Trends in Extraction of Rare Earth Elements from Coal Ashes: A Review
Abstract
1. Introduction
2. Coal Fly Ash
2.1. Properties of Coal Fly Ash
2.1.1. Mineralogy of Coal Fly Ash
2.1.2. Chemical Composition of Coal Fly Ash
2.2. Rare Earth Elements and Yttrium
2.2.1. Recovery of REY from Ores
2.2.2. Recycling of REY
2.3. Capture and Disposal of Coal Fly Ash
3. Physical Processing of Coal Fly Ash
3.1. Air Classification
3.2. Wet Gravity Separation
3.3. Flotation
3.4. Magnetic Separation
3.5. Multi-Step Physical Separation
4. Chemical Processing of Coal Fly Ash
4.1. Leaching
4.2. Recovery from Leachates: Extraction or Adsorption
4.3. Precipitation
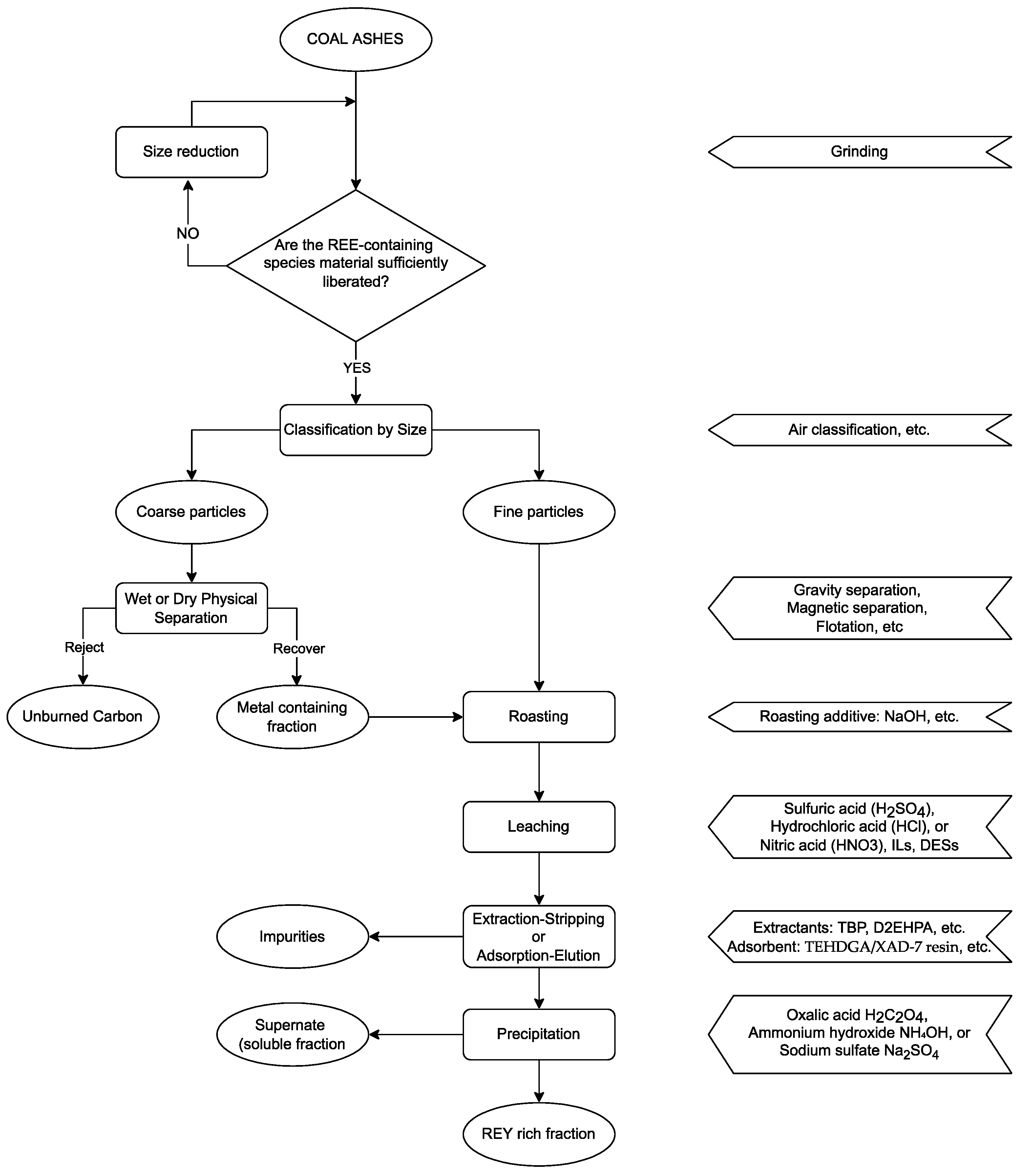
5. Main Flowsheets
6. Conclusions
- Eliminating the need to open new mines and their associated environmental disruption;
- Providing a steady supply of critical elements;
- Avoiding grinding as coal ash has a fine particle size;
- Minimizing the leaching of toxic elements (e.g., As, Hg, Pb, Se, Tl, and F) and their contamination to surface and ground waters from tailings;
- Reducing the environmental burden and the costs of landfilling;
- Lowering the cost of the necessary infrastructure and mining by utilizing a readily available industrial by-product.
Author Contributions
Funding
Conflicts of Interest
References
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef]
- Tuan, L.; Thenepalli, T.; Chilakala, R.; Vu, H.; Ahn, J.; Kim, J. Leaching Characteristics of Low Concentration Rare Earth Elements in Korean (Samcheok) CFBC Bottom Ash Samples. Sustainability 2019, 11, 2562. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, M.K.; Pathak, D.D. Review on the Processes for the Recovery of Rare Earth Metals (REMs) from Secondary Resources. In Rare Metal Technology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 53–65. [Google Scholar] [CrossRef]
- Roy, W.R.; Thiery, R.G.; Schuller, R.M.; Subway, J.J. Coal fly ash: A review of the literature and proposed classification system with emphasis on environmental impacts. Environ. Geol. Notes 1981, 96, EGN-96. [Google Scholar]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Huang, Z.; Fan, M.; Tian, H. Rare earth elements of fly ash from Wyoming’s Powder River Basin coal. J. Rare Earths 2020, 38, 219–226. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Wen, Z.; Zhou, C.; Pan, J.; Cao, S.; Hu, T.; Ji, W.; Nie, T. Recovery of rare-earth elements from coal fly ash via enhanced leaching. Int. J. Coal Prep. Util. 2020, 284, 124725. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorization of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Karan, R.; Sreenivas, T.; Kumar, M.A.; Singh, D.K. Recovery of rare earth elements from coal flyash using deep eutectic solvents as leachants and precipitating as oxalate or fluoride. Hydrometallurgy 2022, 214, 105952. [Google Scholar] [CrossRef]
- Pan, J.; Nie, T.; Vaziri Hassas, B.; Rezaee, M.; Wen, Z.; Zhou, C. Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 2020, 248, 126112. [Google Scholar] [CrossRef] [PubMed]
- Stuckman, M.Y.; Lopano, C.L.; Granite, E.J. Distribution and speciation of rare earth elements in coal combustion by-products via synchrotron microscopy and spectroscopy. Int. J. Coal Geol. 2018, 195, 125–138. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Dwyer, G.S.; Hsu-Kim, H. Trends in the Rare Earth Element Content of U.S.-Based Coal Combustion Fly Ashes. Environ. Sci. Technol. 2016, 50, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A Comprehensive Review of Rare Earth Elements Recovery from Coal-Related Materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Ward, C.R.; Yan, X.; Guo, W.; French, D.; Graham, I.T. Anomalies of rare metals in Lopingian super-high-organic-sulfur coals from the Yishan Coalfield, Guangxi, China. Ore Geol. Rev. 2017, 88, 235–250. [Google Scholar] [CrossRef]
- Ponou, J.; Dodbiba, G.; Anh, J.W.; Fujita, T. Selective recovery of rare earth elements from aqueous solution obtained from coal power plant ash. J. Environ. Chem. Eng. 2016, 4, 3761–3766. [Google Scholar] [CrossRef]
- Peiravi, M.; Dehghani, F.; Ackah, L.; Baharlouei, A.; Godbold, J.; Liu, J.; Mohanty, M.; Ghosh, T. A Review of Rare-Earth Elements Extraction with Emphasis on Non-conventional Sources: Coal and Coal By-products, Iron Ore Tailings, Apatite, and Phosphate By-products. Min. Metall. Explor. 2020, 38, 1–26. [Google Scholar] [CrossRef]
- Kashiwakura, S.; Kumagai, Y.; Kubo, H.; Wagatsuma, K. Dissolution of Rare Earth Elements from Coal Fly Ash Particles in a Dilute H2SO4 Solvent. Open J. Phys. Chem. 2013, 3, 69–75. [Google Scholar] [CrossRef]
- Dvorak, A.J.; Lewis, B.G.; Chee, P.C.; Dettmann, E.H.; Freeman, R.F., III. Impacts of Coal-Fired Power Plants on Fish, Wildlife, and Their Habitats; U.S. Department of Interior: Washington, DC, USA, 1978; p. 260. [Google Scholar]
- Huang, Z.; Fan, M.; Tiand, H. Coal and coal by-products: A large and developable unconventional resource for critical materials—Rare earth elements. J. Rare Earths 2018, 36, 337–338. [Google Scholar] [CrossRef]
- Woszuk, A.; Bandura, L.; Franus, W. Fly ash as low cost and environmentally friendly filler and its effect on the properties of mixed asphalt. J. Clean. Prod. 2019, 235, 493–502. [Google Scholar] [CrossRef]
- Xing, Y.; Guo, F.; Xu, M.; Gui, X.; Li, H.; Li, G.; Xia, Y.; Han, H. Separation of unburned carbon from coal fly ash: A review. Powder Technol. 2019, 353, 372–384. [Google Scholar] [CrossRef]
- Yang, F.; Pranda, P.; Hlavacek, V. Recovery of fly ash carbon by carbochlorination via phosgene route. Powder Technol. 2003, 131, 206–211. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. Int. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Sun, X.; Li, Z. Unburned Carbon from Fly Ash for Mercury Adsorption:, I. Separation and Characterization of Unburned Carbon. J. Miner. Mater. Charact. Eng. 2002, 1, 39–60. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Varnavskaya, A.; Ju, D. Magnetite and Carbon Extraction from Coal Fly Ash Using Magnetic Separation and Flotation Methods. Minerals 2019, 9, 320. [Google Scholar] [CrossRef]
- Peiravi, M.; Ackah, L.; Guru, R.; Mohanty, M.; Liu, J.; Xu, B.; Zhu, X.; Chen, L. Chemical extraction of rare earth elements from coal ash. Miner. Metall. Process. 2017, 34, 170–177. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Shu, C.M. Progressive utilization prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.-L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Hulett, L.D., Jr.; Weinberger, A.J.; Northcutt, K.J.; Ferguson, M. Chemical species in fly ash from coal-burning power plants. Science 1980, 210, 1356–1358. [Google Scholar] [CrossRef]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Hower, J.C.; Johnston, M.N.; Song, W.; Wang, P.; Zhang, S. Petrology, Mineralogy, and Chemistry of Size-Fractioned Fly Ash from the Jungar Power Plant, Inner Mongolia, China, with Emphasis on the Distribution of Rare Earth Elements. Energy Fuels 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- Llorens, J.F.; Fernández-Turiel, J.L.; Querol, X. The fate of trace elements in a large coal-fired power plant. Environ. Geol. 2001, 40, 409–416. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, C.; Tang, M.; Cao, S.; Liu, C.; Zhang, N.; Wen, M.; Luo, Y.; Hu, T.; Ji, W. Study on the modes of occurrence of rare earth elements in coal fly ash by statistics and a sequential chemical extraction procedure. Fuel 2019, 237, 555–565. [Google Scholar] [CrossRef]
- Hower, J.; Groppo, J.; Henke, K.; Hood, M.; Eble, C.; Honaker, R.; Zhang, W.; Qian, D. Notes on the Potential for the Concentration of Rare Earth Elements and Yttrium in Coal Combustion Fly Ash. Minerals 2015, 5, 356–366. [Google Scholar] [CrossRef]
- Mardon, S.M.; Hower, J.C. Impact of coal properties on coal combustion by-product quality: Examples from a Kentucky power plant. Int. J. Coal Geol. 2004, 59, 153–169. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, C.; Liu, C.; Tang, M.; Cao, S.; Hu, T.; Ji, W.; Luo, Y.; Wen, M.; Zhang, N. Modes of Occurrence of Rare Earth Elements in Coal Fly Ash: A Case Study. Energy Fuels 2018, 32, 9738–9743. [Google Scholar] [CrossRef]
- Lin, R.; Stuckman, M.; Howard, B.H.; Bank, T.L.; Roth, E.A.; Macala, M.K.; Lopano, C.; Soong, Y.; Granite, E.J. Application of sequential extraction and hydrothermal treatment for characterization and enrichment of rare earth elements from coal fly ash. Fuel 2018, 232, 124–133. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Liu, P.; Huang, R.; Tang, Y. Comprehensive Understandings of Rare Earth Element (REE) Speciation in Coal Fly Ashes and Implication for REE Extractability. Environ. Sci. Technol. 2019, 53, 5369–5377. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Huang, B.; Dong, Y.; Sun, X. The recovery of rare earth elements from coal combustion products by ionic liquids. Miner. Eng. 2019, 130, 142–147. [Google Scholar] [CrossRef]
- McLellan, B.; Corder, G.; Ali, S. Sustainability of Rare Earths—An Overview of the State of Knowledge. Minerals 2013, 3, 304–317. [Google Scholar] [CrossRef]
- Kim, J.-A.; Dodbiba, G.; Tanimura, Y.; Mitsuhashi, K.; Fukuda, N.; Okaya, K.; Matsuo, S.; Fujita, T. Leaching of Rare-Earth Elements and Their Adsorption by Using Blue-Green Algae. Mater. Trans. 2011, 52, 1799–1806. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.H.; Uchimiya, M.; Kwon, E.E.; Jeon, B.H.; Deep, A.; Yun, S.T. Global demand for rare earth resources and strategies for green mining. Environ. Res. 2016, 150, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Chen, Z. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earths 2011, 29, 1–6. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- (DOE). U.S.D.o.E. 2010. 2010 Critical Materials Strategy. Available online: https://www.energy.gov/eere/amo/2010-critical-materials-strategy (accessed on 30 October 2022).
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Van Acker, K.; Blanpain, B.; Mishra, B.; Apelian, D. Rare-Earth Economics: The Balance Problem. JOM 2013, 65, 846–848. [Google Scholar] [CrossRef]
- Falconnet, P. The economics of rare earths. J. Less Common Met. 1985, 111, 9–15. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Kato, Y.; Fujinaga, K.; Nakamura, K.; Takaya, Y.; Kitamura, K.; Ohta, J.; Toda, R.; Nakashima, T.; Iwamori, H. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat. Geosci. 2011, 4, 535–539. [Google Scholar] [CrossRef]
- Takaya, Y.; Yasukawa, K.; Kawasaki, T.; Fujinaga, K.; Ohta, J.; Usui, Y.; Nakamura, K.; Kimura, J.I.; Chang, Q.; Hamada, M.; et al. The tremendous potential of deep-sea mud as a source of rare-earth elements. Sci. Rep. 2018, 8, 5763. [Google Scholar] [CrossRef] [PubMed]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Fray, D.J. Chemical Engineering: Separating Rare Earth Elements. Science 2000, 289, 2295–2296. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Zhang, W.; Werner, J. Acid Leaching of Rare Earth Elements from Coal and Coal Ash: Implications for Using Fluidized Bed Combustion To Assist in the Recovery of Critical Materials. Energy Fuels 2019, 33, 5971–5980. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Zhang, W.; Werner, J.; Noble, A.; Luttrell, G.H.; Yoon, R.H. Enhancement of a Process Flowsheet for Recovering and Concentrating Critical Materials from Bituminous Coal Sources. Min. Metall. Explor. 2019, 37, 3–20. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef]
- Eggert, R.; Wadia, C.; Anderson, C.; Bauer, D.; Fields, F.; Meinert, L.; Taylor, P. Rare Earths: Market Disruption, Innovation, and Global Supply Chains. Annu. Rev. Environ. Resour. 2016, 41, 199–222. [Google Scholar] [CrossRef]
- Ponou, J.; Garrouste, M.; Dodbiba, G.; Fujita, T.; Ahn, J.-W. Sulfation–Roasting–Leaching–Precipitation Processes for Selective Recovery of Erbium from Bottom Ash. Sustainability 2019, 11, 3461. [Google Scholar] [CrossRef]
- Rumpf, H. Particle Technology; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Shapiro, M.; Galperin, V. Air classification of solid particles: A review. Chem. Eng. Process. Process Intensif. 2005, 44, 279–285. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Pre-processing of coal combustion fly ash by classification for enrichment of rare earth elements. Energy Rep. 2018, 4, 660–663. [Google Scholar] [CrossRef]
- Falconer, A. Gravity separation: Old technique/new methods. Phys. Sep. Sci. Eng. 1970, 12, 31–48. [Google Scholar] [CrossRef]
- Manser, R.J.; Barley, R.W.; Wills, B.A. The shaking table concentrator—The influence of operating conditions and table parameters on mineral separation—The development of a mathematical model for normal operating conditions. Miner. Eng. 1991, 4, 369–381. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low-rank coal by surfactants—A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Drzymala, J.; Gorke, J.T.; Wheelock, T.D. A Flotation Collector for the Separation of Unburned Carbon from Fly Ash. Coal Prep. 2005, 25, 67–80. [Google Scholar] [CrossRef]
- Eisele, T.C.; Kawatra, S.K. Use of froth flotation to remove unburned carbon from fly ash. Miner. Process. Extr. Metall. Rev. 2002, 23, 1–10. [Google Scholar] [CrossRef]
- Harris, T.; Wheelock, T.D. Process Conditions for the Separation of Carbon from Fly Ash by Froth Flotation. Int. J. Coal Prep. Util. 2008, 28, 133–152. [Google Scholar] [CrossRef]
- Sivamohan, R. The problem of recovering very fine particles in mineral processing—A review. Int. J. Miner. Process. 1990, 28, 247–288. [Google Scholar] [CrossRef]
- Walker, A.; Wheelock, T.D. Separation of Carbon from Fly Ash Using Froth Flotation. Coal Prep. 2006, 26, 235–250. [Google Scholar] [CrossRef]
- Zhang, W.; Groppo, J.; Honaker, R. Ash Beneficiation for REE Recovery. In Proceedings of the World of Coal Ash (WOCA) Conference, Nasvhille, TN, USA, 5–7 May 2015. [Google Scholar]
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A Review of the Occurrence and Promising Recovery Methods of Rare Earth Elements from Coal and Coal By-Products. Int. J. Coal Prep. Util. 2015, 35, 295–330. [Google Scholar] [CrossRef]
- Gray, M.L.; Champagne, K.J.; Soong, Y.; Killmeyer, R.P.; Maroto-Valer, M.M.; Andrésen, J.M.; Ciocco, M.V.; Zandhuis, P.H. Physical cleaning of high carbon fly ash. Fuel Process. Technol. 2002, 76, 11–21. [Google Scholar] [CrossRef]
- Ashworth, R.A.; Rodriguez, L.A.; Padila, A.A.; Spake, N.B.; Bery, W.W.; Schmeda, R.A. Method for the Recovery of Minerals and Production of By-Products from Coal Ash. U.S. Patent 4652433A, 24 March 1987. [Google Scholar]
- Lin, R.; Howard, B.H.; Roth, E.A.; Bank, T.L.; Granite, E.J.; Soong, Y. Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel 2017, 200, 506–520. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A. Mineralogy characterization and recovery of rare earth elements from the roof and floor materials of the Guxu coalfield. Fuel 2020, 270, 117533. [Google Scholar] [CrossRef]
- Rozelle, P.L.; Khadilkar, A.B.; Pulati, N.; Soundarrajan, N.; Klima, M.S.; Mosser, M.M.; Miller, C.E.; Pisupati, S.V. A Study on Removal of Rare Earth Elements from, U.S. Coal Byproducts by Ion Exchange. Metall. Mater. Trans. E 2016, 3, 6–17. [Google Scholar]
- Cao, S.; Zhou, C.; Pan, J.; Liu, C.; Tang, M.; Ji, W.; Hu, T.; Zhang, N. Study on Influence Factors of Leaching of Rare Earth Elements from Coal Fly Ash. Energy Fuels 2018, 32, 8000–8005. [Google Scholar] [CrossRef]
- Kumari, A.; Parween, R.; Chakravarty, S.; Parmar, K.; Pathak, D.D.; Lee, J.-C.; Jha, M.K. Novel approach to recover rare earth metals (REMs) from Indian coal bottom ash. Hydrometallurgy 2019, 187, 1–7. [Google Scholar] [CrossRef]
- Arrachart, G.; Couturier, J.; Dourdain, S.; Levard, C.; Pellet-Rostaing, S. Recovery of rare earth elements (REE) using ionic solvents. Processes 2021, 9, 1202. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective Leaching of Rare Earth Elements from Bauxite Residue (Red Mud), Using a Functionalized Hydrophobic Ionic Liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Hsu-Kim, H. Effects of roasting additives and leaching parameters on the extraction of rare earth elements from coal fly ash. Int. J. Coal Geol. 2018, 196, 106–114. [Google Scholar] [CrossRef]
- Yakaboylu, G.A.; Baker, D.; Wayda, B.; Sabolsky, K.; Zondlo, J.W.; Shekhawat, D.; Wildfire, C.; Sabolsky, E.M. Microwave-Assisted Pretreatment of Coal Fly Ash for Enrichment and Enhanced Extraction of Rare-Earth Elements. Energy Fuels 2019, 33, 12083–12095. [Google Scholar] [CrossRef]
- King, J.F.; Taggart, R.K.; Smith, R.C.; Hower, J.C.; Hsu-Kim, H. Aqueous acid and alkaline extraction of rare earth elements from coal combustion ash. Int. J. Coal Geol. 2018, 195, 75–83. [Google Scholar] [CrossRef]
- Manurung, H.; Rosita, W.; Bendiyasa, I.M.; Prasetya, A.; Anggara, F.; Astuti, W.; Djuanda, D.R.; Petrus, H.T.B.M. Recovery of rare earth elements and yttrium from non-magnetic coal fly ash using acetic acid solution. Met. Indones. 2020, 42, 35–42. [Google Scholar] [CrossRef]
- Uda, T.; Jacob, K.T.; Hirasawa, M. Technique for enhanced rare earth separation. Science 2000, 289, 2326–2329. [Google Scholar] [CrossRef]
- Das, S.; Gaustad, G.; Sekar, A.; Williams, E. Techno-economic analysis of supercritical extraction of rare earth elements from coal ash. J. Clean. Prod. 2018, 189, 539–551. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Burgard, M.; Dieleman, C.B.; Matt, D. Rare-earth metal-ion separation using a supported liquid membrane mediated by a narrow rim phosphorylated calix [4]arene. J. Membr. Sci. 1998, 144, 57–64. [Google Scholar] [CrossRef]
- Smith, R.C.; Taggart, R.K.; Hower, J.C.; Wiesner, M.R.; Hsu-Kim, H. Selective Recovery of Rare Earth Elements from Coal Fly Ash Leachates Using Liquid Membrane Processes. Environ. Sci Technol. 2019, 53, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.-Y. Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Park, D.; Middleton, A.; Smith, R.; Deblonde, G.; Laudal, D.; Theaker, N.; Hsu-Kim, H.; Jiao, Y. A biosorption-based approach for selective extraction of rare earth elements T from coal byproducts. Sep. Purif. Technol. 2020, 241, 116726. [Google Scholar] [CrossRef]
- Mondal, S.; Ghar, A.; Satpati, A.K.; Sinharoy, P.; Singh, D.K.; Sharma, J.N.; Sreenivas, T.; Kain, V. Recovery of rare earth elements from coal fly ash using TEHDGA impregnated resin. Hydrometallurgy 2019, 185, 93–101. [Google Scholar] [CrossRef]
- Chi, R.; Xu, Z. A solution chemistry approach to the study of rare earth element precipitation by oxalic acid. Metall. Mater. Trans. B 1999, 30, 189–195. [Google Scholar] [CrossRef]
- Anand Rao, K.; Ram, K.; Madhu Babu, J.; Rama Devi, G.; Sreenivas, T. Development of process scheme for recovery of rare earths from leachate of coal flyash. Clean. Chem. Eng. 2022, 4, 100078. [Google Scholar]
| Category | Method | Concentrator/ Equipment | Medium | Effective Particle Size |
|---|---|---|---|---|
| Physical Separation | Air Classification | Air | +100 μm | |
| Wet Gravity Separation | Hydrocyclone | Water | 5–100 μm | |
| Shaking table | Water | 60–600 μm | ||
| Spiral concentrator | Water | 60–600 μm | ||
| Conical concentrator | Water | 60–600 μm | ||
| Sink-float separation | Water | 55–500 μm | ||
| Dense medium separator | Heavy media | +500 μm | ||
| Magnetic Separation | High-intensity wet magnetic separator | Air or water | +20 μm | |
| Flotation | Flotation machine | Water | 40–71 μm | |
| Chemical Treatment | Leaching | Acid solution | −25 μm | |
| Solvent Extraction | Organic solvents | |||
| Selective Precipitation | Precipitants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodbiba, G.; Fujita, T. Trends in Extraction of Rare Earth Elements from Coal Ashes: A Review. Recycling 2023, 8, 17. https://doi.org/10.3390/recycling8010017
Dodbiba G, Fujita T. Trends in Extraction of Rare Earth Elements from Coal Ashes: A Review. Recycling. 2023; 8(1):17. https://doi.org/10.3390/recycling8010017
Chicago/Turabian StyleDodbiba, Gjergj, and Toyohisa Fujita. 2023. "Trends in Extraction of Rare Earth Elements from Coal Ashes: A Review" Recycling 8, no. 1: 17. https://doi.org/10.3390/recycling8010017
APA StyleDodbiba, G., & Fujita, T. (2023). Trends in Extraction of Rare Earth Elements from Coal Ashes: A Review. Recycling, 8(1), 17. https://doi.org/10.3390/recycling8010017





