Grape Marc Flour as a Horticulture By-Product for Application in the Meat Industry
Abstract
1. Introduction
2. Results
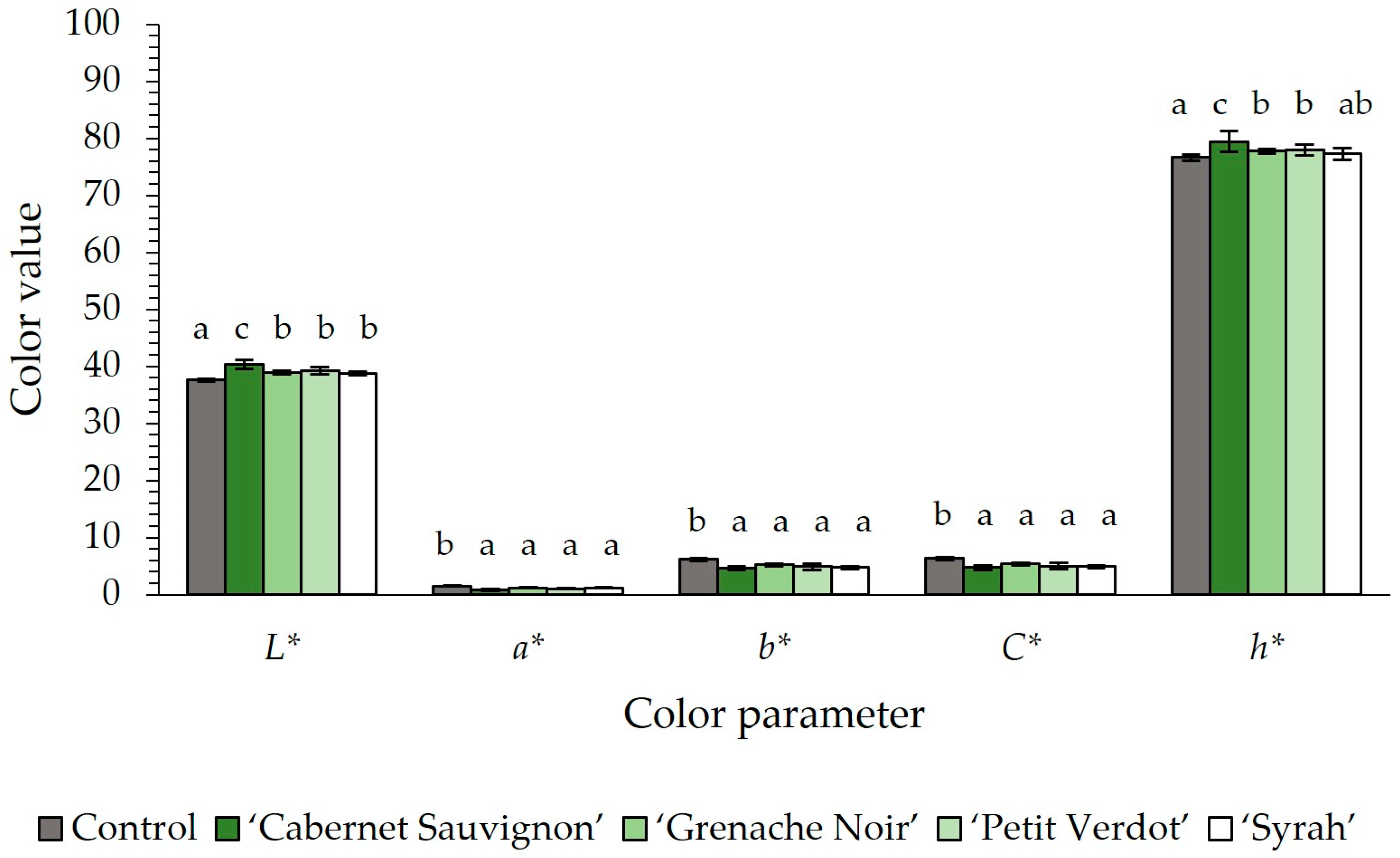
2.1. pH, Color, and Techno-Functional Properties
2.2. Polyphenol Content and Antioxidant Activity
2.3. Oxidative Stability of Extracts Inside a Meat Matrix
3. Discussion
4. Materials and Methods
4.1. Flours and Extracts
4.2. Physicochemical and Techno-Functional Properties
4.3. Antioxidant Activity and Polyphenol Content
4.4. Oxidative Stability of the Extracts Inside a Meat Matrix
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L* | Brightness |
| a* | Redness |
| b* | Yellowness |
| C* | Chroma |
| h* | Hue |
| WRC | Water retention capacity |
| ORC | Oil retention capacity |
| SWC | Swelling capacity |
| EMC | Emulsifying capacity |
| GLC | Gelling capacity |
| TPC | Total phenolic content |
| TTC | Total tannin content |
| TFVC | Total flavonoid content |
| TCGA | Total chlorogenic acid content |
References
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Boff, J.M.; Strasburg, V.J.; Ferrari, G.T.; De Oliveira Schmidt, H.; Manfroi, V.; De Oliveira, V.R. Chemical, technological, and sensory quality of pasta and bakery products made with the addition of grape marc flour. Foods 2022, 11, 3812. [Google Scholar] [CrossRef]
- Silva, M.E.D.S.; Grisi, C.V.B.; Silva, S.P.D.; Madruga, M.S.; Silva, F.A.P.D. The technological potential of agro-industrial residue from grape pulping (Vitis spp.) for application in meat products: A review. Food Biosci. 2022, 49, 101877. [Google Scholar] [CrossRef]
- Yalcin, E.; Ozdal, T.; Gok, I. Investigation of textural, functional, and sensory properties of muffins prepared by adding grape seeds to various flours. J. Food Process. Preserv. 2022, 46, e15316. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Grape marc as innovative flour for the formulation of functional muffins: How particle size affects the nutritional, textural and sensory properties. Foods 2022, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape marc extracts obtained under different extraction systems on meat quality of pork burgers. LWT 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Amin, R.A.; Edris, S.N. Grape seed extract as natural antioxidant and antibacterial in minced beef. ACS FST 2022, 2, 89–96. [Google Scholar]
- López-Marcos, M.C.; Bailina, C.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. Properties of dietary fibers from agroindustrial coproducts as source for fiber-enriched foods. Food Bioprocess Tech. 2015, 8, 2400–2408. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Englert, A.H.; Corrêa, A.P.F.; Brandelli, A.; Ferreira Marczak, L.D.; Tessaro, I.C. Grape marc powder: Physicochemical and microbiological stability during storage and moisture sorption isotherm. Food Bioprocess Tech. 2014, 7, 2500–2506. [Google Scholar] [CrossRef]
- San-Martín-Hernández, C.S.; Martínez-Téllez, M.Á.; Sañudo-Barajas, J.A.; Quintana-Obregón, E.A. Characterization of Cabernet, Grenache, and Syrah grape marc powders produced in Northwestern Mexico. Emir. J. Food Agric. 2021, 33, 846–851. [Google Scholar]
- Torres-Martínez, B.D.M.; Vargas-Sánchez, R.D.; Pérez-Alvarez, J.Á.; Fernández-López, J.; Esqueda, M.C.; Rodríguez-Carpena, J.G.; Ibarra-Arias, F.J.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Recovery of an additive for pork meat from Pleurotus ostreatus grown in agro-industrial wastes. Biotecnia 2025, 27, e2396. [Google Scholar] [CrossRef]
- Zhu, F.-M.; Du, B.; Li, J. Effect of ultrafine grinding on physicochemical and antioxidant properties of dietary fiber from wine grape marc. Food Sci. Technol. Int. 2014, 20, 55–62. [Google Scholar] [CrossRef]
- Alvarez-Ossorio, C.; Orive, M.; Sanmartín, E.; Alvarez-Sabatel, S.; Labidi, J.; Zufia, J.; Bald, C. Composition and techno-functional properties of grape seed flour protein extracts. ACS Food Sci. Technol. 2022, 2, 125–135. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the US: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Pereira, A.; Lee, H.C.; Lammert, R.; Wolberg, C.; Ma, D.; Immoos, C.; Casassa, F.; Kang, I. Effects of red-wine grape marc on the quality and sensory attributes of beef hamburger patty. Int. J. Food Sci. Tech. 2022, 57, 1814–1823. [Google Scholar] [CrossRef]
- Sáez, M.I.; Sabio, J.; Galafat, A.; Vizcaíno, A.J.; Alarcón-López, F.J.; Martínez Moya, T.F. Evaluation of white grape marc extract as an additive to extend the shelf-life of fish fillets. Foods 2025, 14, 1438. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011, 87, 46–53. [Google Scholar] [CrossRef]
- Garzon, A.; Bravo, I.; Barbero, A.J.; Albaladejo, J. Mechanistic and kinetic study on the reactions of coumaric acids with reactive oxygen species: A DFT approach. J. Agric. Food Chem. 2014, 62, 9705–9710. [Google Scholar] [CrossRef]
- Wu, H.; Bak, K.H.; Goran, G.V.; Tatiyaborworntham, N. Inhibitory mechanisms of polyphenols on heme protein-mediated lipid oxidation in muscle food: New insights and advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 4921–4939. [Google Scholar] [CrossRef]
- Kumar, N.; Saxena, D.C.; Singh, S. Techno-Functional, Thermal, Structural, and Morphological Properties of Indian Halim (Lepidium sativum) Seed Flour. J. Food Sci. 2025, 90, e70388. [Google Scholar] [CrossRef]
- Matić, P.; Jakobek, L. Spectrophotometric Folin-Ciocalteu and aluminum chloride method validation for the determination of phenolic acid, flavan-3-ol, flavonol, and anthocyanin content. Croat. J. Food Sci. Technol. 2021, 13, 176–183. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.W.; Bain, H.; Dale, M.F.B. Development of a rapid colorimetric method for the determination of chlorogenic acid in freeze-dried potato tubers. J. Sci. Food Agric. 1992, 58, 41–48. [Google Scholar] [CrossRef]
- Takatsuka, M.; Goto, S.; Kobayashi, K.; Otsuka, Y.; Shimada, Y. Evaluation of pure antioxidative capacity of antioxidants: ESR spectroscopy of stable radicals by DPPH and ABTS assays with singular value decomposition. Food Biosci. 2022, 48, 101714. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Demirata, B.; Apak, R. A Novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. Anal. Methods 2010, 2, 1770. [Google Scholar] [CrossRef]

| Assay | Control | ‘Cabernet Sauvignon’ | ‘Grenache Noir’ | ‘Petit Verdot’ | ‘Syrah’ |
|---|---|---|---|---|---|
| pH | 6.44 ± 0.01 e | 3.77 ± 0.01 c | 3.72 ± 0.01 b | 3.87 ± 0.01 d | 3.66 ± 0.01 a |
| L* | 80.16 ± 0.26 e | 41.96 ± 0.08 c | 51.75 ± 0.60 d | 38.57 ± 0.13 a | 39.34 ± 0.16 b |
| a* | 2.61 ± 0.21 a | 6.43 ± 0.08 d | 7.52 ± 0.23 e | 4.08 ± 0.08 b | 5.01 ± 0.11 c |
| b* | 20.39 ± 0.45 e | 4.37 ± 0.08 c | 9.11 ± 0.25 d | 1.90 ± 0.08 a | 2.47 ± 0.13 b |
| C* | 20.52 ± 0.48 e | 7.77 ± 0.08 c | 11.82 ± 0.33 d | 4.50 ± 0.08 a | 5.58 ± 0.14 b |
| h* | 82.73 ± 0.36 e | 34.17 ± 0.61 c | 50.47 ± 0.42 d | 24.99 ± 0.86 a | 26.21 ± 0.94 b |
| Visual color |
| Assay | Control | ‘Cabernet Sauvignon’ | ‘Grenache Noir’ | ‘Petit Verdot’ | ‘Syrah’ |
|---|---|---|---|---|---|
| WRC | 71.04 ± 0.42 c | 61.55 ± 1.01 a | 70.40 ± 1.64 bc | 61.03 ± 1.95 a | 67.78 ± 2.60 b |
| ORC | 49.63 ± 1.10 a | 48.36 ± 3.83 a | 59.78 ± 4.45 b | 50.08 ± 3.69 a | 59.48 ± 1.65 b |
| SWC | 90.83 ± 1.17 c | 25.33 ± 0.82 a | 34.09 ± 2.49 b | 91.23 ± 0.37 c | 35.00 ± 3.16 ab |
| EMC | n.d. | n.d. | n.d. | n.d. | n.d. |
| GLC | n.d. | n.d. | n.d. | n.d. | n.d. |
| Assay | Control | ‘Cabernet Sauvignon’ | ‘Grenache Noir’ | ‘Petit Verdot’ | ‘Syrah’ |
|---|---|---|---|---|---|
| TPC | 11.48 ± 0.54 a | 30.34 ± 0.52 d | 28.48 ± 1.33 b | 29.18 ± 0.60 bc | 29.12 ± 0.68 bc |
| TTC | 93.09 ± 3.15 a | 326.41 ± 14.53 c | 124.38 ± 13.63 b | 326.42 ± 16.46 c | 383.33 ± 11.86 d |
| TFVC | 56.45 ± 2.51 c | 70.90 ± 0.78 e | 42.35 ± 0.70 a | 47.91 ± 2.58 b | 64.61 ± 1.24 d |
| TCGA | 28.30 ± 0.55 a | 186.30 ± 2.41 d | 101.84 ± 1.47 b | 178.01 ± 2.51 c | 216.00 ± 2.69 e |
| Assay | Control | ‘Cabernet Sauvignon’ | ‘Grenache Noir’ | ‘Petit Verdot’ | ‘Syrah’ |
|---|---|---|---|---|---|
| DPPH | 31.10 ± 0.36 a | 86.71 ± 0.58 e | 71.02 ± 2.65 c | 61.18 ± 4.11 b | 85.87 ± 0.24 d |
| ABTS | 32.77 ± 1.39 a | 86.38 ± 0.42 b | 86.16 ± 0.66 b | 86.53 ± 0.84 b | 85.53 ± 0.95 b |
| FPBP | 0.083 ± 0.003 a | 0.424 ± 0.006 d | 0.165 ± 0.004 b | 0.227 ± 0.003 c | 0.664 ± 0.006 e |
| FRAP | 0.094 ± 0.003 a | 0.449 ± 0.011 d | 0.177 ± 0.008 b | 0.226 ± 0.011 c | 0.530 ± 0.057 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Ortiz, M.A.; Sánchez-Escalante, A.; Torrescano-Urrutia, G.R.; Vargas-Sánchez, R.D.; Torres-Martínez, B.d.M.; Quintana-Obregón, E.A. Grape Marc Flour as a Horticulture By-Product for Application in the Meat Industry. Recycling 2025, 10, 164. https://doi.org/10.3390/recycling10040164
Vargas-Ortiz MA, Sánchez-Escalante A, Torrescano-Urrutia GR, Vargas-Sánchez RD, Torres-Martínez BdM, Quintana-Obregón EA. Grape Marc Flour as a Horticulture By-Product for Application in the Meat Industry. Recycling. 2025; 10(4):164. https://doi.org/10.3390/recycling10040164
Chicago/Turabian StyleVargas-Ortiz, Manuel Alejandro, Armida Sánchez-Escalante, Gastón R. Torrescano-Urrutia, Rey David Vargas-Sánchez, Brisa del Mar Torres-Martínez, and Eber Addí Quintana-Obregón. 2025. "Grape Marc Flour as a Horticulture By-Product for Application in the Meat Industry" Recycling 10, no. 4: 164. https://doi.org/10.3390/recycling10040164
APA StyleVargas-Ortiz, M. A., Sánchez-Escalante, A., Torrescano-Urrutia, G. R., Vargas-Sánchez, R. D., Torres-Martínez, B. d. M., & Quintana-Obregón, E. A. (2025). Grape Marc Flour as a Horticulture By-Product for Application in the Meat Industry. Recycling, 10(4), 164. https://doi.org/10.3390/recycling10040164








