1. Introduction
The continuous growth of the global population has led to a rise in the consumption of disposable goods, leading to copious increases in waste generation. As an example,
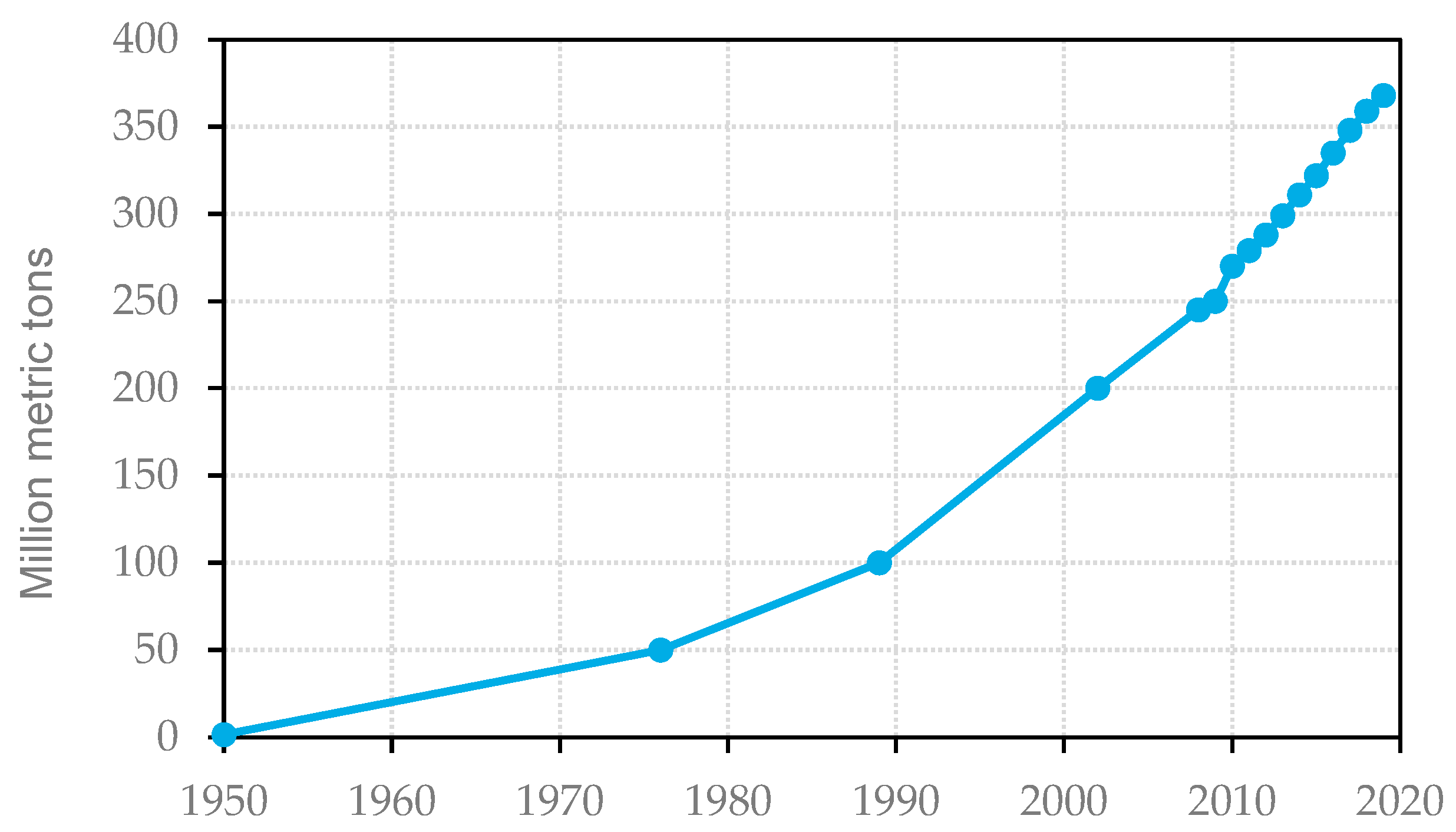
Figure 1 illustrates the worldwide volume in million metric tons of plastic production. Starting in 1950, the annual production of plastic was not relevant. After 1970, the amount of plastic manufacturing started to grow exponentially and reached a value of 270 Mt (million metric tons) in 2010 and more than 368 Mt in 2019 [
1]. This represents a growth of more than 36% in the years between 2010 and 2019. In the case of Europe, which has one of the highest rates of plastic recycling worldwide [
2], the production of plastic resin has reduced by 14% in the last 4 years, and the recyclability has gone up by 22% [
3]. In the year 2018, out of the 61.8 Mt of plastics produced, 29 Mt was recollected as waste, out of which 9.4 Mt was recycled, 12.4 Mt was incinerated and the remaining 7.2 Mt ended up in landfills [
3]. Comparing the recycling rates to the total production, only 15% of plastic was recycled, and compared to the collected waste, 32% was recycled. In other equally developed economies such as the United States of America, which do not have recycling regulations, plastic recyclability is around 10% [
2]. These values demonstrate the potential for the improvement in polymer recycling.
In the case of the United States, 32.52 Mt of plastic waste was produced in 2017 [
4]. Overall, 63% was either waste in the form of polypropylene (PP), low-density polyethylene (LDPE) or high-density polyethylene (HDPE). However, only 18% of this waste was recycled. Leaving the polypropylenes and polyethylenes aside, the fourth most commonly produced polymer in the United States was polyethylene terephthalate (PET), making up 14.4% of the total plastic waste. Out of this 4.7 Mt of PET waste, only 20% was recycled, leaving significant room for improvement [
4].
PET is a polymer which offers multiple recycling options within mechanical and chemical recycling, and therefore has a high recyclability potential [
5]. Mechanical recycling is the simplest and most low-cost way of reusing PET, as the process consists only of washing, drying and melt processing. However, due to multiple degradation processes, melt processing of mechanically recycled PET leads to a decrease in the molecular weight. This reduction is translated into a lower viscosity, which complicates the processing of the polymer melt [
5]. Unlike mechanical recycling, chemical recycling is an option where the polymer chain of the recycled material is fully or partially depolymerized into its monomers and subsequently repolymerized into an equivalent of a virgin resin [
6]. The most common processes for chemical recycling are glycolysis, methanolysis, hydrolysis, aminolysis and ammonolysis, and they depend on the reagent used for the depolymerization [
7]. Slow production rates and cost-intensive processes are still some of the downsides of chemical recycling; however, the usage of new reaction catalysts and the implementation of microwaves or ultrasound waves as a heating source are improving the effectivity of the recycling process drastically [
8].
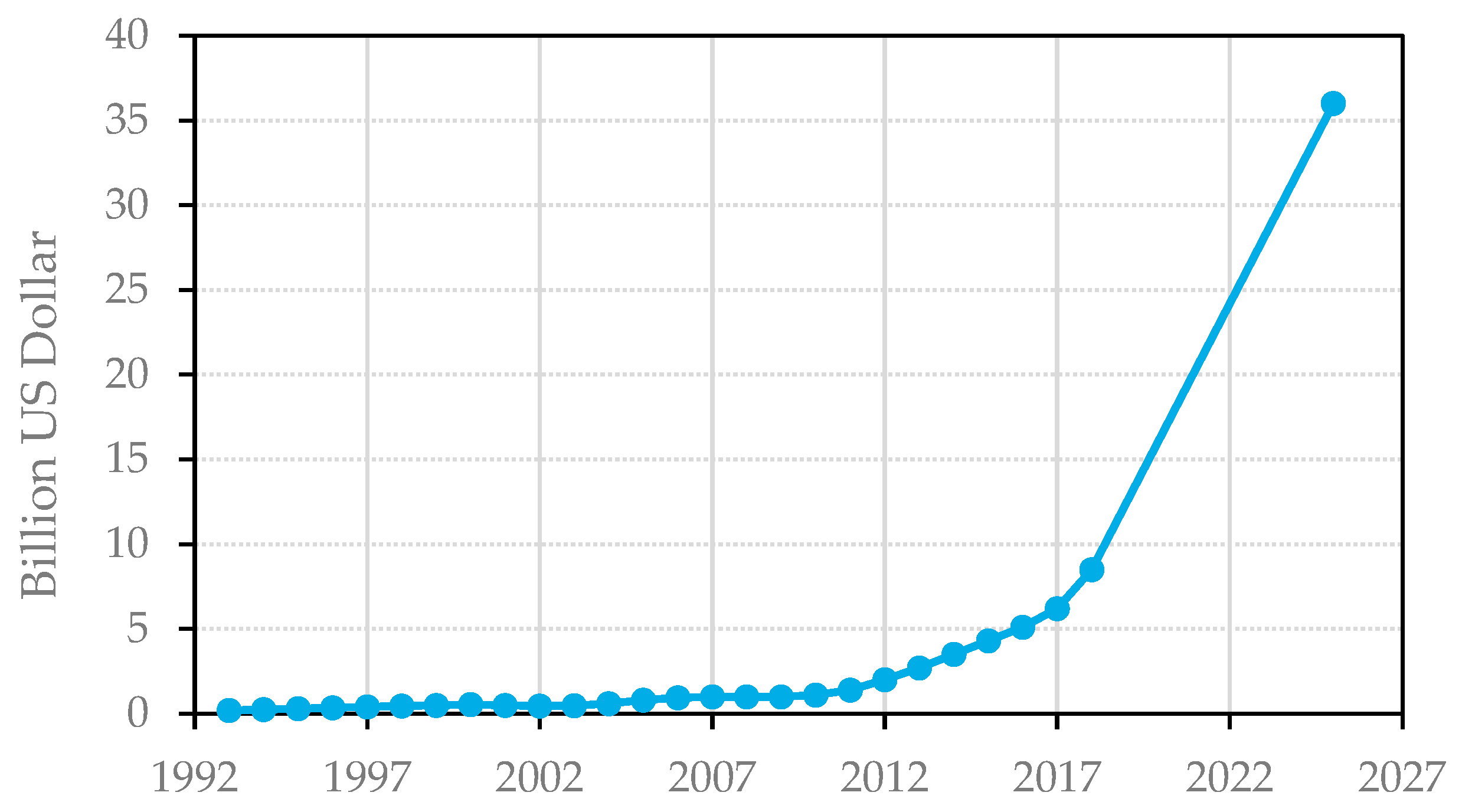
Similarly, the field of additive manufacturing (AM) has seen steady growth since its creation in the late 1980s, as illustrated in
Figure 2, where the total revenues for services and products since 1993 are presented. Between 2012 and 2018, the AM market revenues improved by a factor of more than 8, and they are expected to keep improving until 2025 by a factor of over 36 [
9]. AM techniques possess unique advantages, such as almost unrestricted geometrical options or the absence of molds, which is causing the field to be perceived as a viable mainstream manufacturing process [
10]. Material extrusion (MEX), brought to the market in 1991 by Stratasys under the name fused deposition modeling (FDM), is one of the most widely used and well-known AM technologies. This process uses the extrusion of thermoplastic polymers to create three-dimensional parts. Since being introduced, the MEX process has become one of the most common, simple, and cost-effective AM processes in the industry [
10]. The goal of this work is combining the high potential recyclability of PET with the AM market, specifically through the production of a MEX filament that can be used to produce functional parts. The mechanical recycling of PET and the subsequent extrusion of the recycled material into a filament for the MEX process are analyzed. Compared to the work completed by Zander et al. [
11], where recycled PET is also used as feedstock for MEX, this work places emphasis on different aspects, such as the extrusion process with the importance of the extrusion screw and the extrusion parameters and also the procedures prior the extrusion, such as the influence of drying and the possible degradation of PET.
5. Discussion
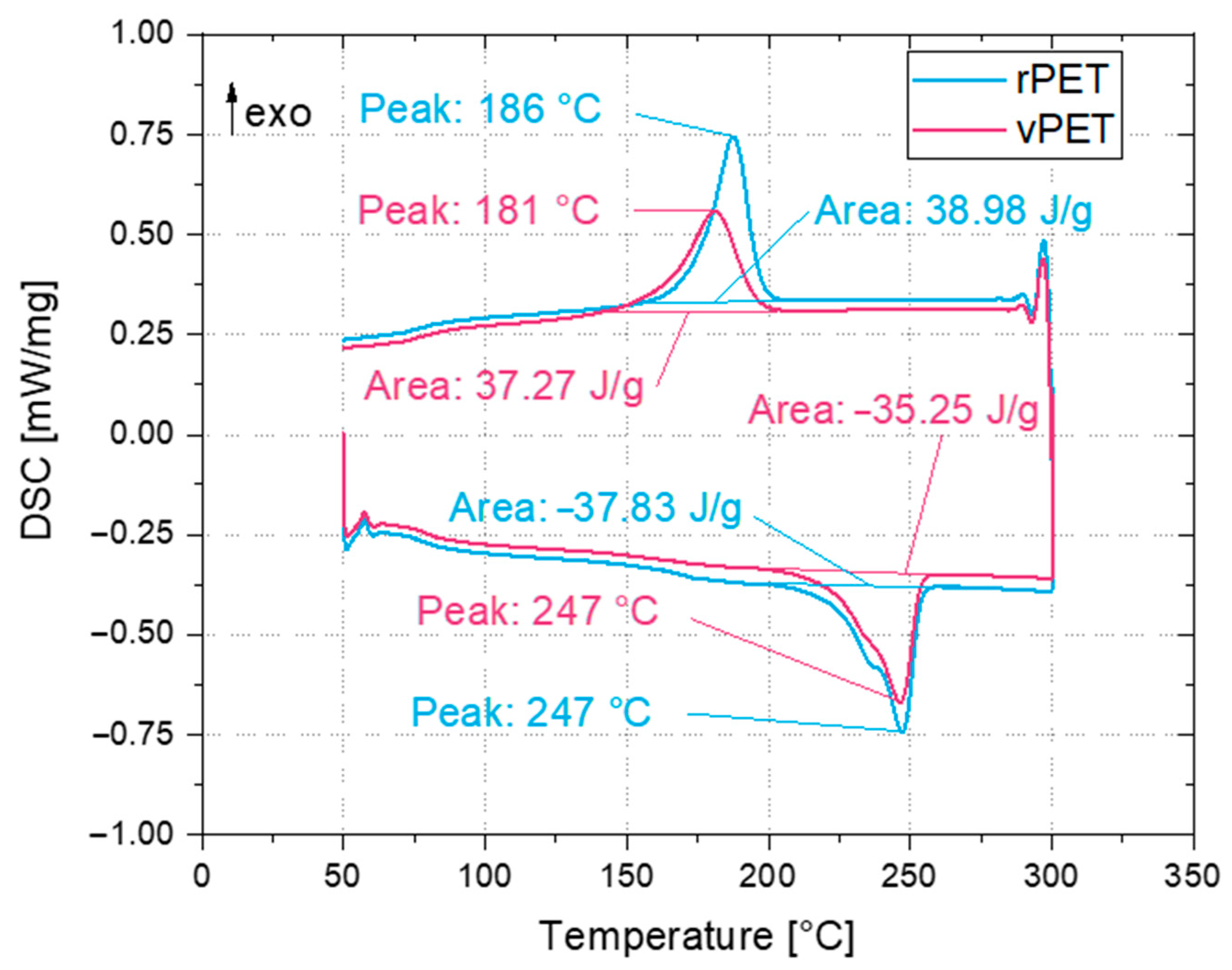
This work focused on recycling bottle-grade PET in order to extrude it into a filament for the MEX process. Compared to other materials used for material extrusion, PET presents higher melting temperatures and the formation of crystallites, which influence the final coloration of the printed parts [
11]. For these reasons, PET is avoided as a MEX material. However, the usage of recycled PET as a filament for additive manufacturing also presents some advantages. The recycled PET, as used in this study, is recycled mechanically. Mechanical or physical recycling, as it is also named, grinds and separates the PET out of the remaining waste due to size, color and density. Afterwards, it reutilizes the PET without intentionally changing the molecular structure of the material [
29,
30]. With the help of the float and sink process, PET and PVC, having a density of approximately 1.4 g/cm
3, are easily separated from the remaining commonly used polymers such as polyethylene (PE) and polypropylene (PP) [
4]. In comparison to chemical recycling, for example the ethylene glycol modification of the PET to generate PETG, the simple steps during mechanical recycling make it a reliable and low-cost process [
29]. In addition, no further materials apart from the PET bottles are needed. Therefore, the filament can be produced basically everywhere where PET bottles are found. A further advantage in comparison to the chemical recycling is the time it takes to depolymerize or chemically modify the PET. Without the use of microwave irradiation, chemical recycling processes can take from four to eight hours [
31,
32]. This implies a high energy consumption to keep the endothermic reaction running.
PET presents a further advantage, as the miscibility between the virgin and the recycled resin is ideal [
33]. The blend between these two variants presents absolutely no phase separation and can therefore be mixed totally, depending on the properties needed. As the results in [
33] present, blends with high contents of recycled PET of up to 90% behave basically as well as the virgin resin in dynamic and static mechanical tests. In addition, the viscosity of the polymer melt can be adapted with different quantities of recycled and virgin material.
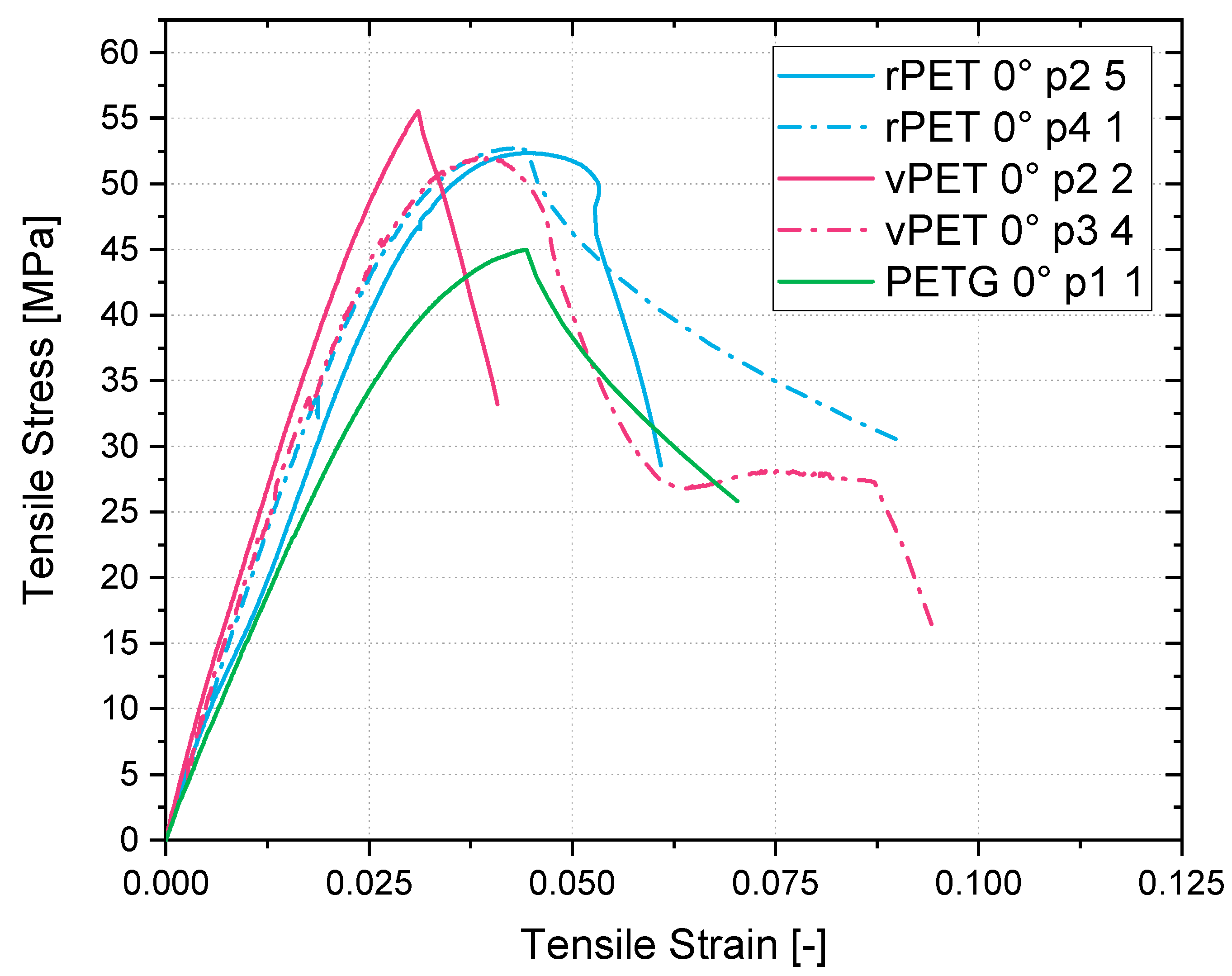
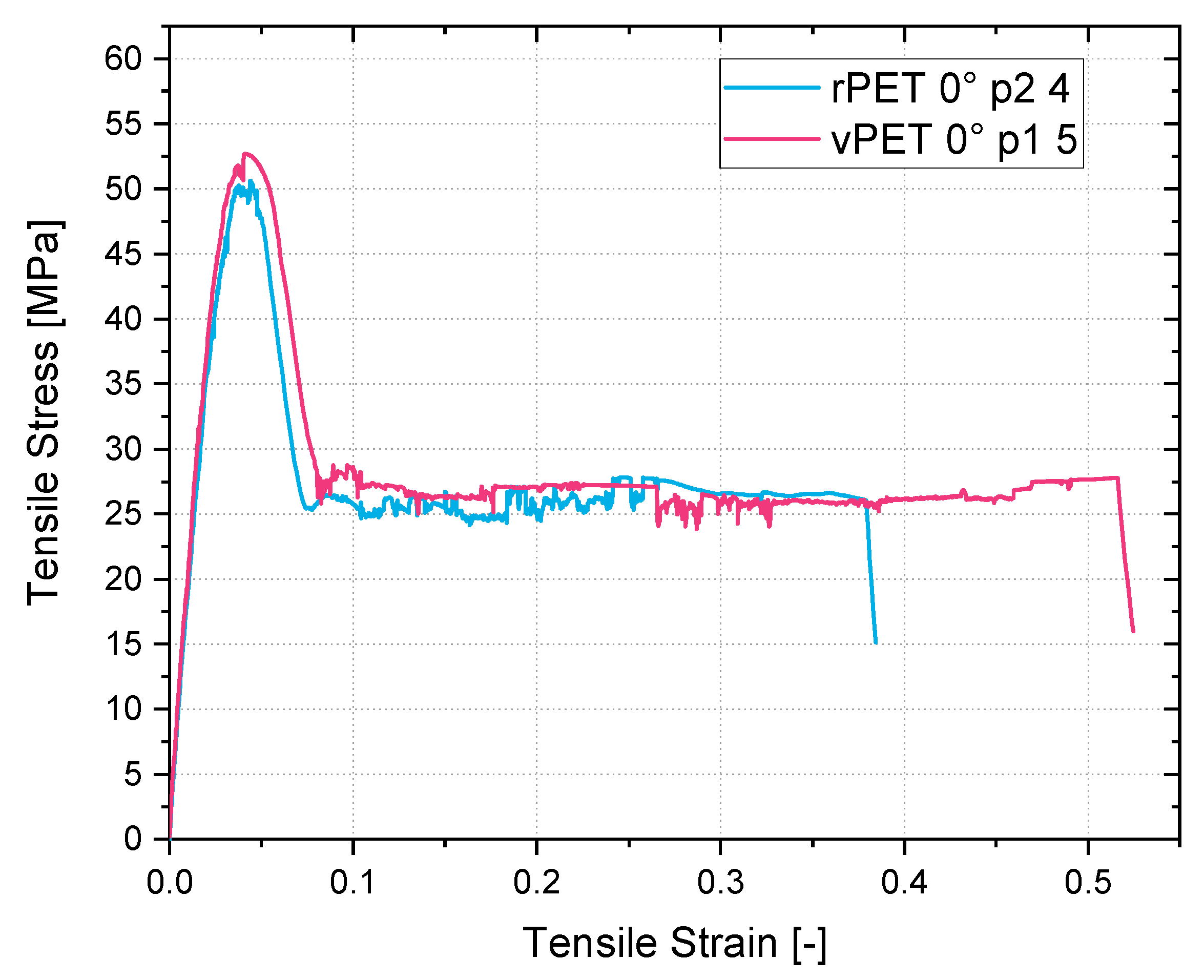
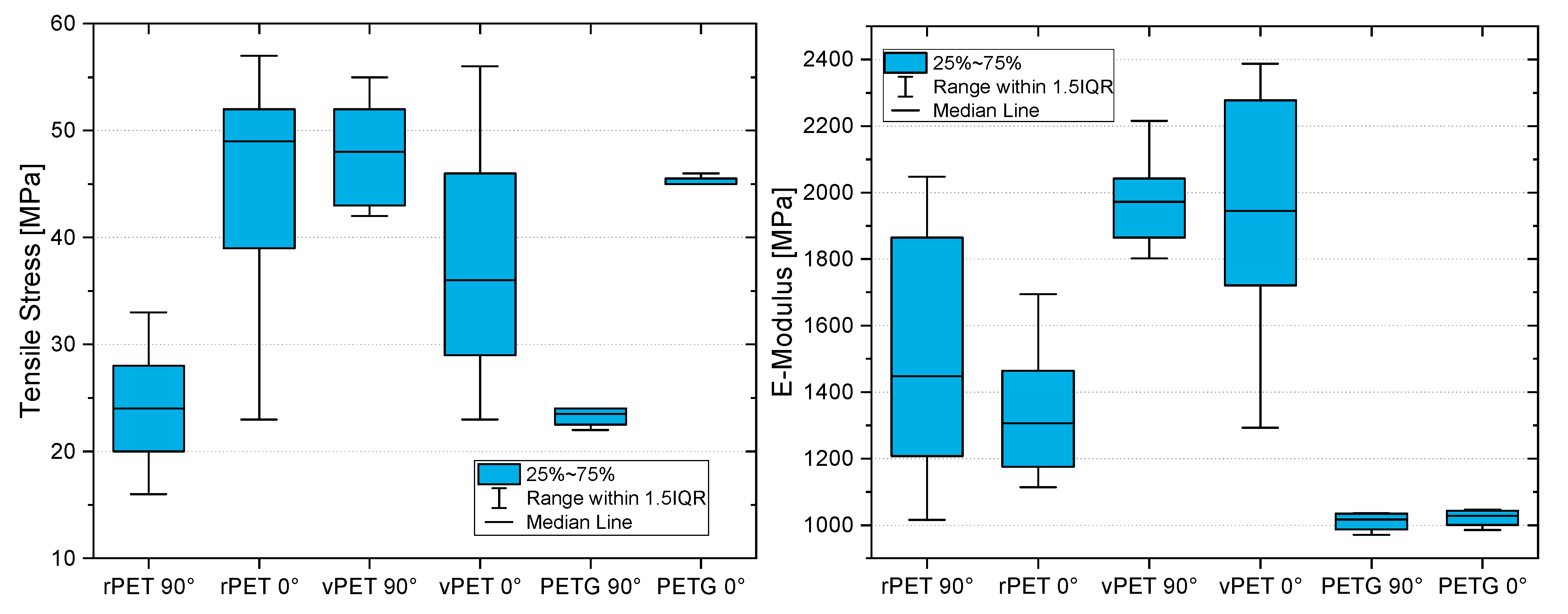
Apart from the advantages in miscibility and simple recyclability, the recycled resin presents higher tensile strengths and E-modulus in comparison to the glycol-modified PETG filament available on the market. As the results in this study clarify, the printed tensile coupons made from PETG presented a maximum tensile strength of 45 MPa with a bead orientation of 0° and of 24 MPa with a bead orientation of 90°. Compared to the PETG, the recycled coupon tests presented results of 53 MPa at a 0° bead orientation and of 33 MPa at a 90° bead orientation. This represents a 17% higher molecular strength and a 38% higher interlaminar strength of the recycled material in comparison to the commonly used filament for MEX.
6. Conclusions
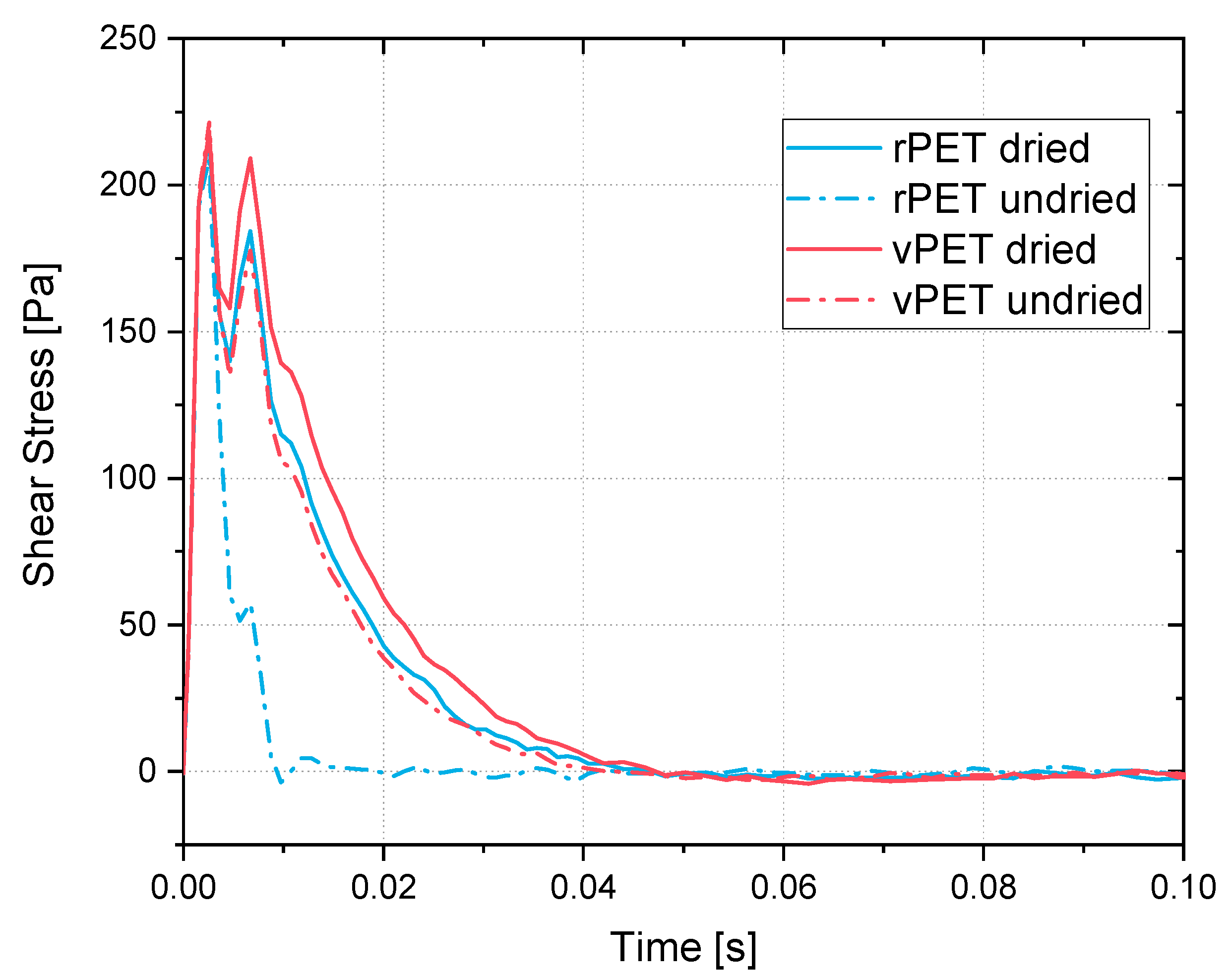
Within a growing additive manufacturing market and a greater need for recycling and reusing materials, the recycling of PET into a filament for the MEX process has proved to be a viable option. Washed and shredded PET bottles can be used as a material input for pellets. Drying the material prior to extrusion proved to be an important step, leading to higher complex viscosities in both vPET and rPET. These produced pellets can then, in turn, be extruded into a filament. Printed tensile coupons out of the recycled PET filament exhibited higher tensile strengths than the PETG filament available in the market. However, the highest tensile strengths were shown by the vPET. The final geometry and function of the recycled material is up to the capacity and imagination of each manufacturer, given the freedom offered by the MEX process. In addition to the potential use for additive manufacturing, the recycling of PET into a filament could also increase the merit of the material. Recycled PET can not only be recycled but even upcycled if being sold as a filament.
In order to improve the results and make the recycling of PET more feasible, further work needs to be carried out. Within the timeframe, some important factors were noted, but not all could be examined in detail. The propositions presented below can be used as guidance for future work.
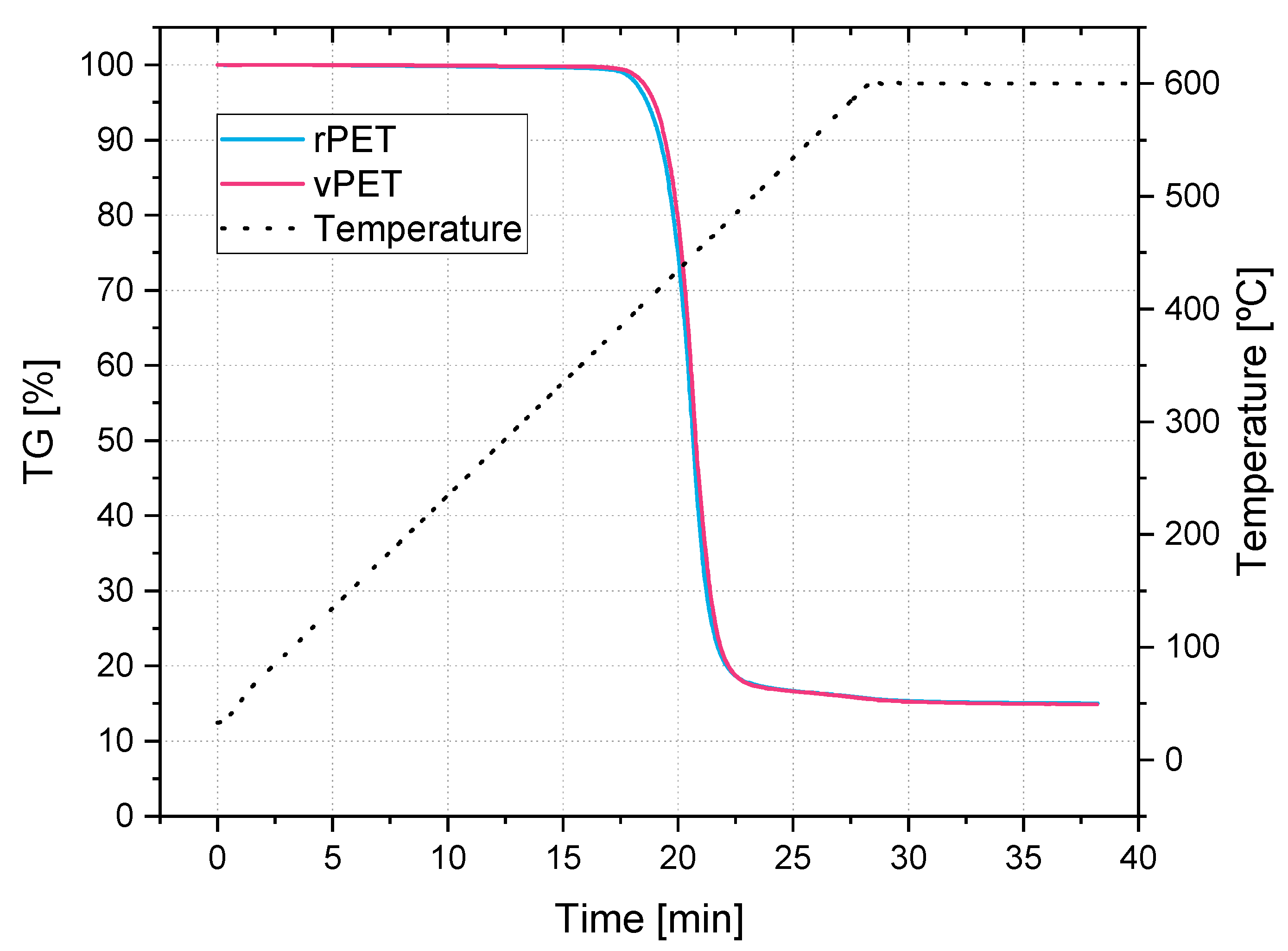
The drying procedure of the material needs to be properly analyzed. PET is sensitive to moisture and therefore needs to be dried before processing. Recommended moisture contents for processing vary in the previous literature on this subject. A better understanding of the drying process, including the drying atmosphere, drying temperature, drying time and resultant moisture content could lead to a better handling of the material and less degradation during processing. A further interesting parameter is the time in between the end of the drying procedure and the start of the extrusion process. Not all laboratories are capable of drying and blowing the material straight into the extruder. So, the influence of recess time and the humidity in the air would need to be considered too.
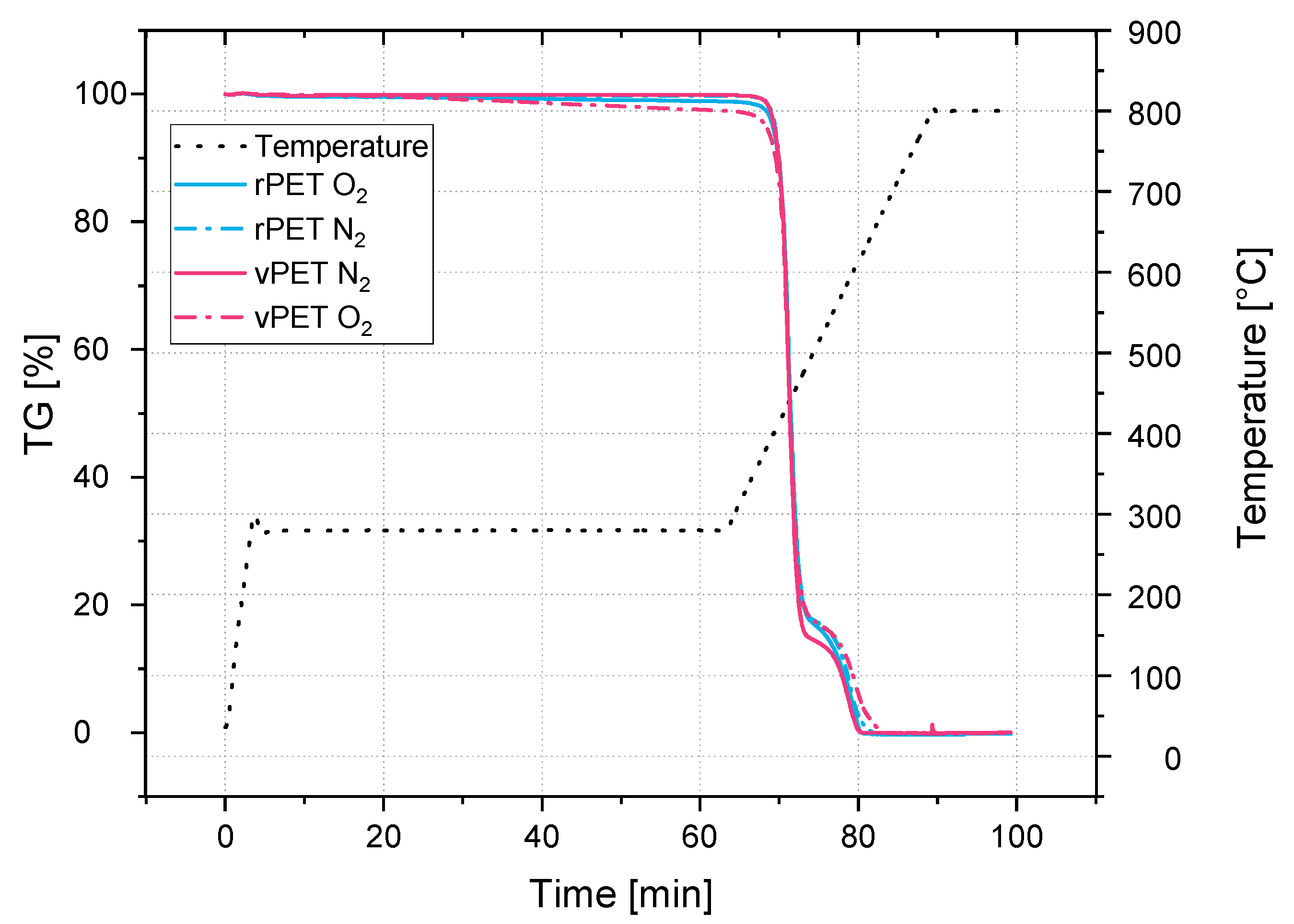
Similar to the point presented previously is the degradation of PET: this material degrades hydrolytically and thermally. Depending on prior drying and the processing temperatures, it undergoes different mechanisms of degradation. Which processing temperatures would be sufficient to obtain a stable rheological behavior and what amount of degraded material could still be tolerated need to be studied. The literature presents some degradation analysis of PET, but the results are not in context with the behavior during processing [
15,
16,
18].
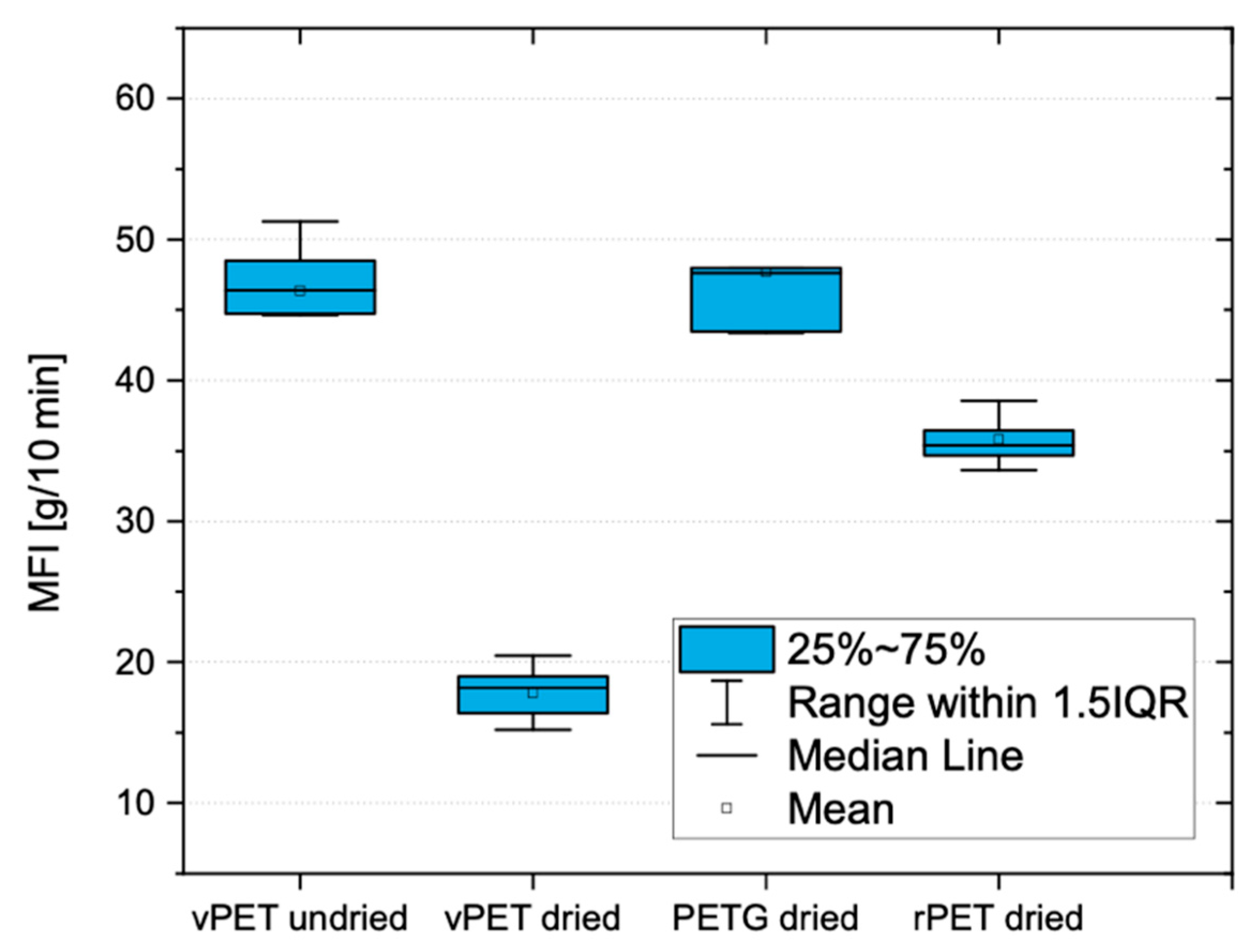
The viscosities of the vPET and the rPET are different in a dried and undried state. During the printing of the filament, different viscosities cause different material throughput through the nozzle. If the printing speed is equal for the different cases, there will be a variation in the amount of printed material. Therefore, the speeds must be adapted depending on the rheological behavior in order to obtain a homogeneous print. This could be complemented with an analysis of the influence of the amount of virgin PET mixed with the recycled one during extrusion. Different ratios could be tested in order to produce a stable and strong filament, using the highest amount of recycled material as possible.