Comparative Study of Manufacturing NdFeB Magnet Wastes Recycling: Oxidative Roasting-Selective Leaching and Whole Leaching Routes
Abstract
1. Introduction
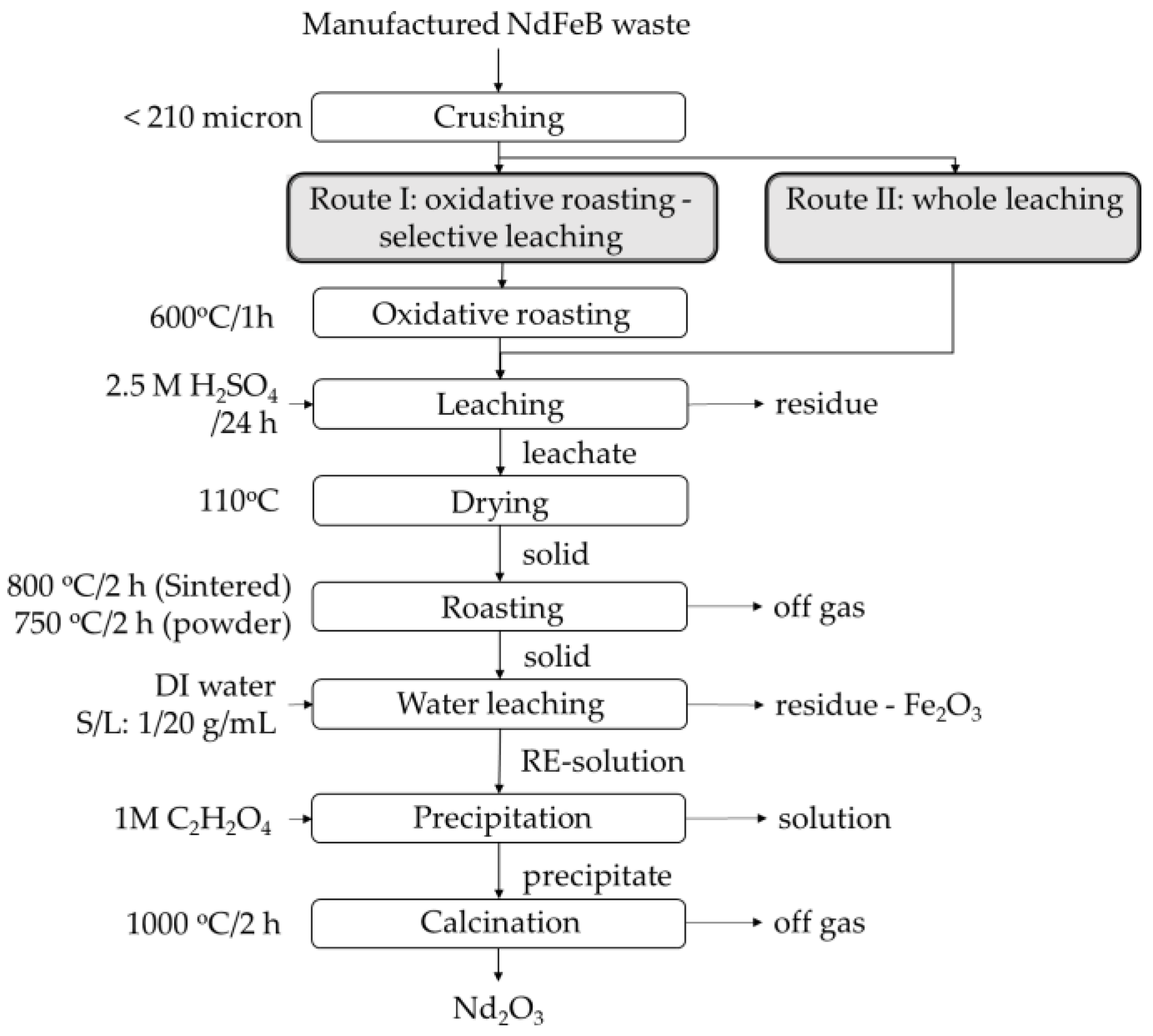
2. Experimental Procedure
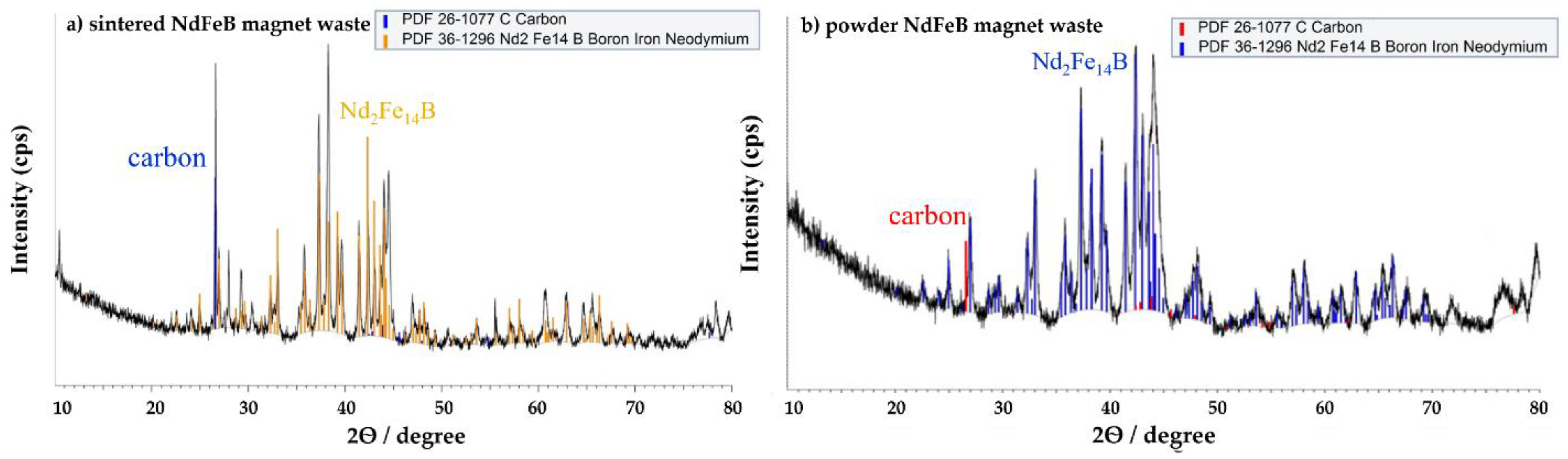
2.1. Waste Preparation
2.2. Recycling of Sintered NdFeB Magnet Wastes
3. Results and Discussion
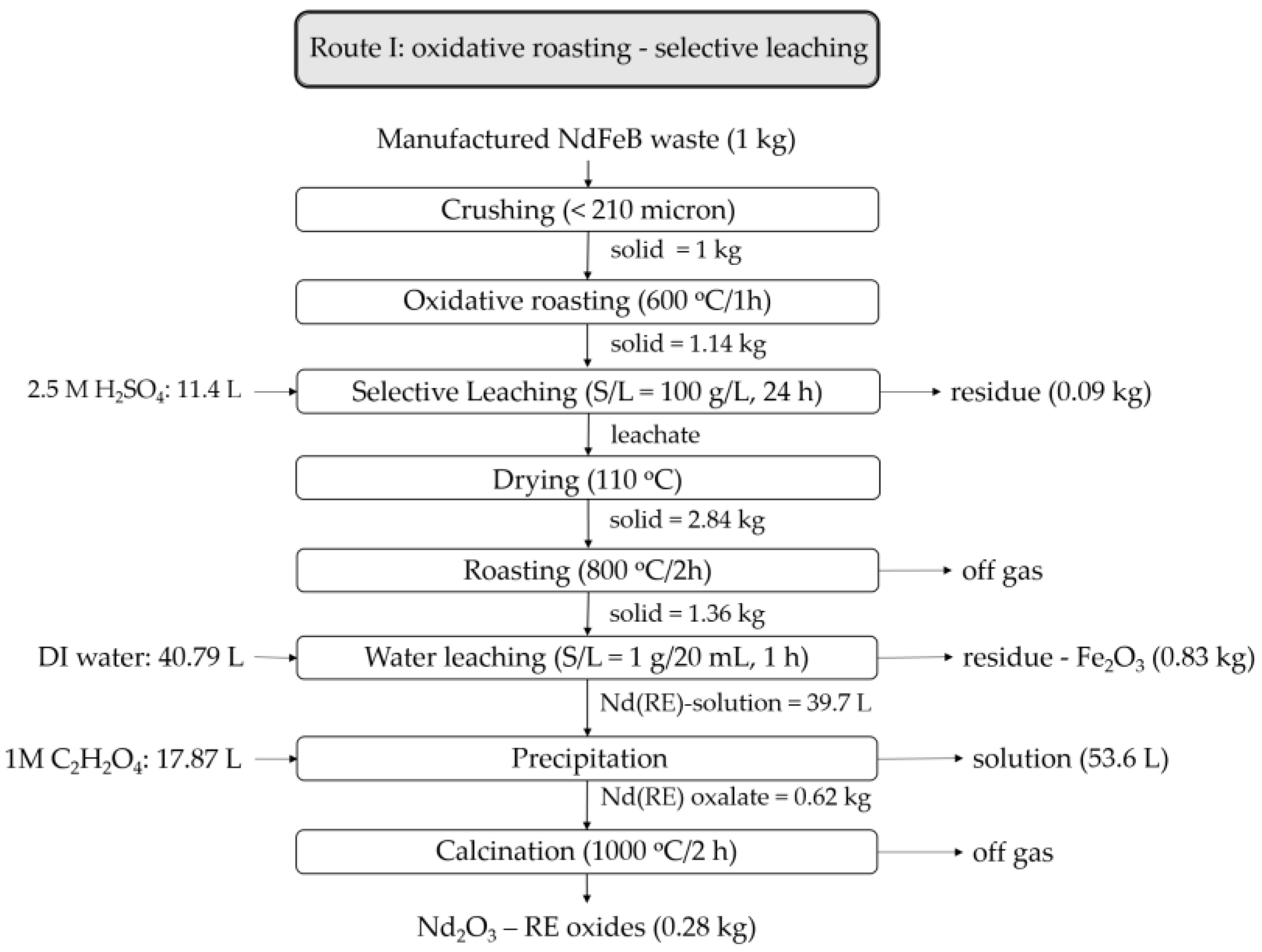
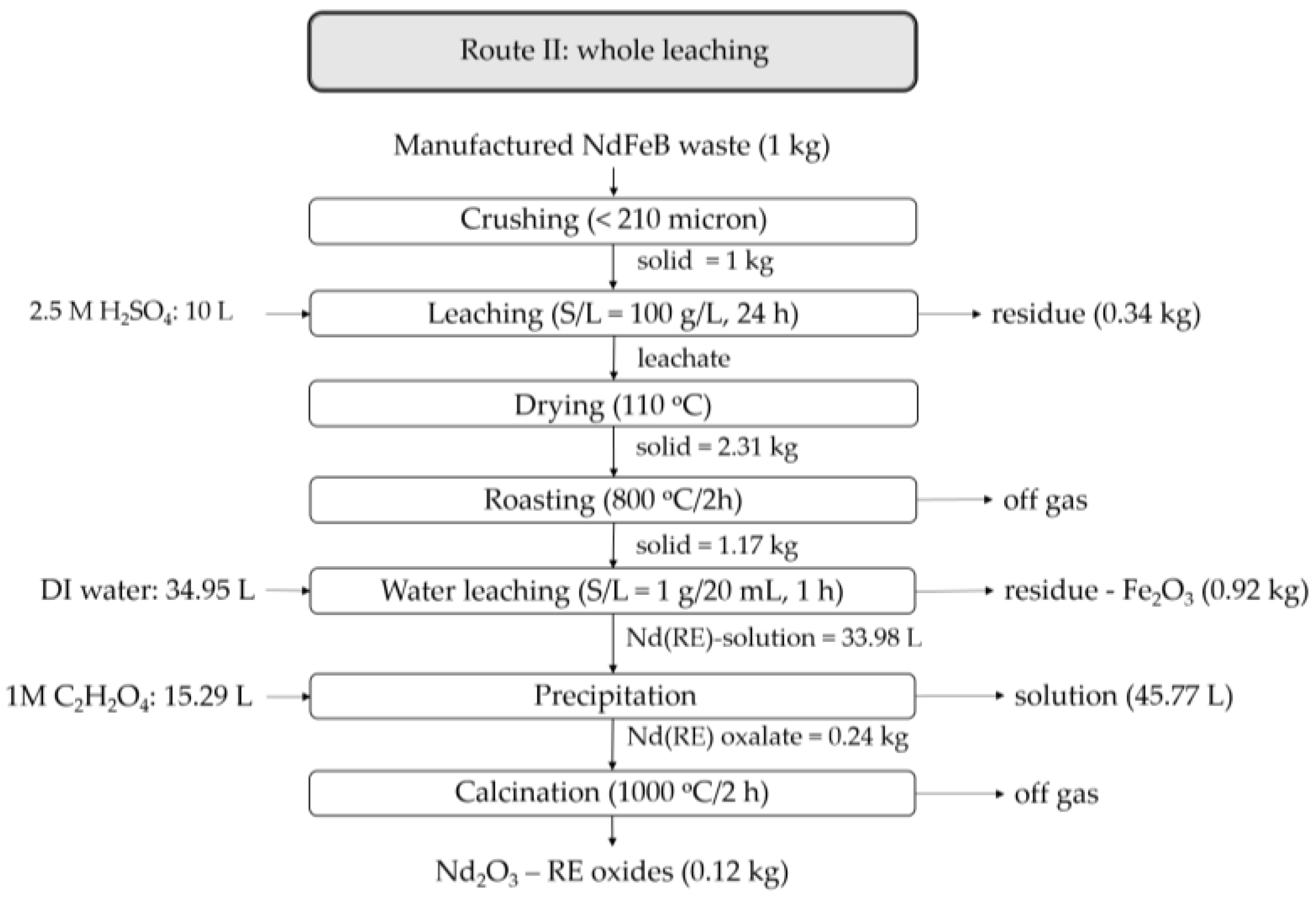
3.1. Phase Evolution and Products along the Course of Oxidative Roasting, Acid Leaching, Roasting, Water Leaching, Precipitation and Calcination
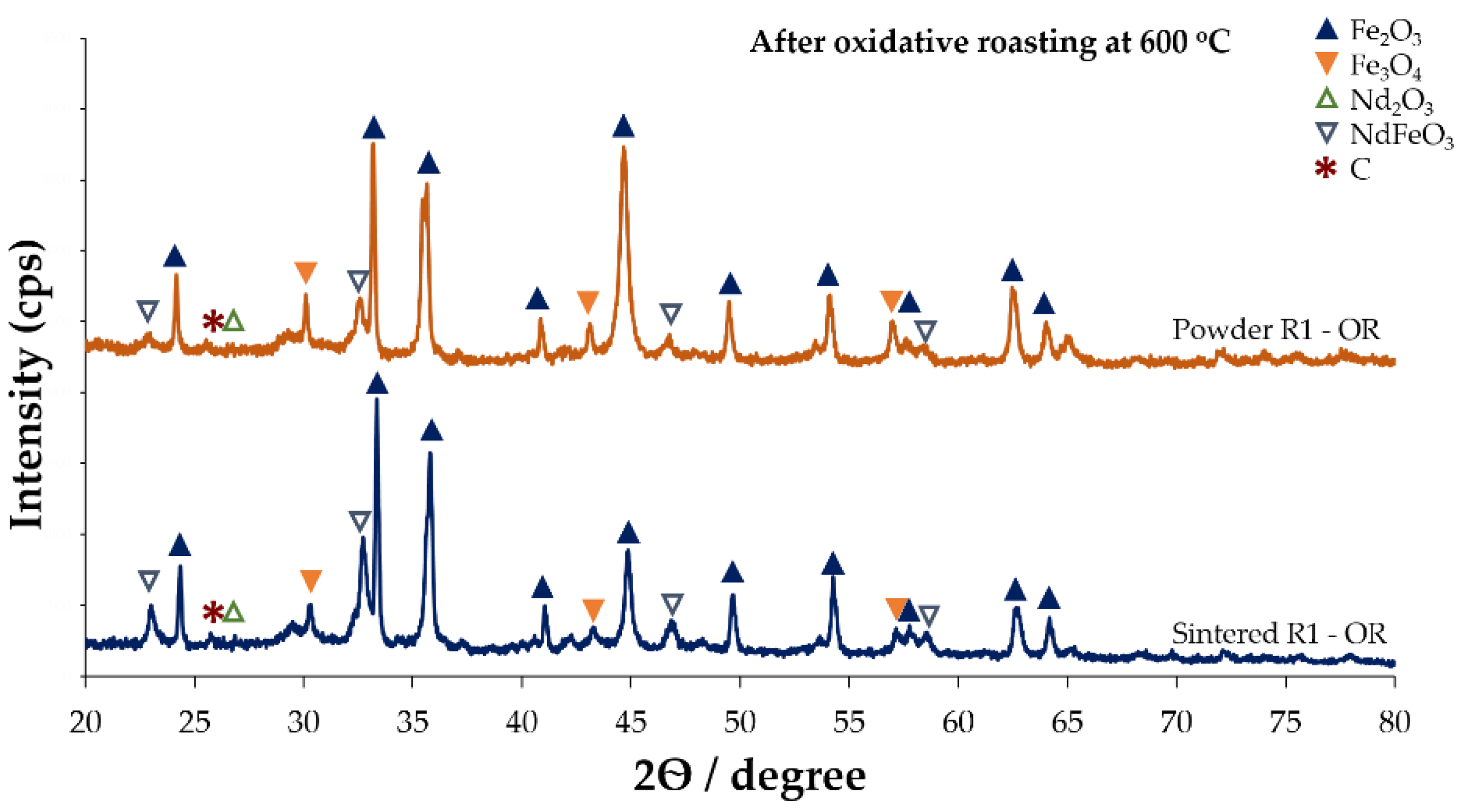
3.1.1. Effects of Oxidative Roasting on Phase Evolution
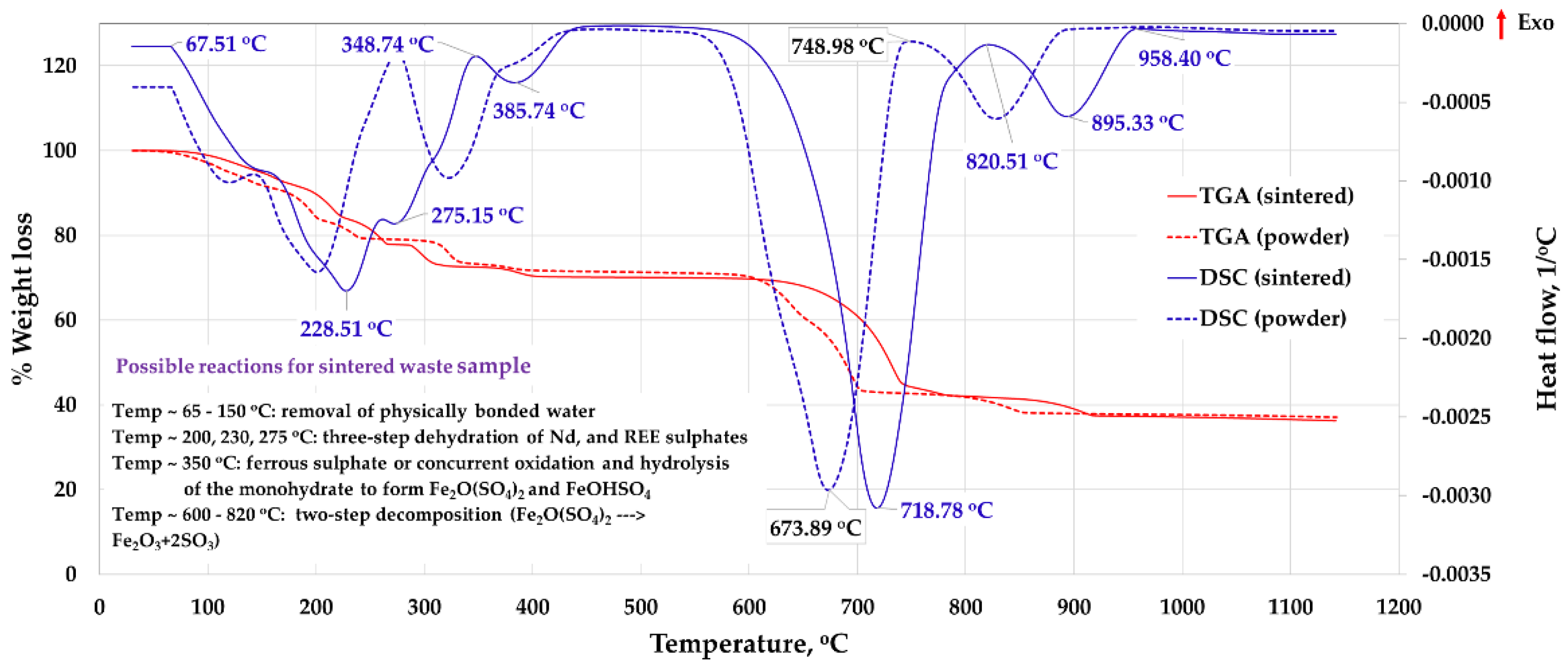
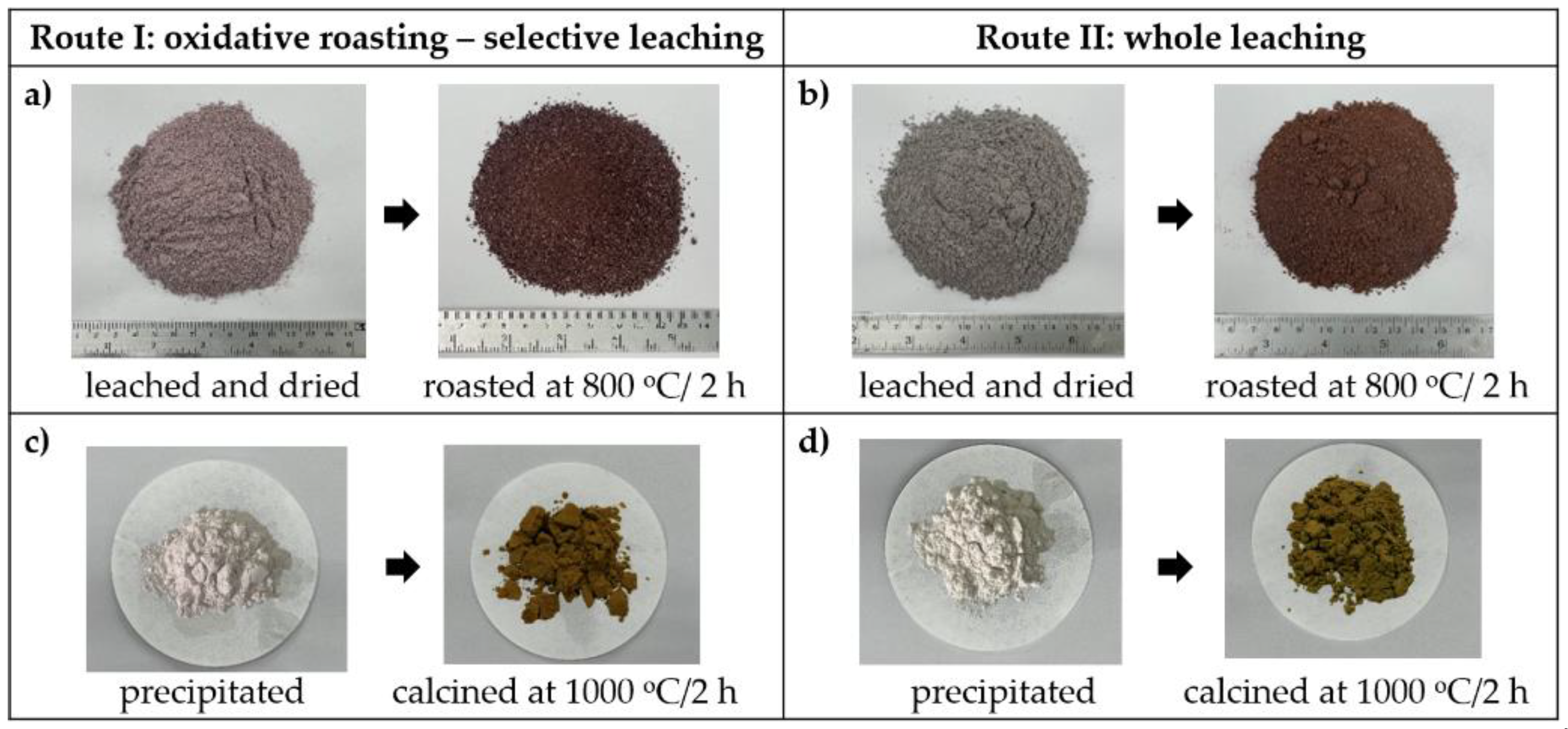
3.1.2. Acid Leaching and Drying
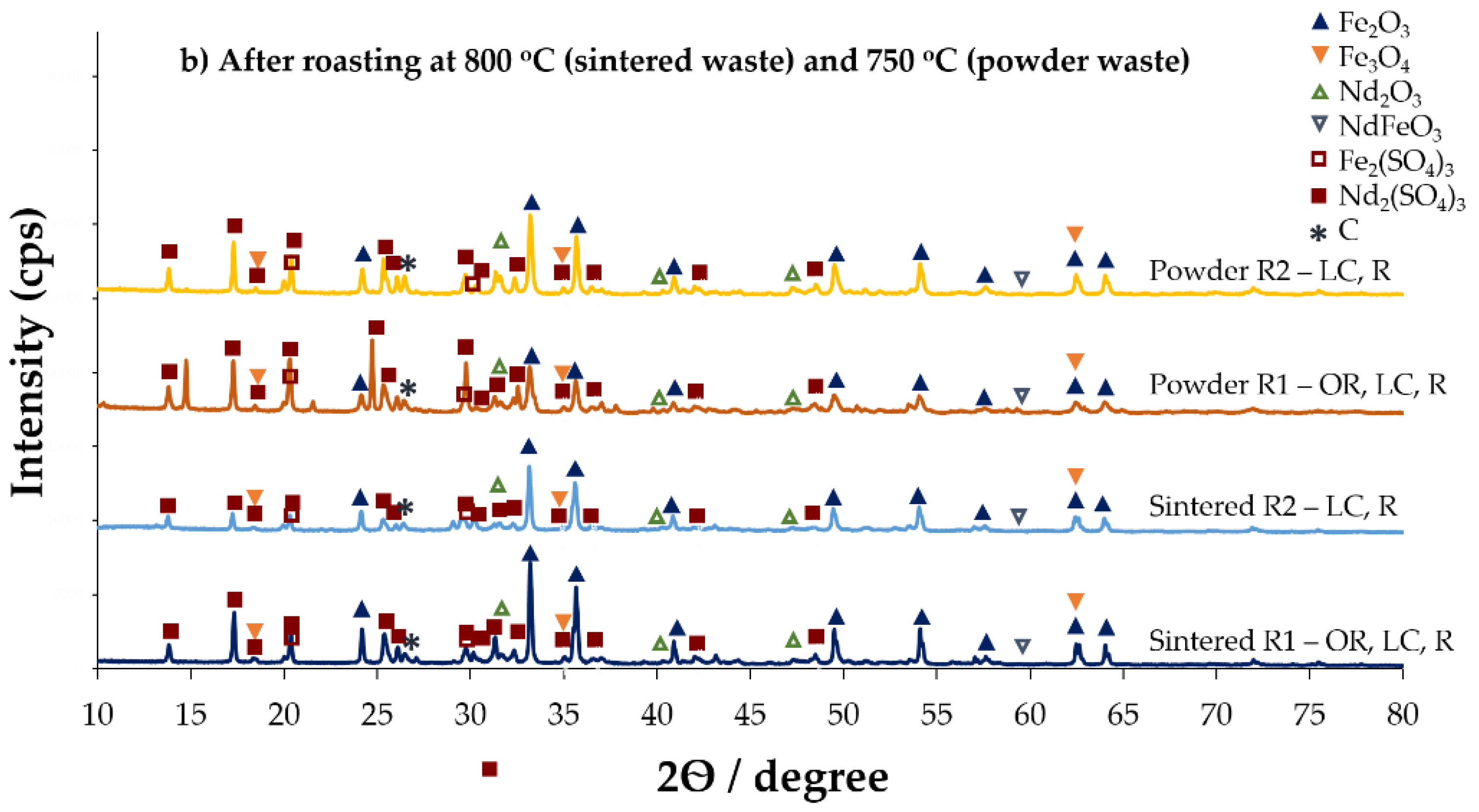
3.1.3. Roasting and Water Leaching
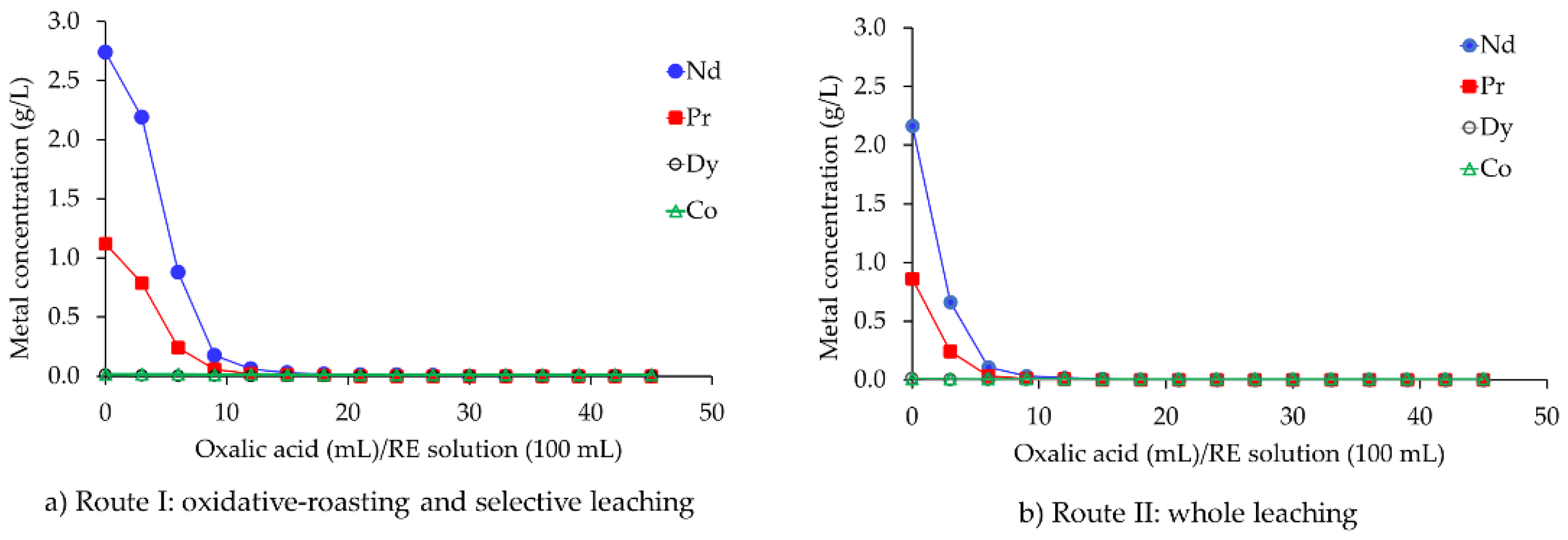
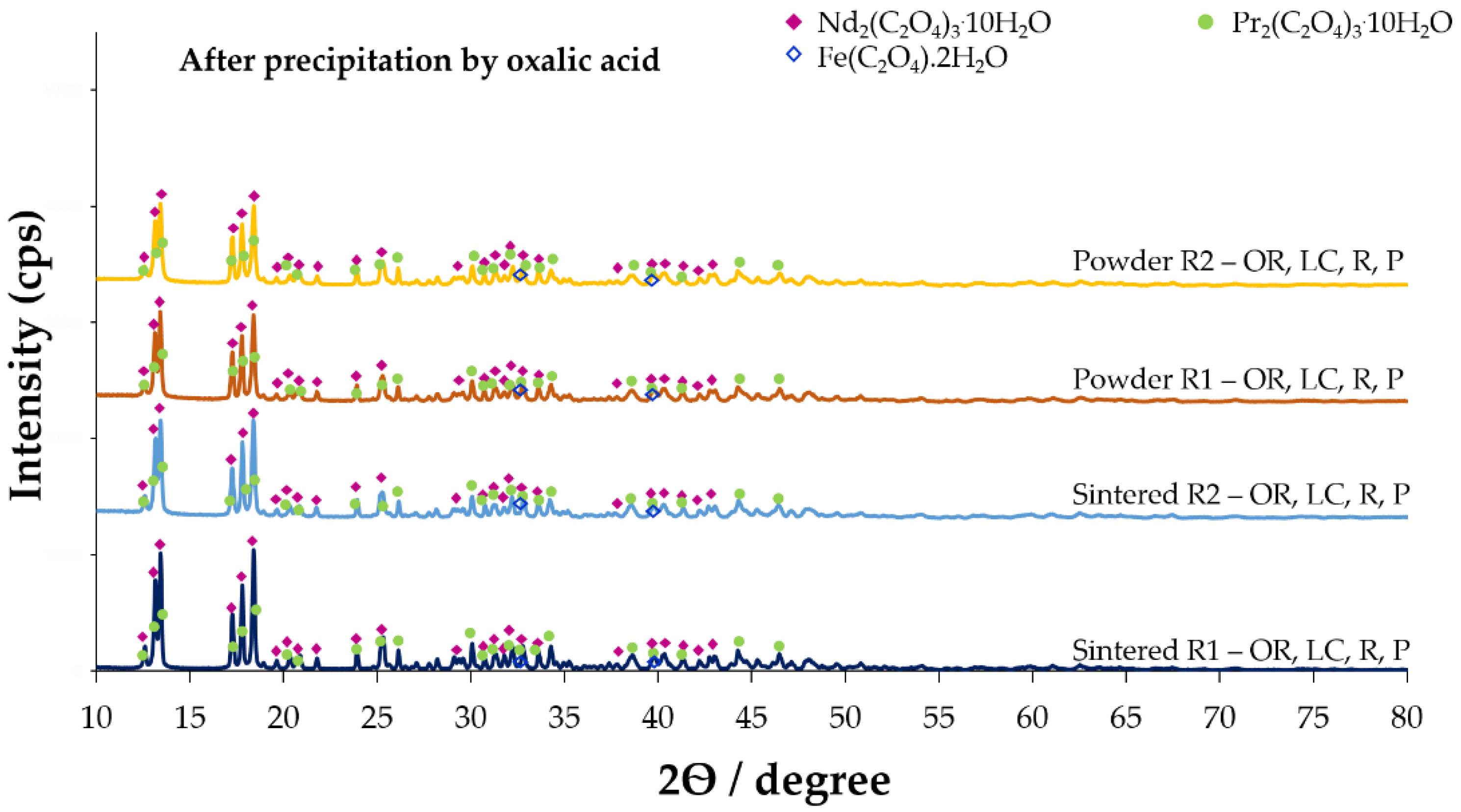
3.1.4. Precipitation of Neodymium Oxalate
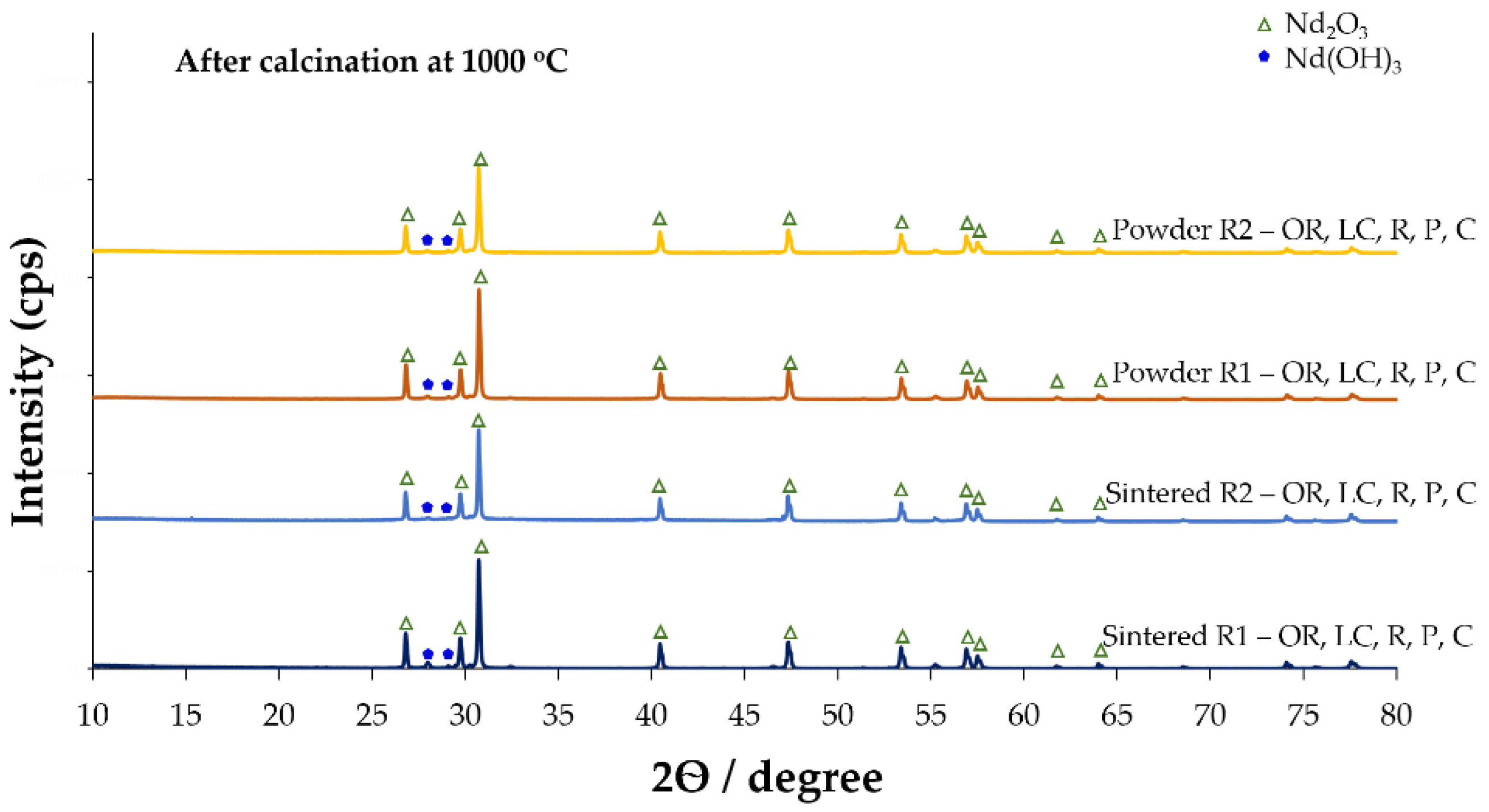
3.1.5. Calcination
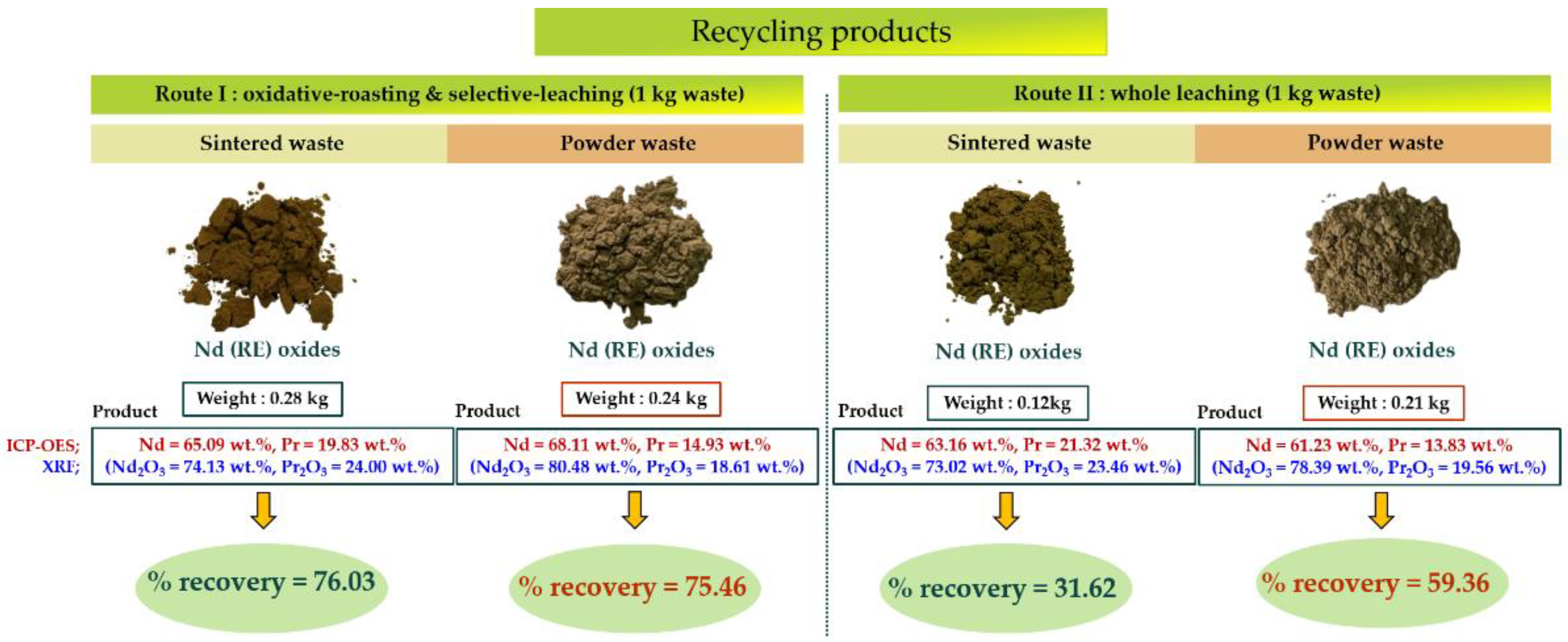
3.2. Recovery and Purity of Neodymium Oxide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baba, K.; Hiroshige, Y.; Nemoto, T. Rare-earth magnet recycling. Hitachi. Rev. 2013, 62, 452–454. [Google Scholar]
- Cui, J.; Ormerod, J.; Parker, D.; Ott, R.; Palasyuk, A.; Mccall, S.; Paranthaman, M.P.; Kesler, M.S.; Mcguire, M.A.; Nelbedim, I.C.; et al. Manufacturing processes for permanent magnets: Part I-sintering and casting. JOM 2022, 74, 1279–1295. [Google Scholar] [CrossRef]
- Schulze, R.; Buchert, M. Estimates of global REE recycling potentials from NdFeB magnet material. Resour. Conserv. Recycl. 2016, 113, 12–27. [Google Scholar] [CrossRef]
- Rademaker, J.H.; Kleijn, R.; Yang, Y. Recycling as a strategy against rare earth element criticality: A systemic evaluation of the potential yield of NdFeB magnet recycling. Environ. Sci. Technol. 2013, 47, 10129–10136. [Google Scholar] [CrossRef]
- Schreiber, A.; Marx, J.; Zapp, P.; Kuckshinrichs, W. Comparative life cycle assessment of neodymium oxide electrolysis in molten salt. Adv. Eng. Mater. 2020, 22, 1901206. [Google Scholar] [CrossRef]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Recycling of rare earths from scrap. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsvier: Amsterdam, The Netherlands, 2013; Volume 43, pp. 159–211. [Google Scholar]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Gerven, T.V. Hydrometallurgical recycling of NdFeB magnets: Complete leaching, iron removal and electrolysis. J. Rare Earths 2017, 35, 574–584. [Google Scholar] [CrossRef]
- Klemettinen, A.; Zak, A.; Chojnacka, I.; Matuska, S.; Leśniewicz, A.; Wełna, M.; Adamski, Z.; Klemettinen, L.; Rycerz, L. Leaching of rare earth elements from NdFeB magnets without mechanical pretreatment by sulfuric (H2SO4) and hydrochloric (HCl) acids. Minerals 2021, 11, 1374. [Google Scholar] [CrossRef]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and recovery of rare-earth elements from neodymium magnet waste using organic acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Loy, S.V.; Önal, M.A.R.; Binnemans, K.; Gerven, T.V. Recovery of valuable metals from NdFeB magnets by mechanochemically assisted ferric sulfate leaching. Hydrometallurgy 2019, 191, 105154. [Google Scholar]
- Yoon, H.-S.; Kim, C.-J.; Lee, J.-Y.; Kim, S.-D.; Kim, J.-S.; Lee, J.-C. Separation of neodymium from NdFeB permanent magnetic scrap. J. Korean Inst. Resour. Recycl. 2003, 12, 57–63. [Google Scholar]
- Liu, F.; Porvali, A.; Halli, P.; Wilson, B.P.; Lundström, M. Comparison of different leaching media and their effect on REEs recovery from spent Nd-Fe-B magnets. JOM 2020, 72, 806–815. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Wang, J.; Wang, H.; Peng, C.; Wilson, B.P.; Lundström, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets. Miner. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- Xin, W.; Deng, Y.; Jiang, Y.; Yuan, Y.; Wang, P. Phase evolution and oxidation characteristics of the Nd-Fe-B and Ce-Fe-B magnet scrap powder during the roasting process. High Temp. Mater. Process. 2020, 39, 477–488. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Gerven, T.V.; Jones, P.T.; et al. REE recovery from end-of-life NdFeB permanent magnet scrap: A critical review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, W.B.; Jeong, W.B.; Yoon, I.J. Extraction of neodymium from Nd-Fe-B magnet scraps by sulfuric acid. J. Korean Inst. Met. Mater. 1998, 36, 967–972. [Google Scholar]
- Asabe, K.; Saguchi, A.; Takahashi, W.; Suzuki, R.O.; Ono, K. Recycling of rare earth magnet scraps: Part I carbon removal by high temperature oxidation. Mater. Trans. 2001, 42, 2487–2491. [Google Scholar] [CrossRef]
- Saguchi, A.; Asabe, K.; Takahasi, W.; Suzuki, R.O.; Ono, K. Recycling of rare earth magnet scraps part III carbon removal from Nd magnet grinding sludge under vacuum heating. Mater. Trans. 2002, 43, 256–260. [Google Scholar] [CrossRef]
- Saguchi, A.; Asabe, K.; Fukuda, T.; Takahashi, W.; Suzuki, R.O. Recycling of rare earth magnet scraps: Carbon and oxygen removal from Nd magnet scraps. J. Alloys. Compd. 2006, 408–412, 1377–1381. [Google Scholar] [CrossRef]
- Kritsarikan., W.; Patcharawit, T.; Yingkakorn, T.; Khumkoa, S. Recycling of sintered NdFeB magnet waste via oxidative roasting and selective leaching. Int. J. Miner. Metall. Mater. 2021, 15, 181–186. [Google Scholar]
- Salmon, O.N. High temperature thermodynamics of the iron oxide systems. J. Phys. Chem. A 1960, 65, 550–556. [Google Scholar] [CrossRef]
- Kim, C.-J.; Yoon, H.-S.; Chung, K.W.; Lee, J.-Y.; Kim, S.-D.; Shin, S.M.; Kim, H.-S.; Cho, J.-T.; Kim, J.-H.; Lee, E.-J.; et al. Leaching kinetics of praseodymium in sulfuric acid of rare earth elements (REE) slag concentrated by pyrometallurgy from magnetic ore. Korean Chem. Eng. Res. 2015, 53, 46–52. [Google Scholar] [CrossRef][Green Version]
- Almeida, L.D.; Grandjean, S.; Vigier, N.; Patisson, F. Insights into the thermal decomposition of lanthanide (III) and actinide (III) oxalates—From neodymium and cerium to plutonium. Eur. J. Inorg. Chem. 2012, 2012, 4986–4999. [Google Scholar] [CrossRef]
- Ekthammathat, N.; Phuruangrat, A.; Kuntalue, B.; Thongtem, S.; Thongtem, T. Preparation of neodymium hydroxide nanorods and neodymium oxide nanorods by a hydrothermal method. Dig. J. Nanomater. Biostructures 2015, 10, 715–719. [Google Scholar]
- Feldhaus, D.; Chvetković, V.S.; Vulkićević, N.M.; Barudžija, T.S.; Friedrich, B.; Jovićević, J.N. Electrochemical deposition of Nd and Pr on Mo from molten fluoride. In Proceedings of the 15th International Conference on Fundamental and Applied Aspects of Physical Chemistry, Virtual Meeting, 20–24 September 2021. [Google Scholar]
- Feldhaus, D.; Tschauner, M.-T.; Friedrich, B. Influence of LiF on the synthesis of the neodymium & praseodymium molten salt electrolysis. In Proceedings of the European Metallurgical Conference, Salzburg Congress, Salzburg, Austria, 27–30 June 2021. [Google Scholar]





| Type | Element (wt.%) | |||||
|---|---|---|---|---|---|---|
| Fe | Nd | Pr | B | Dy | Other | |
| sintered waste | 59.81 | 23.97 | 7.124 | 0.261 | 0.062 | balanced |
| powder waste | 66.33 | 21.66 | 4.760 | - | 0.056 | balanced |
| Recycling Step | Element (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Nd | Pr | Co | O | S | Other | |
| Crushed magnet | 67.50 | 18.705 | 6.29 | 3.85 | 0.63 | 0.02 | balanced |
| Route I: Oxidative roasting—selective leaching | |||||||
| OR | 74.75 | 14.80 | 4.66 | 3.39 | 0.85 | 0.02 | balanced |
| OR, LC | 68.42 | 10.37 | 3.31 | 4.65 | 1.34 | 11.17 | balanced |
| OR, LC residue | 94.93 | 1.72 | 0.58 | 0.74 | 0.33 | 1.01 | balanced |
| OR, LC, R | 77.07 | 11.28 | 3.68 | 5.45 | 0.79 | 1.40 | balanced |
| OR, LC, R, WL residue | 92.15 | 0.41 | 0.11 | 6.31 | 0.61 | 0.17 | balanced |
| OR, LC, R, WL, PP | 0.50 | 71.89 | 22.63 | - | 3.19 | 0.18 | balanced |
| OR, LC, R, WL, PP, C | 0.31 | 73.81 | 23.80 | - | 0.83 | 0.07 | balanced |
| Route II: whole leaching | |||||||
| LC | 71.49 | 7.65 | 2.40 | 4.33 | 0.69 | 13.06 | balanced |
| LC residue | 0.92 | 67.12 | 20.29 | - | 0.87 | 8.70 | balanced |
| LC, R | 83.81 | 7.40 | 2.45 | 5.03 | - | 1.06 | balanced |
| LC, R, WL residue | 93.14 | 0.71 | 0.21 | 5.42 | 0.26 | 0.07 | balanced |
| LC, R, WL, PP | 0.45 | 73.80 | 24.47 | - | 0.56 | 0.12 | balanced |
| LC, R, WL PP, C | 1.20 | 72.59 | 23.22 | - | 1.45 | 0.13 | balanced |
| Waste | Route | Composition, wt.% | % Recovery | |||
|---|---|---|---|---|---|---|
| Nd | Pr | Fe | Others | |||
| Sintered | I | 65.09 | 19.83 | 0.31 | balanced | 76.03 |
| II | 63.16 | 21.32 | 1.20 | balanced | 31.62 | |
| Powder | I | 68.11 | 14.93 | 0.30 | balanced | 75.46 |
| II | 61.23 | 13.83 | 0.51 | balanced | 59.36 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patcharawit, T.; Kritsarikan, W.; Yingnakorn, T.; Khumkoa, S. Comparative Study of Manufacturing NdFeB Magnet Wastes Recycling: Oxidative Roasting-Selective Leaching and Whole Leaching Routes. Recycling 2022, 7, 68. https://doi.org/10.3390/recycling7050068
Patcharawit T, Kritsarikan W, Yingnakorn T, Khumkoa S. Comparative Study of Manufacturing NdFeB Magnet Wastes Recycling: Oxidative Roasting-Selective Leaching and Whole Leaching Routes. Recycling. 2022; 7(5):68. https://doi.org/10.3390/recycling7050068
Chicago/Turabian StylePatcharawit, Tapany, Woranittha Kritsarikan, Tanongsak Yingnakorn, and Sakhob Khumkoa. 2022. "Comparative Study of Manufacturing NdFeB Magnet Wastes Recycling: Oxidative Roasting-Selective Leaching and Whole Leaching Routes" Recycling 7, no. 5: 68. https://doi.org/10.3390/recycling7050068
APA StylePatcharawit, T., Kritsarikan, W., Yingnakorn, T., & Khumkoa, S. (2022). Comparative Study of Manufacturing NdFeB Magnet Wastes Recycling: Oxidative Roasting-Selective Leaching and Whole Leaching Routes. Recycling, 7(5), 68. https://doi.org/10.3390/recycling7050068







