissue simulation in maxillofacial surgery using preoperative and postoperative CT scans. Int. Congr. Ser. 2004, 1268, 419–424. [Google Scholar] [CrossRef]
- Ulusoy, İ.; Akagunduz, E.; Sabuncuoglu, F.; Gorgulu, S.; Ucok, O. Use of the dynamic volume spline method to predict facial soft tissue changes associated with orthognathic surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, e17–e23. [Google Scholar] [CrossRef]
- Terzic, A.; Combescure, C.; Scolozzi, P. Accuracy of Computational Soft Tissue Predictions in Orthognathic Surgery from Three-Dimensional Photographs 6 Months After Completion of Surgery: A Preliminary Study of 13 Patients. Aesthetic Plast. Surg. 2014, 38, 184–191. [Google Scholar] [CrossRef]
- Khambay, B.; Ullah, R. Current methods of assessing the accuracy of three-dimensional soft tissue facial predictions: Technical and clinical considerations. Int. J. Oral Maxillofac. Surg. 2015, 44, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Bianchi, A.; Bassi, M.; Gori, R.; Lamberti, C.; Sarti, A. Mathematical Modeling and Numerical Simulation in Maxillofacial Virtual Surgery. J. Craniofacial. Surg. 2007, 18, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, F.; Borghi, A.; Bevini, M.; Badiali, G.; Lunari, O.; Dunaway, D.; Marchetti, C. Soft tissue prediction in orthognathic surgery: Improving accuracy by means of anatomical details. PLoS ONE 2023, 18, e0294640. [Google Scholar] [CrossRef]
- Fang, X.; Kim, D.; Xu, X.; Kuang, T.; Lampen, N.; Lee, J.; Deng, H.H.; Liebschner, M.A.K.; Xia, J.J.; Gateno, J.; et al. Correspondence attention for facial appearance simulation. Med. Image Anal. 2024, 93, 103094. [Google Scholar] [CrossRef]
- Swennen, G.R.J. 3D Virtual Treatment Planning of Orthognathic Surgery: A Step-by-Step Approach for Orthodontists and Surgeons; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Iblher, N.; Gladilin, E.; Stark, B.G. Soft-Tissue Mobility of the Lower Face Depending on Positional Changes and Age. Plast. Reconstr. Surg. 2013, 131, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Martínez, R.; Swennen, G.R.J. Cone-beam computerized tomography imaging and analysis of the upper airway: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2011, 40, 1227–1237. [Google Scholar] [CrossRef]
- Stratemann, S.; Huang, J.; Maki, K.; Miller, A.; Hatcher, D. Comparison of cone beam computed tomography imaging with physical measures. Dentomaxillofacial Radiol. 2008, 37, 80–93. [Google Scholar] [CrossRef]
- Eidson, L.; Cevidanes, L.H.S.; de Paula, L.K.; Hershey, H.G.; Welch, G.; Rossouw, P.E. Three-dimensional evaluation of changes in lip position from before to after orthodontic appliance removal. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 410–418. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, N.-K.; Moon, S.-W.; Jang, M.-J.; Kim, H.-S.; Yun, P.-Y. Evaluation of soft tissue changes around the lips after bracket debonding using three-dimensional stereophotogrammetry. Angle Orthod. 2015, 85, 833–840. [Google Scholar] [CrossRef]
- van der Vlis, M.; Dentino, K.M.; Vervloet, B.; Padwa, B.L. Postoperative Swelling After Orthognathic Surgery: A Prospective Volumetric Analysis. J. Oral Maxillofac. Surg. 2014, 72, 2241–2247. [Google Scholar] [CrossRef]
- Baan, F.; Liebregts, J.; Xi, T.; Schreurs, R.; de Koning, M.; Bergé, S.; Maal, T. A New 3D Tool for Assessing the Accuracy of Bimaxillary Surgery: The OrthoGnathicAnalyser. PLoS ONE 2016, 11, e0149625. [Google Scholar] [CrossRef]
- Singh, G.D.; Singh, M. Virtual Surgical Planning: Modeling from the Present to the Future. J. Clin. Med. 2021, 10, 5655. [Google Scholar] [CrossRef] [PubMed]
- Lampen, N.; Kim, D.; Fang, X.; Xu, X.; Kuang, T.; Deng, H.H.; Barber, J.C.; Gateno, J.; Xia, J.; Yan, P. Deep learning for biomechanical modeling of facial tissue deformation in orthognathic surgical planning. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Knoops, P.G.M.; Papaioannou, A.; Borghi, A.; Breakey, R.W.F.; Wilson, A.T.; Jeelani, O.; Zafeiriou, S.; Steinbacher, D.; Padwa, B.L.; Dunaway, D.J.; et al. A machine learning framework for automated diagnosis and computer-assisted planning in plastic and reconstructive surgery. Sci. Rep. 2019, 9, 13597. [Google Scholar] [CrossRef] [PubMed]
- Bouletreau, P.; Makaremi, M.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Artificial Intelligence: Applications in orthognathic surgery. J. Stomatol Oral Maxillofac. Surg. 2019, 120, 347–354. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar]
- Almukhtar, A.; Ju, X.; Khambay, B.; McDonald, J.; Ayoub, A. Comparison of the Accuracy of Voxel Based Registration and Surface Based Registration for 3D Assessment of Surgical Change following Orthognathic Surgery. PLoS ONE 2014, 9, e93402. [Google Scholar] [CrossRef]
- Holte, M.B.; Sæderup, H.; Pinholt, E.M. Comparison of surface- and voxel-based registration on the mandibular ramus for long-term three-dimensional assessment of condylar remodelling following orthognathic surgery. Dentomaxillofac. Radiol. 2022, 51, 20210499. [Google Scholar] [CrossRef]
- Andriola, F.d.O.; Haas Junior, O.L.; Guijarro-Martínez, R.; Hernández-Alfaro, F.; Oliveira, R.B.D.; Pagnoncelli, R.M.; Swennen, G.R. Computed tomography imaging superimposition protocols to assess outcomes in orthognathic surgery: A systematic review with comprehensive recommendations. Dentomaxillofac. Radiol. 2022, 51, 20210340. [Google Scholar] [CrossRef] [PubMed]
- Maal, T.J.J.; Plooij, J.M.; Rangel, F.A.; Mollemans, W.; Schutyser, F.A.C.; Bergé, S.J. The accuracy of matching three-dimensional photographs with skin surfaces derived from cone-beam computed tomography. Int. J. Oral Maxillofac. Surg. 2008, 37, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Plooij, J.M.; Swennen, G.R.J.; Rangel, F.A.; Maal, T.J.J.; Schutyser, F.A.C.; Bronkhorst, E.M.; Kuijpers–Jagtman, A.M.; Bergé, S.J. Evaluation of reproducibility and reliability of 3D soft tissue analysis using 3D stereophotogrammetry. Int. J. Oral Maxillofac. Surg. 2009, 38, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Marlière, D.A.A.; Demétrio, M.S.; Verner, F.S.; Asprino, L.; Chaves Netto, H.D.d.M. Feasibility of iterative closest point algorithm for accuracy between virtual surgical planning and orthognathic surgery outcomes. J. Cranio-Maxillofac. Surg. 2019, 47, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Almukhtar, A.; Ayoub, A.; Khambay, B.; McDonald, J.; Ju, X. State-of-the-art three-dimensional analysis of soft tissue changes following Le Fort I maxillary advancement. Br. J. Oral Maxillofac. Surg. 2016, 54, 812–817. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Almukhtar, A.; Keeling, A.; Hsung, T.-C.; Ju, X.; McDonald, J.; Ayoub, A.; Khambay, B.S. The Accuracy of Conformation of a Generic Surface Mesh for the Analysis of Facial Soft Tissue Changes. PLoS ONE 2016, 11, e0152381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).