Waste Valorization Technologies in Tannery Sludge, Chromite, and Magnesite Mining
Abstract
1. Introduction
2. Mining Waste—The Chromite Case Study
2.1. Valorization of Ultrabasic Rocks
2.1.1. Refractory Raw Material
2.1.2. Neutralization of Acidic Wastewater
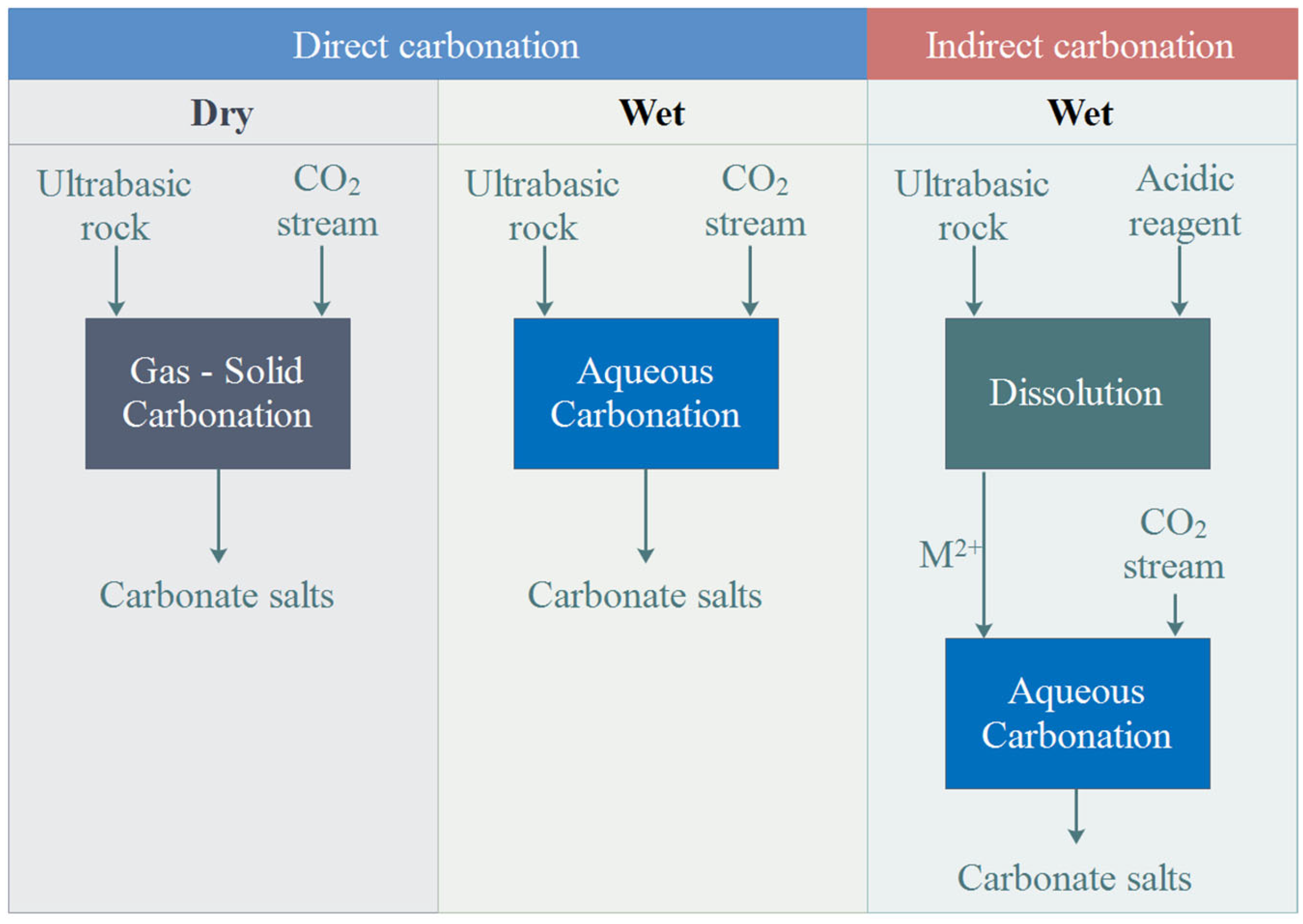
2.1.3. Carbon Dioxide Storage
3. Mining Waste—The Magnesite Case Study
3.1. Sustainable Binder
3.2. Neutralizing of Acid Mine Drainage
3.3. SOx Absorption
3.4. Refractories and Insulating Material
4. Industrial Waste—The Tannery Sludge Case Study
4.1. Energy Recovery
4.2. Chromium Recovery
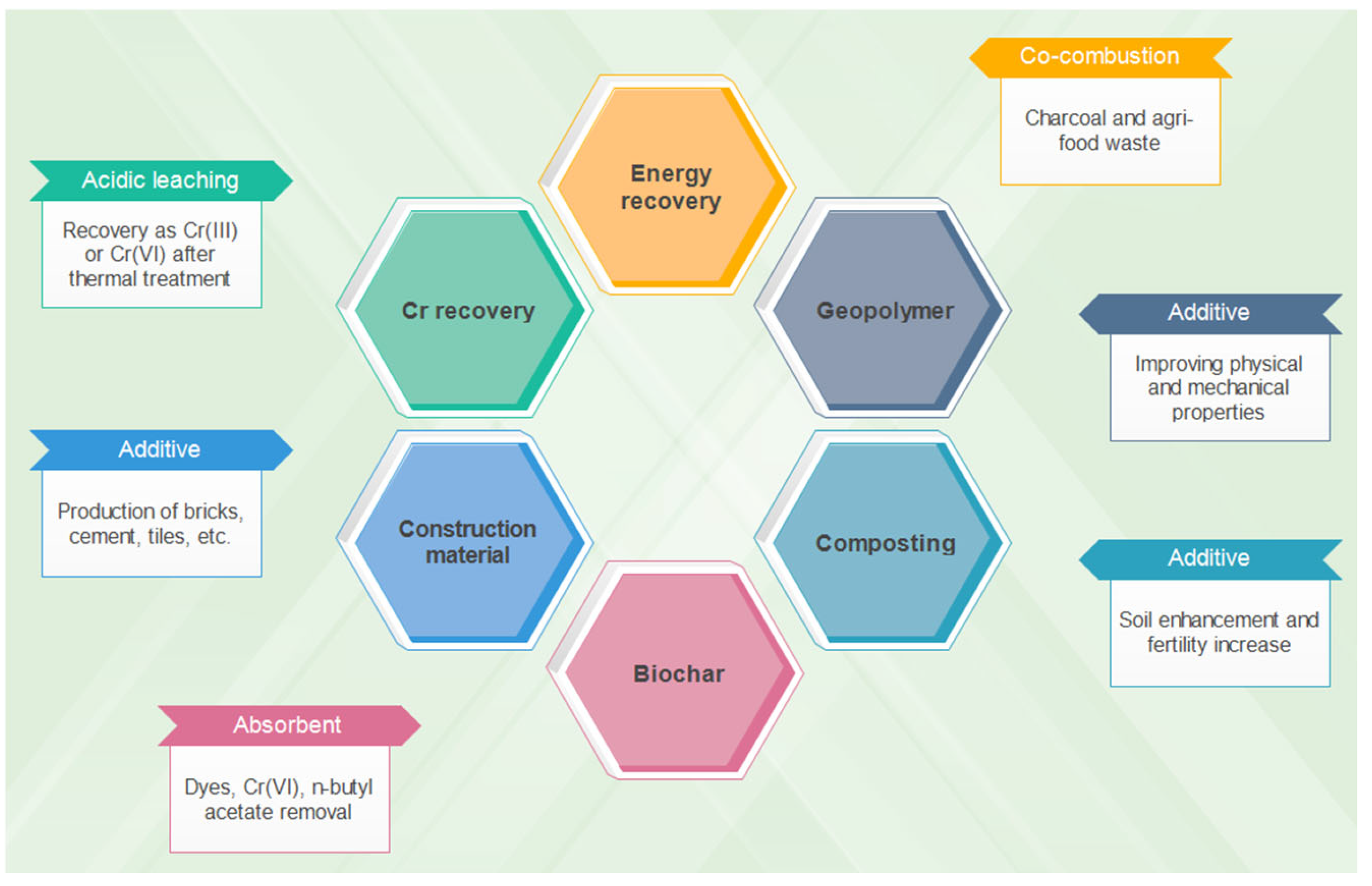
4.3. Other Valorization Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment Programme and International Resource Panel. Global Material Flows and Resource Productivity: Assessment Report for the UNEP International Resource Panel. Available online: https://wedocs.unep.org/20.500.11822/21557 (accessed on 31 March 2025).
- Schandl, H.; Fischer-Kowalski, M.; West, J.; Giljum, S.; Dittrich, M.; Eisenmenger, N.; Geschke, A.; Lieber, M.; Wieland, H.; Schaffartzik, A.; et al. Global material flows and resource productivity forty years of evidence. J. Ind. Ecol. 2018, 22, 827–838. [Google Scholar] [CrossRef]
- European Commission. Development of a Guidance Document on Best Practices in the Extractive Waste Management Plans Circular Economy Action; European Union: Luxembourg, 2019; ISBN 9789276000372. [Google Scholar]
- Bag, S.; Pretorius, J.H.C. Relationships between industry 4.0, sustainable manufacturing and circular economy: Proposal of a research framework. Int. J. Organ. Anal. 2022, 30, 864–898. [Google Scholar] [CrossRef]
- Silvério, A.C.; Ferreira, J.; Fernandes, P.O.; Dabić, M. How does circular economy work in industry? Strategies, opportunities, and trends in scholarly literature. J. Clean. Prod. 2023, 412, 137312. [Google Scholar] [CrossRef]
- Zaman, A.U. A comprehensive study of the environmental and economic benefits of resource recovery from global waste management systems. J. Clean. Prod. 2016, 124, 41–50. [Google Scholar] [CrossRef]
- Yu, Z.; Khan, S.A.R.; Ponce, P.; Zia-ul-haq, H.M.; Ponce, K. Exploring essential factors to improve waste-to-resource recovery: A roadmap towards sustainability. J. Clean. Prod. 2022, 350, 131305. [Google Scholar] [CrossRef]
- Khan, A.H.; López-Maldonado, E.A.; Khan, N.A.; Villarreal-Gómez, L.J.; Munshi, F.M.; Alsabhan, A.H.; Perveen, K. Current solid waste management strategies and energy recovery in developing countries—State of art review. Chemosphere 2022, 291, 133088. [Google Scholar] [CrossRef]
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A. Electronic waste generation, regulation and metal recovery: A review. Environ. Chem. Lett. 2021, 19, 1347–1368. [Google Scholar] [CrossRef]
- Bu, X.; Danstan, J.K.; Hassanzadeh, A.; Behrad Vakylabad, A.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching—An overview. Miner. Process. Extr. Metall. Rev. 2024, 45, 28–45. [Google Scholar] [CrossRef]
- Woźniak, J.; Pactwa, K. Overview of polish mining wastes with circular economy model and its comparison with other wastes. Sustainability 2018, 10, 3994. [Google Scholar] [CrossRef]
- Dziike, F.; Franklyn, P.J.; Hlekelele, L.; Durbach, S. Recovery of waste gold for the synthesis of gold nanoparticles supported on radially aligned nanorutile: The growth of carbon nanomaterials. RSC Adv. 2020, 10, 28090–28099. [Google Scholar] [CrossRef]
- Gedam, V.V.; Raut, R.D.; Lopes de Sousa Jabbour, A.B.; Agrawal, N. Moving the circular economy forward in the mining industry: Challenges to closed-loop in an emerging economy. Resour. Policy 2021, 74, 102279. [Google Scholar] [CrossRef]
- Upadhyay, A.; Laing, T.; Kumar, V.; Dora, M. Exploring barriers and drivers to the implementation of circular economy practices in the mining industry. Resour. Policy 2021, 72, 102037. [Google Scholar] [CrossRef]
- Mateus, A.; Martins, L. Challenges and opportunities for a successful mining industry in the future. Boletín Geológico Min. 2019, 130, 99–121. [Google Scholar] [CrossRef]
- Kaźmierczak, U.; Blachowski, J.; Górniak-Zimroz, J. Multi-criteria analysis of potential applications of waste from rock minerals mining. Appl. Sci. 2019, 9, 441. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-thinking mining waste through an integrative approach led by circular economy aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef]
- International Council on Mining and Metals. Mining and Metals and the Circular Economy. Available online: https://www.icmm.com/website/publications/pdfs/mining-metals/2016/research_circular-economy.pdf?cb=7827 (accessed on 31 March 2025).
- Banerjee, S.; Ghosh, S.; Jha, S.; Kumar, S.; Mondal, G.; Sarkar, D.; Datta, R.; Mukherjee, A.; Bhattacharyya, P. Assessing pollution and health risks from chromite mine tailings contaminated soils in India by employing synergistic statistical approaches. Sci. Total Environ. 2023, 880, 163228. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Ullah, Z.; Ali, A.; Sardar, T.; Iqbal, J.; Gao, X.; Bundschuh, J.; Li, C.; Khattak, S.A.; et al. Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model. Int. J. Environ. Res. Public Health 2023, 20, 2113. [Google Scholar] [CrossRef]
- Shams, M.; Tavakkoli Nezhad, N.; Dehghan, A.; Alidadi, H.; Paydar, M.; Mohammadi, A.A.; Zarei, A. Heavy metals exposure, carcinogenic and non-carcinogenic human health risks assessment of groundwater around mines in Joghatai, Iran. Int. J. Environ. Anal. Chem. 2022, 102, 1884–1899. [Google Scholar] [CrossRef]
- Adhikari, S.; Marcelo-Silva, J.; Beukes, J.P.; van Zyl, P.G.; Coetsee, Y.; Boneschans, R.B.; Siebert, S.J. Contamination of useful plant leaves with chromium and other potentially toxic elements and associated health risks in a polluted mining-smelting region of South Africa. Environ. Adv. 2022, 9, 100301. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef]
- Nanda, S.P.; Panda, B.P.; Pradhan, A. Mining activities influencing the nutritional status of soil and water near a chromite mining site of Odisha, India. Environ. Qual. Manag. 2022, 32, 151–159. [Google Scholar] [CrossRef]
- Mishra, H.; Sahu, H.B. Environmental scenario of chromite mining at Sukinda Valley—A review. Int. J. Environ. Eng. Manag. 2013, 4, 287–292. [Google Scholar]
- Zhang, X.; Li, G.; Wu, J.; Xiong, N.; Quan, X. Leaching of Valuable Elements from the Waste Chromite Ore Processing Residue: A Kinetic Analysis. ACS Omega 2020, 7, 4110–4120. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, I.; Kuldeyev, Y.; Serzhanova, N.; Sadykov, N.; Tastanova, A. The process of beneficiation of fine chrome sludges on concentration tables. Metalurgija 2022, 61, 381–384. [Google Scholar]
- Koleli, N.; Demir, A. Chromite. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Prasad, M.N.V., Shih, K., Eds.; Academic Press: London, UK, 2016; pp. 245–264. [Google Scholar]
- Freese, K.; Miller, R.; Cutright, T.; Senko, J. Review of Chromite Ore Processing Residue (COPR): Past Practices, Environmental Impact and Potential Remediation Methods. Curr. Environ. Eng. 2014, 1, 82–90. [Google Scholar] [CrossRef]
- Beukes, J.P.; du Preez, S.P.; van Zyl, P.G.; Paktunc, D.; Fabritius, T.; Päätalo, M.; Cramer, M. Review of Cr(VI) environmental practices in the chromite mining and smelting industry—Relevance to development of the Ring of Fire, Canada. J. Clean. Prod. 2017, 65, 874–889. [Google Scholar] [CrossRef]
- Nayak, S.; Rangabhashiyam, S.; Balasubramanian, P.; Kale, P. A review of chromite mining in Sukinda Valley of India: Impact and potential remediation measures. Int. J. Phytoremediation 2020, 22, 804–818. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Cavallo, A. Serpentinitic waste materials from the dimension stone industry: Characterization, possible reuses and critical issues. Resour. Policy 2018, 59, 17–23. [Google Scholar] [CrossRef]
- Torre, F.; Farina, V.; Taras, A.; Pistidda, C.; Santoru, A.; Bednarcik, J.; Mulas, G.; Enzo, S.; Garroni, S. Room temperature hydrocarbon generation in olivine powders: Effect of mechanical processing under CO2 atmosphere. Powder Technol. 2020, 364, 915–923. [Google Scholar] [CrossRef]
- Kokkinos, E.; Peleka, E.; Zouboulis, A. Thermal Treatment of Serpentinized Olivine Wastes, Obtained from Chromite Mineral Enrichment Operations, as an Example of Circular Economy in the Mining Sector. Mater Proc. 2023, 15, 38. [Google Scholar] [CrossRef]
- Acar, İ. Sintering properties of olivine and its utilization potential as a refractory raw material: Mineralogical and microstructural investigations. Ceram. Int. 2020, 46, 28025–28034. [Google Scholar] [CrossRef]
- Nemat, S.; Ramezani, A.; Emami, S.M. Possible use of waste serpentine from Abdasht chromite mines into the refractory and ceramic industries. Ceram. Int. 2016, 42, 18479–18483. [Google Scholar] [CrossRef]
- Emami, S.M.; Ramezani, A.; Nemat, S. Sintering behavior of waste serpentine from abdasht chromite mines Abdasht chromite mines and kaolin blends. Ceram. Int. 2017, 43, 15189–15193. [Google Scholar] [CrossRef]
- van Noort, R.; Mørkved, P.T.; Dundas, S.H. Acid neutralization by mining waste dissolution under conditions relevant for agricultural applications. Geosciences 2018, 8, 380. [Google Scholar] [CrossRef]
- Erguler, Z.A.; Kalyoncu Erguler, G. The effect of particle size on acid mine drainage generation: Kinetic column tests. Miner. Eng. 2015, 76, 154–167. [Google Scholar] [CrossRef]
- García-Valero, A.; Martínez-Martínez, S.; Faz, A.; Rivera, J.; Acosta, J.A. Environmentally sustainable acid mine drainage remediation: Use of natural alkaline material. J. Water Process Eng. 2020, 33, 101064. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Du, S.; Zhang, Z.; Lu, W.; Su, P.; Jiao, Y.; Zhao, Y. A review: The formation, prevention, and remediation of acid mine drainage. Environ. Sci. Pollut. Res. 2023, 30, 111871–111890. [Google Scholar] [CrossRef]
- Kokkinos, E.; Kotsali, V.; Tzamos, E.; Zouboulis, A. Acid Mine Drainage Neutralization by Ultrabasic Rocks: A Chromite Mining Tailings Evaluation Case Study. Sustainability 2024, 16, 8967. [Google Scholar] [CrossRef]
- Gerogianni, N.; Magganas, A.; Stamatakis, M.; Pomonis, P. Effectiveness of Olivine-Rich Ultrabasic Rocks from Greece on Acid Mine Drainage and Dairy Wastewater Treatment. In Proceedings of the International Conference IWWATV, Athens, Greece, 21–23 May 2015. [Google Scholar]
- King, H.E.; Plümper, O.; Geisler, T.; Putnis, A. Experimental investigations into the silicification of olivine: Implications for the reaction mechanism and acid neutralization. Am. Mineral. 2011, 96, 1246–1253. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Storti, G.; Seward, T.M.; Mazzotti, M. Dissolution kinetics of fosteritic olivine at 90–150 °C including effects of the presence of CO2. Geochim. Cosmochim. Acta 2006, 70, 4403–4416. [Google Scholar] [CrossRef]
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Reaction mechanism for the aqueous-phase mineral carbonation of heat-activated serpentine at low temperatures and pressures in flue gas conditions. Environ. Sci. Technol. 2014, 48, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Aines, R.; Bennett, E.; Benson, S.M.; Carter, E.; Coggon, J.A.; De Obeso, J.C.; Evans, O.; Gadikota, G.; Dipple, G.M.; et al. In situ carbon mineralization in ultramafic rocks: Natural processes and possible engineered methods. Energy Procedia 2018, 146, 92–102. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Petallidou, K.C.; Vasiliades, M.A.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. Carbon dioxide storage in olivine basalts: Effect of ball milling process. Powder Technol. 2015, 273, 220–229. [Google Scholar] [CrossRef]
- Li, J.; Jacobs, A.D.; Hitch, M. Direct aqueous carbonation on olivine at a CO2 partial pressure of 6.5 MPa. Energy 2019, 173, 902–910. [Google Scholar] [CrossRef]
- Marín, O.; Valderrama, J.O.; Kraslawski, A.; Cisternas, L.A. Potential of tailing deposits in chile for the sequestration of carbon dioxide produced by power plants using ex-situ mineral carbonation. Minerals 2021, 11, 320. [Google Scholar] [CrossRef]
- Veetil, S.P.; Pasquier, L.C.; Blais, J.F.; Cecchi, E.; Kentish, S.; Mercier, G. Direct gas–solid carbonation of serpentinite residues in the absence and presence of water vapor: A feasibility study for carbon dioxide sequestration. Environ. Sci. Pollut. Res. 2015, 22, 13486–13495. [Google Scholar] [CrossRef]
- Haug, T.A.; Munz, I.A.; Kleiv, R.A. Importance of dissolution and precipitation kinetics for mineral carbonation. Energy Procedia 2011, 4, 5029–5036. [Google Scholar] [CrossRef]
- Turri, L.; Muhr, H.; Rijnsburger, K.; Knops, P.; Lapicque, F. CO2 sequestration by high pressure reaction with olivine in a rocking batch autoclave. Chem. Eng. Sci. 2017, 171, 27–31. [Google Scholar] [CrossRef]
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Parameters optimization for direct flue gas CO2 capture and sequestration by aqueous mineral carbonation using activated serpentinite based mining residue. Appl. Geochem. 2014, 50, 66–73. [Google Scholar] [CrossRef]
- Saran, R.K.; Arora, V.; Yadav, S. CO2 sequestration by mineral carbonation: A review. Glob. Nest J. 2018, 20, 497–503. [Google Scholar] [CrossRef]
- Haug, T.A.; Kleiv, R.A.; Munz, I.A. Investigating dissolution of mechanically activated olivine for carbonation purposes. Appl. Geochem. 2010, 25, 1547–1563. [Google Scholar] [CrossRef]
- Pagona, E.; Kalaitzidou, K.; Zouboulis, A.; Mitrakas, M. Effects of additives on the physical properties of magnesite ore mining by-products for the production of refractories. Miner. Eng. 2021, 147, 107247. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Pagona, E.; Mitrakas, M.; Zouboulis, A. MagWasteVal Project-Towards Sustainability of Mining Waste. Sustainability 2023, 15, 1648. [Google Scholar] [CrossRef]
- Vembu, P.R.S.; Ammasi, A.K. Sustainable use of magnesite mine waste in self-compacting concrete and its study on strength, microstructure, cost and CO2 emission. Mater. Res. Express 2024, 11, 066506. [Google Scholar] [CrossRef]
- Shanmugasundaram, V.; Shanmugam, B. Application of cement treated magnesite mine tailings as subgrade. Constr. Build. Mater. 2023, 365, 130064. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Masindi, V. A novel technology for neutralizing acidity and attenuating toxic chemical species from acid mine drainage using cryptocrystalline magnesite tailings. J. Water Process Eng. 2016, 10, 67–77. [Google Scholar] [CrossRef]
- Del Valle-Zermeño, R.; Formosa, J.; Aparicio, J.A.; Chimenos, J.M. Reutilization of low-grade magnesium oxides for flue gas desulfurization during calcination of natural magnesite: A closed-loop process. Chem. Eng. J. 2014, 254, 63–72. [Google Scholar] [CrossRef]
- Hycnar, E.; Sęk, M.; Ratajczak, T. Magnesite as a Sorbent in Fluid Combustion Conditions—Role of Magnesium in SO2 Sorption Process. Minerals 2023, 13, 442. [Google Scholar] [CrossRef]
- Sadik, C.; Moudden, O.; El Bouari, A.; El Amrani, I.E. Review on the elaboration and characterization of ceramics refractories based on magnesite and dolomite. J. Asian Ceram. Soc. 2016, 4, 219–233. [Google Scholar] [CrossRef]
- Yu, C.; Dong, B.; Chen, Y.F.; Ma, B.Y.; Ding, J.; Deng, C.J.; Zhu, H.X.; Di, J.H. Enhanced oxidation resistance of low-carbon MgO–C refractories with ternary carbides: A review. J. Iron Steel Res. Int. 2022, 29, 1052–1062. [Google Scholar] [CrossRef]
- Ren, X.; Ma, B.; Wang, L.; Liu, G.; Yu, J. From magnesite directly to lightweight closed-pore MgO ceramics: The role of Si and Si/SiC. Ceram. Int. 2021, 47, 31130–31137. [Google Scholar] [CrossRef]
- Salomão, R.; Arruda, C.C.; Pandolfelli, V.C.; Fernandes, L. Designing high-temperature thermal insulators based on densification-resistant in situ porous spinel. J. Eur. Ceram. Soc. 2021, 41, 2923–2937. [Google Scholar] [CrossRef]
- Ma, B.; Zan, W.; Liu, K.; Mu, X.; Deng, C.; Huang, A. Preparation and properties of porous MgO based ceramics from magnesite tailings and fused magnesia. Ceram. Int. 2023, 49, 19072–19082. [Google Scholar] [CrossRef]
- Kokkinos, E.; Zouboulis, A.I. The Chromium Recovery and Reuse from Tanneries: A Case Study According to the Principles of Circular Economy. In Leather and Footwear Sustainability Textile Science and Clothing Technology, 1st ed.; Muthu, S.S., Ed.; Springer: Singapore, 2020; Volume 6, pp. 123–157. [Google Scholar] [CrossRef]
- Erdem, M. Chromium recovery from chrome shaving generated in tanning process. J. Hazard. Mater. 2006, B129, 143–146. [Google Scholar] [CrossRef]
- Wystalska, K.; Sobik-Szołtysek, J. Sludge from tannery industries. In Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource Recovery, 1st ed.; Prasad, M.N.V., de Campos Favas, P.J., Vithanage, M., Mohan, S.V., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; Volume 2, pp. 31–46. ISBN 9780128159071. [Google Scholar]
- Kokkinos, E.; Zouboulis, A. Hydrometallurgical recovery of CR(III) from tannery waste: Optimization and selectivity investigation. Water 2020, 12, 719. [Google Scholar] [CrossRef]
- Kokkinos, E.; Proskynitopoulou, V.; Zouboulis, A. Chromium and energy recovery from tannery wastewater treatment waste: Investigation of major mechanisms in the framework of circular economy. J. Environ. Chem. Eng. 2019, 7, 103307. [Google Scholar] [CrossRef]
- Beshah, D.A.; Tiruye, G.A.; Mekonnen, Y.S. Characterization and recycling of textile sludge for energy-efficient brick production in Ethiopia. Environ. Sci. Pollut. Res. 2021, 28, 16272–16281. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, F.; Migliaccio, R.; Ruoppolo, G.; Balsamo, M.; Montagnaro, F.; Imperiale, E.; Caracciolo, D.; Urciuolo, M. Tannery Sludge Gasification in a Fluidized Bed for Its Energetic Valorization. Ind. Eng. Chem. Res. 2022, 61, 16972–16979. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, F.; Ye, Z.; He, F.; Qin, L.; Lv, G. Acid gas emission and ash fusion characteristics of multi-component leather solid waste incineration in bubbling fluidized bed. Environ. Pollut. 2023, 335, 122249. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Ma, L.; Li, J.; Tan, P.; Fang, Q.; Chen, G. Effects of temperature on the migration behaviour of arsenic and chromium in tannery sludge under CO2 gasification. J. Hazard. Mater. 2024, 461, 132663. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, F.; Kanhar, A.H. Sludge acts as a catalyst for coal during the co-combustion process investigated by thermogravimetric analysis. Energies 2017, 10, 1993. [Google Scholar] [CrossRef]
- Dudyński, M.; Dudyński, K.; Kluska, J.; Ochnio, M.; Kazimierski, P.; Kardaś, D. Gasification of leather waste for energy production: Laboratory scale and industrial tests. Int. J. Energy Res. 2021, 45, 18540–18553. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Zhang, G.; Kang, G.; Liu, Z.; Yu, J.; Gao, S. Research on the Influence of Combustion Methods on NOx Emissions from Co-combustion of Various Tannery Wastes. ACS Omega 2022, 7, 4110–4120. [Google Scholar] [CrossRef]
- Cai, H.; Liu, J.; Kuo, J.; Xie, W.; Evrendilek, F.; Zhang, G. Ash-to-emission pollution controls on co-combustion of textile dyeing sludge and waste tea. Sci. Total Environ. 2021, 794, 148667. [Google Scholar] [CrossRef]
- Khan, A.; Ali, I.; Naqvi, S.R.; AlMohamadi, H.; Shahbaz, M.; Ali, A.M.; Shahzad, K. Assessment of thermokinetic behaviour of tannery sludge in slow pyrolysis process through artificial neural network. Chemosphere 2023, 337, 139226. [Google Scholar] [CrossRef]
- Abbas, N.; Jamil, N.; Hussain, N. Assessment of key parameters in tannery sludge management: A prerequisite for energy recovery. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2656–2663. [Google Scholar] [CrossRef]
- Kluska, J.; Turzyński, T.; Ochnio, M.; Kardaś, D. Characteristics of ash formation in the process of combustion of pelletised leather tannery waste and hardwood pellets. Renew. Energy 2020, 149, 1246–1253. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, X.; Lv, G.; Wang, F.; Huang, Q.; Chi, Y.; Yan, J.; Yuan, W.; Chen, X.; Luo, W. Co-Combustion of Tannery Sludge in a Bench-Scale Fluidized-Bed Combustor: Gaseous Emissions and Cr Distribution and Speciation. Energy Fuels 2017, 31, 11069–11077. [Google Scholar] [CrossRef]
- Zhan, M.; Sun, C.; Chen, T.; Li, X. Emission characteristics for co-combustion of leather wastes, sewage sludge, and coal in a laboratory-scale entrained flow tube furnace. Environ. Sci. Pollut. Res. 2019, 26, 9707–9716. [Google Scholar] [CrossRef] [PubMed]
- Staszak, K.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; Góra, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Advances in the Removal of Cr(III) from Spent Industrial Effluents—A Review. Materials 2023, 16, 378. [Google Scholar] [CrossRef]
- Pantazopoulou, E.; Zouboulis, A. Chromium recovery from tannery sludge and its ash, based on hydrometallurgical methods. Waste Manag. Res. 2020, 38, 19–26. [Google Scholar] [CrossRef]
- Raguraman, R.; Sailo, L. Efficient chromium recovery from tannery sludge for sustainable management. Int. J. Environ. Sci. Technol. 2017, 14, 1473–1480. [Google Scholar] [CrossRef]
- Kokkinos, E.; Banti, A.; Mintsouli, I.; Touni, A.; Sotiropoulos, S.; Zouboulis, A. Combination of thermal, hydrometallurgical and electrochemical tannery waste treatment for Cr(III) recovery. Appl. Sci. 2021, 11, 532. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, C.; Wang, H.; Zhang, G.; Li, Q.; Wang, M. Extraction of chromium from tannery sewage sludge. Can. Metall. Q. 2022, 61, 183–189. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, H.; Chen, X.; Zhu, C.; Li, X. Effect of incineration temperature on chromium speciation in real chromium-rich tannery sludge under air atmosphere. Environ. Res. 2020, 183, 109159. [Google Scholar] [CrossRef]
- Juel, M.A.I.; Mizan, A.; Ahmed, T. Sustainable use of tannery sludge in brick manufacturing in Bangladesh. Waste Manag. 2017, 60, 259–269. [Google Scholar] [CrossRef]
- Shen, D.; Huang, M.; Feng, H.; Li, N.; Zhou, Y.; Long, Y. Effect of waste addition points on the chromium leachability of cement produced by co-processing of tannery sludge. Waste Manag. 2017, 61, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Malaiškiene, J.; Kizinievič, O.; Kizinievič, V. A study on tannery sludge as a raw material for cement mortar. Materials 2019, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Lian, F.; Xia, R.; Wang, Z. Formulation and durability of a geopolymer based on metakaolin/tannery sludge. Waste Manag. 2018, 79, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Genua, F.; Lancellotti, I.; Leonelli, C. Geopolymer-Based Stabilization of Heavy Metals, the Role of Chemical Agents in Encapsulation and Adsorption: Review. Polymers 2025, 17, 670. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Wang, D.; Ju, X.; Fu, S. Cyano and acylamino group modification for tannery sludge bio-char: Enhancement of adsorption universality for dye pollutants. J. Environ. Chem. Eng. 2021, 9, 104939. [Google Scholar] [CrossRef]
- Ramya, V.; Murugan, D.; Lajapathirai, C.; Saravanan, P.; Sivasamy, A. Removal of toxic pollutants using tannery sludge derived mesoporous activated carbon: Experimental and modelling studies. J. Environ. Chem. Eng. 2019, 7, 102798. [Google Scholar] [CrossRef]
- Rossi, D.; Cappello, M.; Antognoli, M.; Brunazzi, E.; Seggiani, M. Pyrolyzed tannery sludge as adsorbent of volatile organic compounds from tannery air emissions. Chem. Eng. J. 2023, 454, 140320. [Google Scholar] [CrossRef]
- de Sousa, R.S.; Santos, V.M.; de Melo, W.J.; Nunes, L.A.P.L.; van den Brink, P.J.; Araújo, A.S.F. Time-dependent effect of composted tannery sludge on the chemical and microbial properties of soil. Ecotoxicology 2017, 26, 1366–1377. [Google Scholar] [CrossRef]
- de Moraes Cunha Gonçalves, M.; de Almeida Lopes, A.C.; Gomes, R.L.F.; de Melo, W.J.; Araujo, A.S.F.; Pinheiro, J.B.; Marin-Morales, M.A. Phytotoxicity and cytogenotoxicity of composted tannery sludge. Environ. Sci. Pollut. Res. 2020, 27, 34495–34502. [Google Scholar] [CrossRef]
- Miranda, A.R.L.; Mendes, L.W.; Rocha, S.M.B.; Van den Brink, P.J.; Bezerra, W.M.; Melo, V.M.M.; Antunes, J.E.L.; Araujo, A.S.F. Responses of soil bacterial community after seventh yearly applications of composted tannery sludge. Geoderma 2018, 318, 1–8. [Google Scholar] [CrossRef]
| Material | Equilibrium pH | Acidic Reagent | Neutralization Medium | Highlight | Ref. |
|---|---|---|---|---|---|
| Dunite | 4 | Oxalic acid | Soil | CO2 release prevention | [39] |
| Natural carbonates | 6.5 | Field AMD sample | AMD | Toxic metal removal | [41] |
| Serpentine | 8 | Simulated AMD | AMD | Toxic metal removal Magnesium dissolution | [43] |
| Dunite | 8 | Simulated AMD | AMD | Toxic metal removal | [44] |
| Olivine | 2.3 | Sulfuric acid | AMD | Nickel dissolution | [45] |
| Method | Efficiency (Kg CO2/Kg) | Enhancing Factor | Ref. |
|---|---|---|---|
| Wet direct carbonation | - | NaCl addition | [52] |
| Dry direct carbonation | 0.07 | Water vapor (10 vol.%) | [54] |
| Wet direct carbonation | - | NaHCO3, oxalic, and ascorbic acid | [56] |
| Wet direct carbonation | 0.28 | Pressure (10.2 bar) | [57] |
| Temperature | Process | Additive | Impact on Mineralogy and Refractory Properties |
|---|---|---|---|
| 650–680 °C | Serpentine decomposition | - | Nearly complete decomposition of serpentine |
| 850 °C | Recrystallization in olivine and pyroxenes | - | Initial formation of olivine and pyroxenes |
| 1300 °C | Enhanced pyroxene formation due to excess Si | - | Olivine formation reduced—more pyroxenes due to available Si |
| >1300 °C | Depending on the additive | Chromite | Promotes forsterite formation—improves refractory behavior |
| Alumina | Forms MgAl2O4 spinel—reduces forsterite and refractory properties | ||
| Maghemite | Increases bulk density—facilitates sintering at 5 wt% | ||
| Chromite + Maghemite | Forms spinels Mg(Cr,Fe,Al)2O4 and MgFe2O4—enhances refractory properties |
| Application | Positives | Negatives |
|---|---|---|
| Acidic medium neutralization |
|
|
| CO2 sequestration |
|
|
| Refractories and insulating material |
|
|
| Binder in cement production |
|
|
| SOx absorption- |
|
|
| Organic Carbon (wt%) | Total Carbon (wt%) | Ca (wt%) | CaCO3 (wt%) | HHV (MJ/Kg) | Ref. |
|---|---|---|---|---|---|
| 12.2 | - | 14.8 | 26 | 2 | [77] |
| 20.7 | 18.8 | 1.2 | - | 3 | [78] |
| - | 15.3 | - | - | 5.9 | [82] |
| - | 44.2 | 0.6 | - | 7.25 | [83] |
| - | 39.6 | 15.1 1 | - | 8.5 | [84] |
| - | 21 | - | - | 9.12 | [85] |
| - | 35.2 | 18.9 | - | 9.27 | [86] |
| - | 33.6 | - | Verified, no quantified | 14.9 | [79] |
| - | 21.9 | - | Verified, no quantified | 15.1 | [80] |
| - | 54.6 | - | Verified, no quantified | 21.9 | [81] |
| Initial HHV (MJ/Kg) | Co-Combustion Material | Proportion of Materials | Additive’s HHV (MJ/Kg) | HHV (MJ/Kg) | Ref. |
|---|---|---|---|---|---|
| 10.6 | Coal | 1:1 | 31.8 | 21.8 | [87] |
| 10.6 | Rice husk | 1:1 | 15.7 | 12.4 | [87] |
| 16.6 | Hardwood pellets | 1:1 | 19.6 | 18.1 | [88] |
| Initial Cr Content (wt%) | Pre-Treatment | Leaching Reagent | Recovery (%) | Recovered Cr Valence | Final Step/Product | Ref. |
|---|---|---|---|---|---|---|
| 8.6 | No | H2SO4 (pH 1) | 97 | III | Precipitation (NaOH-pH 8)/Cr(OH)3 (59 wt% Cr) | [92] |
| ~0.9 | No | H2SO4 (4% v/v) | 90 | III | Cr(III)oxidation to Cr(IV) by H2O2 + UV Cr(VI) reduction to Cr(III) by Na2SO3/Cr2(SO4)3 | [93] |
| 14.1 | No | H2SO4 (1 N) | 93 | III | Precipitation (NaOH-pH 8)/Cr(OH)3 (36 wt% Cr) | [76] |
| 29 | Thermal (700 °C) | H2SO4 (1 N) | 90 | VI | Electrochemical Cr(VI) reduction to Cr(III) | [94] |
| 18.9 | Thermal (600 °C) and 50 wt% Na2CO3 | H2SO4 (42 wt%) | 99.7 | VI | - | [95] |
| Application | Positives | Negatives |
|---|---|---|
| Energy recovery |
|
|
| Chromium recovery |
|
|
| Building materials |
|
|
| Absorption- |
|
|
| Fertilizer |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinos, E.; Peleka, E.; Tzamos, E.; Zouboulis, A. Waste Valorization Technologies in Tannery Sludge, Chromite, and Magnesite Mining. Recycling 2025, 10, 123. https://doi.org/10.3390/recycling10040123
Kokkinos E, Peleka E, Tzamos E, Zouboulis A. Waste Valorization Technologies in Tannery Sludge, Chromite, and Magnesite Mining. Recycling. 2025; 10(4):123. https://doi.org/10.3390/recycling10040123
Chicago/Turabian StyleKokkinos, Evgenios, Effrosyni Peleka, Evangelos Tzamos, and Anastasios Zouboulis. 2025. "Waste Valorization Technologies in Tannery Sludge, Chromite, and Magnesite Mining" Recycling 10, no. 4: 123. https://doi.org/10.3390/recycling10040123
APA StyleKokkinos, E., Peleka, E., Tzamos, E., & Zouboulis, A. (2025). Waste Valorization Technologies in Tannery Sludge, Chromite, and Magnesite Mining. Recycling, 10(4), 123. https://doi.org/10.3390/recycling10040123









