Abstract
During the last half-century, the CO2 concentration in the world’s atmosphere has increased from 310 p.p.m. to over 380 p.p.m. This is due to the widespread usage of fossil fuels as a main source of energy. Modeling forecasts have shown that this trend will continue to rise and reducing CO2 emissions is a challenging task for multi-stakeholders, including research institutions. The UN Climate Change Conference in Glasgow (COP26) has stressed that stakeholders need to work together to achieve a NetZero target. Technologies involving absorbents for the capture of CO2 from a gas mixture are energy-intensive. Carbon adsorption and conversion (CAC) approaches have been gaining attention recently since these technologies can mitigate CO2 emissions. In this review, materials ranging from advanced carbon-based materials to natural resources-based materials will be reviewed. Adsorption and conversion capacities as well as the scalability possibility of these technologies for solving the CO2 emission problem will be investigated. The review, therefore, is timely and meaningful concerning the net zero emission targets set by countries and developmental organizations worldwide.
1. Introduction
Recently, anthropogenic global warming and climate change have become global issues. Carbon dioxide (CO2) is a greenhouse element, and evidence indicates that human-caused CO2 emissions are a major contributor to global warming [1]. It is forecasted that fuel-based power plants alone will continue to increase in number. Drastic changes in the global climate and a significant increase in global temperature have been reported. There were 315 natural catastrophes in 2018 worldwide, most of which were weather-related. About 68.5 million people were impacted by natural catastrophes such as cyclones, tornadoes, forest fires, and droughts, resulting in a total estimated cost of USD 131.7 billion in damages [2]. It is extremely worrisome that 2018’s economic losses from fires are nearly equal to those suffered due to wildfires over the entire previous decade. Food, water, health, the environment, and infrastructure are some sectors most affected by climate change [2].
Fossil fuels are currently some main sources of artificial CO2 emissions [3], and capturing CO2 is becoming very important globally. In 1977, it was proposed that CO2 could be removed from the exhaust of a coal power station and stored in a rock formation. This idea gave rise to the field of carbon capture and storage. According to the International Energy Agency, this method can cut emissions of CO2 by 17 percent by the year 2050, so it should be incorporated into the policies of every country to lessen the impact of global warming. Adsorption, membrane separation technology, absorption, and cryogenic processes are common methods that can be used to store and treat CO2 [4]. Studies on CO2 adsorption and conversion have been completed and reported in the literature (details provided in Table 1 below). In addition, activated carbon, porous silica [5], zeolite [6], sepiolite [7], metal–organic frameworks (MOFs) [8], boron nitride (BN), Mxenes [9], ionic liquids, and porous adsorbents are some of the known materials for CO2 adsorption [10]. However, a thorough review of carbon-based materials synthesized from bio-sources, their adsorption mechanism, preparation methods, their characteristics, and some industrial scale-up of these technologies are still lacking and require further investigation. This review aims to shed light on these points as well as to discuss state--of-the-art technologies in CO2 capture and conversion fields.

Table 1.
Reviews of bio-based synthesized materials.
2. CO2 Adsorption Mechanism
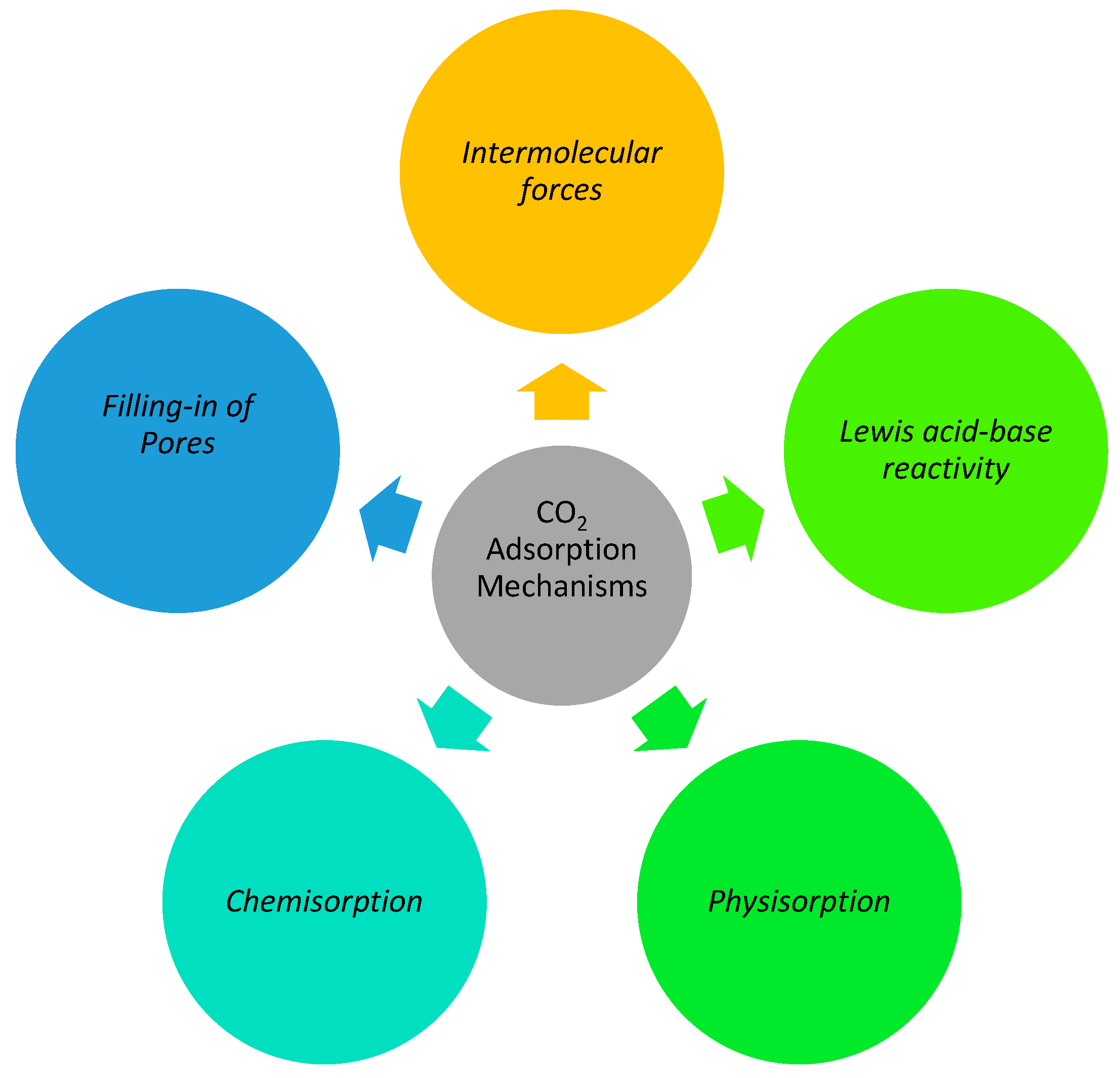
To investigate adsorption processes, it is important to learn about the adsorption mechanism of different materials. The quantum sieve impact, kinetic impact, dynamic impact, and molecular sieve impact are some of the adsorption techniques suggested [16]. When separating H2 and D2 using a quantum sieve, for example, the effect is strongly related to the de Broglie wavelength and the diffusion velocities of the gas molecules [17,18]. When all of the molecules in the gas can travel through the adsorbent pores, the polarizability, dipole moment, and magnetic sensitivity of the adsorbate and adsorbent surfaces play a much larger role in the capture of specific species [19,20]. This is because the dynamic effect dominates the adsorption behavior under these circumstances. The kinetic diameter (i.e., collision distance) is the minimum distance between the adsorbate and adsorbent molecules approaching each other with zero kinetic energy [21,22]. This distance determines the efficiency of CO2 capture under the carefully regulated pore size of adsorbents (such as zeolites and carbons). Because of its molecular filter mechanism, this process is extremely temperature dependent. In air separation and CO2 capture, separating CO2 from CH4 requires fine-tuning the adsorbents’ particle size [23,24]. There are two main categories of contact between carbonaceous adsorbents and CO2 molecules: physical and molecular. Typically, carbons physically adsorb CO2 via the van der Waals force, which is closely linked with temperature and pressure [25] (i.e., the process by which pores are filled). High pressure and low temperature significantly improve the adsorption ability [26,27]. The CO2 molecules are chemically bound to the adsorbent surface, making the connection greater than that of physical capture. For chemical adsorption, the formation of hydrogen bonds and the interaction between acids and bases are two important processes. The graphical mechanism of CO2 adsorption is illustrated in Figure 1.
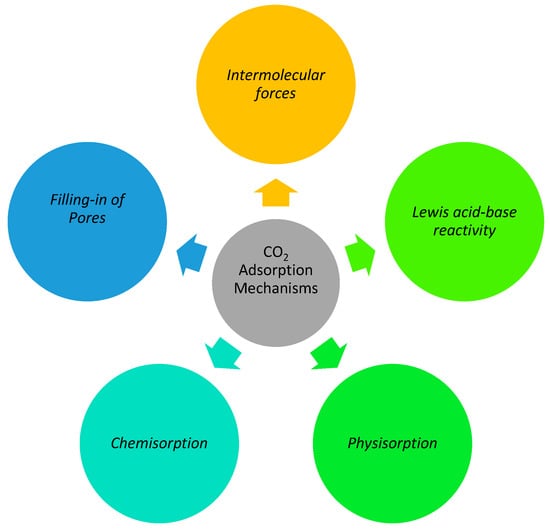
Figure 1.
Mechanisms of CO2 Adsorption.
2.1. Filling-In of Pores Mechanism
When the pore size of the adsorbent is about the same as the CO2 molecule, more CO2 will be absorbed [28] The kinetic diameter of CO2 is predicted to be 0.33 nm, making it significantly lower than that of CH4 (0.38 nm) and N2 (0.5 nm) (0.364 nm). For optimal CO2 selectivity, the adsorbent’s pore size should be controlled to be between 0.33 and 0.36 nm. On the other hand, it was discovered that CO2 adsorption increases in proportion to total micropore volume [29]. An increase in CO2 adsorption was seen at a decreased pore volume, indicating that pore filling regulates CO2 adsorption by ultra micropores. In the experiment by Parshetti et al. [25], the adsorption capacity for CO2 increased with both the surface area and the micropore volume. This suggests a unique pore-filling process and physical adsorption behavior.
2.2. Intermolecular Forces
Hydrogen atoms form covalent links known as hydrogen bonds when they join with other elements that can accept hydrogen. The oxygen atoms in CO2 are negatively charged because of their large electronegativity gap with the carbon atom, enabling them to make weak hydrogen bonds with CH on the carbon’s surface [26]. The behavior of CO2 adsorption on N-doped carbon surfaces was analyzed using Niwa’s model [30]. The high electronegativity of oxygen in CO2 makes the CHO hydrogen bond weaker than the OHO and NHO bonds. The redshift of the expanded CH anti-symmetric vibration point in the FT-IR spectra provided further evidence of hydrogen bonding. The surface hydroxyl group on the polybenzoxazine-based porous carbon used in another study was found to be responsible for the enhanced CO2 adsorption capacity at reduced binder energy due to the presence of hydrogen bonding between the OH and the O atoms of CO2 [31].
2.3. Lewis Acid-Base Reactivity
When forming a covalent coordinate connection, Lewis acids and bases interact with one another through the transfer of lone electron pairs from the acid to the base. For instance, carbon dioxide (CO2) binds strongly to a nitrogen-rich carbon surface created by the alkaline activation of an N-containing polymer. This is because the nitrogen-rich carbon surface’s enhanced basicity and higher electron density bind the electron-deficient CO2 molecules via a Lewis acid-base interaction [32]. Similar to N-doped carbon, an acid-pretreated biomass carbon adsorbent has several oxygenated groups on its surface. The adsorbent was able to bind additional CO2 molecules because of the Lewis acid-base interaction since these groups took electrons from their neighbors to create a donor. The surface decorating of electronegative atoms created by in situ breakdown or pretreatment, as well as the addition of metal oxides, allows for the modification of the basicity and electron density of carbon [33]. Interactions between CO2 and the low coordination number of O2 ions found in metal oxides resulted in the formation of surface carbonates [34,35].
2.4. Physisorption
Physisorption, also known as physical adsorption, is the primary process accountable for CO2 collection on activated carbon and char surfaces. Intermolecular contacts, such as the weak van der Waals force, are responsible for the adsorption of CO2. These results are bolstered by the reality that adsorption can be undone. No initial energy is needed for the adsorption of CO2 onto a solid char surface, and the physisorption of CO2 molecules on the solid char surface is temperature dependent. At a lower temperature, more CO2 is adsorbed at equilibrium). CO2 has a significant quadrupole moment but no dipole. Since CO2 has a straight structure with polar bonds at both ends, it can engage with active sites on the char surface. CO2 adsorption via the physisorption adsorption mechanism is highly surface area dependent. It is important to note that the adsorption process also involves nitrogenous compounds such as amines, amides, and nitriles. Because of the powerful interactions between the acidic CO2 and the basic nitrogenous surface functional groups, the presence of nitrogenous functional groups on char surfaces can improve the physisorption of CO2 [36].
2.5. Chemisorption
An acidic CO2 molecule forms a covalent connection with the active site of the adsorbent during chemisorption. Many different chemisorption mechanisms are seen in adsorbents. CO2 adsorption, for example, is mediated by metal sites and surface functional groups in metal-organic frameworks (MOFs). The incorporation of nitrogen-containing functional groups into the char structure by the addition of amine-containing molecules can also lead to chemisorption [37]. This happens when water is added to CO2, turning it into bicarbonate and producing a zwitterion (either carbamate or ammonium zwitterion). Biochar and hydrochar both have functional groups that may chemisorb CO2, and these groups can be present in the biomass naturally or added by chemical modification, such as the addition of metal oxides [33,38].
3. Biomass-Derived Carbonaceous Materials
3.1. Various Bio-Based Synthesized Materials Have Been Developed
Carbon materials derived from biomass, agricultural by-products, and organic sources can selectively and efficiently capture CO2, and the possibility that nitrogen is a major factor hindering selectivity is very interesting and meaningful to study. Chen Jin [39] studied the adsorption of CO2 from sawdust waste at ambient conditions and increased the adsorption capacity by increasing N and microalgae (at a rate of microalgae per poplar sawdust = 0.5:1), and noted an adsorption result of 4.14 mmol g−1 for CO2 uptake, which is almost 1.7 times higher than non-N-enhanced adsorption (N-doping). Karolina Kiełbasaa et al. [40] studied the conversion of olive pulp into activated biocarbon for CO2 adsorption by thermochemical methods, in which the author developed three types of porous activated bio carbons prepared for CO adsorption. Xuping Yang [41] studied the adsorption properties of biochar synthesized from seaweed-based biochar for CO2 gas. Various carbon feedstock, pre-treatment methods, and CO2 uptake are described in Table 2.

Table 2.
Carbonaceous materials synthesized from biomass, organic sources, and adsorption capacity.
3.2. Synthesized Carbonaceous Materials for CO2 Adsorption
CO2 levels have risen dramatically as a result of industrialization and economic development, contributing significantly to climate change and global warming. To counteract this, scientists have developed and begun using carbon-based adsorbents that are both effective and long-lasting in their ability to reduce CO2 levels [45]. High adsorption capacity, long-term stability, high selectivity [45,46], high porous volume, and recycling ability at low chemical and energy usage costs are all desirable qualities in a CO2 adsorbent substance. Carbonaceous materials are promising for carbon dioxide (CO2) capture and storage due to their exceptional potential as adsorbents for CO2 due to their morphological tunability, high specific surface area, functional groups, ample porous structure, exceptional gas storage capacity, reproducibility, and electronic properties [47,48].
To increase CO2 capture efficiency, carbon-based materials can be modified in several ways, such as through material activation or N-doping. Activated carbon, carbon nanotubes, carbons derived from coal (e.g., bio-chars), carbons derived from metal-organic frameworks, carbon aerogels, carbons derived from polymers, and graphene oxide are just some of the carbonaceous materials that have been fabricated and used as CO2 adsorbents [49].
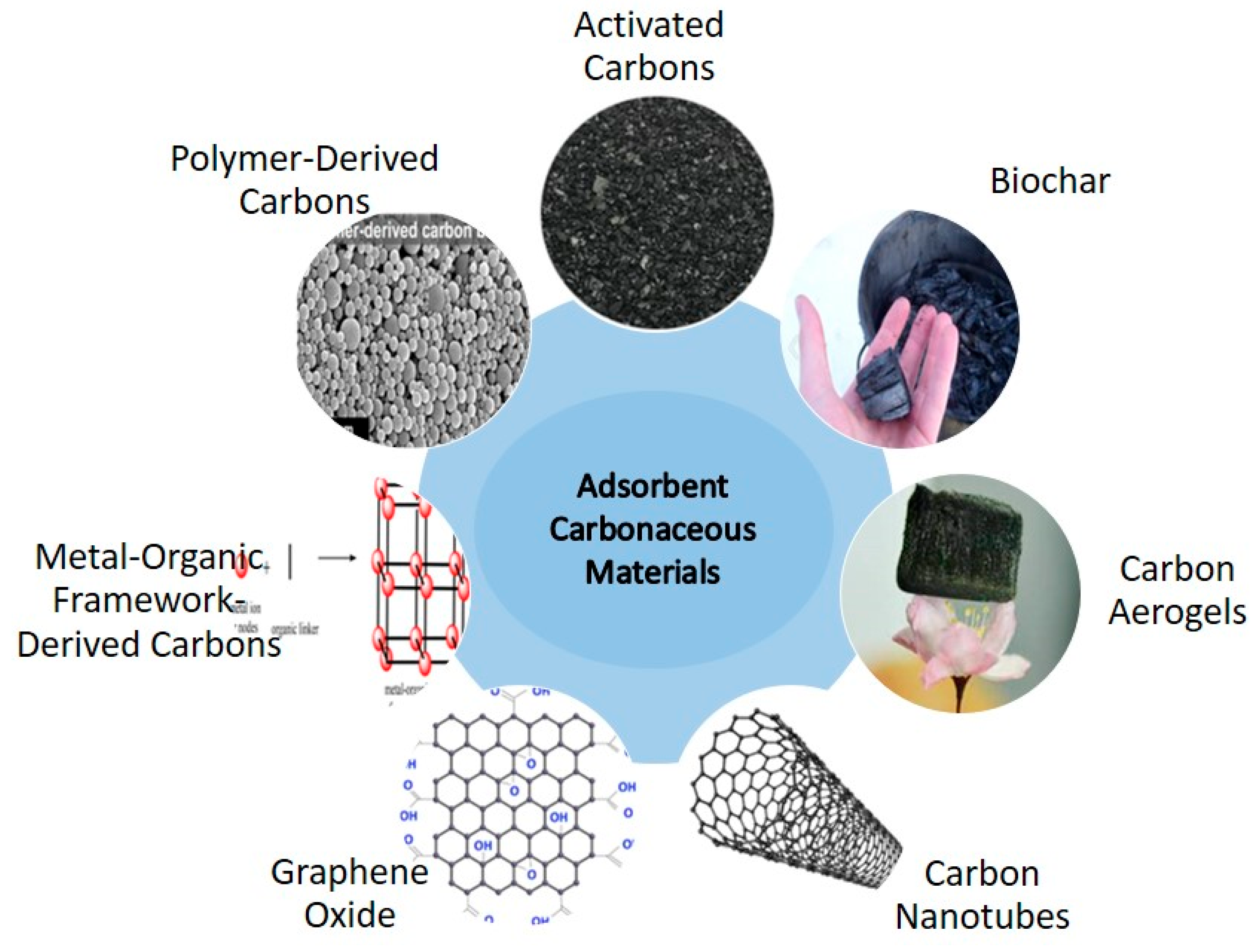
Activated carbon (AC) is a porous substance that is widely used, and it can be produced from bio-waste. AC is a very potential for CO2 capture, especially in humid environment due to its synthesis technique, low cost, high surface area, and hydrophobic characteristics [49,50]. To improve the structural properties of carbon materials and thus their CO2 uptake performance, two steps are required in its production processes: (i) the carbonization of the precursor materials, which can be accomplished through the pyrolysis of biomass or hydrothermal treatment, and (ii) activation, which can be accomplished through physical or chemical methods [51]. Precursors such as pine nut shells [52], pine sawdust [43], water chestnut [53], the stone of the yellow mombin fruit, the stone of the acai fruit [51,53], Amazonian nutshells [43], and cotton stem agricultural residue [44] have been researched. Serafin et al. [54] used a one-step carbonization method supplemented with a chemical activation process using potassium hydroxide (KOH) to extract inexpensive carbons from walnut shells. The specific surface area increased up to 1868 cm2/g, the micropore concentration increased to 0.94 cm3/g, and CO2 uptake capacity increased to 9.54, 5.17, and 4.33 mmol/g at temps of 0, 25, and 40 °C, respectively, in the resulting activated carbon (AC-800). Synthesized carbonaceous materials used for CO2 adsorption are illustrated in Figure 2.
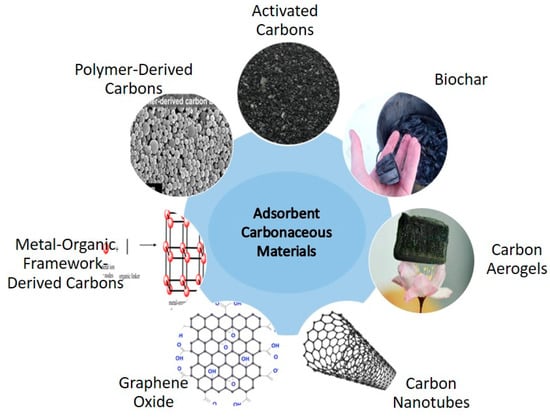
Figure 2.
Synthesized carbonaceous materials used for CO2 adsorption.
- (a)
- Carbon nanotubes (CNTs) are hollow cylinders that have attracted attention as potential CO2 adsorbents due to their exceptional water resistance, high mechanical strength, and efficacy in adsorbing CO2 under high-pressure circumstances [55]. However, under low pressure, their adsorption capacity drops. The CO2 adsorption efficacy of CNTs can be improved through chemical functionalization or composite adding. Including amine groups in CNTs resulted in more binding sites for CO2. In the same way, the CO2 adsorption capacity of CNTs can be improved by introducing other species, such as diamine or tetraethylenepentamine (TEPA) [55].
- (b)
- Carbons produced from coal such as coal, coal tar, and pitch materials are inexpensive, readily available, and have a high tensile strength. These features make them promising materials for producing activated carbon (AC), as they have extremely large pores [56]. In addition to the efficient conversion of industrial byproducts, petroleum wastes have also been successfully changed into ACs using well-defined activation conditions. These ACs’ hierarchical porous structures make them excellent CO2 adsorbents over a broad pressure range. When used in a low-pressure range (1–35 bar) and at 25 °C, the activated adsorbents produced from anthracite coal significantly increase the CO2 adsorption capacity [57]. The adsorption CO2 uptake capacities are up to 10.51 mmol/g and 27.58 mmol/g [57]. This shows that ACs made from anthracite coal can improve their CO2 adsorption capacity as a result of its high surface area.
- (c)
- Carbons produced from metal-organic frameworks (MOFs): The use of MOFs in gas capture has received considerable attention due to their surface chemistry and extraordinary porosity [58]. A high concentration of metal sites within a MOF’s framework significantly increases its binding capacity [59]. The existence of metal nodes and organic linking groups in a MOF allows the hole sizes and the 3D network structure to be tuned [60].
- (d)
- Carbon aerogels (CAs) are porous materials that are perfect for gas adsorption because of their uniform pore distribution and tiny microporous structures. A study [61] showed that CAs’ binding characteristics can be enhanced by the incorporation of heteroatoms and metals. For these reasons, CAs are a promising material for gas adsorption. The effectiveness of CAs depends on two factors: (i) the techniques used to prepare them, and (ii) the precursors chosen. By using inexpensive bio-resources, Geng et al. [62] were able to create monolithic biocarbon aerogels with a meshed asymmetrical, hierarchical porosity structure. The bio-based carbon adsorbent was shown to have an adsorption capacity of 4.49 mmol g−1 at 298 K and 100 kPa after the material’s processing conditions were optimized [63]. Big mesopores (24.7 nm) and the distributed N moieties [64] formed after the surface modification of CAs (with N functionalities) with tetraethylenepentamine (TEPA) were found to increase the CO2 adsorption by 4.1 mmol/g.
- (e)
- Carbons produced from polymers have attracted a lot of interest because of their malleability in terms of structure, permeability, and functionalization [65]. Utilizing proper carbonization and activation techniques, a wide range of porous and heteroatom-doped carbon adsorbents can be synthesized from polymers. Using polymeric precursors containing nitrogen-based functional groups, a series of nitrogen-doped carbonaceous materials were synthesized. Post-polymer alteration [66] and monomer selection for high N content can also increase the nitrogen content of the final carbons [67].
- (f)
- Graphene oxide (GO): GO has been proven to be a superior adsorbent. GO is created through an oxidation process, and it has a high porosity, a large surface area, and an excess of oxygen groups [68]. Basic groups introduced to the GO surface, on the other hand, can increase its CO2 adsorption capability [69]. Polymers, such as polyetherimide, can have their binding capability increased by incorporating them with amine groups. Because of the N-rich surface created when polyetherimide (PEI) was impregnated onto the GO, its CO2 adsorption capacity was enhanced via electron acceptor-donor interaction; this effect was further amplified when more PEI was added because more chemical linkages were created in the form of the carbamate complex [70]. Due to its powerful acid-base interaction, PEIGO was found to have a large uptake of CO2 (84 mg/g). CO2 adsorption enhancement through the creation of GO/metal heterostructures has been demonstrated [71]. Chen et al. [72] deposited Li and Al onto GO because of their lower toxicity, lower cost, and lower environmental impact compared to Ti. A higher CO2 binding energy was achieved by anchoring the metals to the GO surface with urethane and hydroxyl groups.
Though those materials are promising concerning CO2 adsorption in principle at research and lab scales, and some CO2 adsorption setups have been used at the industrial scale, the specific CO2 adsorption performance of those materials at industrial scales is t yet to be determined and needs to be tested and verified before being used on a larger scale.
4. Carbon-Based Materials for CO2 Conversion
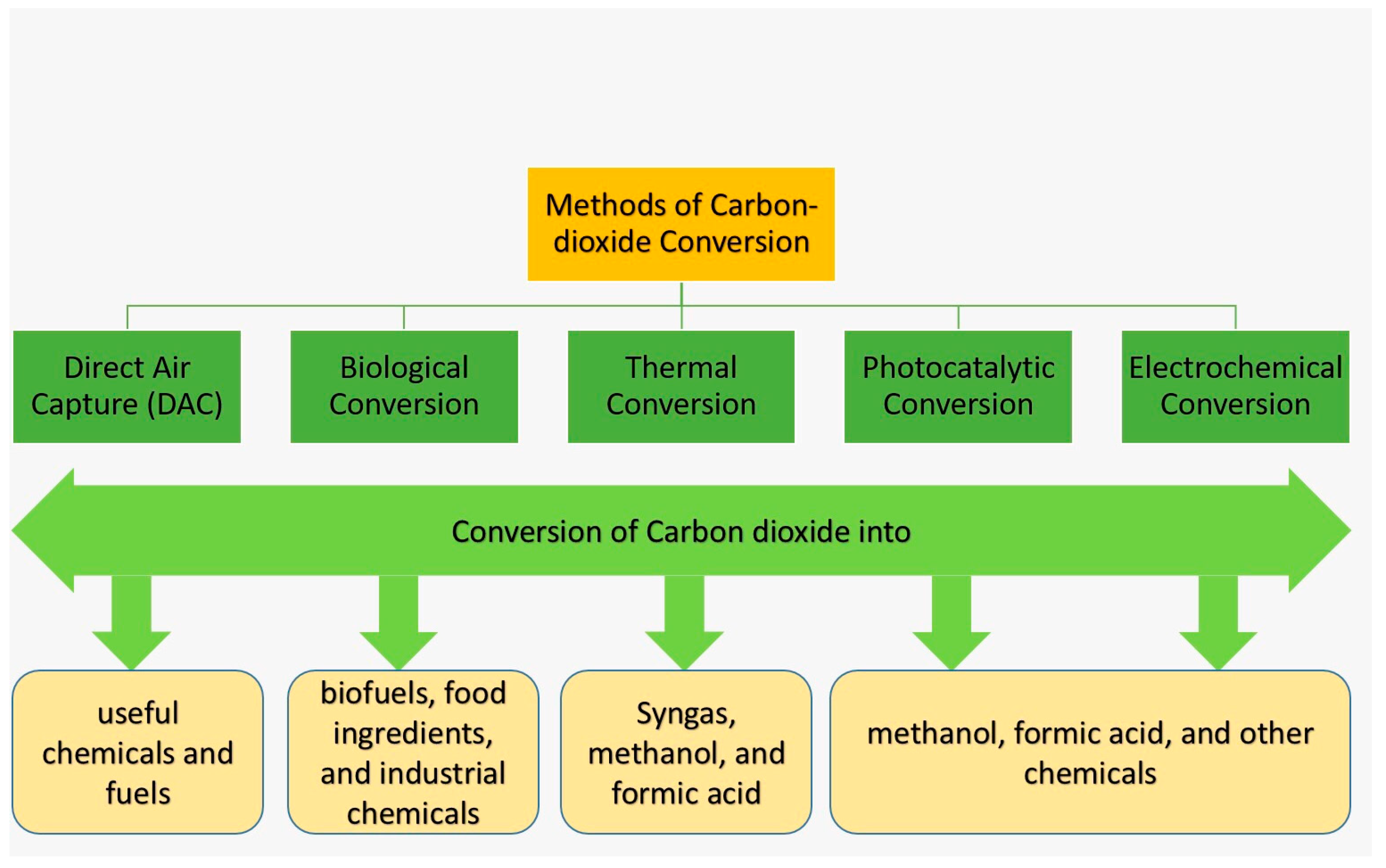
The global climate is changing unprecedentedly, and the primary reason behind this catastrophic change is the significant rise in global CO2 emissions. It is critically important to lower the CO2 concentration in the atmosphere, and this can be accomplished by CO2 conversion technology where carbon-based materials are used. CO2 conversion refers to the process of converting CO2 into useful chemicals and fuels, and some common conversion methods are illustrated in Figure 3:
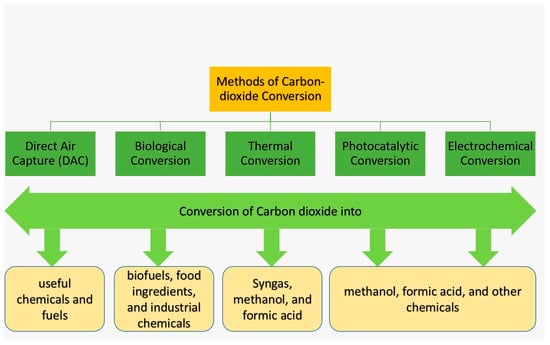
Figure 3.
CO2 conversion methods.
- Direct Air Capture (DAC): This is a potential technology that directly captures CO2 from the air, and then converts it into useful chemicals and fuels. Industrial applications and the details of this technology are discussed in Section 5 of this paper.
- Biological conversion: This method uses microorganisms or enzymes to convert CO2 into useful products (biofuels, food ingredients, and industrial chemicals) [73].
- Thermal conversion: This method uses heat to convert CO2 into syngas (mainly H2 and CO), methanol, and formic acid [74].
- Photocatalytic conversion: This method uses light energy and catalysts to convert CO2 into various chemicals such as methanol, formic acid, and others [75].
- Electrochemical conversion: This method uses electricity and catalysts to convert CO2 into various chemicals. This is the most widely studied and developed method that converts CO2 into value-added chemicals and fuels with the help of an electro-catalyst, often a metal or metal oxide [76].
Different types of materials are potentially used for CO2 conversions into useful chemicals and fuels, including carbon-based materials, namely activated carbon, graphene, and carbon nanotubes. These materials possess a high surface area and can act as catalysts, making them promising for use in CO2 reduction reactions. However, more research is needed to optimize their performance and make the process more efficient and cost-effective.
4.1. Graphene
Graphene has been studied for its potential in converting CO2 into useful chemicals and fuels. One way for CO2 conversion using graphene is through graphene-based catalyst usage, which can facilitate chemical reactions that convert CO2 into CH3OH or formic acid. Another application is membranes synthesized from graphene, which can selectively separate CO2 from other gasses. Graphene has several qualities that make it a promising material for CO2 conversion, and some of them are as follows [77]:
- High surface area: This characteristic makes graphene useful for catalytic reactions;
- High conductivity: Graphene is an excellent conductor of electricity and heat, which makes it useful in electrochemical reactions;
- Chemical stability: Graphene is chemically stable, and can be used in harsh environments and high-temperature reactions;
- Selectivity: Graphene membranes can be made to be highly selective, which makes graphene useful for CO2 separation;
- Low cost: Graphene is made of carbon, which is abundant and inexpensive;
- Durability: Graphene is a strong and durable materials that can be used in long-term applications. These qualities make graphene a highly promising material for CO2 conversion.
4.2. Carbon Aerogels (CAs)
These materials are highly porous, lightweight, have a high surface area, are usually made from carbon nanostructures, and have been shown to have the potential for CO2 conversion [49,77]. Their high surface areas allow these materials to adsorb large amounts of CO2, and they, therefore, can be used in a variety of chemical reactions to convert CO2 into other compounds [77].
One of the main ways ACs have been used for CO2 conversion is in the form of adsorbents. CO2 adsorption on carbon aerogels can be done through physisorption or chemisorption processes, which are then followed by the release of CO2 by heating or by changing the pressure.
High surface area: CAs possess a very high surface area, typically from 300 to 2000 square meters per gram. This property allows them to adsorb large amounts of gases, liquids, and other substances [78];
- Low density: CAs are extremely lightweight, with densities as low as 0.003 g per cubic centimeter;
- High thermal conductivity: CAs have high thermal conductivity, which makes them useful for thermal insulation and heat dissipation;
- Mechanical strength: CAs have low compressive strength, but their mechanical properties can be improved by adding a binder or by using a different manufacturing process [79];
- High electrical conductivity: CAs can be made to be highly conductive, which makes them useful in applications such as super-capacitors and batteries;
- Porous structure: CAs have a highly porous structure and large pore volumes, which allows for the easy diffusion of gases and liquids;
- Low cost: CAs can be made from inexpensive, abundant materials, and their production process is relatively simple, which makes them a cost-effective material. However, these properties can vary depending on the specific type of carbon aerogel and how it was manufactured;
- Chemical stability: CAs are chemically stable and can withstand high temperatures and harsh environments [80].
4.3. Activated Carbons (ACs)
Activated carbons, usually known as activated charcoal, are carbon-based materials with a highly porous surface area which makes them useful for CO2 conversion. ACs can also be used as a catalyst to convert CO2 into other chemical compounds. For example, they have been used to catalyze the conversion of CO2 into formic acid and methanol, which are useful chemicals for other industrial processes [81].
ACs can be used as a CO2 adsorbent, which involves both the physical and chemical adsorption processes. The CO2 can then be released by heating or by changing the pressure, making it possible to capture and store CO2 in this way. ACs are particularly effective at adsorbing CO2 at high pressures and temperatures, making them a potential material for capturing CO2 from industrial processes [82].
Though those materials are promising in CO2 conversion at research and lab scales, the CO2 conversion performance of those materials at industrial scales still need to be tested and verified.
Yellow tuff, a natural tuff, and low-cost adsorption material, has been reported to be conveniently employed in a vacuum swing for CO2 adsorption processes [83]. MOFs are proving to be effective adsorbents for CO2 capture due to their microporous structure and chemical and thermal stabilities [84]. MOFs are capable of providing both physical and chemical interactions with CO2 [83], while some other chemical adsorbents, such as amine-functionalized adsorbents, are capable of interacting strongly with the acidic CO2 molecules [83]. Zeolites have been shown to have significant potential for CO2 adsorption due to their high porosity, ultra-small pores, structural diversity, high stability, excellent recyclability, and chemical reactivity [35].
5. Carbon Capture Technologies for Climate Change Mitigation
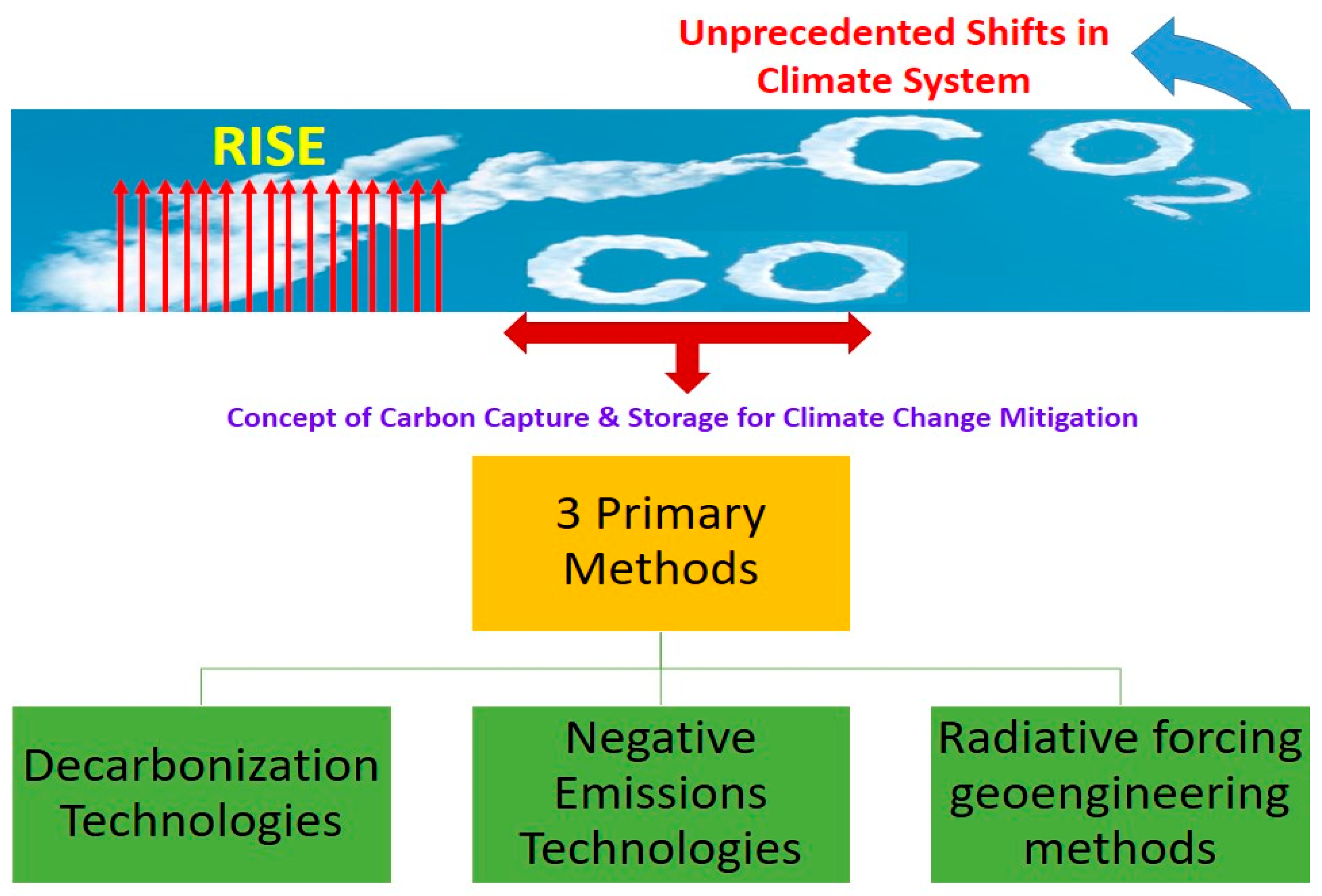
Three main strategies exist for mitigating the consequences of climate change (illustrated in Figure 4). Traditional mitigation efforts aim to reduce carbon dioxide emissions through the use of de-carbonization technologies and methods such as renewable energy, fuel switching [85], efficiency increases, nuclear power, and carbon capture, storage, and usage. The risks associated with these devices are generally low and have been around for some time [86,87,88]. The second possibility involves the use of novel tools and methods. It is possible to remove CO2 from the atmosphere and store it using negative emissions devices [86]. Numerous negative emissions techniques are already in use, including bioenergy carbon capture and storage, bio-char, enhanced weathering, direct air carbon capture and storage, ocean fertilization, ocean alkalinity enhancement, soil carbon sequestration, afforestation/reforestation, wetland construction/restoration, and mineral carbonation/biomass use in construction [89,90,91]. The third and final choice involves controlling the amount of radiation coming from the sun and the planet. The goal of using geoengineering techniques that rely on radiative forcing is to either keep our world’s average temperature stable or to make our planet slightly cooler. The main geoengineering techniques for radiative forcing are stratospheric aerosol injection, marine sky brightening, cirrus cloud thinning, space-based mirrors, surface-based brightness, and various radiation management approaches [92,93].
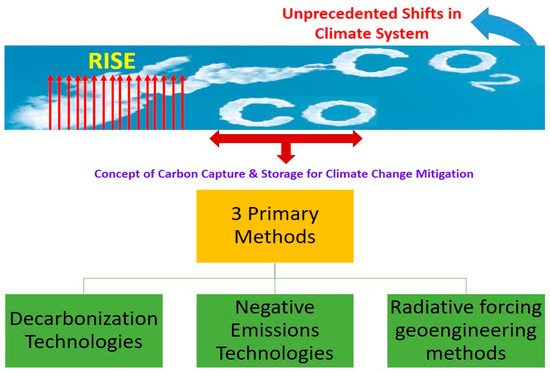
Figure 4.
CO2 conversion methods for climate change mitigation.
As a means of decarbonizing the energy and manufacturing industries, carbon capture and storage holds a great deal of potential. The burning of solid fuels such as coal, oil, or gas can produce carbon dioxide gas, which can now be collected and isolated using modern technology. The CO2 is then transported through pipes to subterranean storage facilities, where it will stay for thousands of years. Eliminating or at least greatly lowering the pollution from burning fossil fuels is priority number one. The three possible capturing times are before, after, and during oxyfuel combustion. When it comes to CO2 capture and storage, each method has its specific process. Post-combustion capture devices, on the other hand, are well-suited for retrofitted uses. CO2 is captured, liquefied, and then transported via ship or conduit to long-term holding sites. It has been found that depleted oil and gas areas, coal beds, and underground saline reservoirs that are not used for potable water are all viable options.
Direct Air Carbon Capture Technology
One of the emerging technologies for artificially removing CO2 from the atmosphere is direct air carbon capture and storage (DACCS). This technology uses molecular bonding to extract CO2 from the atmosphere, and it is then either kept in geological reservoirs or put to some other use, such as in the production of compounds or mineral carbonates adsorption. The stability of CO2 storage is a major issue for this technology as well, just as it is for carbon capture and storage and bioenergy carbon capture and storage. Capturing CO2 from atmospheric air is much more challenging than capturing CO2 from highly concentrated combustion gas streams since direct air carbon capture and storage requires more energy and materials. If unforeseen issues with large-scale implementation can be resolved, the global capacity for CO2 removal will be between 0.5 and 5 GtCO2 per year by 2050 and could increase to 40 GtCO2 per year by the end of the century. The cost of CO2 removal is estimated to run from USD 600 to 1000 per metric ton of moved (tCO2) at the beginning but to drop to USD 100 to 300 per metric ton of CO2 removed in the future [94]. Several companies have developed and implemented direct air capture technology at an industrial scale. These companies include Carbon Engineering, Climeworks, Global Thermostat, Hydro Cell, Sky Tree, and Infinitree. Carbon Engineering has constructed a DAC plant located in the Permian Basin in Texas, USA, with a capture capacity of 1 MtonCO2/year [95]. There are no legislative instruments in place to aid this technology, as is the case with many of the other negative emissions options that have been investigated.
The direct air capture of CO2 by physisorbent materials, namely TEPA-SBA-15 (amine-modified mesoporous silica, chemisorbent, and Zeolite 13X (inorganic), HKUST-1, Mg-MOF-74/Mg-dobdc, and SIFSIX-3-Ni (a hybrid ultra-microporous material including four physisorbents), were found to be able to capture carbon from CO2-rich gas mixtures [96]. The greenhouse gas removal efficiency was 79–91%, while in the best case, the removal efficacy can be as high as 97% [97]. However, competition and reaction with atmospheric moisture significantly reduced the direct air capture (DAC) performance [96], and the drawback of DAC lies in the significant amount of energy required for capturing CO2 from the atmosphere [98]. Amine-based cellulose adsorbents or silica gel are normally used in the DAC system [99,100]. An estimate of US$610–780/tC CO2 was reported for the facility using sodium hydroxide, which is a strong base [95]. The thermal energy required for a DAC adsorption system is between 1400 and 2000 kWhth/ton CO2 [100].
6. Carbon Recycling through CO2 Conversion
Circular economy is a model that recycles all waste and byproducts discharged from a production process and generates no waste at the production end pipe. A cycle c economy is a process that involves the transforming of CO2 from a linear economy into value-added chemicals and fuels. CO2 is converted into methanol with enzymes or methane via electrodes and hydrogenophilic methanogenic cultures [101]. Catalysts can also be used in converting CO2 into other chemicals. For example, activated carbons have been used as a catalyst in converting CO2 into formic acid and methanol, as mentioned above, both of which are useful chemicals that can be used as feedstock for other industrial processes. About 200 million tons of CO2 are utilized in different processes each year, and then CO2 will react with ammonia to produce urea for fertilizers, while petroleum companies are also injecting CO2 underground to help recover fossil oil. Methanol and H2 are some of the key products produced from CO2 conversion processes, and those chemicals can be green or gray methanol and H2. When methanol is produced from renewable energy sources, a new term, “e-methanol”, is used. In terms of environmental impact, H2, and methanol are known as the lowest carbon intensity chemicals, and this is best for the climate. Methanol is one of the most practical alternatives to conventional fuels in the maritime area globally, and its use in a ship’s operation can reduce SOx emissions by 99%, PM emissions by 95%, NOx by 60%, and CO2 by 25% [102].
Recently, there has been an increasing number of projects that utilize sustainable feedstock such as captured CO2 from industrial emitters and green hydrogen produced from municipal solid waste (MSW), forestry residues, or agricultural waste. In terms of market impact, methanol is available in over 100 ports today. Conventionally, by 55% of global consumption of methanol is used in the production of downstream chemicals. Increasingly, the fastest growing segment is where it is in the numerous applications where it is consumed as a fuel (~45%). Captured CO2 can also be used for enhanced oil recovery [103].
Using methanol as a fuel would produce CO2 emissions of 54.7 tons per day at service speed, compared to 64.7 tons per day for diesel, and even less when using renewable or bio-methanol blends. Methanol is known to be a fuel that has no sulfur emissions, very low particulate matter, and CO2 emissions 15% lower than conventional marine fuel oil. Methanol can even blend with water to meet IMO NOx Tier III requirements, removing the need for expensive exhaust gas treatment [104]. Renewable methanol can also be produced from renewable H2 and captured CO2. Globally, methanol was valued at USD 30.7 billion in 2021 and is forecasted to reach USD 36.3 billion by 2026. The option to recycle CO2 could reduce industrial costs related to CO2 certification, and in Germany alone, the production costs of e-MeOH or methanol produced from electricity will vary between EUR 608 and 1453 per ton.
Hydrogen can also be produced from captured CO2. An electrolyte is used in this technology. When CO2 is injected into the aqueous electrolyte, the CO2 will react with the cathode, making the solution more acidic, which eventually generates electricity and creates H2. This is a new trend and has a great environmental and market impact since captured CO2 can be recycled and turned into a clean energy source [105].
7. Techno-Economic Analysis and Life Cycle Assessment
CO2 capture is a potential approach, though its techno-economic feasibility and life cycle vary, depending on technology used. Some carbon-based synthesized adsorbents used in its CO2 capture facility are expensive, while some adsorbents synthesized from bio-sources such as activated carbon seem to be less expensive due to the availability of bio-sources in various countries. Physical adsorbents such as zeolites are widely used in the oil and gas industry, and can adsorb as much as 6.18 mol CO2/kg adsorbent at 25 °C and 1 bar [106]; however, these adsorbents have a low selectivity of CO2 compared to other gases, they become negligible above 200 °C, and their adsorption capacity declines quickly with increasing temperatures above 30 °C [106]. For zeolites, the CO2- capture efficiency could reach up to 96% [107]. Multi-walled CNT-synthesized adsorbents are cost-effective for CO2 capture from flue gases, are stable for prolonged adsorption-desorption cyclic operation, can run for up to 20 cycles, and have a maximum CO2 adsorption capacity of 2.61 mol CO2/kg adsorbent at 20 °C and 1 bar [106]. ACs synthesized from the stones of yellow mombin are found to have 10 cycles [50].
Several CO2 capture and conversion processes have been implemented, such as to produce synthetic natural gas [108], and this process is effective in reducing both the net CO2 emission to the atmosphere by 45% and to have a cost of EUR 2.39 € per kg Raw-SNG [108]. The direct conversion of CO2 into liquid fuels and natural synthetic gases [109] also shows that this technology is promising, efficient, and profitable. It was found that DAI units normally have a lifetime of 20 years [98]. A study by Sai Gokul Subravet [110] proved that the CO2 avoided costs for a two single-stage pressure–vacuum swing adsorption (PVSA) system ranges from EUR 87.1 to 10.4 € per ton of CO2 avoided; the PVSA system is attractive for high CO2 concentration streams such as emission streams from steel and cement plants, while ideal adsorbents should have a wide range of CO2 affinity and a negligible N2 affinity [110]. A work by Anshuman Sinha on DAC [111] shows that the cost of DAC lies between USD 86 to 221 per tCO2, while the thermal energy ranges between 3.4 to 4.8 GJ per tCO2 captured, and the electrical energy ranges between 0.55 to 1.12 GJ per tCO2 captured [111].
8. Challenges and Perspectives
CO2 capture and CO2 conversion have remained a trendy topic of discussion, research, and technology development of late due to the urgent need to address the problem of climate change. Achievements in the field have been reported. In terms of the conventional treatment of CO2 in industry, polluted CO2 is usually treated by the absorption method, which involves the use of a liquid phase solvent sprayed against the gas phase in a wet scrubber system. However, the technologies remain under development because of several challenges, some of which are as follows:
- (a)
- The mismatched scale of operations: CO2 capture and conversion require a significant upfront investment, particularly for transmission. The storage infrastructure and conversion are also a challenge. The key to success (expressed in adsorption capacity or CO2 separation efficiency) often depends very much on the adsorbent or membrane materials.
- (b)
- In the adsorption-based CO2 capture processes, the choice of the gas-solid contact system (fixed bed, fluidized bed, moving bed) is crucial. Merely choosing the most promising adsorbent (i.e., the adsorbent providing the best combination of required properties) is not enough for the commercial deployment of the adsorption technology; the potential of each sorbent can be fully exploited only by using the most suitable combination of a gas–solid reactor configuration and regeneration mode [112,113,114].
- (c)
- The need for a social license to capture, transport, and store CO2, in addition to ongoing storage liabilities, are some other challenges that need to be overcome to make these technologies fully usable in real applications. Capturing and converting CO2 into other gasses remains a challenging task for many agencies, organizations, and stakeholders. There are several issues with carbon capture and storage, such as insufficiently protected storage capacity and the possibility of leakage. The environmental consequences of accidental leaks at coastal storage sites have been reported [115]. Additional concerns with this technology include public acceptability [94,116] and expensive execution costs. CO2 capture enables a wide variety of applications, including chemical production, fuel generation, microalga cultivation, concrete production, and oil recovery [93,117].
CO2 capture and conversion technologies have an important role in capturing and reducing CO2 and combating climate change at the global level. CO2 capture performed by biological approaches such as microalgae utilization to convert CO2 into valuable products [118,119,120,121,122,123] and novel catalysts for improving the adsorption capacities of existing and bio-based materials might be new areas of research and development in this important field.
9. Conclusions
CO2 is continuously released into the atmospheric environment via different routes, but mainly via industrial activities such as energy production, transportation, and oil and gas production. Increasing CO2 emissions cause anthropogenic global warming. COP26 set a target for countries concerning their CO2 emissions. CO2 capture and conversion can be attainable by both conventional and advanced technologies, while adsorption and electrochemical methods have certain advantages. Synthesized carbonaceous materials such as silica-based adsorbents, polymeric materials, clay, biomass-based materials, carbon nanotubes and graphene oxide, and other materials have shown to be promising CO2 adsorbents. Activated carbon can be prepared and activated by different methods such as physical or chemical activation processes, while carbon nanotubes can be produced through (i) arc discharge, (ii) laser ablation, and (iii) chemical vapor deposition. Some physical properties (surface area, porosity) and chemical properties (functional groups of the materials) are significant factors determining the performance of these carbonaceous materials. The availability of feedstocks such as agricultural wastes for producing these materials, and catalysts modifying and increasing adsorption capacities of these materials are prospective areas of research and development for CO2 capture and conversion technology in the future.
Author Contributions
Conceptualization, T.-D.H. and S.A.B.; methodology, T.-D.H. and S.A.B.; validation, T.-D.H. and S.A.B.; formal analysis, T.-D.H., S.B. and F.A.M.; investigation, T.-D.H. and S.A.B.; resources, T.-D.H. and S.A.B.; data curation, T.-D.H. and S.B.; writing—original draft preparation, T.-D.H., S.B., F.A.M., I.Q. and S.B.P.; writing—review and editing, T.-D.H., S.A.B., F.A.M. and A.H.; visualization, T.-D.H. and S.A.B.; project administration, T.-D.H. and S.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I would like to acknowledge the generous advisory support and opinion provided by Nhuan Nghiem of Clemson University, USA, and Le Minh Thang of the School of Chemistry and Life Science, Hanoi University of Science and Technology, who have encouraged me to structure and prepare this manuscript for submission. Sincere thanks also go to the RoHan Project funded by the German Academic Exchange Service (DAAD, No. 57315854) and to the Federal Ministry for Economic Cooperation and Development (BMZ) of Germany inside the framework of the “SDG Bilateral Graduate School program” for providing a. research scholarship to T.D.H so that he could focus on and complete this work.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Factsheet: What Is the IPCC; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Khdary, N.H.; Alayyar, A.S.; Alsarhan, L.M.; Alshihri, S.; Mokhtar, M. Metal oxides as catalyst/supporter for CO2 capture and conversion, review. Catalysts 2022, 12, 300. [Google Scholar] [CrossRef]
- Jena, K.K.; Panda, A.P.; Verma, S.; Mani, G.K.; Swain, S.; Alhassan, S.M. MWCNTs-ZnO-SiO2 mesoporous nano-hybrid materials for CO2 capture. J. Alloys Compd. 2019, 800, 279–285. [Google Scholar] [CrossRef]
- Chen, S.; Tao, Z.; Fu, Y.; Zhu, M.; Li, W.; Li, X. CO2 separation from offshore natural gas in quiescent and flowing states using 13X zeolite. Appl. Energy 2017, 205, 1435–1446. [Google Scholar] [CrossRef]
- Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; Ruíz, B.; Rosário, M. Carbon dioxide/methane separation by adsorption on sepiolite. J. Nat. Gas Chem. 2007, 16, 235–243. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, P.; Qi, S.C.; Liu, X.Q.; Yan, J.H.; Fan, F.; Sun, L.B. Metal–organic frameworks with target-specific active sites switched by photoresponsive motifs: Efficient adsorbents for tailorable CO2 capture. Angew. Chem. Int. Ed. 2019, 58, 6600–6604. [Google Scholar] [CrossRef]
- Lu, C.; Shi, X.; Liu, Y.; Xiao, H.; Li, J.; Chen, X. Nanomaterials for adsorption and conversion of CO2 under gentle conditions. Mater. Today 2021, 50, 385–399. [Google Scholar] [CrossRef]
- Omodolor, I.S.; Otor, H.O.; Andonegui, J.A.; Allen, B.J.; Alba-Rubio, A.C. Dual-Function Materials for CO2 Capture and Conversion: A Review. Ind. Eng. Chem. Res. 2020, 59, 17612–17631. [Google Scholar] [CrossRef]
- Nakhate, P.; Van Der Meer, Y. A systematic review on seaweed functionality: A sustainable bio-based material. Sustainability 2021, 13, 6174. [Google Scholar] [CrossRef]
- AliAkbari, R.; Ghasemi, M.H.; Neekzad, N.; Kowsari, E.; Ramakrishna, S.; Mehrali, M.; Marfavi, Y. High value add bio-based low-carbon materials: Conversion processes and circular economy. J. Clean. Prod. 2021, 293, 126101. [Google Scholar] [CrossRef]
- Vinayak, A.; Sharma, S.; Singh, G.B. Bioinspired Materials for CO2 Capture and Conversion, in CO2-Philic Polymers, Nanocomposites and Chemical Solvents; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–76. [Google Scholar]
- Jha, M.K.; Joshi, S.; Sharma, R.K.; Kim, A.A.; Pant, B.; Park, M.; Pant, H.R. Surface modified activated carbons: Sustainable bio-based materials for environmental remediation. Nanomaterials 2021, 11, 3140. [Google Scholar] [CrossRef]
- Abbas, Y.; Yun, S.; Wang, Z.; Zhang, Y.; Zhang, X.; Wang, K. Recent advances in bio-based carbon materials for anaerobic digestion: A review. Energy Rev. 2021, 135, 110378. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Garberoglio, G. Quantum sieving in organic frameworks. Chem. Phys. Lett. 2009, 467, 270–275. [Google Scholar] [CrossRef]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem. Eng. J. 2019, 360, 250–259. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Qiao, X.; Shen, X.; Guo, J.; Xiang, J.; Jin, Y. Experiment and regeneration kinetic model study on CO2 adsorbent prepared from fly ash. Chem. Eng. J. 2021, 421, 127865. [Google Scholar] [CrossRef]
- Joerin, F.; Desthieux, G.; Beuze, S.B.; Nembrini, A. Participatory diagnosis in urban planning: Proposal for a learning process based on geographical information. J. Environ. Manag. 2009, 90, 2002–2011. [Google Scholar] [CrossRef]
- Morali, U.; Demiral, H.; Sensoz, S. Preparation of new carbon molecular sieves for optimized carbon dioxide adsorption and product yield. New Carbon Mater. 2020, 35, 209–219. [Google Scholar] [CrossRef]
- Seredych, M.; Jagiello, J.; Bandosz, T.J. Complexity of CO2 adsorption on nanoporous sulfur-doped carbons–Is surface chemistry an important factor? Carbon 2014, 74, 207–217. [Google Scholar] [CrossRef]
- Querejeta Montes, N.; Gil Matellanes, M.V.; Pevida García, C.; Álvarez Centeno, T. Standing out the key role of ultramicroporosity to tailor biomass-derived carbons for CO2 capture. J. CO2 Util. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.J.T. Theoretical Studies for Lewis Acid-Base Interactions and C–H…O Weak Hydrogen Bonding in Various CO2 Complexes. J. Phys. Chem. A 2008, 112, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Hong, L.; Ju, S.; Liu, X.; Zhuang, Q.; Zhan, G.; Yu, X. Highly selective CO2 uptake in novel fishnet-like polybenzoxazine-based porous carbon. Energy Fuels 2019, 33, 11454–11464. [Google Scholar] [CrossRef]
- Sivadas, D.L.; Vijayan, S.; Rajeev, R.; Ninan, K.; Prabhakaran, K. Nitrogen-enriched microporous carbon derived from sucrose and urea with superior CO2 capture performance. Carbon 2016, 109, 7–18. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Malla, F.A.; Khan, S.; Gupta, N. Application of alkaline chemicals for biogas methane enrichment. Econ. Environ. Conserv. 2016, 22, 475–484. [Google Scholar]
- Bui, M.; Adjiman, C.; Bardow, A.; Anthony, E.; Boston, A.; Brown, S.; Mac Dowell, N. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Khan, S.A.; Malla, F.A.; Rashmi; Malav, L.C.; Gupta, N.; Kumar, A. Potential of wastewater treating Chlorella minutissima for methane enrichment and CO2 sequestration of biogas and producing lipids. Energy 2018, 150, 153–163. [Google Scholar] [CrossRef]
- Jin, C.; Sun, J.; Bai, S.; Zhou, Z.; Sun, Y.; Guo, Y.; Wang, R.; Zhao, C. Sawdust wastes-derived porous carbons for CO2 adsorption. Part 2. Insight into the CO2 adsorption enhancement mechanism of low-doping of microalgae. J. Environ. Chem. Eng. 2022, 10, 108265. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Ge, S.; Xiong, L.; Sun, Q. Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind. Crops Prod. 2016, 83, 346–352. [Google Scholar] [CrossRef]
- Paul, S.; Bhagobaty, R.; Nihalani, M.; Joshi, S. Characterization of oleaginous endophytic fungi of biodiesel plants as potential biofuel minifactories. Bioenergy 2020, 142, 105750. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Sun, J.; Chen, Y.; Guo, Y.; Han, D.; Wang, R.; Zhao, C. Sawdust wastes-derived porous carbons for CO2 adsorption. Part 1. Optimization preparation via orthogonal experiment. Sep. Purif. Technol. 2021, 276, 119270. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Bayar, Ş.; Varol, E.A.; Sreńscek-Nazzal, J.; Bosacka, M.; Michalkiewicz, B. Thermochemical conversion of lignocellulosic biomass-olive pomace-into activated biocarbon for CO2 adsorption. Ind. Crop. Prod. 2022, 187, 115416. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, D.; Cheng, X.; Yuan, C.; Wang, S.; He, Z.; Esakkimuthu, S. Adsorption properties of seaweed-based biochar with the greenhouse gases (CO2, CH4, N2O) through density functional theory (DFT). Biomass Bioenergy 2022, 163, 106519. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Louis-Therese, J.; Gaspard, S.; Szilágyi, P.Á.; Titirici, M.M. Biomass derived carbon materials: Synthesis and application towards CO2 and H2S adsorption. Nano Sel. 2022, 3, 165–177. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Junior, O.F.C.; Sreńscek-Nazzal, J. Preparation of low-cost activated carbons from amazonian nutshells for CO2 storage. Bioenergy 2021, 144, 105925. [Google Scholar] [CrossRef]
- Pramanik, P.; Patel, H.; Charola, S.; Neogi, S.; Maiti, S. High surface area porous carbon from cotton stalk agro-residue for CO2 adsorption and study of techno-economic viability of commercial production. J. CO2 Util. 2021, 45, 101450. [Google Scholar] [CrossRef]
- Gao, X.; Yang, S.; Hu, L.; Cai, S.; Wu, L.; Kawi, S. Carbonaceous materials as adsorbents for CO2 capture: Synthesis and modification. Carbon Capture Sci. Technol. 2022, 3, 100039. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, S.-J. Imidazolium-optimized conductive interfaces in multilayer graphene nanoplatelet/epoxy composites for thermal management applications and electroactive devices. Polymer 2019, 168, 53–60. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.-J.; Lee, J.W.; Park, S.-J. Functionalized carbon materials for electronic devices: A review. Micromachines 2019, 10, 234. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E. An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 443–453. [Google Scholar] [CrossRef]
- Fiuza, R.A., Jr.; de Jesus Neto, R.M.; Correia, L.B.; Andrade, H.M.C. Preparation of granular activated carbons from yellow mombin fruit stones for CO2 adsorption. J. Environ. Manag. 2015, 161, 198–205. [Google Scholar] [CrossRef]
- De Souza, L.K.; Gonçalves, A.A.; Queiroz, L.S.; Chaar, J.S.; Filho, G.N.D.R.; da Costa, C.E. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO2 capture. J. Environ. Manag. 2020, 25, e00168. [Google Scholar] [CrossRef]
- Quan, C.; Su, R.; Gao, N. Preparation of activated biomass carbon from pine sawdust for supercapacitor and CO2 capture. Int. J. Energy Res. 2020, 44, 4335–4351. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Fu, N.; Chen, H.; Lin, H.; Han, S. Biomass-derived nitrogen-doped porous carbon with superior capacitive performance and high CO2 capture capacity. Electrochim. Acta 2018, 266, 161–169. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Junior, O.F.C.; Sreńscek-Nazzal, J. Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Chung, A.-J.; Liao, C.-H. CO2 capture with amine-loaded carbon nanotubes via a dual-column temperature/vacuum swing adsorption. Appl. Energy 2014, 113, 706–712. [Google Scholar] [CrossRef]
- Singh, G.; Bahadur, R.; Lee, J.M.; Kim, I.Y.; Ruban, A.M.; Davidraj, J.M.; Semit, D.; Karakoti, A.; Al Muhtaseb, A.H.; Vinu, A. Nanoporous activated biocarbons with high surface areas from alligator weed and their excellent performance for CO2 capture at both low and high pressures. Chem. Eng. J. 2021, 406, 126787. [Google Scholar] [CrossRef]
- Hamyali, H.; Nosratinia, F.; Rashidi, A.; Ardjmand, M.J. Anthracite coal-derived activated carbon as an effectiveness adsorbent for superior gas adsorption and CO2/N2 and CO2/CH4 selectivity: Experimental and DFT study. J. Environ. Chem. Eng. 2022, 10, 107007. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Cota, I.; Martinez, F.F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption. Co-Ord. Chem. Rev. 2017, 351, 189–204. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Yeon, S.-H.; Park, S.-J. Defining contribution of micropore size to hydrogen physisorption behaviors: A new approach based on DFT pore volumes. Carbon 2019, 143, 288–293. [Google Scholar] [CrossRef]
- Geng, S.; Maennlein, A.; Yu, L.; Hedlund, J.; Oksman, K. Monolithic carbon aerogels from bioresources and their application for CO2 adsorption. Microporous Mesoporous Mater. 2021, 323, 111236. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Chen, L.; Gong, M.; Cheng, Y.; Liu, Y.; Yin, S.; Luo, D. Effect of Pore Structure of Supports on CO2 Adsorption of Tetraethylenepentamine/Carbon Aerogels Prepared by Incipient Wetness Impregnation Method. Pol. J. Environ. Stud. 2019, 28, 4127–4137. [Google Scholar] [CrossRef]
- Alabadi, A.; Abbood, H.A.; Li, Q.; Jing, N.; Tan, B. Imine-linked polymer based nitrogen-doped porous activated carbon for efficient and selective CO2 capture. Sci. Rep. 2016, 6, 38614. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.-Z.; Qi, S.-C.; Liu, X.; Xue, D.-M.; Peng, S.-S.; Yu, G.-X.; Liu, X.-Q.; Sun, L.-B. Fabrication of N-doped porous carbons for enhanced CO2 capture: Rational design of an ammoniated polymer precursor. Chem. Eng. J. 2019, 369, 170–179. [Google Scholar] [CrossRef]
- Peng, A.-Z.; Qi, S.-C.; Liu, X.; Xue, D.-M.; Peng, S.-S.; Yu, G.-X.; Liu, X.-Q.; Sun, L.-B. N-doped porous carbons derived from a polymer precursor with a record-high N content: Efficient adsorbents for CO2 capture. Chem. Eng. J. 2019, 372, 656–664. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of graphene oxide synthesis method on properties and performance of polysulfone-graphene oxide mixed matrix membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Das, S.K.; Patra, A.K.; Bhaumik, A. Functionalized graphene oxide as an efficient adsorbent for CO2 capture and support for heterogeneous catalysis. RSC Adv. 2016, 6, 72055–72068. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Cui, Y.; Zhu, J.-H.; Han, B.-H. Preparation of three-dimensional graphene oxide–polyethylenimine porous materials as dye and gas adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef]
- Shin, G.-J.; Rhee, K.; Park, S.-J. Improvement of CO2 capture by graphite oxide in presence of polyethylenimine. Int. J. Hydrog. Energy 2016, 41, 14351–14359. [Google Scholar] [CrossRef]
- Chen, C.; Xu, K.; Ji, X.; Miao, L.; Jiang, J. Enhanced adsorption of acidic gases (CO2, NO2 and SO2) on light metal decorated graphene oxide. Phys. Chem. Chem. Phys. 2014, 16, 11031–11036. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Muraza, A.G.O.J.R.; Galadima, A. Reviews, Catalytic thermal conversion of CO2 into fuels: Perspective and challenge. Renew. Sustain. Energy Rev. 2019, 115, 109333–109353. [Google Scholar]
- Bhattacharya, A.; Selvaraj, A. Photocatalytic conversion of CO2 into beneficial fuels and chemicals—A new horizon in atmospheric CO2 mitigation. Process Saf. Environ. Prot. 2021, 156, 256–287. [Google Scholar] [CrossRef]
- Malkhandi, S.; Yeo, B.S. Electrochemical conversion of carbon dioxide to high value chemicals using gas-diffusion electrodes. Curr. Opin. Chem. Eng. 2019, 26, 112–121. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ponrasu, T.; Chandel, S.; Dixit, M.; Muthuvijayan, V. Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. R. Soc. Open Sci. 2018, 5, 172017. [Google Scholar] [CrossRef] [PubMed]
- Milow, B. Carbon Aerogels Synthesis, Properties and Applications. In Proceedings of the Department Chemistry and Physics of Materials at the Paris-Lodron University Salzburg, Salzburg, Austria, 12 September 2019. [Google Scholar]
- Hsieh, T.-H.; Huang, Y.-S.; Shen, M.-Y. Mechanical properties and toughness of carbon aerogel/epoxy polymer composites. J. Mater. Sci. 2015, 50, 3258–3266. [Google Scholar] [CrossRef]
- Pinelli, F.; Piras, C.; Rossi, F. A perspective on graphene based aerogels and their environmental applications. Flatchem 2022, 36, 100449. [Google Scholar] [CrossRef]
- Ramezanipour Penchah, H.; Ghaemi, A.; Jafari, F. Piperazine-modified activated carbon as a novel adsorbent for CO2 capture: Modeling and characterization. Environ. Sci. Pollut. Res. 2021, 29, 5134–5143. [Google Scholar] [CrossRef] [PubMed]
- Pellerano, M.; Pré, P.; Kacem, M.; Delebarre, A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia 2009, 1, 647–653. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R.; Miccio, F. Fixed bed adsorption as affected by thermodynamics and kinetics: Yellow tuff for CO2 capture. Powder Technol. 2020, 373, 446–458. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Hoang, T.-D.; Nghiem, N. Recent developments and current status of commercial production of fuel ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Bustreo, C.; Giuliani, U.; Maggio, D.; Zollino, G. How fusion power can contribute to a fully decarbonized European power mix after 2050. Fusion Eng. Des. 2019, 146, 2189–2193. [Google Scholar] [CrossRef]
- Palmer, C. Mitigating Climate Change Will Depend on Negative Emissions Technologies; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yan, J.; Obersteiner, M.; Möllersten, K.; Moreira, J.R. Negative Emission Technologies–NETs; Elsevier: Amsterdam, The Netherlands, 2019; p. 113749. [Google Scholar]
- Goglio, P.; Williams, A.; Balta-Ozkan, N.; Harris, N.; Williamson, P.; Huisingh, D.; Zhang, Z.; Tavoni, M. Advances and challenges of life cycle assessment (LCA) of greenhouse gas removal technologies to fight climate changes. J. Clean. Prod. 2020, 244, 118896. [Google Scholar] [CrossRef]
- Lin, A.C. Carbon dioxide removal after Paris. Ecol. Law Q. 2019, 45, 533–582. [Google Scholar]
- Pires, J. Negative emissions technologies: A complementary solution for climate change mitigation. Sci. Total Environ. 2019, 672, 502–514. [Google Scholar] [CrossRef]
- Shinnar, R.; Citro, F. Decarbonization: Achieving near-total energy independence and near-total elimination of greenhouse emissions with available technologies. Technol. Soc. 2008, 30, 1–16. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Tian, D.J. Farmland degradation caused by radial diffusion of CO2 leakage from carbon capture and storage. J. Clean. Prod. 2020, 255, 120059. [Google Scholar] [CrossRef]
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. Public perception of carbon capture and storage: A state-of-the-art overview. Heliyon 2019, 5, e02845. [Google Scholar] [CrossRef]
- Arning, K.; Heek, J.O.-V.; Linzenich, A.; Kaetelhoen, A.; Sternberg, A.; Bardow, A.; Ziefle, M. Same or different? Insights on public perception and acceptance of carbon capture and storage or utilization in Germany. Energy Policy 2019, 125, 235–249. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Pastore, C. Biotechnology to develop innovative syntheses using CO2. Environ. Chem. Lett. 2005, 3, 113–117. [Google Scholar] [CrossRef]
- Leonzio, G.; Fennell, P.S.; Shah, N. Analysis of technologies for carbon dioxide capture from the air. Appl. Sci. 2022, 12, 8321. [Google Scholar] [CrossRef]
- Gambhir, A.; Tavoni, M. Direct air carbon capture and sequestration: How it works and how it could contribute to climate-change mitigation. One Earth 2019, 1, 405–409. [Google Scholar] [CrossRef]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.-J.; Daniels, E.A.; Curtin, T.; Perry, J.J., IV; Zaworotko, M.J. Direct air capture of CO2 by physisorbent materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef]
- Terlouw, T.; Treyer, K.; Bauer, C.; Mazzotti, M. Life cycle assessment of direct air carbon capture and storage with low-carbon energy sources. Environ. Sci. Technol. 2021, 55, 11397–11411. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life cycle assessment of carbon dioxide removal technologies: A critical review. Energy Environ. Sci. 2021, 14, 1701–1721. [Google Scholar] [CrossRef]
- Elfving, J.; Bajamundi, C.; Kauppinen, J. Characterization and performance of direct air capture sorbent. Energy Procedia 2017, 114, 6087–6101. [Google Scholar] [CrossRef]
- Leonzio, G.; Mwabonje, O.; Fennell, P.S.; Shah, N. Environmental performance of different sorbents used for direct air capture. Sustain. Prod. Consum. 2022, 32, 101–111. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Carbon Recycling Through CO2-Conversion for Stepping Toward a Cyclic-C Economy. A Perspective. Front. Energy Res. 2022, 8, 159. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wright, L.A. A comparative review of alternative fuels for the maritime sector: Economic, technology, and policy challenges for clean energy implementation. World 2021, 2, 456–481. [Google Scholar] [CrossRef]
- Al-Shargabi, M.; Davoodi, S.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Carbon dioxide applications for enhanced oil recovery assisted by nanoparticles: Recent developments. ACS Omega 2022, 7, 9984–9994. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, Y.; Feng, Y.; Yang, J.; Xia, C. A Prompt Decarbonization Pathway for Shipping: Green Hydrogen, Ammonia, and Methanol Production and Utilization in Marine Engines. Atmosphere 2023, 14, 584. [Google Scholar] [CrossRef]
- Hank, C.; Gelpke, S.; Schnabl, A.; White, R.J.; Full, J.; Wiebe, N.; Smolinka, T.; Schaadt, A.; Henning, H.-M.; Hebling, C. Economics & carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide–power-to-methanol. Sustain. Energy Fuels 2018, 2, 1244–1261. [Google Scholar]
- Lee, B.; Lee, H.; Lim, D.; Brigljević, B.; Cho, W.; Cho, H.-S.; Kim, C.-H.; Lim, H. Renewable methanol synthesis from renewable H2 and captured CO2: How can power-to-liquid technology be economically feasible? Appl. Energy 2020, 279, 115827. [Google Scholar] [CrossRef]
- Hasan, M.F.; Rossi, L.M.; Debecker, D.P.; Leonard, K.C.; Li, Z.; Makhubela, B.C.; Zhao, C.; Kleij, A. Can CO2 and renewable carbon be primary resources for sustainable fuels and chemicals? ACS Sustain. Chem. Eng. 2021, 9, 12427–12430. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Gonzalez-Olmos, R.; Gutierrez-Ortega, A.; Sempere, J.; Nomen, R. Zeolite versus carbon adsorbents in carbon capture: A comparison from an operational and life cycle perspective. J. CO2 Util. 2022, 55, 101791. [Google Scholar] [CrossRef]
- Chauvy, R.; Verdonck, D.; Dubois, L.; Thomas, D.; De Weireld, G. Techno-economic feasibility and sustainability of an integrated carbon capture and conversion process to synthetic natural gas. J. CO2 Util. 2021, 47, 101488. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, R.; Jun, K.-W.; Kim, S.K.; Hwang, S.-M.; Park, H.-G.; Guan, G. Direct conversion of carbon dioxide to liquid fuels and synthetic natural gas using renewable power: Techno-economic analysis. J. CO2 Util. 2019, 34, 293–302. [Google Scholar] [CrossRef]
- Subraveti, S.G.; Roussanaly, S.; Anantharaman, R.; Riboldi, L.; Rajendran, A. How much can novel solid sorbents reduce the cost of post-combustion CO2 capture? A techno-economic investigation on the cost limits of pressure–vacuum swing adsorption. Appl. Energy 2022, 306, 117955. [Google Scholar] [CrossRef]
- Sinha, A.; Realff, M.J. A parametric study of the techno-economics of direct CO2 air capture systems using solid adsorbents. AIChE J. 2019, 65, e16607. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaabout, A.; Cloete, S.; Amini, S. Review on reactor configurations for adsorption-based CO2 capture. Ind. Eng. Chem. Res. 2021, 60, 3779–3798. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. Sound-assisted fluidization for temperature swing adsorption and calcium looping: A review. Materials 2021, 14, 672. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Huang, D.; Li, M.-J.; Wang, R.-L.; Yang, Y.-W.; Tao, W.-Q. Advanced carbon sequestration by the hybrid system of photobioreactor and microbial fuel cell with novel photocatalytic porous framework. Bioresour. Technol. 2021, 333, 125182. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-scale cultivation of microalgae Scenedesmus almeriensis for CO2 capture and lutein production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).