Recycled Glass and Plastic Waste in Sustainable Geopolymer Systems for Affordable Housing Solutions
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Sample Preparation
2.3. Performance Evaluation and Characterisation
3. Results and Discussion
3.1. Phase Changes and Crystallinity Characterisation via XRD

3.2. Compressive Strength
3.3. Water Absorption
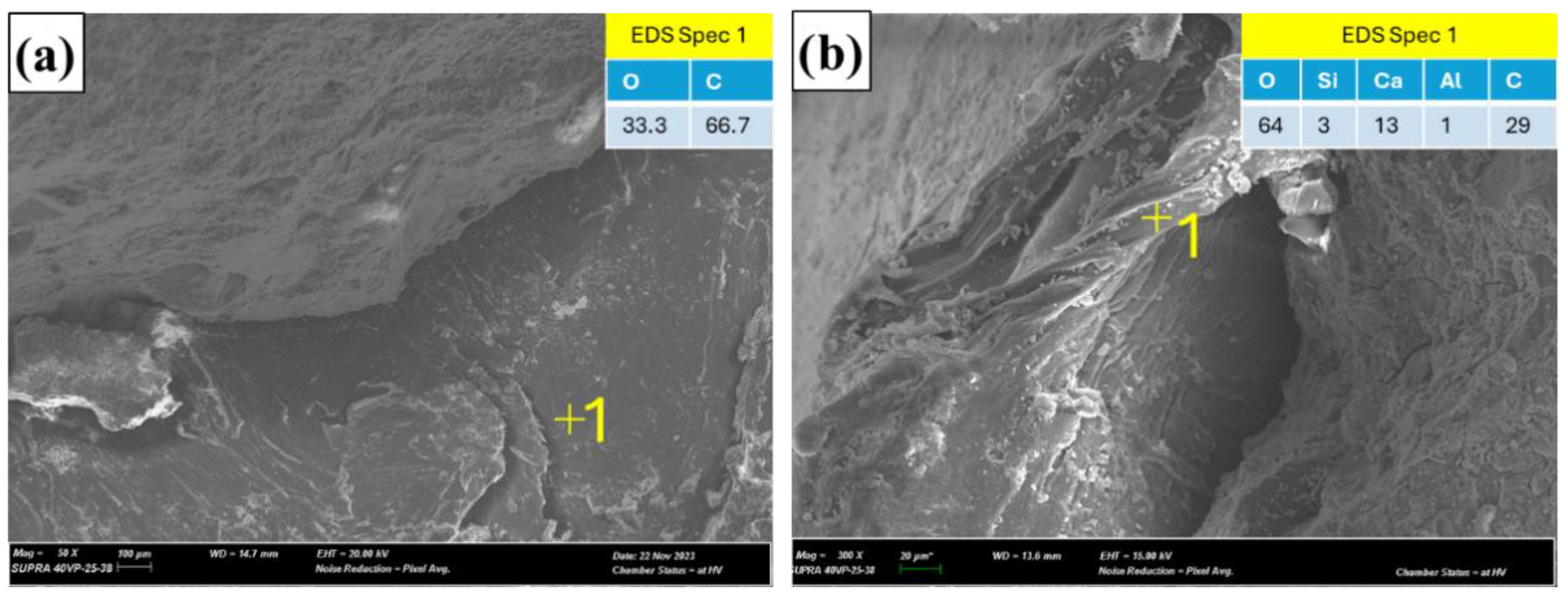
3.4. Microstructure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makinde, O.O. Housing: Central city slums, a case study of Ibadan. J. Environ. Earth Sci. 2012, 2, 21–31. [Google Scholar]
- Chowdhury, R.B.; Moore, G.A. Floating agriculture: A potential cleaner production technique for climate change adaptation and sustainable community development in Bangladesh. J. Clean. Prod. 2017, 150, 371–389. [Google Scholar] [CrossRef]
- Adabre, M.A.; Chan, A.P.; Darko, A.; Osei-Kyei, R.; Abidoye, R.; Adjei-Kumi, T. Critical barriers to sustainability attainment in affordable housing: International construction professionals’ perspective. J. Clean. Prod. 2020, 253, 119995. [Google Scholar] [CrossRef]
- Rahmawati, D.; Rahadi, R.A.; Putri, A.D.; Bandung, E. The Current State of Property Development in Indonesia During the Covid-19 Pandemic. Int. J. Innov. Creat. Change 2021, 15, 2021. [Google Scholar]
- Satoto, E.B. Boosting Homeownership Affordability for Low-Income Communities in Indonesia. Int. J. Sustain. Dev. Plan. 2023, 18, 1365–1376. [Google Scholar] [CrossRef]
- Odoyi, E.J.; Riekkinen, K. Housing policy: An analysis of public housing policy strategies for low-income earners in Nigeria. Sustainability 2022, 14, 2258. [Google Scholar] [CrossRef]
- Ebekozien, A.; Abdul-Aziz, A.-R.; Jaafar, M. Low-cost housing policies and squatters struggles in Nigeria: The Nigerian perspective on possible solutions. Int. J. Constr. Manag. 2021, 21, 1088–1098. [Google Scholar] [CrossRef]
- Purnamasari, L. Effect of Government’s Policy to Build 1 Million Houses on Performance of Housing and Apartment Credits of Banks Listed in Indonesia Stock Exchange During Period 2015–2017. J. Ilm. Ilmu Adm. Publik 2021, 11, 40–50. [Google Scholar] [CrossRef]
- Zuraida, S.; Dewancker, B.; Margono, R.B. Application of non-degradable waste as building material for low-cost housing. Sci. Rep. 2023, 13, 6390. [Google Scholar] [CrossRef]
- Shamsaei, E.; Tang, Z.Q.; de Souza, F.B.; Hosseini, E.; Benhelal, E.; Korayem, A.H.; Duan, W. Zeolitic imidazolate framework nanoleaves (ZIF-L) enhancement of strength and durability of portland cement composites. Constr. Build. Mater. 2021, 272, 122015. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; Van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. Strength development and acid resistance of geopolymer based on waste clay brick powder and phosphorous slag. Struct. Concr. 2019, 20, 1596–1606. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahmad, T.; Husain, D.; Majdi, A.; Alam, M.T.; Husain, N.; Wayal, A.K.S. Ecological footprint and economic assessment of conventional and geopolymer concrete for sustainable construction. J. Clean. Prod. 2022, 380, 134910. [Google Scholar] [CrossRef]
- Rintala, A.; Havukainen, J.; Abdulkareem, M. Estimating the cost-competitiveness of recycling-based geopolymer concretes. Recycling 2021, 6, 46. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Saha, A.K.; Sarker, P.K. Reuse of waste glass as a supplementary binder and aggregate for sustainable cement-based construction materials: A review. J. Build. Eng. 2020, 28, 101052. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass waste for development of Geopolymer composites–Mechanical properties and rheological characteristics: A review. Constr. Build. Mater. 2019, 220, 547–564. [Google Scholar] [CrossRef]
- Qian, Y.; Sheikh, M.N.; Feng, H.; Hadi, M.N. Use of waste glass as fine aggregate in ambient cured alkali-activated mortars. Struct. Concr. 2023, 24, 4145–4160. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Tapas, M.J.; Thomas, P.; Vessalas, K.; Nsiah-Baafi, E.; Martin, L.; Sirivivatnanon, V. Comparative study of the efficacy of fly ash and reactive aggregate powders in mitigating alkali-silica reaction. J. Build. Eng. 2023, 63, 105571. [Google Scholar] [CrossRef]
- Lei, J.; Fu, J.; Yang, E.-H. Alkali-silica reaction resistance and pore solution composition of low-calcium fly ash-based geopolymer concrete. Infrastructures 2020, 5, 96. [Google Scholar] [CrossRef]
- Neo, E.R.K.; Soo, G.C.Y.; Tan, D.Z.L.; Cady, K.; Tong, K.T.; Low, J.S.C. Life cycle assessment of plastic waste end-of-life for India and Indonesia. Resour. Conserv. Recycl. 2021, 174, 105774. [Google Scholar] [CrossRef]
- Darus, N.; Tamimi, M.; Tirawaty, S.; Muchtazar, M.; Trisyanti, D.; Akib, R.; Condorini, D.; Ranggi, K. An overview of plastic waste recycling in the urban areas of Java Island in Indonesia. J. Environ. Sci. Sustain. Dev. 2020, 3, 402–415. [Google Scholar] [CrossRef]
- Pawar, P.R.; Shirgaonkar, S.S.; Patil, R.B. Plastic marine debris: Sources, distribution and impacts on coastal and ocean biodiversity. PENCIL Publ. Biol. Sci. 2016, 3, 40–54. [Google Scholar]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Al-Tulaian, B.; Al-Shannag, M.; Al-Hozaimy, A. Recycled plastic waste fibers for reinforcing Portland cement mortar. Constr. Build. Mater. 2016, 127, 102–110. [Google Scholar] [CrossRef]
- Asokan, P.; Osmani, M.; Price, A.D. Assessing the recycling potential of glass fibre reinforced plastic waste in concrete and cement composites. J. Clean. Prod. 2009, 17, 821–829. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Saikia, N. Influence of curing conditions on the durability-related performance of concrete made with selected plastic waste aggregates. Cem. Concr. Compos. 2013, 35, 23–31. [Google Scholar] [CrossRef]
- Ferreira, L.; De Brito, J.; Saikia, N. Influence of curing conditions on the mechanical performance of concrete containing recycled plastic aggregate. Constr. Build. Mater. 2012, 36, 196–204. [Google Scholar] [CrossRef]
- Mohammadinia, A.; Wong, Y.C.; Arulrajah, A.; Horpibulsuk, S. Strength evaluation of utilizing recycled plastic waste and recycled crushed glass in concrete footpaths. Constr. Build. Mater. 2019, 197, 489–496. [Google Scholar] [CrossRef]
- Wong, Y.C.; Perera, S.; Zhang, Z.; Arulrajah, A.; Mohammadinia, A. Field study on concrete footpath with recycled plastic and crushed glass as filler materials. Constr. Build. Mater. 2020, 243, 118277. [Google Scholar] [CrossRef]
- Sing, M.; Love, P.; Tam, C.-M. Review and exploration of river sand substitutes for concrete production in Asian countries. In Advances in Civil Engineering and Building Materials; CRC Press: Boca Raton, FL, USA, 2012; pp. 115–117. [Google Scholar]
- Arulrajah, A.; Perera, S.; Wong, Y.C.; Maghool, F.; Horpibulsuk, S. Stabilization of PET plastic-demolition waste blends using fly ash and slag-based geopolymers in light traffic road bases/subbases. Constr. Build. Mater. 2021, 284, 122809. [Google Scholar] [CrossRef]
- Kang, S.-H.; Jeong, Y.; Tan, K.H.; Moon, J. The use of limestone to replace physical filler of quartz powder in UHPFRC. Cem. Concr. Compos. 2018, 94, 238–247. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Sui, H.; de Souza, F.B.; Sagoe-Crentsil, K.; Duan, W. Silane-modified graphene oxide in geopolymer: Reaction kinetics, microstructure, and mechanical performance. Cem. Concr. Compos. 2023, 139, 104997. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z.; Wang, H. Mechanical property and structure of alkali-activated fly ash and slag blends. J. Sustain. Cem.-Based Mater. 2012, 1, 167–178. [Google Scholar] [CrossRef]
- Singh, G.B.; Subramaniam, K.V. Quantitative XRD study of amorphous phase in alkali activated low calcium siliceous fly ash. Constr. Build. Mater. 2016, 124, 139–147. [Google Scholar] [CrossRef]
- Moujoud, Z.; Sair, S.; Ousaleh, H.A.; Amadine, O.; Ayouch, I.; Zahouily, M.; El Bouari, A.; Tanane, O. High-performance geopolymer from brick wastes and metakaolin: Alkali treatment optimization, phase transformation, and property analysis. Struct. Concr. 2024, 26, 1477–1497. [Google Scholar] [CrossRef]
- Bourzik, O.; Baba, K.; Akkouri, N. Eco-friendly geopolymer mortar prepared from geopolymer waste: Mechanical and thermal properties. Environ. Qual. Manag. 2024, 33, 411–419. [Google Scholar] [CrossRef]
- Zhu, X.; Luan, M.; Tang, D.; Yang, K.; Yang, C. Understanding the setting behaviours of alkali-activated slag from the dissolution-precipitation point of view. Cem. Concr. Compos. 2024, 148, 105474. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Z.; Wang, J.; Zang, J.; Tang, L.; Ma, F.; Qian, B.; Ma, B.; Wang, L. Investigation into the influence of calcium compounds on the properties of micropore-foamed geopolymer. J. Build. Eng. 2022, 45, 103521. [Google Scholar] [CrossRef]
- Tailby, J.; MacKenzie, K.J. Structure and mechanical properties of aluminosilicate geopolymer composites with Portland cement and its constituent minerals. Cem. Concr. Res. 2010, 40, 787–794. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Sanalkumar, K.U.A.; Lahoti, M.; Yang, E.-H. Investigating the potential reactivity of fly ash for geopolymerization. Constr. Build. Mater. 2019, 225, 283–291. [Google Scholar] [CrossRef]
- Tang, Z.Q.; De Souza, F.B.; Mulder, R.J.; Duan, W. Multistep nucleation and growth mechanism of aluminosilicate gel observed by cryo-electron microscopy. Cem. Concr. Res. 2022, 159, 106873. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Lee, B.; Kim, G.; Kim, R.; Cho, B.; Lee, S.; Chon, C.-M. Strength development properties of geopolymer paste and mortar with respect to amorphous Si/Al ratio of fly ash. Constr. Build. Mater. 2017, 151, 512–519. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S.-L. Strength development in clay–fly ash geopolymer. Constr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Mechanical and microstructural evolutions of fly ash/slag-based geopolymer at high temperatures: Effect of curing conditions. Ceram. Int. 2023, 49, 2091–2101. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; van Deventer, J.S. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Thorneycroft, J.; Orr, J.; Savoikar, P.; Ball, R. Performance of structural concrete with recycled plastic waste as a partial replacement for sand. Constr. Build. Mater. 2018, 161, 63–69. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Ma, L.; Wang, L. Restrained shrinkage behavior of internally-cured UHPC using calcined bauxite aggregate in the ring test and UHPC-concrete composite slab. Cem. Concr. Compos. 2022, 134, 104805. [Google Scholar] [CrossRef]
- Brough, A.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Klima, K.; Schollbach, K.; Brouwers, H.; Yu, Q. Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Constr. Build. Mater. 2022, 314, 125574. [Google Scholar] [CrossRef]
- Kalinkin, A.; Kumar, S.; Gurevich, B.; Alex, T.; Kalinkina, E.; Tyukavkina, V.; Kalinnikov, V.; Kumar, R. Geopolymerization behavior of Cu–Ni slag mechanically activated in air and in CO2 atmosphere. Int. J. Miner. Process. 2012, 112, 101–106. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S. Effect of activator concentration on the strength, ITZ and drying shrinkage of fly ash/slag geopolymer concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- He, S.; Yang, E.-H. Strategic strengthening of the interfacial transition zone (ITZ) between microfiber and cement paste matrix with carbon nanofibers (CNFs). Cem. Concr. Compos. 2021, 119, 104019. [Google Scholar] [CrossRef]
- Khodr, M.; Law, D.W.; Gunasekara, C.; Setunge, S.; Brkljaca, R. Compressive strength and microstructure evolution of low calcium brown coal fly ash-based geopolymer. J. Sustain. Cem.-Based Mater. 2020, 9, 17–34. [Google Scholar] [CrossRef]




| Compound | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | SO3 | Na2O | MgO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| % | 1.38 | 72.6 | 11.7 | 0.5 | 0.4 | 0.1 | 13.1 | 0.6 | 0.22 |
| Compound | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | SO3 | Na2O | MgO | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|
| % | 4.5 | 20.3 | 62.9 | 4.6 | 0.3 | 2.6 | 0.3 | 1.2 | 3.3 |
| Compound | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | TiO2 | Na2O | MgO | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|
| % | 12.4 | 32.6 | 45.06 | 0.25 | 0.28 | 0.57 | 0.25 | 5.4 | 3.19 |
| Compound | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | TiO2 | Na2O | MgO | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|
| % | 18.6 | 70.7 | 1.95 | 2.82 | 1.50 | 0.75 | 0.48 | 0.43 | 2.77 |
| Mixes | ID | Total Binder Content |
|---|---|---|
| OPC only (control) | C | 100% OPC |
| FA-geopolymer mix | 10FA | 40%FA + 60%OPC |
| 15FA | 60%FA + 40%OPC | |
| 25FA | 100%FA | |
| S-geopolymer mix | 10S | 40%S + 60%OPC |
| 15S | 60%S + 40%OPC | |
| 25S | 100%S | |
| (FA + S)-geopolymer mix | 10FA + S | 40%(FA + S) + 60%OPC |
| 15FA + S | 60%(FA + S) + 40%OPC | |
| 25FA + S | 100%(FA + S) |
| ID | Curing Age (d) | %-Crystallinity | %-Amorphous |
|---|---|---|---|
| 25FA | 7 | 16.0 | 84.0 |
| 28 | 19.9 | 80.9 | |
| 25S | 7 | 25.9 | 74.1 |
| 28 | 31.7 | 68.3 | |
| 25FA + S | 7 | 19.1 | 80.9 |
| 28 | 25.6 | 74.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.Q.; Wong, Y.C.; Li, Y.; Masli, E.K. Recycled Glass and Plastic Waste in Sustainable Geopolymer Systems for Affordable Housing Solutions. Recycling 2025, 10, 147. https://doi.org/10.3390/recycling10040147
Tang ZQ, Wong YC, Li Y, Masli EK. Recycled Glass and Plastic Waste in Sustainable Geopolymer Systems for Affordable Housing Solutions. Recycling. 2025; 10(4):147. https://doi.org/10.3390/recycling10040147
Chicago/Turabian StyleTang, Zhao Qing, Yat Choy Wong, Yali Li, and Eryadi Kordi Masli. 2025. "Recycled Glass and Plastic Waste in Sustainable Geopolymer Systems for Affordable Housing Solutions" Recycling 10, no. 4: 147. https://doi.org/10.3390/recycling10040147
APA StyleTang, Z. Q., Wong, Y. C., Li, Y., & Masli, E. K. (2025). Recycled Glass and Plastic Waste in Sustainable Geopolymer Systems for Affordable Housing Solutions. Recycling, 10(4), 147. https://doi.org/10.3390/recycling10040147







