Abstract
A significant part of fruit production is wasted annually, a material of high value without use, causing environmental and social damage. These residues from agro-industrial processes, or those that can no longer be used in the market, can be recycled and generate value-added products by pretreatments/hydrolysis. One of the important pretreatments is acid hydrolysis, which can produce xylooligosaccharides (XOS) from biomass, a product of great commercial value in the food and pharmaceutical markets, mainly due to its prebiotic potential. Bananas, oranges, and guava generate a large volume of waste and represent much of Brazil’s fruit production. The dilute acid hydrolysis resulted in XOS production of 37.69% for banana peel, 59.60% for guava bagasse, 28.70% for orange bagasse, and 49.64% for restaurant residue. XOS were quantified by a liquid chromatograph system with a Bio-Rad Aminex HPX-87C column. The results show that, for this type of material and hydrolysis, the ideal conditions to produce XOS are high temperature, low time, and high acid concentration for banana peel residue (160 °C, 15 min, and 3% H2SO4), low temperature, low time, and high acid concentration for guava bagasse (100 °C,15 min and 3% H2SO4), high temperature and acid concentration with low time for orange bagasse (160 °C,15 min and 3% H2SO4) and high temperature and time and high acid concentration for restaurant waste (160 °C, 55 min and 3% H2SO4). This study identified acid hydrolysis conditions that maximized XOS production with a low amount of xylose production using agro-industrial and food residues, also showing the high potential of the chosen residues through the high yields of XOS production.
1. Introduction
Total waste generation in Brazil in 2020 reached 225.965 tons/day, according to the 2021 Panorama of Solid Waste in Brazil, prepared by the Brazilian Association of Public Cleaning and Special Waste Companies (ABRELPE). Of this total, approximately 52% is made up of organic material (fruit, vegetables, and food in general), and 39.8% of waste was not disposed of correctly. The big waste of food, solid waste generation, and a lack of proper food management plans have become an increasing problem. Especially in restaurants, the variety and quantity of food offered exceeds the need for consumption, resulting in the occurrence of leftovers and waste, which contributes to the increase in solid waste generation [1]. This scenario shows that it is essential to properly plan food preparation and conscientiously use the resources, implementing preventive measures for solid waste generation. Besides reducing losses and waste in the food chain, it is possible to recycle them, rather than throw them away, by processing them and obtaining products with value added [2,3].
The consumption of processed foods generates residues that require alternatives for their recycling and reuse. Food industries also produce tons of by-products during food processing. Fruit and vegetable by-products, which can be derived from preparation and processing, represent the most abundant part. Generally, food by-products are used as animal feed or for the production of biofuels, biogas, and biomaterials. However, due to their active compounds, they have been recycled and transformed for use in other industrial fields such as cosmetics, pharmaceuticals, and food [4,5].
Among the main fruits produced in Brazil, orange leads in volume, totaling 16.7 million tons from orchards in 2015, which corresponded to 40.9% of the total fruit crops. Among the Brazilian states, São Paulo leads the production, with 12.3 million tons, representing 73.3% of the volume produced [6]. The second most-produced fruit in Brazil, in volume, is the banana, which had a volume of about 6.7 million tons in 2017, with the main producers in the states of São Paulo and Bahia [7]. Another fruit of increasing national production is guava, which grew by 11% in its planted area in the year 2018 [8], with growing consumption of fresh fruit and also industrialized derivatives from guava fruit [9]. In addition to this growth, the greatest diversity of guava species is found in the country, which occupies a prominent position among the world’s guava producers [10,11]. The industrial process of food production and local consumption in a restaurant are sources of waste. The main challenge regards food waste from restaurants is its chemical composition, which can vary according to the food components/ingredients (vegetables). However, a selection of waste from pre-prepared food was characterized as a material similar to lignocellulosic biomass components [1].
The use of fruit and restaurant waste (applying lignocellulosic biomass technology) is fundamental as an economical and sustainable alternative. However, the development of fractionation processes is necessary for the use of compounds present in biomass [12]. In order to fractionate the lignocellulosic material, several methods are used, such as pretreatments in acid, alkaline, organic solvents, and ionic liquids, each with its advantages and disadvantages [12,13,14]. Given the costs involved and sugar recovery at the end of the process, dilute acid, steam explosion and alkaline pretreatments are the most used for biomass fractionation [15]. In addition, acid pretreatment/hydrolysis has the advantage of short reaction time but, in contrast, degradation products (furfural) and monosaccharide (mainly xylose) formation occur [16]. Pretreatment with diluted acid allows for the fractionation of hemicellulose into oligosaccharides and monosaccharides by cleavage of the xylan chain glycosidic bonds. This breakdown depends on variables such as the type of acid used, the concentration, reaction time, and temperature [17]. Acid hydrolysis was applied to sugarcane bagasse evaluating acetic and sulfuric acids. The optimized process indicated 1% acetic acid resulting in 18.41% of XOS, and 2% sulfuric acid resulting in 90.13% of XOS [18]. The strong acid showed better action in the xylan hydrolysis, probably due to the partial dissociation of the acetic acid, and its lower pH modification in comparison to sulfuric acid.
Xylooligosaccharides are a product of great industrial interest, and they can be obtained from xylan-rich lignocellulosic materials such as agro-industrial waste (abundant sources of lignocellulosic biomass) [19,20]. XOS have food and pharmaceutical importance as they can stimulate beneficial microorganisms’ growth [21]. For the solubilization and/or hydrolysis of xylan present from the hemicellulosic fraction, different processes can be performed, among which is acid hydrolysis of biomass [18,22], applied in this study. Acid hydrolysis can collaborate for the feasibility of the process due to low cost compared to enzymatic hydrolysis. The present study evaluated the main fruit/food wastes produced in Brazil: banana peel, guava bagasse, orange pomace, and restaurant pre-prepared residue. The direct application of the acid in the biomass was evaluated instead of previous xylan solubilization, which is traditionally applied for XOS production. Industrial fruit waste was evaluated and we identified its potential for bioactive compound production, contributing to recycling waste and producing added value compounds. The biomass was submitted to dilute acid pretreatment to produce XOS, determining the experimental conditions that maximize XOS content with low xylose production.
2. Results and Discussion
2.1. Xylooligosaccharide Production
The xylooligosaccharides production (yield) was reported as the percentage of XOS produced in relation to the mass of the original hemicellulose. The chemical composition and total hemicellulose were reported in a previous study [23]. The highest yield was obtained under the following conditions: for banana peels, the highest XOS yield produced was 37.69%, at high temperature, short reaction time, and high acid concentration (160 °C, 15 min and 3% H2SO4); for guava bagasse the yield was 59.60% of XOS at low temperature, short reaction time, and high acid concentration (100 °C, 15 min and 3% H2SO4); for orange bagasse, the highest XOS yield occurred at a high temperature, short reaction time and high acid concentration (160 °C, 15 min and 3% H2SO4), which was 28.70%; for restaurant pre-prepared waste, the highest XOS yield was 49.64%, occurring under the highest experimental conditions (160 °C, 55 min and 3% H2SO4) (Table 1). Each of the biomasses showed a different maximum XOS yield, which was dependent on a specific experimental condition. Sulfuric acid was necessary at 3% concentration for the maximum XOS yield of all the biomass. The temperature was dependent on the material, varying from 100 to 160 °C. Reaction time was identified from 15 to 55 min, also depending on the biomass. Each of the biomasses needs a specific condition to reach a maximum XOS yield.

Table 1.
Xylooligosaccharides and xylose yield by acid hydrolysis of biomasses using a factorial design 23 with acid treatment (H2SO4 %, m/v, reaction time (min) and temperature (°C)).
XOS yield was reported to be 44.5% for sugarcane bagasse and 45.18% for sugarcane pulp residual hemicellulose [24,25]. Under the optimal conditions for the brewery’s spent grain (temperature of 170 °C, acetic acid concentration of 5%, and reaction time of 30 min), a yield of 6.9% of XOS and 8.9% of xylose were reported (Wen et al., 2019). With tobacco stalk (temperature of 100 °C, acid concentration of 0.25 mol/L of H2SO4, and reaction time of 30 min), a yield of 13% of XOS and 14% of monosaccharides were reported [26]. These results show the great potential of the fruit waste biomasses used in the present study since the optimum conditions for each biomass presented higher XOS contents with much lower xylose concentrations (Table 1). Corroborating the present study, the literature showed a specific condition for each biomass. This fact is probably related to the recalcitrance of the biomass, involving the organization of the plant cell wall and chemical composition [12].
After 24 h, enzymatic hydrolysis of xylan, the highest yield of XOS produced from banana peels, guava bagasse, orange bagasse, and restaurant waste were 53.38%, 57.38%, 49.24%, and 47.25%, respectively [1]. For banana peel and orange bagasse via enzymatic hydrolysis, the XOS yield was higher than in the present study; however, for guava bagasse and restaurant waste, XOS yield after diluted acid hydrolysis was similar. Therefore, considering only XOS yield, there would be no need for solubilization of the xylan present in the last two residues for there to be an improvement in XOS yield. The different yields based on the enzymatic hydrolysis versus acid hydrolysis should consider the need for xylan previous solubilization, enzyme production, and enzyme possible purification (with the aim of to avoid xylose release). With acid hydrolysis, xylose will be produced; however, specific conditions can minimize its production. Depending on the xylose concentration, the purification of the XOS could be avoided.
In addition, liquid hot water (LWH) treatment was applied for the same residues, and the highest XOS yield obtained were 32.28% using banana peel after 160 °C treatment and 15 min reaction time, only 8.21% using guava bagasse with 172.43 °C treatment and 35 min reaction time, 66.13% using orange bagasse after 130 °C treatment and 35 min reaction time, and 33.42% using restaurant waste with 130 °C treatment and 6.72 min reaction time [23]. The same temperature and reaction time led to a higher XOS yield using banana peel residue after diluted acid hydrolysis, which also occurred for guava bagasse using a low temperature and shorter reaction time. Orange bagasse XOS yield was significantly higher after LWH treatment using a lower temperature and higher reaction time. However, restaurant waste presented higher XOS yield after diluted acid hydrolysis using a higher temperature and reaction time.
With the objective of XOS production, a low formation of xylose is desired. For this reason, it is important to optimize the acid hydrolysis to maximize XOS content with low xylose production. In this study, acid was applied at diluted conditions considering the percentage of the mass of acid per mass of material. For xylose yield, the maximum value for banana peel was 2.64%, at a high temperature, short reaction time, and high concentration of acid (160 °C,15 min, and 3% H2SO4); for guava bagasse, the highest content was 5.69% at a high temperature, short reaction time and high concentration of acid (160 °C,15 min and 3% H2SO4); for orange bagasse, the highest xylose content was 3.10% that occurred at a medium temperature and reaction time and high acid concentration (130 °C, 35 min and 3.68% H2SO4—star point); for restaurant waste, the highest value was 4.58%, occurring under the highest conditions (160 °C, 55 min and 3% H2SO4). For sugarcane bagasse, contents of 7 to 13% of xylose were reported using acid hydrolysis [27]; and 8.9% of xylose was reported for brewery’s spent grain [28], higher values than those found in the present study, which shows the potentiality of the studied biomass, considering that the lower xylose formation is advantageous for the study objective.
2.2. Central Composite Design Model Fitting
The acid concentration variable had a significant positive effect on the XOS content for all biomasses. In the case of restaurant residue, the interaction of temperature and reaction time variables also had positive significance (Table 2 and Table 3). The effect of acid concentration had its most pronounced standardized value for all biomasses when compared to the effects obtained by the other independent variables. These results could show that for banana peels, guava bagasse and orange bagasse, the model using the axial points did not lead to the best result, and a first-order model could be more appropriate for the analysis of these biomasses. With a first-order model for banana peel, it was possible to observe the significance of acid concentration, temperature, reaction time, and curvature. Although the curvature is significant, the analysis with the rotational points did not lead to a second-order model adjustment. For this reason, the statistical analysis followed the standard design data. For guava and orange bagasse, temperature, acid concentration, and the interaction between the two parameters were statistically significant. The same happened for orange bagasse.

Table 2.
Analysis of variance (ANOVA) for acid hydrolysis of biomass of banana peels and guava bagasse.

Table 3.
Analysis of variance (ANOVA) for acid hydrolysis of biomass of orange bagasse restaurant residue.
For the analysis, the response variables used were the contents of the oligomers of interest (XOS and xylose) in the hydrolysate fraction. The contents of these oligomers are directly related to the potential of the residue since XOS production can provide prebiotic formulation. On the other hand, xylose content can disrupt this objective. High production of XOS with low xylose release is ideal for the goal of the present study. The study focused on the analysis of acid hydrolysis conditions that would allow for an effective release of XOS with a small generation of xylose. In addition, analyzing the potential of the biomass was chosen for this purpose since not enough studies of this biomass were found. The statistically significant effects of XOS production as the dependent variable and the independent variables (T, t, and acid concentration) of greatest pretreatment significance for each biomass are presented in Figure 1 and Figure 2.
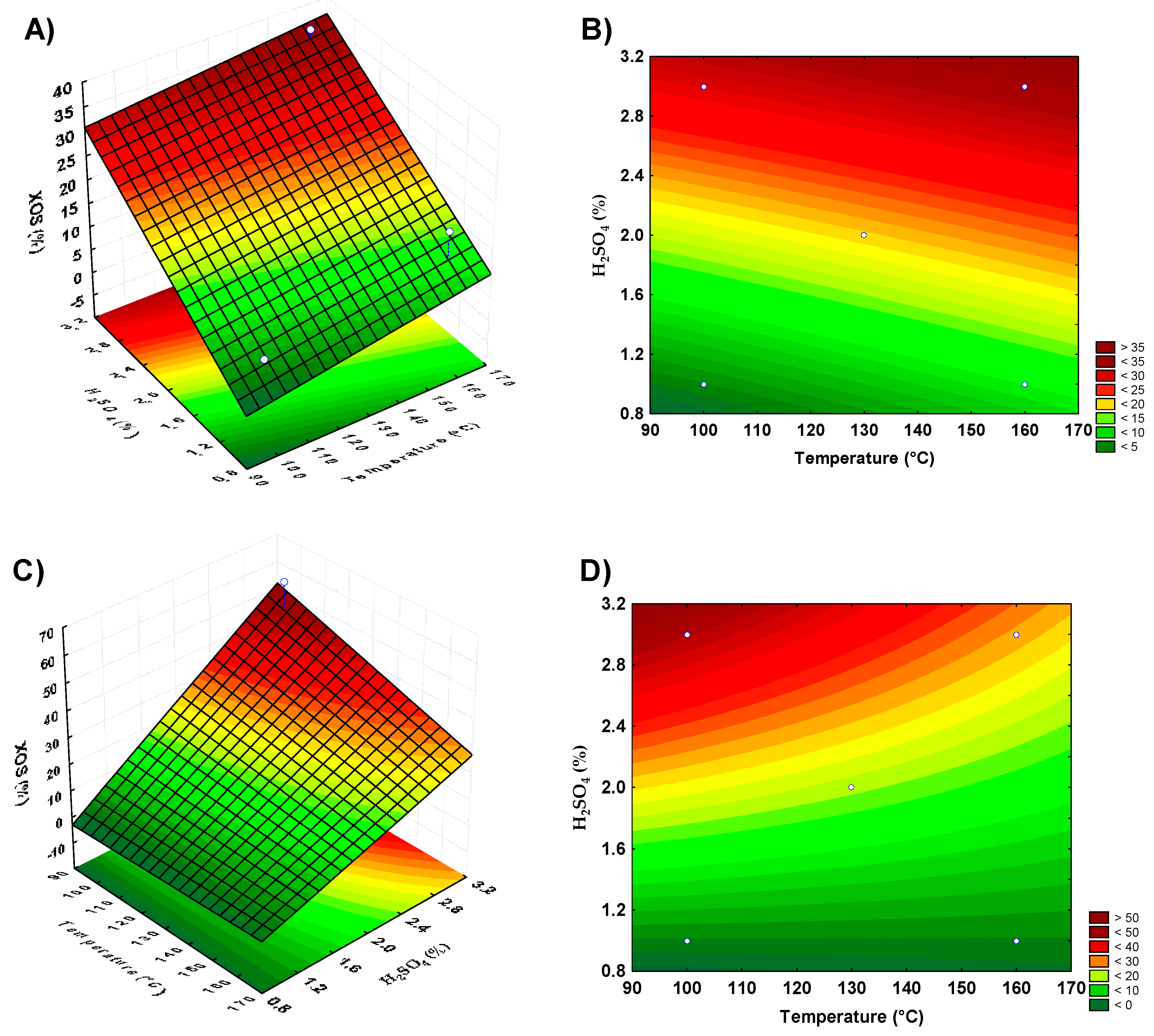
Figure 1.
Response surface and contour plots showing xylooligosaccharides production by acid hydrolysis of banana peels and guava bagasse. (A,B) represent the banana peel residue, (C,D) the guava bagasse.
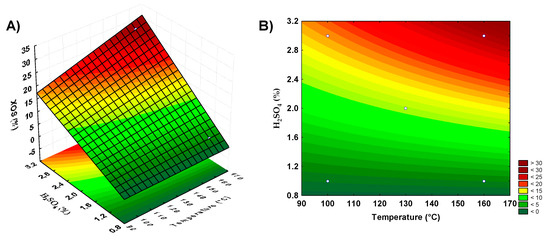
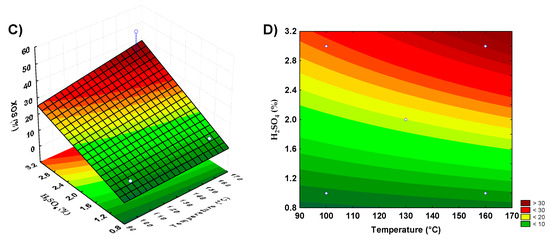
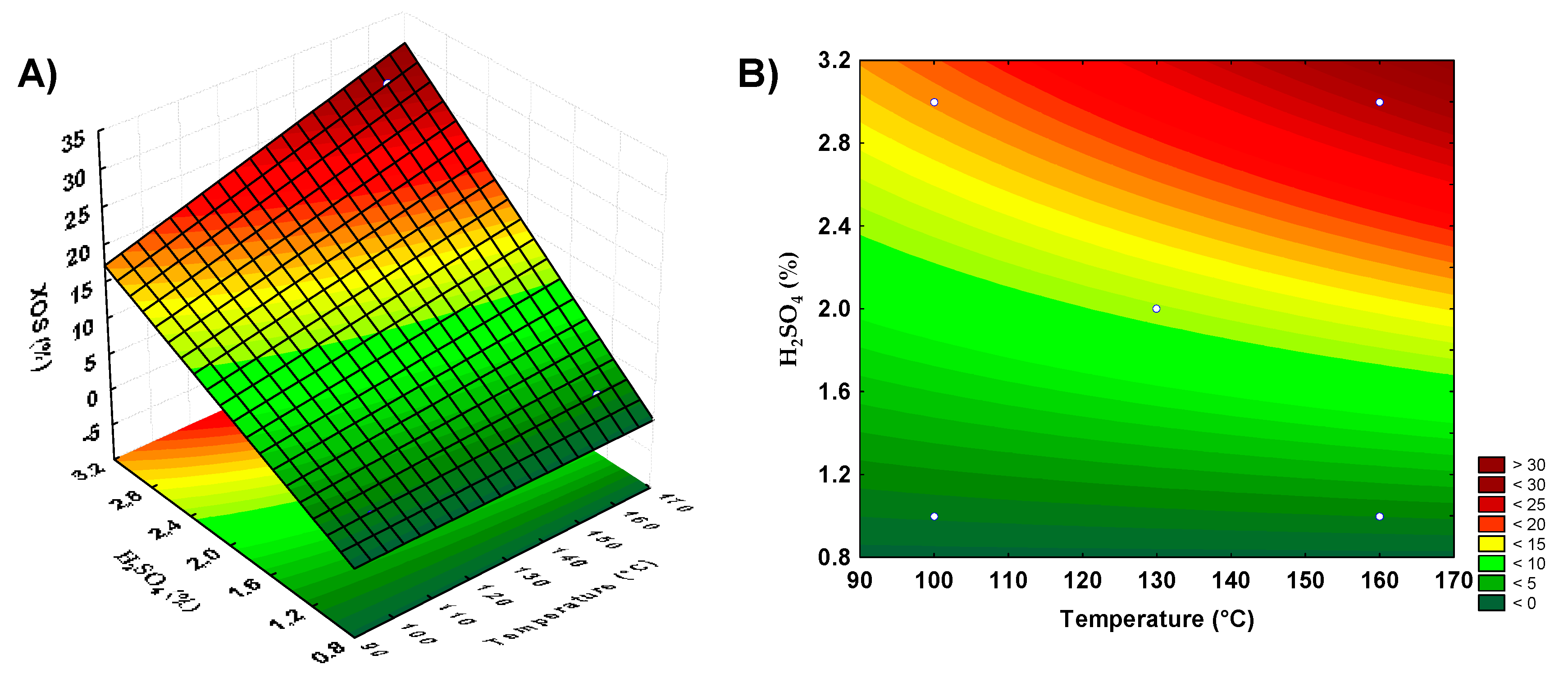
Figure 2.
Response surface and contour plots showing xylooligosaccharides production by acid hydrolysis of orange bagasse and restaurant residue. (A,B) represent the orange bagasse, (C,D) the restaurant residue.
For restaurant waste, the model using the axial points resulted in the best yield, as the second-order model was more appropriate for the analysis of this biomass acid hydrolysis for XOS production. The parameters that showed significance for this biomass were the acid concentration and the interaction between temperature and reaction time. Surface graphs were prepared to analyze the XOS production according to the significant parameters. With banana peel residue, a higher yield was observed in the combined regions of the highest acid concentrations and medium to high temperatures (Figure 1A,B). For guava bagasse residue (Figure 1C,D), the best conditions of XOS production were under the conditions of the highest acid concentrations and lower to medium temperature. For orange bagasse residue (Figure 2A,B), the higher XOS production was at the highest concentrations of acid and temperature. For restaurant waste (Figure 2C,D), the higher XOS production was under the conditions of higher temperature and acid concentration.
The combination of acid concentration and temperature, considering increasing the level of the acid in the pretreatment, could contribute to the banana peels, orange bagasse, and restaurant residue hydrolysis increasing the release of XOS. For guava bagasse, the increase in acid concentration and decrease in temperature could result in a better XOS yield by dilute acid pretreatment. However, these conditions based on acid concentration increase certainly result in higher xylose release, which is not desirable.
3. Material and Methods
3.1. Agro-Industrial and Food Waste
The materials used were fruit and restaurant waste: banana peel, guava bagasse, orange pomace, and restaurant residue consisting of leaves and vegetable peel/leaf. The residues of guava and orange were donated by the companies Predilecta and Selial, respectively. The banana peel was recovered in the local market. The residues were oven-dried at 60 °C, ground with a knife mill, and selected with a 20-mesh sieve [29,30].
The biomasses were chemically characterized to determine cellulose/glucan, lignin, and hemicellulose content as reported elsewhere [31]. The chemical characterization was performed with 300 mg of extractive-free biomass, hydrolyzed by 3 mL of 72% (m/m) sulfuric acid at 30 °C for 1 h. The reaction was stopped by the addition of 82 mL of distilled water and then autoclaved at 121 °C for 1 h. After cooling the hydrolyzed solution was filtered through a previously tared crucible porous plate. The solid residue was washed with distilled water, and oven-dried at 105 °C to determine insoluble lignin. The liquid fraction obtained was quantified by high-performance liquid chromatography (HPLC) to determine glucose, xylose, arabinose, and acetic acid.
The amount of total hemicellulose in the biomass was calculated with the sum of xylan contents, arabinan, and acetyl groups. The sum of the components resulted in the average of hemicellulose, which was 38.42, 28.33, 31.04 and 20.58% for banana peel, guava bagasse, orange bagasse, and restaurant waste, respectively [23].
3.2. Acid Hydrolysis
The acid hydrolysis of the biomass was performed by applying a 2³ experimental design with star rotational points (Table 4). The acid hydrolysis independent variables studied were temperature (°C), reaction time (min), and acid concentration (H2SO4 %, m/v—acid with purity > 95%) [15,32]. The response-dependent variables studied were xylooligosaccharides and xylose.

Table 4.
Experimental design 23 with rotational star points, central and coded, and real levels of the independent variables for biomass acid hydrolysis.
Around 5 g of each biomass was placed in 50 mL stainless steel reactors and heated in a thermostated bath, according to each condition of the experimental design (Table 4). Acid hydrolysis was performed with 15 different conditions with triplicates under central point conditions. After the reaction time, the reactor was cooled in an ice bath and the hydrolysate was filtered with filter paper, separating the solid and liquid fractions, which was prepared for HPLC analysis [30].
The yield in XOS was calculated by the relation between the mass of generated oligomers and the mass of hemicellulose present in the biomass. The yield of the xylose produced from the hemicellulose was calculated by the relationship between the generated xylose mass and the initial hemicellulose mass. The concentration of XOS (mg/mL) was obtained by summing the concentrations of X2 + X3 + X4 + X5 +X6 present in the hydrolysate, determined by liquid chromatography and calculated the yield in percentage, with X2 as the nomenclature for xylobiose (X2), xylotriose (X3), xylotetrose (X4), xylopentose (X5) and xylohexose (X6).
3.3. Determination of XOS
After the acid hydrolysis, the hydrolysate and the solid residue were vacuum-filtered using filter paper. The pH of the samples was adjusted between 5 and 9 with 1 mol/L sodium hydroxide (NaOH). The hydrolysate was filtered using a syringe filter with 0.22 μm pore size and xylose and XOS quantified by HPLC. High-performance liquid chromatography (HPLC—Shimadzu, model NEXERA XR) under the following conditions: BIO-RAD Aminex HPX-87C (300 × 7.8 mm) column; temperature: 80 °C; eluent: ultrapure water with 0.6 mL/min flow; sample volume: 20 μL; detector: refractive index at 80 °C (Shimadzu, RID model) with an analysis time of 15 min. Xylose (X1) (Sigma), xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6) (Megazyme-Ireland) solutions were used as standards.
3.4. Design Experiment for Biomass Pretreatment
The results were analyzed using STATISTIC 7 software, generating ANOVA, surface graphs, and data for the evaluation of the dependent and independent variables in the production of xylose and xylooligosaccharides.
4. Conclusions
The acid hydrolysis resulted in high production of XOS with low xylose yield using fruit and restaurant pre-prepared waste. The acid concentration was the most significant variable since the highest XOS percentages occurred under the conditions of higher acid concentrations. XOS maximization in relation to xylose production is a fundamental point to use acid hydrolysis. The production of XOS was about 25 times higher than the production of xylose for banana peel residue, 147 times higher for guava bagasse, 94 higher for orange bagasse, and 38 times higher for restaurant waste. All the biomass waste presented high XOS yields by acid treatment, with emphasis on guava bagasse, which presented higher XOS production, with low xylose production. In terms of a future study on the XOS production from industrial fruit waste, understanding the prebiotic effect is of great interest for application.
Author Contributions
Conceptualization, B.S.P. and M.B.; methodology, B.S.P., F.M, and M.B.; software, B.S.P. and C.d.F.; validation, B.S.P., C.d.F. and A.A.S.; formal analysis, B.S.P. and A.A.S.; investigation, B.S.P., C.d.F. and A.A.S.; resources, F.M. and M.B.; data curation, B.S.P., C.d.F. and A.A.S.; writing—original draft preparation, B.S.P.; writing—review and editing, B.S.P., C.d.F., A.A.S., F.M and M.B.; visualization, B.S.P., C.d.F., A.A.S., F.M and M.B.; supervision, M.B.; project administration, M.B.; funding acquisition, M.B., and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the São Paulo Research Foundation (FAPESP, process 2017/22401-8).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors state that there is no conflict of interest.
References
- Pereira, B.S.; Castrisana, R.N.; de Freitas, C.; Contiero, J.; Brienzo, M. Chemical composition determines the bioenergy potential of food waste from pre- and post-production. J. Mater. Cycles Waste Manag. 2021, 23, 1365–1373. [Google Scholar] [CrossRef]
- Salimi, E.; Taheri, M.E.; Passadis, K. Valorisation of restaurant food waste under the concept of a biorefinery. Biomass Conv. Bioref. 2021, 11, 661–671. [Google Scholar] [CrossRef]
- Kwan, T.H.; Ong, K.L.; Haque, M.A.; Kwan, W.H.; Kulkarni, S.; Lin, C.S.K. Valorisation of food and beverage waste via saccharification for sugars recovery. Bioresour. Technol. 2018, 255, 67–75. [Google Scholar] [CrossRef]
- Abe, M.; Branciforti, M.; Brienzo, M. Biodegradation of hemicellulose-cellulose-starch based bioplastics and microbial polyesters. Recycling 2021, 6, 22. [Google Scholar] [CrossRef]
- Nardella, S.; Conte, A.; Del Nobile, A.M. State-of-Art on the recycling of by-products from fruits and vegetables of Mediterranean countries to prolong food shelf life. Foods 2022, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- SEAB. Secretaria da Agricultura e do Abastecimento do Paraná. 2017. Available online: http://www.agricultura.pr.gov.br/arquivos/File/deral/Prognosticos/2017/Fruticultura_2016_17.pdf (accessed on 15 December 2020).
- Kist, B.B. Brazilian Fruit Yearbook, 2018. Santa Cruz do Sul: Editora Gazeta Santa Cruz. 2018. Available online: http://www.editoragazeta.com.br/sitewp/wp-content/uploads/2018/04/FRUTICULTURA_2018_dupla.pdf. (accessed on 10 December 2020).
- Globo. Guava Crop Should Increase by 9.26% and Matão Producers Invest in Cultivation. 2018. Available online: https://g1.globo.com/sp/sao-carlos-regiao/noticia/safra-de-goiaba-deve-aumentar-926-e-produtores-de-matao-investem-no-cultivo.ghtml (accessed on 9 June 2021).
- Quintal, S.S.R.; Viana, A.P.; Campos, B.M.; Vivas, M.; Amaral Junior, A.T. Selection via mixed models in segregating guava families based on yield and quality traits. Rev. Bras. Frutic. Jaboticabal 2017, 39, e866. [Google Scholar] [CrossRef]
- Vitti, K.A.; Lima, L.M.; Filho, J.G.M. Agricultural and economic characterization of guava production in Brazil. Rev. Bras. De Frutic. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Pommer, C.V.; Murakami, K.R.N.; Watlington, F. The Agronomic—Guava in the World, Campinas. 2006. Available online: http://www.iac.sp.gov.br/publicacoes/agronomico/pdf/v58_Goiaba_no_mundo.pdf (accessed on 9 June 2021).
- Melati, R.B.; Shimizu, F.L.; Oliveira, G.; Pagnocca, F.C.; Souza, W.; Sant’Anna, C.; Brienzo, M. Key factors affecting the recalcitrance and conversion process of biomass. BioEnergy Res. 2019, 12, 1–20. [Google Scholar] [CrossRef]
- Zamora, H.D.Z.; Freitas, C.; Bueno, D.; Shimizu, F.L.; Contiero, J.; Brienzo, M. Biomass fractionation based on enzymatic hydrolysis for biorefinery systems. In Biorefineries: A Step Towards Renewable and Clean Energy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 217–254. [Google Scholar] [CrossRef]
- Shimizu, F.L.; Zamora, H.D.Z.; Schmatz, A.A.; Melati, R.B.; Bueno, D.; Brienzo, M. Biofuels generation based on technical process and biomass quality. In Biofuel Production Technologies: Critical Analysis for Sustainability; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–64. [Google Scholar] [CrossRef]
- Shimizu, F.L.; Monteiro, P.Q.; Ghiraldi, P.H.C.; Melati, R.B.; Pagnocca, F.C.; Brienzo, M. Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind. Crops Prod. 2018, 115, 62–68. [Google Scholar] [CrossRef]
- Santos, C.; Bueno, D.; Sant’Anna, C.; Brienzo, M. High xylose yield from stem and external fraction of sugarcane biomass by diluted acid pretreatment. Biomass Convers. Biorefinery 2020, 1–9. [Google Scholar] [CrossRef]
- Brienzo, M.; Carvalho, A.F.A.C.; Figueiredo, F.C.; Neto, P.O. Sugarcane bagasse hemicellulose properties, extraction technologies and xylooligosaccharides production. In Food Waste: Practices, Management and Challenges; Riley, G.L., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 155–188. [Google Scholar]
- Forsan, C.F.; Freitas, C.; Masarin, F.; Brienzo, M. Xylooligosaccharide production from sugarcane bagasse and leaf using Aspergillus versicolor endoxylanase and diluted acid. Biomass Convers. Biorefinery 2021, 1–16. [Google Scholar] [CrossRef]
- Freitas, C.; Carmona, E.C.; Brienzo, M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre 2019, 18, e100184. [Google Scholar] [CrossRef]
- Brienzo, M.; Carvalho, C.; Milagres, A.M.F. Xylooligosaccharides production from alkali-pretreated sugarcane bagasse using xylanases from Thermoascus aurantiacus. Appl. Biochem. Biotechnol. 2010, 162, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Terrone, C.C.; Masarin, F.; Carmona, E.C.; Brienzo, M. In vitro study of the effect of xylooligosaccharides obtained from banana pseudostem xylan by enzymatic hydrolysis on probiotic bacteria. Biocatal. Agric. Biotechnol. 2021, 33, 101973. [Google Scholar] [CrossRef]
- Qing, Q.; Li, H.; Kumar, R.; Wyman, C.E. Xylooligosaccharides production, quantification, and characterization in context of lignocellulosic biomass pretreatment. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 391–415. [Google Scholar] [CrossRef]
- Pereira, B.S.; de Freitas, C.; Masarin Brienzo, M. Xylooligosaccharides from Industrial Fruit and Restaurant Waste Produced by Liquid Hot Water Treatment. Bioenerg. Res. 2022. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhang, X.; Xu, Y. An eco-friendly biorefinery strategy for xylooligosaccharides production from sugarcane bagasse using cellulosic derived gluconic acid as efficient catalyst. Bioresour. Technol. 2019, 289, 21755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xuefei, C.; Ruochen, Z.; Lin, X.; Tongqi, Y.; Quentin, S.; Runcang, S. Evaluation of xylooligosaccharide production from residual hemicelluloses of dissolving pulp by acid and enzymatic hydrolysis. RSC Adv. 2018, 8, 35211–35217. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, O.; Erdoğan-Tokatlı, K.; Bakir, U.; Yilmaz, L. Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. Food Sci. Technol. 2010, 43, 119–125. [Google Scholar] [CrossRef]
- Jacobsen, E.; Wyman, C.E. Xylose monomer and oligomer yields for uncatalyzed hydrolysis of sugarcane bagasse hemicellulose at varying solids concentration. Ind. Eng. Chem. Res. 2002, 41, 1454–1461. [Google Scholar] [CrossRef]
- Wen, P.; Zhang, T.; Wang, J.; Lian, Z.; Zhang, J. Production of xylooligosaccharides and monosaccharides from poplar by a two-step acetic acid and peroxide/acetic acid pretreatment. Biotechnol Biofuels 2019, 12, 87. [Google Scholar] [CrossRef]
- Alves, R.C.; Melati, R.B.; Casagrande, G.M.S.; Contiero, J.; Pagnocca, F.C.; Brienzo, M. Sieving process selects sugarcane bagasse with lower recalcitrance to xylan solubilization. J. Chem. Technol. Biotechnol. 2020, 96, 327–334. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Bueno, D.; Pagnocca, F.C.; Brienzo, M. Minor biomass particle size for an efficient cellulose accessibility and enzymatic hydrolysis. ChemistrySelect 2020, 5, 7627–7631. [Google Scholar] [CrossRef]
- Brazilian standard method for sugarcane bagasse chemical characterization. NBR 16550-2016; Brazilian Association of Technical Standards: São Paulo, Brazil, 2016.
- Neto, F.S.P.P.; Roldán, I.U.M.; Galán, J.P.M.; Monti, R.; Oliveira, S.C.; Masarin, F. Model-based optimization of xylooligosaccharides production by hydrothermal pretreatment of Eucalyptus by-product. Ind. Crops Prod. 2020, 154, 112707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).