Abstract
Hydrothermal carbonization (HTC) technology converts biomass into a carbon-rich, oxygen-containing solid fuel. Most studies have focused on hydrochar produced under laboratory conditions, leaving a gap in understanding the performance of industrially produced hydrochar. This study comprehensively analyzes three types of industrially produced hydrochar for blast furnace (BF) injection. The results indicate that hydrochar has a higher volatile and lower fixed carbon content. It has a lower high heating value (HHV) than coal and contains more alkali matter. Nevertheless, hydrochar exhibits a better grindability and combustion performance than coal. Blending hydrochar with anthracite significantly enhances the combustion reactivity of the mixture. The theoretical conversion rate calculations reveal a synergistic effect between hydrochar and anthracite during co-combustion. Environmental benefit calculations show that replacing 40% of bituminous coal with hydrochar can reduce CO2 emissions by approximately 145 kg/tHM, which is equivalent to an annual reduction of 528 kton of CO2 and 208 kton of coal in BF operations. While industrially produced hydrochar meets BF injection requirements, its low ignition point and high explosivity necessitate the careful control of the blending ratio.
1. Introduction
The iron and steel industry plays an essential role in increasing the industrial output, enhancing the national economy, and strengthening the country’s comprehensive strength. China contributed roughly 869 million tons, or 64.18%, of the 1354 million tons of hot metal produced globally in 2021. China has been the largest producer of steel in the world and has maintained that position since 1996 [1,2]. The classic long-flow process structure (BOF) dominates the manufacture of steel in China, and the energy structure is high-carbon, with coal and coke accounting for around 90% of the energy input. A BF consumes fossil coal and coke, accumulating CO2 in the atmosphere and promoting the greenhouse effect. About 7% of the carbon emissions from energy systems come from the steel industry, with China responsible for more than 60%. Minimizing energy consumption in the steel industry is critical since China is the top user of coal and the top emitter of greenhouse gases globally [3,4].
According to the REN21 report [5], renewable energy was estimated to contribute around 19.3% of the ultimate energy consumption of the world. Renewable energy sources include geothermal, hydro, solar, wind, and biomass. Biomass energy accounts for around 9% of the world’s primary energy supply. Biomass, which is mainly made up of cellulose (C6H10O5)x, hemicellulose (C5H8O4)m, and lignin [C9H10O3(COH3)0.9-1.7]n, is broadly described as any renewable or recyclable organic matter, including all plants and animals, microbes, and any organic matter created by them for their life activities [6,7]. Additionally, biomass, also referred to as the “carbon neutral fuel”, has zero net CO2 emissions during combustion [8] and is the potential fuel substitute for fossil reducers, garnering attention in the steel industry [9]. The steel industry might emit no CO2 by switching to renewable energy sources. China has abundant biomass resource reserves. According to origins, biomass used as fuel can be divided into domestic sewage, industrial organic waste residues and waste liquids, forestry residues, agricultural residues, and solid municipal and rural wastes [10]. The yearly production of primary biomass resources in China is currently at 3.49 billion tons. Still, their potential for usage as an energy source is only about 0.46 billion tons of standard coal, with just 0.06 billion tons of standard coal currently being used. Utilizing biomass energy in the future to produce hot metal in BFs has enormous potential. Biomass has a low nitrogen, sulfur, and ash content. Its application as the fuel or reductant in the processes of sintering [11], pelleting [12], injecting [13,14], and coking [15] is crucial to the creation of a green, low-carbon, and sustainable BF ironmaking industry.
However, biomass differs from coal in terms of composition and structure, and the direct use of biomass raw materials in BFs is primarily restricted by their low calorific value and fixed carbon content, poor grindability, low energy density, and high moisture and volatile content [16,17]. Utilizing biochar produced during the carbonization process for BF injection is one of the primary applications of biomass resources to produce steel [18,19]. The two main biomass carbonization techniques currently used are pyrolysis and hydrothermal carbonization. Pyrolytic carbonization is defined as the thermal decomposition of biomass in the temperature range from 350 °C to 650 °C under anoxic conditions, primarily yielding biochar as the solid product, which has a high calorific value, high fixed carbon content, and ease of fragmentation as advantages. However, it also has a high energy consumption, high emissions, and high alkali metals as negatives [20]. In contrast to pyrolysis carbonization, hydrothermal carbonization uses water as the medium to transform biomass into biocarbon after reacting for a predetermined amount of time at a lower temperature (150–350 °C) [21]. The reaction process has the advantages of a low energy consumption and low pollution, and the product has an abundant surface functional groups and good chemical activity. Furthermore, hydrothermal carbonization (HTC) has been demonstrated to effectively mitigate the concentration of hazardous constituents in biomass feedstocks, including alkali metals (e.g., potassium), water-soluble salts, and chloride-containing compounds under controlled process conditions. [22]. Hydrothermal carbonization can better apply biomass in BF ironmaking, thus effectively reducing steel enterprises’ carbon reduction pressure and energy burden.
The preparation of hydrochar for BF injection has significant advantages by recycling various biomass raw materials, such as agricultural and forestry waste and urban solid waste [23,24,25]. Firstly, this process provides a sustainable and resource-efficient way for waste utilization, reducing environmental issues caused by landfilling or incineration while achieving resource recycling [26,27,28]. Secondly, hydrochar has a high specific surface area and good reaction activity, which can improve the gas–solid reaction efficiency in BFs, thereby enhancing the combustion efficiency. Low-content sulfur and phosphorus elements can reduce pollutant emissions in the BF flue gas and reduce the production of environmental pollution [29]. In addition, the addition of hydrochar can partially replace traditional coke or coal, reducing production costs and carbon emissions, which meets the development requirements of green metallurgy [30,31,32].
To ensure optimal utilization, it is necessary to determine the nature and combustion characteristics of the hydrochar before using it for BF injection. Additionally, the hydrochar produced from the preparation of various types of biomasses may differ significantly. Extensive experimental investigations have systematically evaluated the combustion characteristics of hydrochar, with particular focus on their thermal reaction behavior and co-combustion kinetics with conventional fossil fuels [33,34,35]. The mass loss rate curve of the combustion process of hydrochar had two significant peaks for volatile fraction and fixed carbon combustion, and only one peak for fixed carbon combustion was for coal combustion. The starting combustion temperature and burnout temperature of hydrochar were lower than those of coal, and the maximum combustion rate and average combustion rate were higher, indicating that the co-combustion of coal and hydrochar can effectively improve the combustion characteristics of the blends. According to the study on the co-combustion of various hydrochar types with coal [36,37,38], the hydrochar was more reactive in combustion, and the ratio of hydrochar had a more significant impact on the combustibility of the co-blends. As the hydrochar ratio increased, the ignition points of the co-blends decreased, their combustion ranges shortened, and their combustion properties improved. The structural and combustion differences between coal and hydrochar make it possible for synergistic effects to exist between the two in the co-combustion process [39,40]. However, most of the hydrochar used in the studies was produced by reactors under laboratory conditions, and there is a gap with industrially produced ones. Further research on the co-combustion of coal and industrially produced hydrochar is needed to provide theoretical guidance for its practical implementation in BF injection.
The proximate analysis, ultimate analysis, ignition points and explosivity, ash composition, grindability, and combustibility of three types of biowaste-derived hydrochar provided by an HTC plant were compared with coal, and the similarities and differences between hydrochar and coal were obtained. In addition, the coal blending scheme was determined based on the ignition points and explosivity of the blended coal with different ratios of hydrochar, and a thermogravimetric analysis (TGA) was used to analyze the combustion characteristics of various blended coal samples. At the same time, the theoretical combustion conversions of the blended coal were calculated, and the experimental values of different blended coal samples were plotted against the computed conversions, which were used to verify the synergistic effect of the co-combustion of the blended coal. Finally, the environmental benefits of hydrochar were calculated. The results of this study further deepen the knowledge of the co-combustion of hydrochar and coal to provide theoretical references for the application of biocarbon for BF injection.
2. Results and Discussion
2.1. Physical and Chemical Characteristics
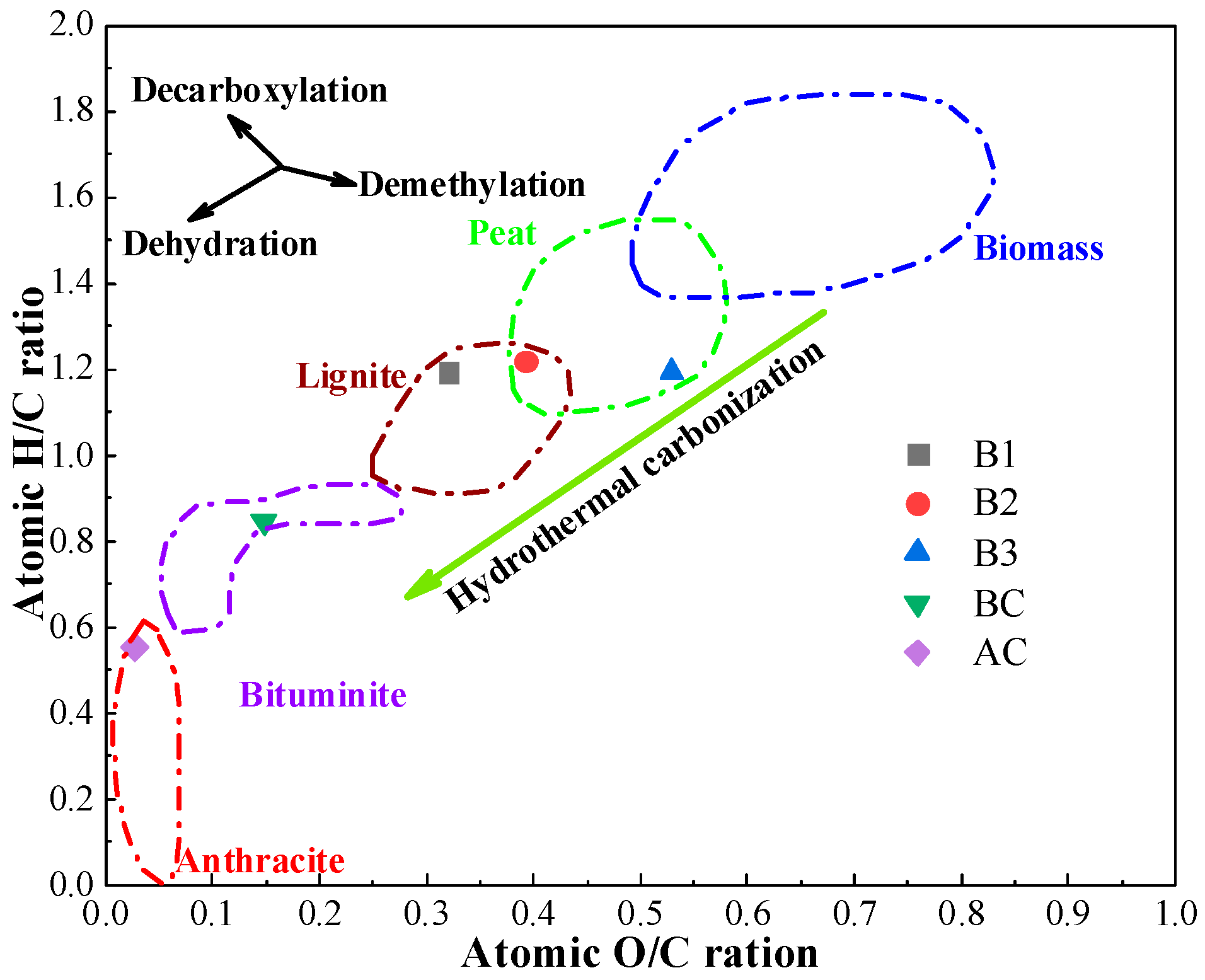
The results of the proximate analysis, ultimate analysis, and HHV of coal and hydrochar are shown in Table 1. The hydrochar produced exhibited significantly reduced fixed carbon content (23–32%) and substantially elevated volatile matter (53–64%) compared to conventional pulverized coal, with these contrasting characteristics attributed to the carbonization process preserving labile organic components while promoting aromatic condensation. Meanwhile, hydrochar was more reactive and likely to experience combustion reactions based on its higher H and O contents [41,42]. Therefore, blending hydrochar with coal can enhance the performance of the ignition of the blend before the air outlet, which is advantageous for increasing the smelting intensity and decreasing the amount of air needed to produce hot metal when injecting in the BF, as stated in the previous study [43]. The ash content varies widely depending on the type of biomass. As shown in Table 1, B1 and B2 have a high ash content, 18.55% and 14.44%, respectively. The ash content in B3 is low, which is similar to BC. As shown in the Van Krevelen diagram (Figure 1) [44], the H/C and O/C atomic ratios of hydrochar were identical to BC. This confirms that HTC significantly improves the fuel quality of the biomass and is consistent with the results of Kambo et al. [45], who found that HTC-treated miscanthus exhibited an ash content and O/C–H/C ratios comparable to lignite. However, the HHV of hydrochar was lower than that of coal due to the low C content and high O content, with a maximum of only 21.17 MJ/kg, meaning that the thermal stability of the BF needs to be taken into consideration when using hydrochar for BF injection.

Table 1.
Proximate and ultimate analyses and high heating value of coal and hydrochar.
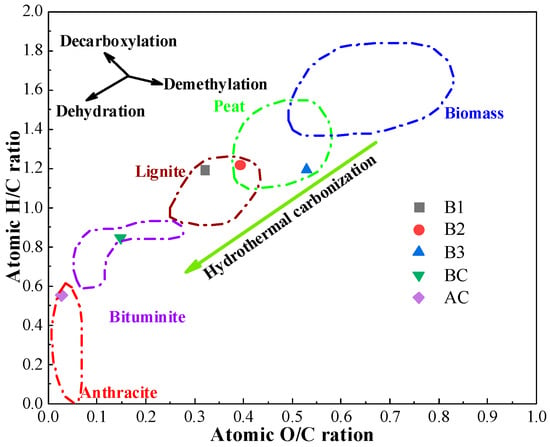
Figure 1.
Van Krevelen diagram of coal and hydrochar.
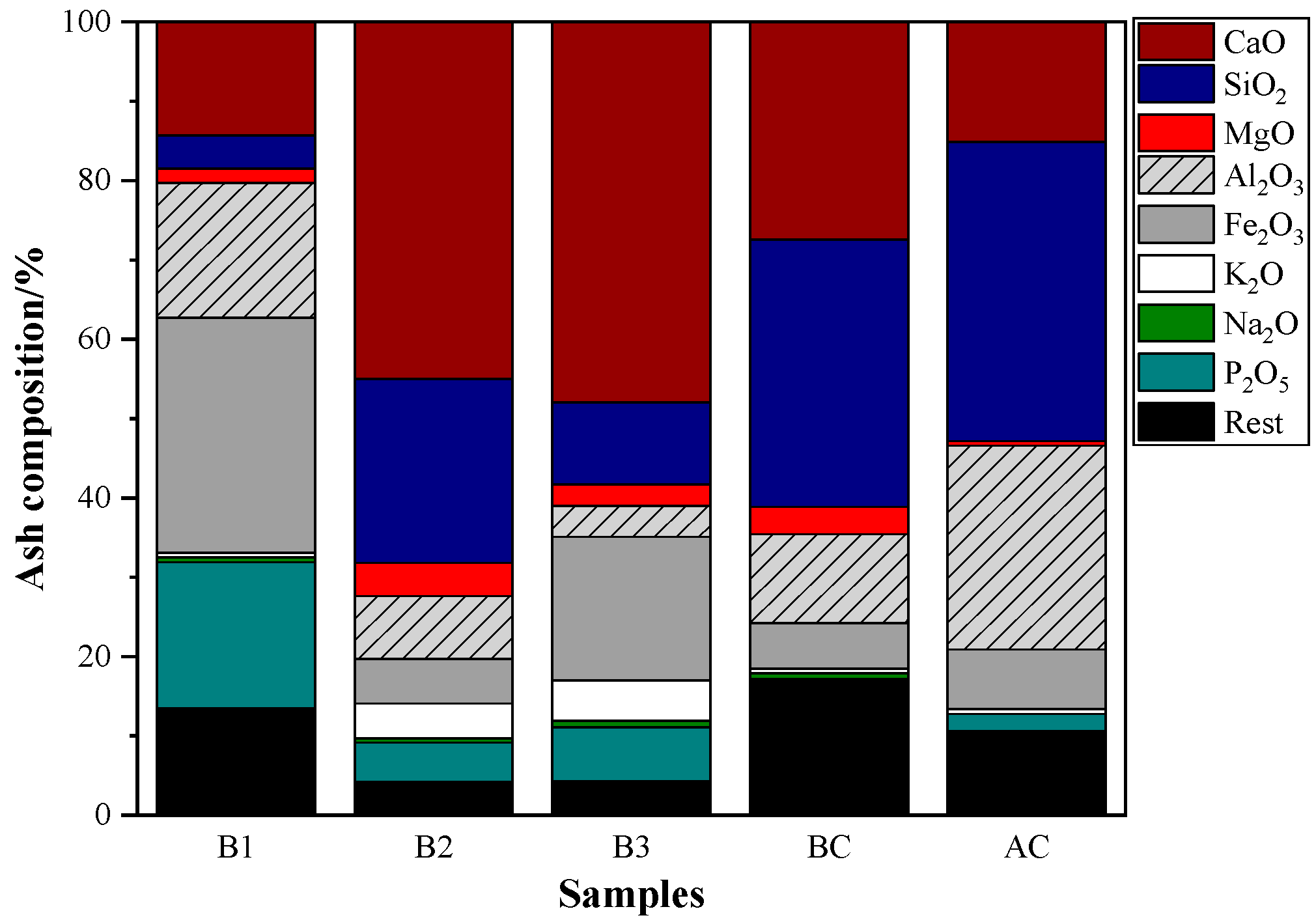
From the composition analysis of ash (Figure 2), it could be found that the alkali matter in hydrochar is higher than that in coal. The high alkali metal element content of hydrochar is a significant barrier to its use in BF injection, and the cyclic enrichment of alkali metals within the BF can have a detrimental effect on the metallurgical characteristics of coke and ore as well as the durability of refractory materials [46,47]. Even though the alkali matter could function as the catalyst in the combustion process, it has the disadvantage of a low melting temperature that could lead to agglomeration in the combustion process [48]. Meanwhile, a higher content of SiO2 and Al2O3 in coal ash could improve the softening and melting characteristics of hydrochar ash.
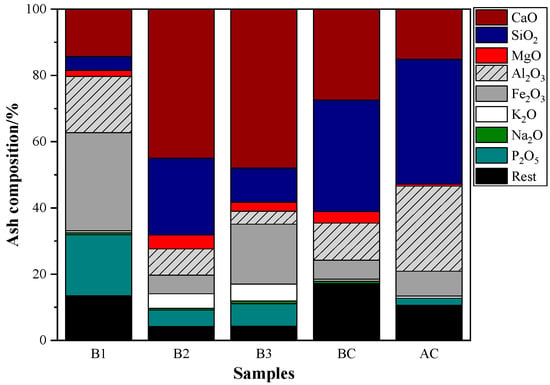
Figure 2.
Ash composition of coal and hydrochar.
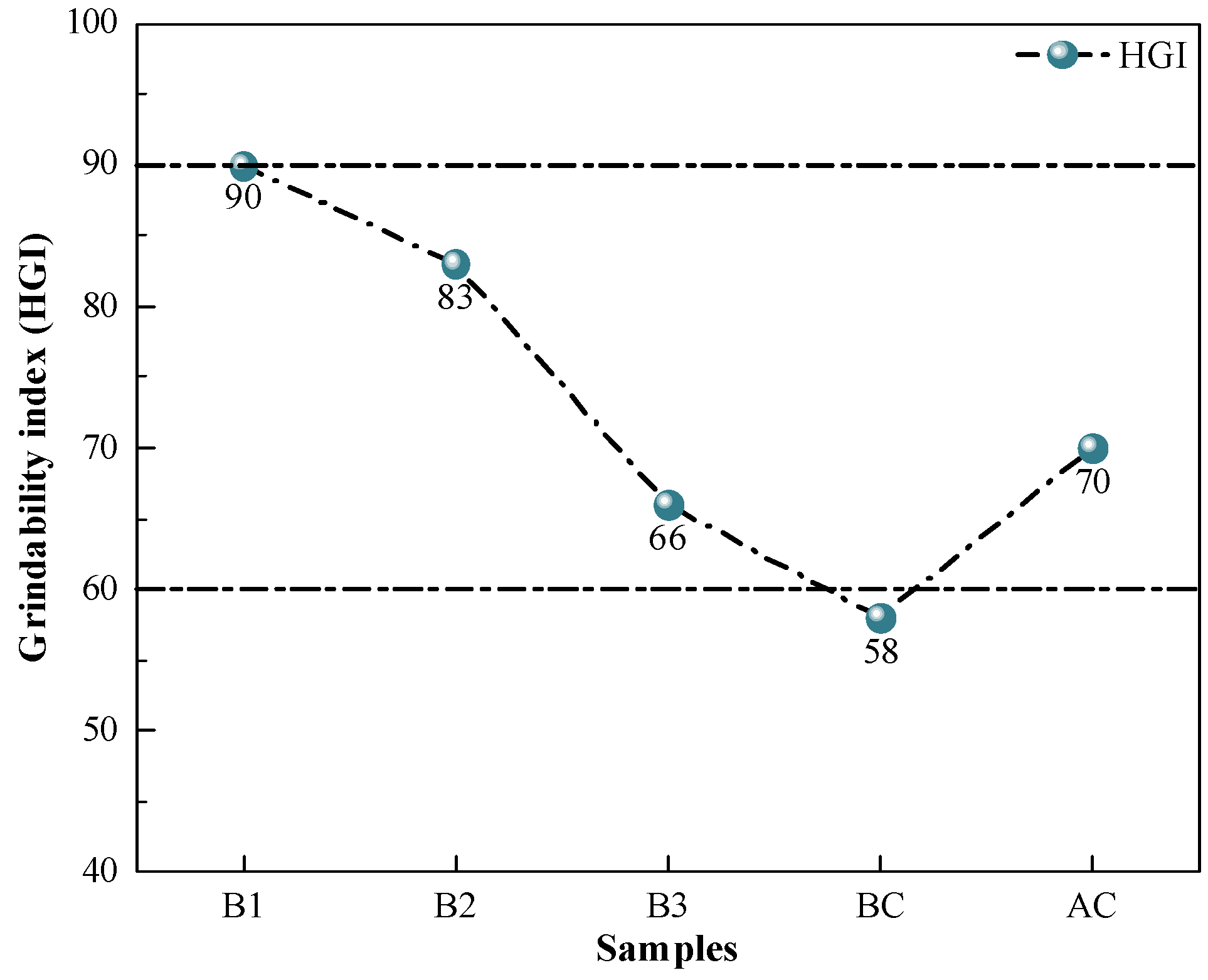
The grindability index characterizes the difficulty of the sample pulverization. The biomass sample has a different structure from coal pulverization, which is more ductile, making it impossible to crush it directly using pulverization equipment like ball mills [49]. The cellulose, hemicellulose, and lignin of biomass undergo condensation after the HTC process to produce carbon compounds. The hydrochar obtained from the preparation then turns black, loses hardness, becomes more brittle, is easily crushed under external extrusion, and has a significantly improved grindability [50]. The results of the grindability index measurements of the samples are shown in Figure 3. The hydrochar had grindability indices above 65, which were sufficient to meet the present demands of the pulverizing machinery of steel firms for the pulverizing performance of injecting fuel. On the one hand, the pulverizing process does not have a large loss to the mill, which reduces the corresponding pulverizing cost; on the other hand, it can reduce the problem of uneven coal powder blending, which causes the blockage of the injecting system and unstable BF smelting.
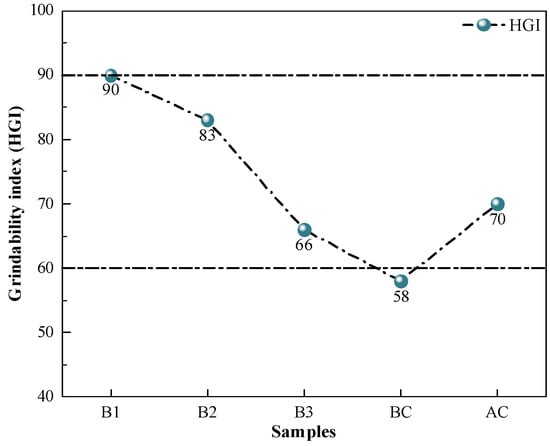
Figure 3.
Grindability index of coal and hydrochar.
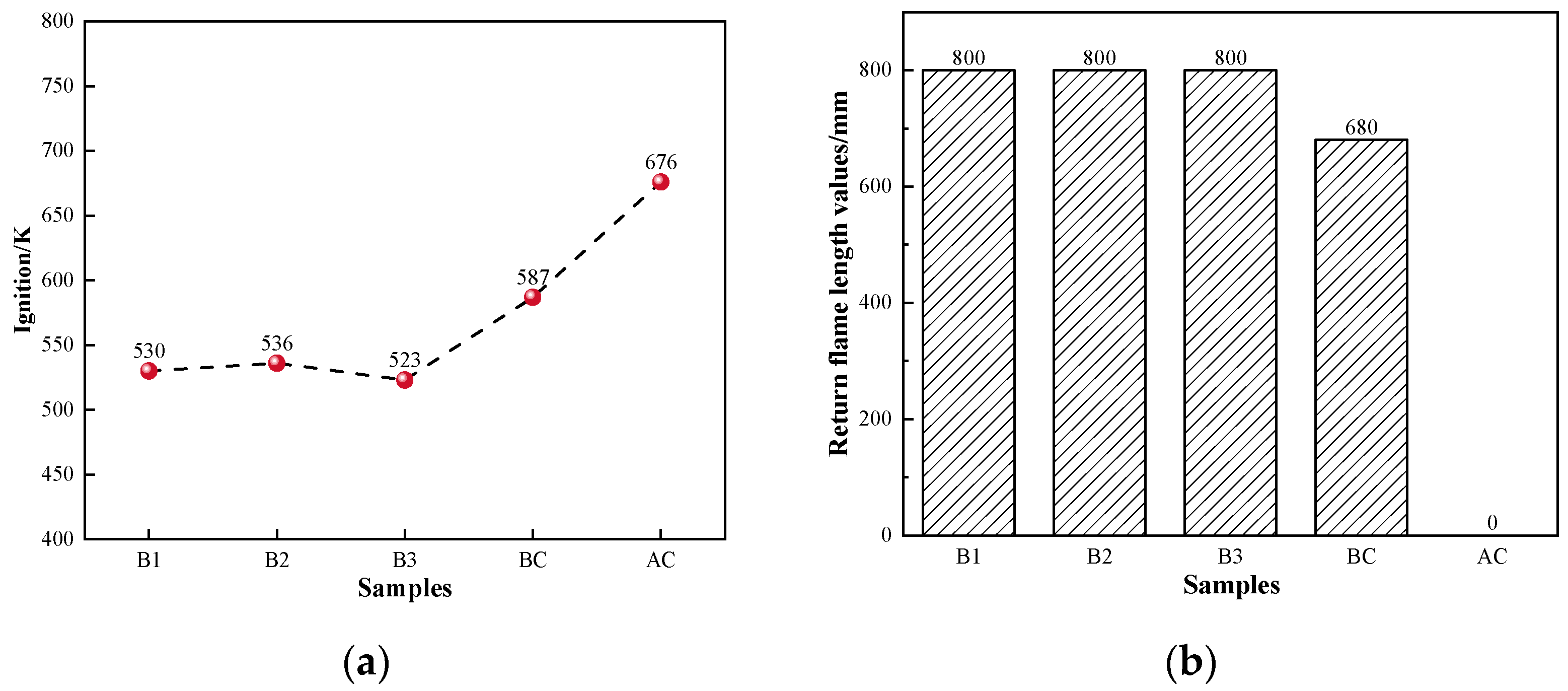
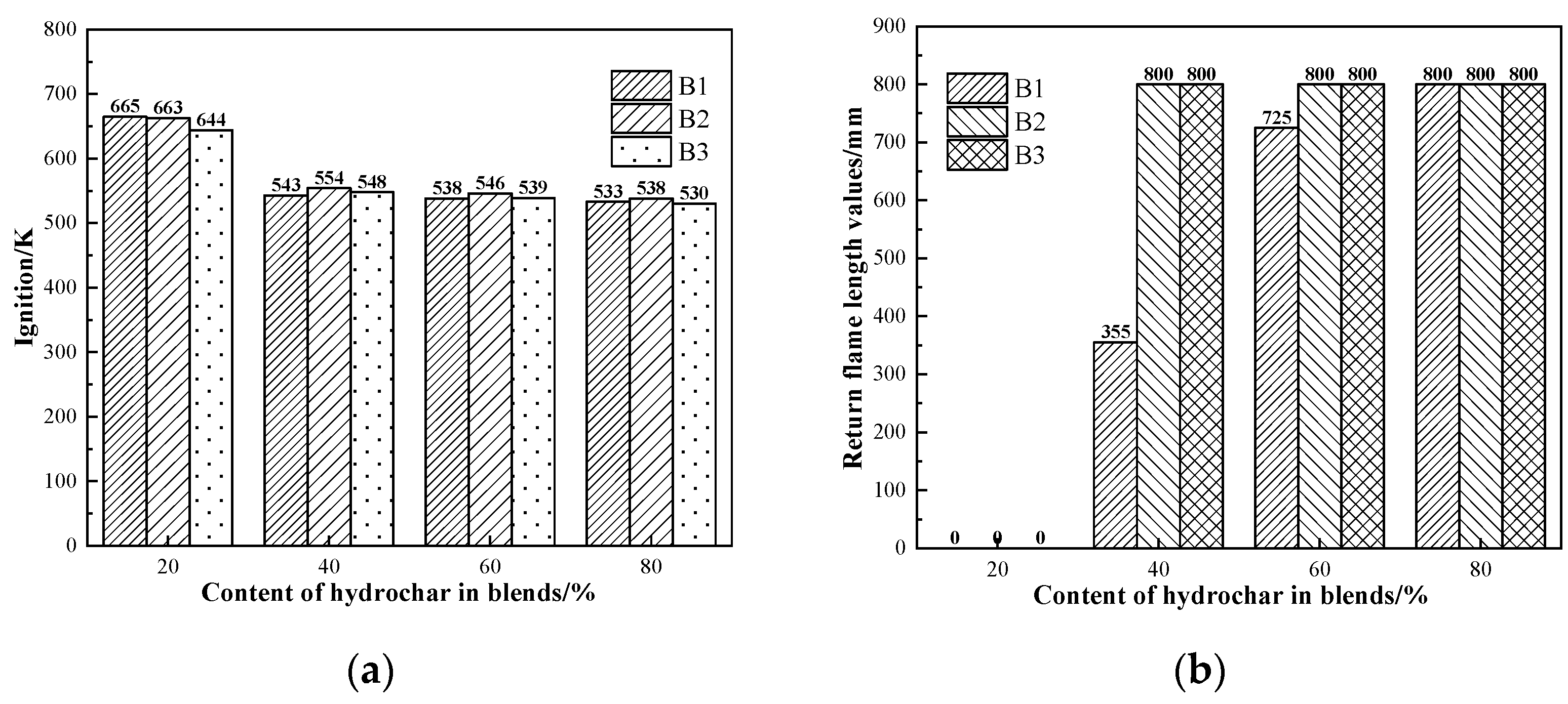
The ignition points and explosivity of coal are important indicators for evaluating the safety performance of BF injection fuel [51]. To ensure safe production, the BF injection pulverized coal process requires combustion with a suitable ignition point and weak explosivity. The ignition points and explosivity data of hydrochar in this study are shown in Figure 4a. Compared to coal, hydrochar had a lower ignition point temperature of 523–538 K. This was mainly due to the higher elemental H and O content of hydrochar, which facilitates the combustion reaction. It was evident that hydrochar had a return flame length greater than 800 mm and high explosive capabilities, as shown in Figure 4b. The primary explanation was that hydrochar had a high volatile content (all above 50%). The combustion process of biochar was likely to occur at lower temperatures when a lot of volatile matter was released into the air, leading to explosions [52,53,54,55]. Therefore, it is critical to ensure hydrochar can be used safely for BF injection in steel factories.
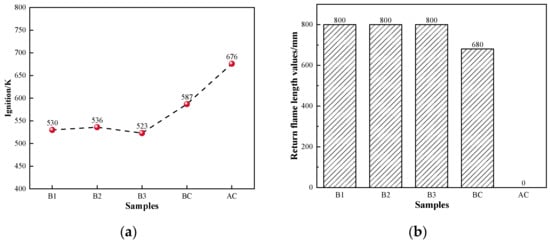
Figure 4.
Safety performance indicators of coal and hydrochar: (a) ignition and (b) explosivity.
2.2. Thermogravimetric Analysis of Coal and Hydrochar
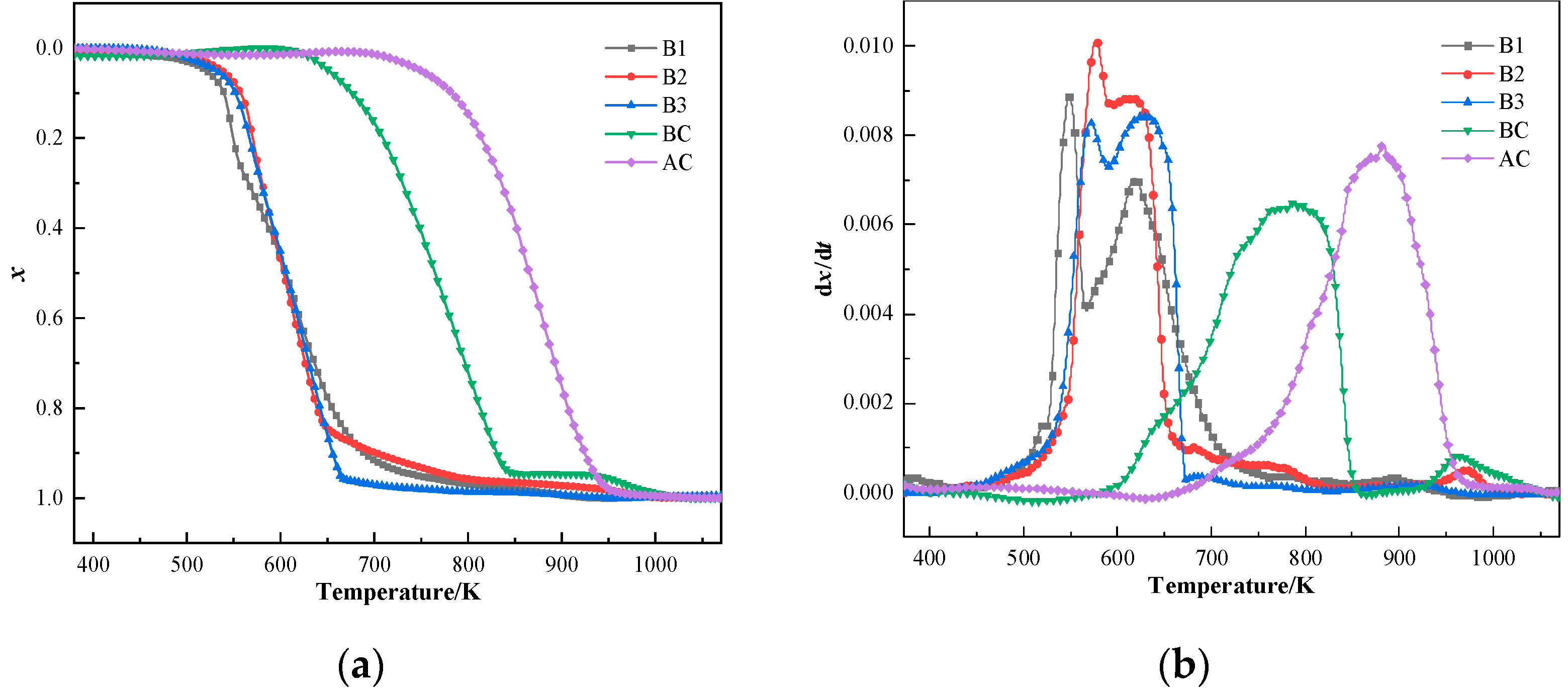
The conversion and reaction rate curves of samples at the heating rate of 20°C/min are shown in Figure 5. The conversion curves of samples showed strikingly comparable curves, with combustion behavior clearly separated into two distinct stages. The combustion characteristics of hydrochar exhibited three distinct thermal decomposition regimes compared to coal’s monophasic behavior. (1) The low-temperature regime (400–450 K) showed moisture-dominated minor mass loss. (2) The primary devolatilization stage (500–600 K) displayed sharp mass loss peaks corresponding to rapid volatile release, attributed to inherent volatile matter content of hydrochar. (3) The carbon combustion phase (600–700 K) presented broadened reaction rate peak with sustained mass loss. In contrast, coal combustion demonstrated a single high-temperature peak combining volatile release and carbon combustion in the reaction rate curve (750–950 K). Liang et al. [56] and Ma et al. [57] also explained the reason for the multi-peak phenomenon in the combustion curves of hydrochar. Comparing the combustion curves of various samples, the combustibility of three hydrochar was better than that of coal, and their use in BF injection can enhance the combustibility of blended coal in front of the tuyere zone. The TG curves of the blends move toward a low-temperature region.
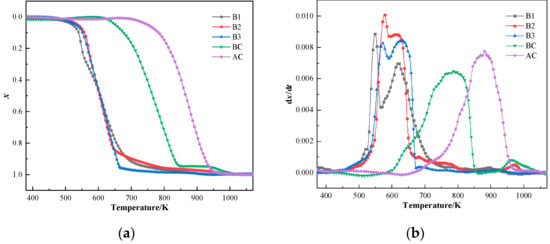
Figure 5.
Combustion curves of coal and hydrochar: (a) conversion rate curves and (b) reaction rate curves.
As shown in Table 2, hydrochar exhibited significantly lower initial combustion temperature (Ti = 520.76 K) and burnout temperature (Tf = 663.95 K) compared to coal. This disparity arises from two synergistic factors: (1) the elevated volatile matter content in hydrochar, which accelerates the onset of combustion reactions, and (2) the enhanced alkali metal content in its ash matrix, which acts as a catalyst to lower the burnout temperatures [58,59]. Notably, the Rmean values for hydrochar surpassed 8.40 × 10−3 s−1, exceeding those of coal (≤7.80 × 10−3 s−1), indicating superior kinetic reactivity. The comprehensive combustion index (S) and ignition index (C) for hydrochar were also markedly higher, reflecting its enhanced combustion efficiency and ease of ignition. These findings corroborate the superior combustion performance of hydrochar relative to coal, consistent with the results based on the physical and chemical characteristics of hydrochar.

Table 2.
Combustion characteristic indices of coal and hydrochar.
2.3. Hydrochar/Coal Blending Scheme
According to the experimental analysis in the preceding section, hydrochar can partially substitute bituminous coal as injection coal via tuyeres in BFs because it has a lower ignition point temperature, high explosive capabilities, and superior combustibility. The hydrochar (B1, B2, and B3) and anthracite coal blends were prepared with four different hydrochar ratios (i.e., 20 wt.%, 40 wt.%, 60 wt.%, and 80 wt.%) to clarify the changing pattern of the safety performance after blending the two materials.
Figure 6 shows the ignition points and explosivity of the blends, respectively. According to the figure, the ignition points of the samples ranged from about 640 K to 670 K when the proportion of hydrochar was 20%; it dropped sharply to about 90 K to 110 K when the proportion of hydrochar was increased to 40%; and finally, as the proportion increased, the ignition points of the samples showed a gentle decreasing trend and the range was maintained at about 5 K. According to the return flame length data of the samples, the samples were all non-explosive when the hydrochar content was 20%; when the hydrochar content was 40%, the B1 and AC blends exhibited weak explosivity; the B2 and AC and B3 and AC blends exhibited a strong explosivity; and when the hydrothermal carbon content is or over 60%, the hydrochar and AC blend exhibited strong explosivity. When hydrochar is used as the injection fuel in the BF, the proportion of hydrochar should be carefully controlled to be less than 40%.
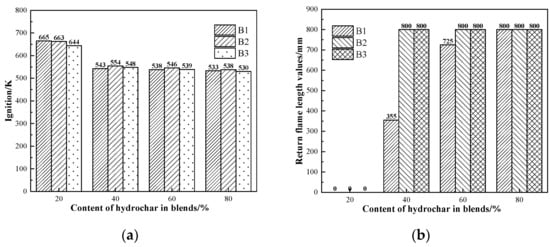
Figure 6.
Safety performance indicators of the blends: (a) ignition and (b) explosivity.
2.4. Co-Combustion Characteristics of the Blends
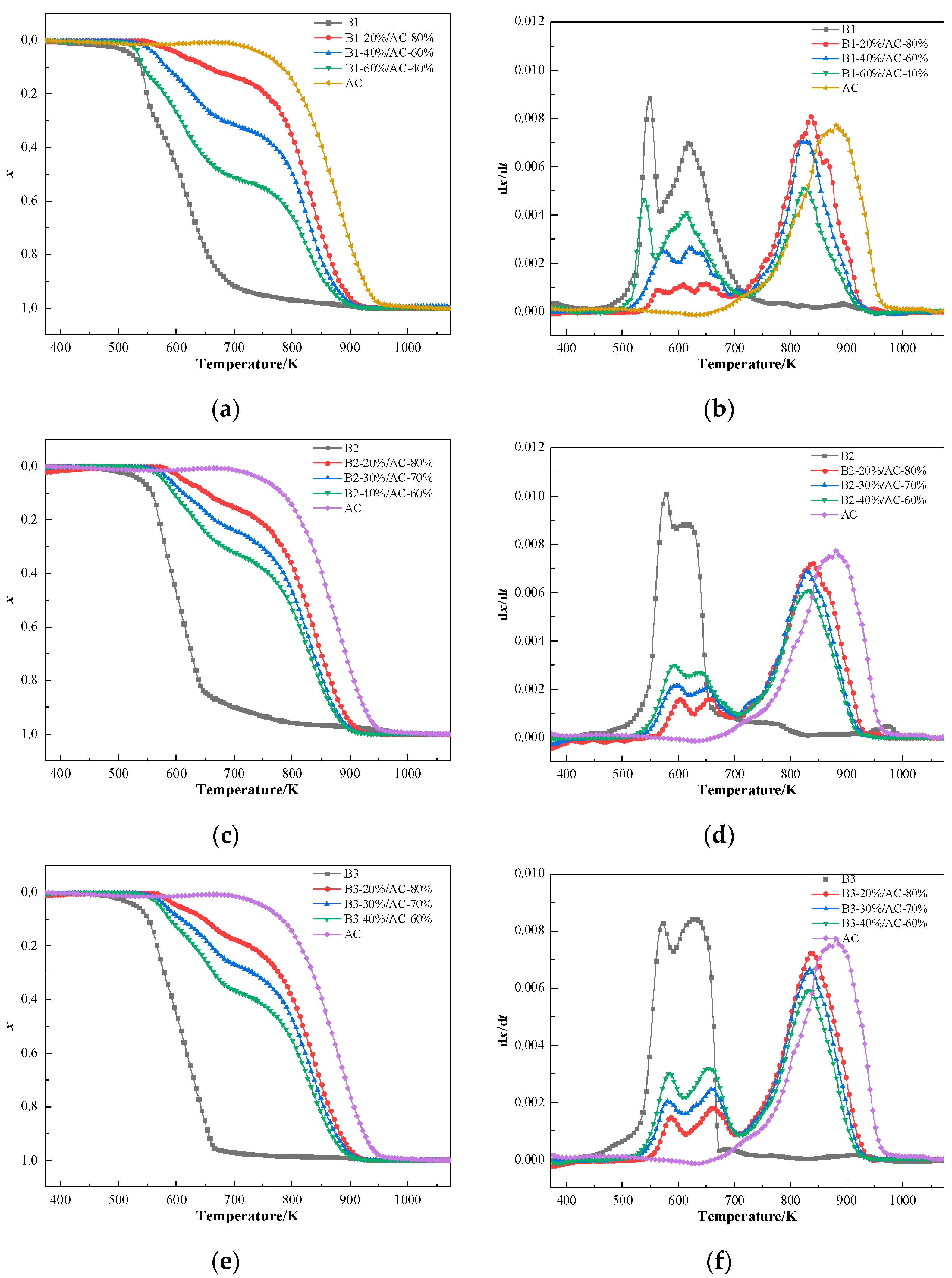
According to the test results of the ignition points and the explosivity of the blends in the preceding section, hydrochar ratios B1 (20%, 40%, and 60%), B2 (20%, 30%, and 40%), and B3 (20%, 30%, and 40%) were chosen to conduct combustibility experiments. The results are shown in Figure 7.
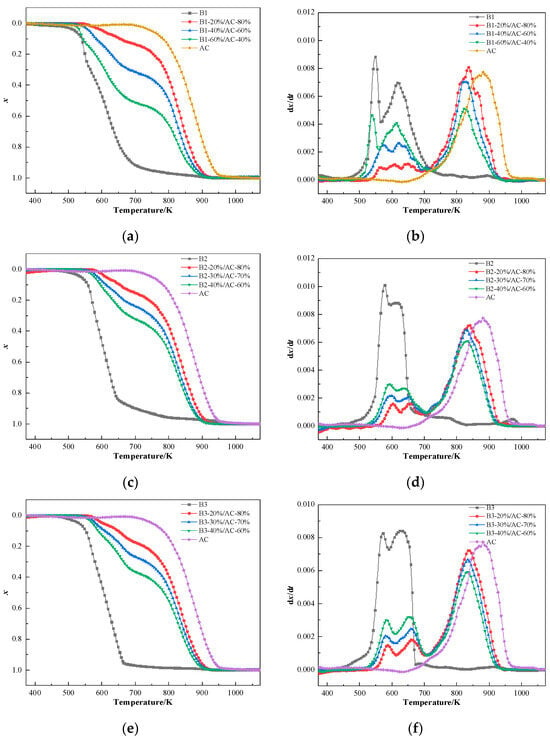
Figure 7.
Combustion curves of the blends: (a,c,e) conversion rate curves of B1, B2, and B3 and (b,d,f) reaction rate curves of B1, B2, and B3.
The hydrochar proportion critically governs the co-combustion process of coal-hydrochar blends, as evidenced by thermal decomposition curves. The TG curves of the blends are located between the individual combustion curves of coal and hydrochar and vary with the hydrochar contents in blends. With the increase in the hydrochar content, the TG curves of the blends move toward a low-temperature region, the combustion reactivity of blends is effectively improved, and the combustibility becomes closer to that of hydrochar. The DTG curves show that the mass loss peaks are located between the individual hydrochar and AC combustion zones for the blends.
Thermogravimetric analysis revealed triphasic combustion curves in DTG curves, attributable to distinct thermal reaction pathway. The initial peak (500–600 K) corresponded to volatile release and combustion from the labile functional groups of hydrochar, while the secondary peak (600–700 K) arose from carbon combustion of hydrochar. A tertiary peak (800–900 K) was ascribed to slow-burning anthracite char residues. Blends exhibited phase-dependent combustion behavior: at low hydrochar loadings (20%), the TG curves closely resembled pristine anthracite, with indistinct hydrochar-specific peaks obscured by dominant coal combustion signatures. Conversely, high-hydrochar blends (>60%) displayed pronounced low-temperature peaks, reflecting accelerated devolatilization kinetics. Notably, the combustion curves shifted dually with increasing hydrochar content: peaks near 534 K intensified due to enhanced volatile cracking, while those at 623 K broadened as carbon combustion kinetics converged. This non-linear thermal response aligns with prior findings on biomass-coal synergies [60,61].
Table 3 shows the results of estimating the combustion characteristic parameters of the blended coal. Alkalis had a considerable catalytic effect on the burning of coal and semicoke in the study by Farrow et al. [62], and the catalytic impact grew with an increasing alkaline content [63]. Because hydrochar contains significantly more K2O than AC, as shown in Figure 4, the catalytic effect became more pronounced as the proportion of the dispensed hydrochar increased. As a result, the Ti and Tf of the blended coal showed a decreasing trend, with the Ti showing a more significant decrease than the Tf. When B1 was increased to 80%, the Ti decreased by 359.74 K and the Tf decreased by 308.71 K only; when B2 and B3 were increased to 40%, the Ti decreased by 307.80 K and 304.43 K and the Tf decreased by 288.87 K and 287.58 K, respectively. These results showed that hydrochar had a more significant influence on the start of the combustion of the coal blends and can be burned at a lower temperature to release heat and promote the combustion process of the coal blends. The S and C of the samples increased to different degrees, indicating that the hydrochar significantly contributed to the combustion performance of AC. The maximum combustion rate for the blends is located at different temperatures with different ratios. The Rmean decreases with an increase in the hydrochar content, and Rmax increases with a rise in the hydrochar content. Other researchers have also found similar results [64].

Table 3.
Combustion characteristic parameters for the blends.
2.5. Synergistic Effects of Blended Coal Combustion
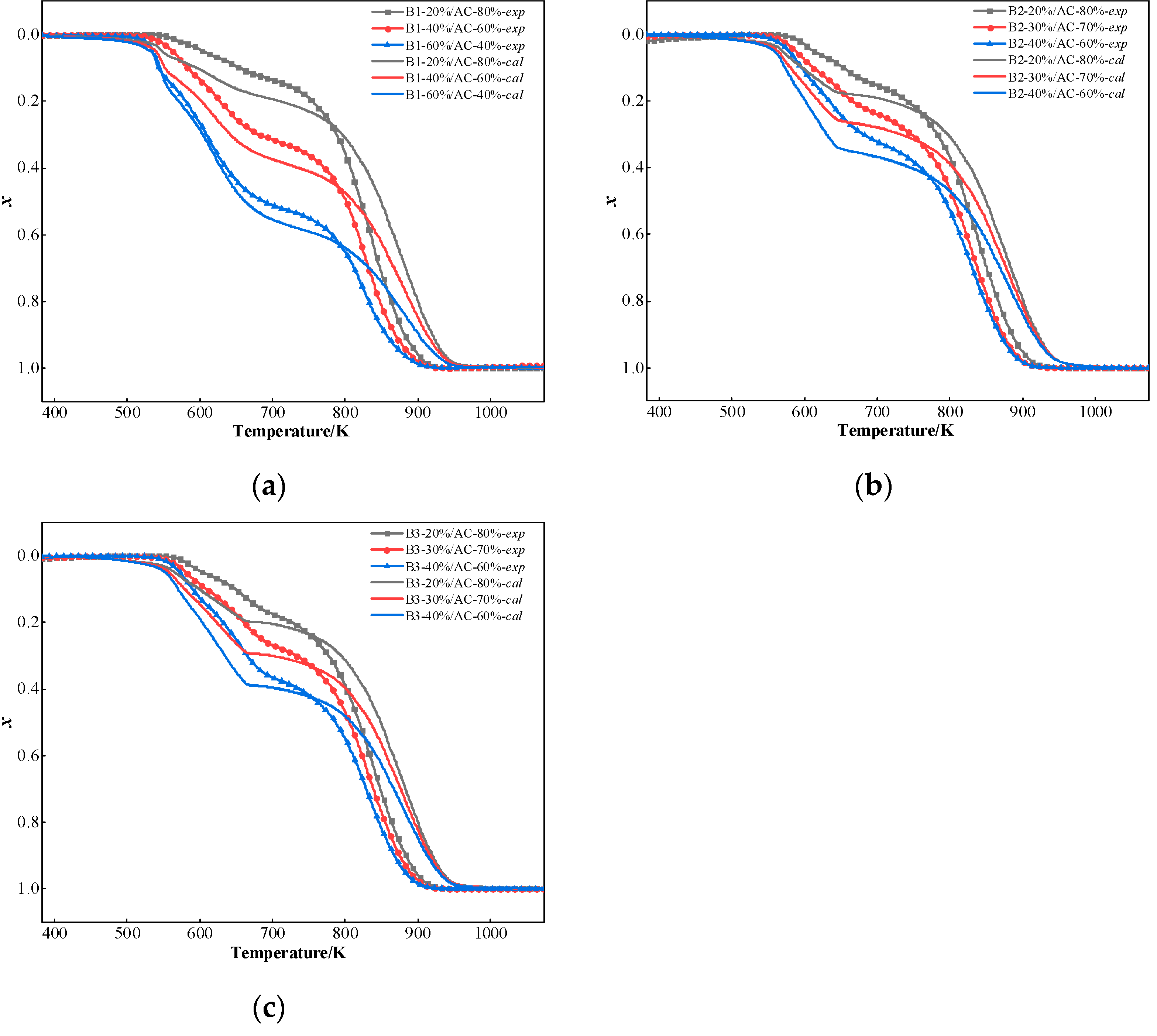
The conversion rate curves of the blended coal were between the two samples separately, and the Ti, Tf, S, and C did not increase linearly by increasing the proportion of hydrochar dispensing. According to the study of Wang et al. [37], the presence of alkali metals in palm kernel shell char has a synergistic effect on the co-combustion of palm kernel shell char and anthracite. The synergistic interactions between hydrochar and AC were quantified through comparative assessment of experimental and theoretical combustion thermogravimetric experiment. The theoretical conversion values were derived via Equation (1) using individual combustion parameters obtained under identical thermal conditions. Deviation analysis revealed three distinct interaction regimes: (1) Positive deviation (experimental > theoretical) indicates hydrochar promotes the combustion of the blend; (2) Negative deviation (experimental < theoretical) suggests hydrochar has an inhibitory effect on the combustion of the blend; (3) Null deviation (experimental = theoretical) confirms no synergistic effect in the combustion of hydrochar and coal blended. [65].
where a and b are the mass ratios of hydrochar and AC, respectively, in the blend, and XB and XAC are the corresponding conversion rates of the individual sample.
The comparative assessment of experimental and calculated conversion rate curves at 20 K/min heating rate (Figure 8) confirming synergistic interactions between hydrochar and coal during combustion process. At 775 K, there is a definite intersection between the calculated and experimental curves. The experimental curves were lower than the calculated curves when the temperature was below 775 K. In comparison, the experimental curves were higher than the calculated curves when the temperature was above 775 K. These findings reveal that there is a synergistic effect in the co-combustion of hydrochar and AC. Below 775 K, the volatilization and combustion of the volatile matter were limited by AC of blends. AC has a higher degree of coalification and low volatile content, which requires a higher temperature for combustion and will inhibit the combustion of the blended coal in the beginning stage. When two samples are blended, the heat transfer is influenced by the component of the blended coal. This can create a temperature gradient that volatile matter decomposition and subsequent combustion processes migrate preferentially toward high-temperature zones. In the high-temperature region (above 775 K), the combustion of carbon residue in hydrochar is faster than that of AC. This is because the release of the volatile matter from hydrochar at low temperatures and the following combustion generates heat, which promotes the combustion of AC. In the meantime, carbon combustion can be catalyzed by the alkali metal oxides in the hydrochar ash. Farrow et al. [62] found that the synergistic catalytic effect of biochar is nearly totally lost when alkali and alkaline earth metals are removed from the biomass. This demonstrates even more how important alkaline metals are in accelerating coal burning.
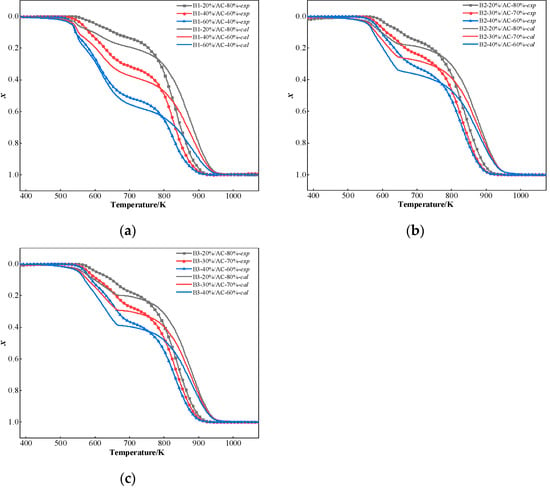
Figure 8.
Comparison of experimental curves and calculated curves result: (a) B1; (b) B2; and (c) B3.
The co-combustion mechanism analysis demonstrates that AC presence suppresses ignition initiation of blends, while hydrochar-derived alkali metal oxides (K2O, Na2O) synergistically enhance char combustion by promoting surface oxygen diffusion. This dual-mode interaction aligns with Zhang et al. [38] findings on miscanthus hydrochar-lignite co-combustion, wherein alkaline earth metals accelerated devolatilization phase of lignite.
2.6. Energy Conservation and Emission Reduction Benefit Analysis
An energy–mass balance model of the BF was established to investigate the effect of injected hydrochar on BF smelting. The 60:40 ratio of anthracite and bituminous coal for BF injection was selected, which is the most basic blending ratio for steel plants. Hydrochar is considered a green fuel with net zero CO2 emissions, and it was decided that B3 would replace BC for BF injection in the ratios of 10%, 20%, 30%, or 40%. Equation (2) [66] was used to determine the carbon reduction effect of substituting hydrochar for bituminous coal in varying fractions for BF injection when the fuel ratio was 200 kg/tHM and the combustion rate was 100%. The computation results are displayed in Figure 9.
where Ecom is the CO2 emissions reduced by using hydrochar to replace BC, ton; NCV is the heating value of BC, GJ/ton; FC is the replaced amount of BC, ton; CC is the carbon content of BC, ton/GJ; and OF is the carbon oxidation rate of BC, %.
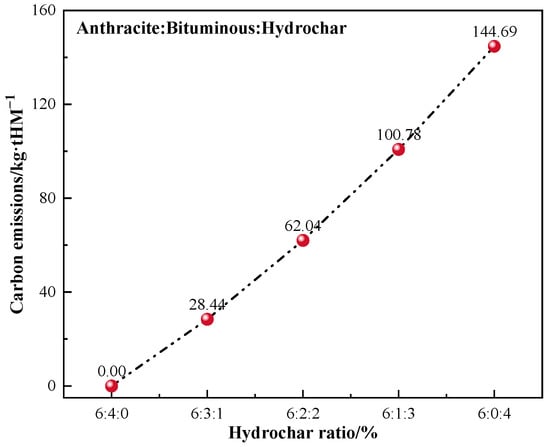
Figure 9.
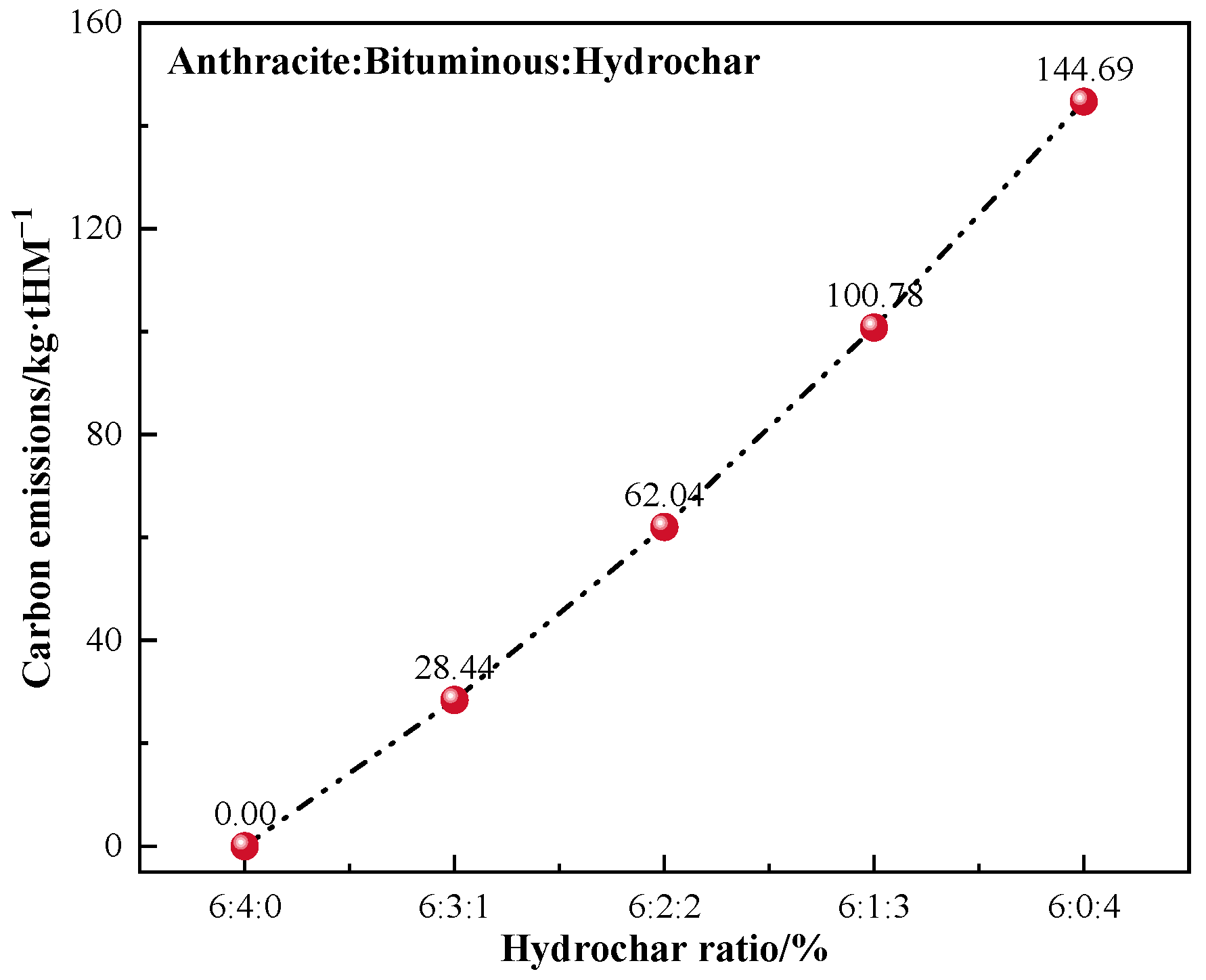
Reduction in CO2 emission by B3 replacing bituminous coal for BF injection.
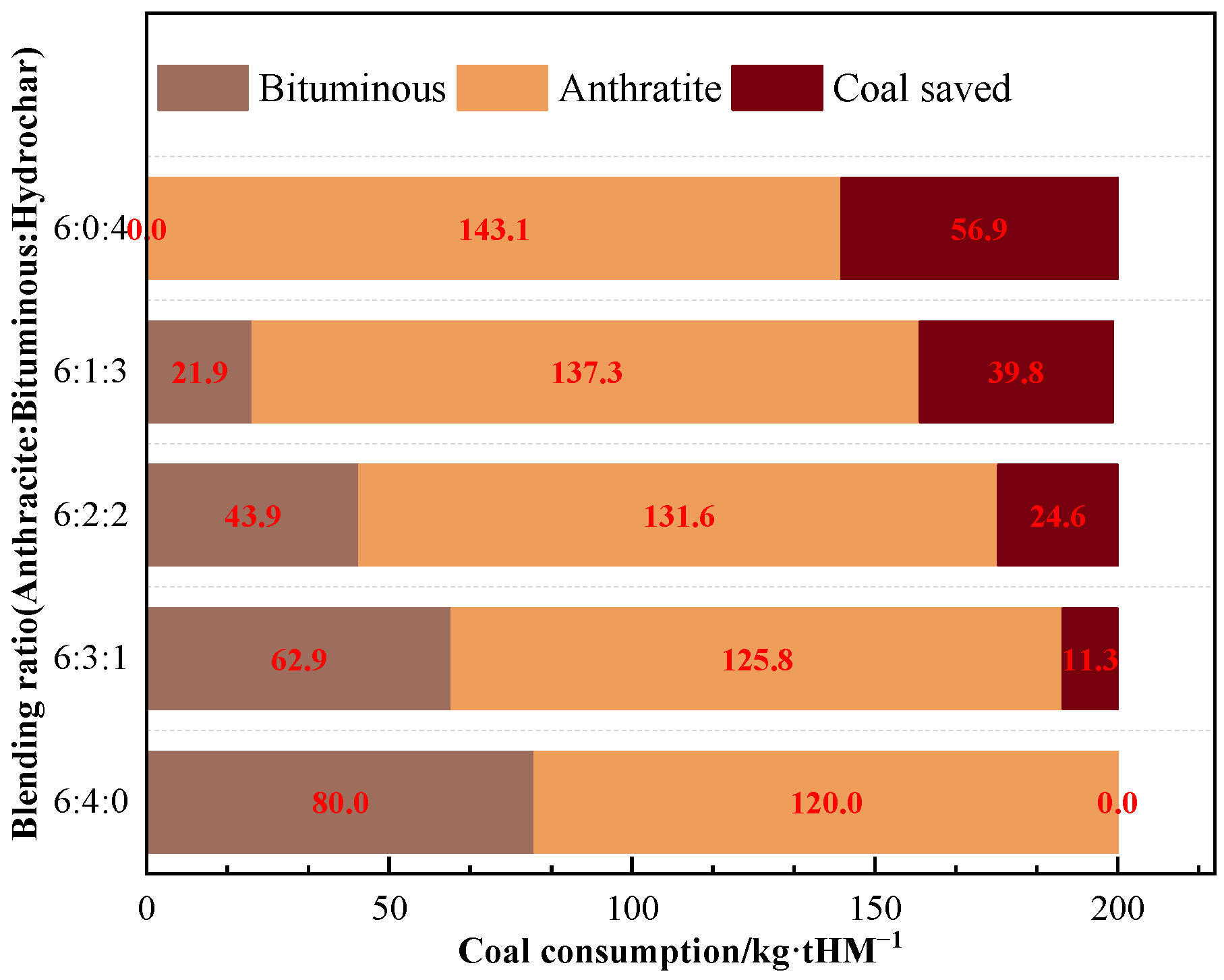
As the ratio of hydrochar injection increased, the reduction in CO2 emissions grew steadily. The reduction of CO2 emissions was approximately 145 kg/tHM when bituminous coal was totally substituted with B3 for BF injection. The annual CO2 reduction via injecting hydrochar instead of bituminous might be approximated as 528 kton, assuming that the BF produces 10 kton of hot metal every day. The annual coal consumption reduction is estimated at 208 kton (Figure 10).
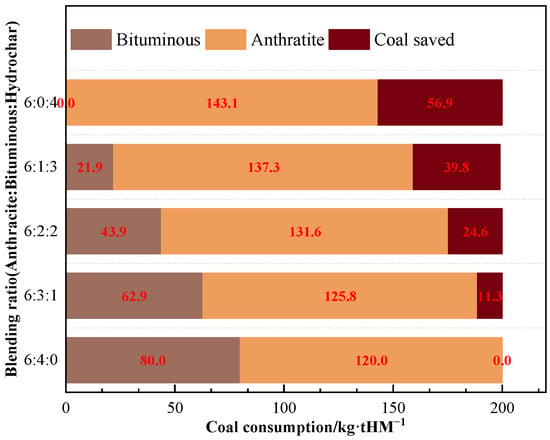
Figure 10.
Coal consumption results of B3 replacing bituminous coal for BF injection.
Based on the analysis above, hydrochar proves to be a viable alternative to pulverized coal for blast furnace injection. It can reduce the mining output of coal and BF CO2 emissions, which helps the country achieve the strategic goal of “double carbon” set by the Chinese government.
3. Materials and Methods
3.1. Material Preparation
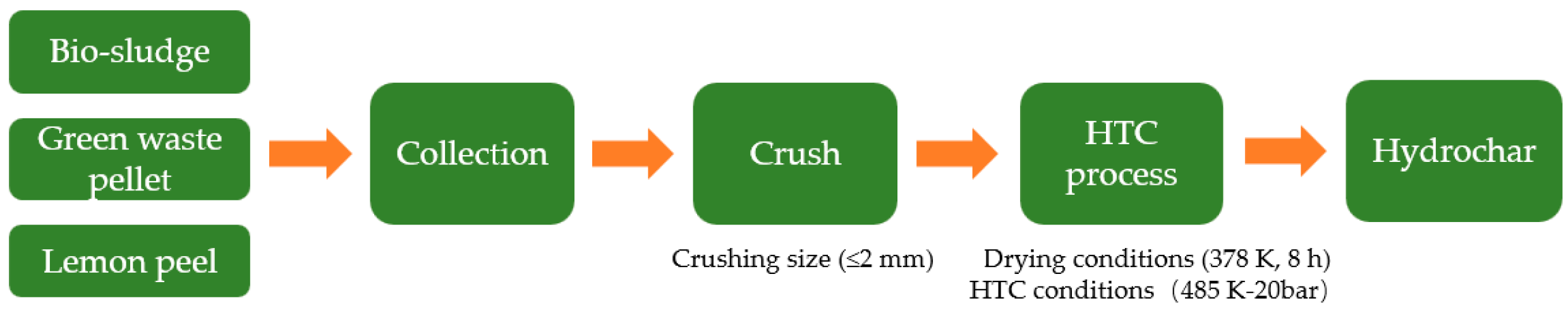
Experimental studies were conducted using hydrochar and coal as raw materials in this paper. The three types of hydrochar were produced by the hydrothermal carbonization process with the numbers B1 (bio-sludge hydrochar), B2 (green waste pellet hydrochar), and B3 (lemon peel hydrochar), respectively. A schematic illustration of the production process of hydrochar is shown in Figure 11. The HTC process demonstrates around 60% quality conversion efficiency under standardized moderate operational parameters (20 bar, 485 K) as implemented in the Ingelia commercial system. Bituminous coal (BC) is a typical low-rank coal with a low calorific value and high volatile matter, which is commonly used for BF injection in China, and anthracite coal (AC) is a high-quality fuel to replace coke for BF injection. Before the studies, the samples were crushed to a size of 2 mm and dried for 8 h at 378 K in an oven. Table 1 lists the proximate and ultimate analysis of the material, and the morphology of the hydrochar is presented in Figure 12.
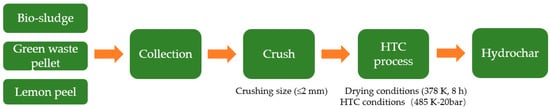
Figure 11.
Schematic illustration of production process of hydrochar.

Figure 12.
The morphology of three types of hydrochar: B1, B2, and B3.
3.2. Material Analysis
The Chinese national standard GB/T B212-2008 [67] was followed in the proximate analysis of the samples. The elemental (C/H/N/S) content of the samples was determined using elemental analyzers. The oxygen content was calculated using the difference, as shown in Equation (3). The HHV of samples was calculated using Equation (4) combined with the ultimate analysis results.
where C, H, S, O, N, and Ad denote the mass percentages of carbon, hydrogen, sulfur, oxygen, nitrogen, and ash in the sample, respectively.
The raw material ash was prepared in the air oxidation at 1088 K in a muffle furnace according to GB/T212-2008 [67]. The XRF-18000 (Shimadzu, Japan) was utilized to ascertain the chemical makeup of the ash. The Hardgrove grindability meter was used to determine the grindability qualities of samples according to the guidelines in GB/T 34534-2017 [68], “Method for Determination of Grindability Index of Coal” (Hardgrove method). Based on the national standard GB/T 18511-2017 and AQ 1045-2007 [69,70], the solid oxidant method and a long tube experimental apparatus were used to determine the ignition points and explosivity.
3.3. Thermogravimetric Analysis
The combustibility of the samples was studied analytically by using the thermogravimetric analyzer (HCT-3) produced by the Beijing Hengjiu Optical Instrument Company (Beijing, China). Each experiment employed a sample with a particle size of less than 0.074 mm and a mass of approximately 5 mg. The weight loss of the sample was measured from ambient temperature to 1173 K under the heating rate of 20 K/min in an alumina crucible that measured 5 mm by 4 mm, and the reaction atmosphere was air. The experimental data obtained from the thermogravimetric analyzer can be used to calculate the reaction rate x by using Equation (5). The conversion and reaction rate curves of the samples under the experimental heating rate were plotted using Origin software (Origin 2018).
where m0 is the initial mass of the sample, mg; mt is the mass of the sample at time t; m∞ is the final mass at the end of the experiment, mg; and x is the combustion conversion of the sample, %.
The TG-DTG analysis [71] enabled quantitative assessment of key combustion parameters, including initial combustion temperature (Ti), burnout temperatures (Tf), maximum reaction rate (Rmax), average reaction rate (Rmean), ignition index (C), and comprehensive combustion characteristics index (S). Among them, the C and S are calculated by Equations (6) and (7) [72,73].
4. Conclusions
HTC is a promising biomass thermal treatment technology for upgrading biomass wastes into green solid biofuel (hydrochar), which can be injected into BFs to replace fossil coal, thus reducing the fossil carbon emissions of the steel industry.
In this paper, the performance of industrially produced hydrochar for BF injection was evaluated systematically for the first time. The thermogravimetric analysis was used to examine the co-combustion properties of the blends of hydrochar and pulverized coal. The following are the primary findings from this study:
- (1)
- The mass fraction of hydrogen and oxygen in the hydrochar was higher than that in BC and AC; the alkali metal content was higher than that in coal. The grindability and the ignition point met the requirements for BF injection, and it also has strong explosive properties. The combustibility of the three hydrochars was better than that of the coal.
- (2)
- Blending AC with hydrochar can increase its combustion reactivity. Two types of synergistic effects were identified in the combustion process of blends: ignition com-bustion of hydrochar is restricted by the presence of AC, but hydrochar can promote the combustion of the fixed carbon in AC.
- (3)
- The reduction in CO2 emissions by replacing bituminous coal with hydrochar can reach 145 kg per ton of hot metal. The annual CO2 reduction can reach 528 kton, and the annual coal reduction is 208 kton of BF, which reduces the fossil fuel use and increases the BF smelting efficiency.
Author Contributions
Conceptualization, G.W. and X.N.; methodology, C.W. and A.K.; validation, J.W., and H.L.; formal analysis, J.W.; investigation, G.W. and J.W.; resources, C.W. and A.K.; data curation, G.W. and J.W.; writing—original draft preparation, J.W.; writing—review and editing, G.W. and J.W.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Baowu Low Carbon Metallurgy Innovation Foundation (Nos. BWLCF202215, BWLCF202305), the National Natural Science Foundation of China (No. 52174295), and the EU RFCS project of BioReSteel (No. 101112383).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The acquisition of the experimental samples in this study was supported by Ingelia in Spain.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BF | blast furnace |
| HHV | high heating value |
| HTC | hydrothermal carbonization |
| BC | bituminous coal |
| AC | anthracite coal |
References
- Available online: https://worldsteel.org/steel-topics/statistics/world-steel-in-figures-2022/ (accessed on 20 September 2024).
- Crude Steel Production in 2021 of China. Available online: https://data.stats.gov.cn/ (accessed on 20 September 2024).
- Sarkodie, S.A.; Strezov, V.; Weldekidan, H.; Asamoah, E.F.; Owusu, P.A.; Doyi, I.N.Y. Environmental sustainability assessment using dynamic autoregressive-distributed lag simulations–nexus between greenhouse gas emissions, biomass energy, food and economic growth. Sci. Total Environ. 2019, 668, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Huang, W.J.; Chen, Z.F. The peak of CO2 emissions in China: A new approach using survival models. Energy Econ. 2019, 81, 1099–1108. [Google Scholar] [CrossRef]
- REN21 Secretariat, Paris. REN21, Renewables Global Status Report. Available online: http://www.ren21.net/wp-content/uploads/2017/06/17-8399-GSR-2017-Full-Report-0621-Opt.pdf (accessed on 21 June 2018).
- Liu, Y.; Wei, J.; Li, J. Progress in hydrothermal carbonization of waste biomass and application of biochar in waste water treatment. Chem. Bioeng. 2019, 36, 1–10. [Google Scholar]
- Zhang, J.L.; Fu, H.Y.; Liu, Y.X.; Dang, H.; Ye, L.; Albero, N.C.; Xu, R.S. Review on biomass metallurgy: Pretreatment technology, metallurgical mechanism and process design. Int. J. Miner. Metall. Mater. 2022, 29, 1133–1149. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Xie, G.H.; Fang, Y.; Ling, S.; Li, M.; Yang, Y.; Fu, T. Review of the definition, classification, and resource assessment of biowaste. J. China Agric. Univ. 2019, 24, 1–9. [Google Scholar]
- Fernández, M.J.; Mediavilla, I.; Barro, R.; Borjabad, E.; Ramos, R. Sintering reduction of herbaceous biomass when blended with woody biomass: Predictive and combustion tests. Fuel 2019, 239, 1115–1124. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Dacres, O.D.; Wang, J.J.; Wei, B.; Jian, Y.M.; Zhang, M.; Liu, J.M.; Ma, F.Y.; Rahman, N. Combustion kinetics and mechanism of biomass pellet. Energy 2020, 205, 117909. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Wen, C.; Liu, T.Y.; Xu, M.H.; Ling, P.P. Combustion and co-combustion of biochar: Combustion performance and pollutant emissions. Appl. Energy 2024, 376, 124292. [Google Scholar] [CrossRef]
- Campos de Assis, C.F.; Leal, E.M.; Assis, P.S.; Nascimento, L.M.; Konishi, H.; Usui, T. Experimental analysis of injecting different blends of biomass materials and charcoal in a blast furnace. Ironmak. Steelmak. 2020, 47, 284–289. [Google Scholar] [CrossRef]
- Seo, M.W.; Jeong, H.M.; Lee, W.J.; Yoona, S.J.; Ra, H.W.; Kim, Y.K.; Lee, D.; Han, S.W.; Kim, S.D.; Lee, J.G.; et al. Carbonization characteristics of biomass/coking coal blends for the application of bio-coke. Chem. Eng. J. 2020, 394, 124943. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of agriculture residue to enhance combustible properties. Energy Fuels. 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Long, H.M.; Wei, R.F.; Li, N.; Zhou, D.; Meng, Q.M.; Li, J.X. Disposal of city combustible solid waste by blast furnace. Iron Steel 2018, 53, 1–9. [Google Scholar]
- Zhang, S.H.; Shao, J.N.; Lan, C.C.; Bi, Z.X.; Lv, Q. Application status and prospect of biomass energy in ironmaking process. Iron Steel 2022, 57, 13–22. [Google Scholar]
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Minaret, J. Review on comparative study of dry and wet torrefaction. Sustain. Energy Technol. Assess. 2015, 12, 26–37. [Google Scholar] [CrossRef]
- Li, B.Q.; Liu, J.; Li, R.Y. Biochars Preparation and Its Applications in Energy and Environment Field. Biomass Chem. Eng. 2012, 46, 34–38. [Google Scholar]
- Liu, Z.G.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.; Chappell, M.A.; Bae, S. Hydrothermal carbonization of municipal waste streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Donar, Y.O.; Çağlar, E.; Sınağ, A. Preparation and characterization of agricultural waste biomass based hydrochars. Fuel 2016, 183, 366–372. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates–Structure and energetic properties of the solid products. Fuel 2020, 275, 117837. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Cara-Jiménez, J.; Baena-Moreno, F.M.; Zhang, Z. Hydrothermal carbonization of biomass and waste: A review. Environ. Chem. Lett. 2022, 20, 211–221. [Google Scholar] [CrossRef]
- Cavali, M.; Junior, N.L.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Belli Filho, P.; de Castilhos Junior, A.B. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Xu, R.S.; Zhang, J.L.; Wang, M.Y.; Li, J.H. Cross-upgrading of biomass hydrothermal carbonization and pyrolysis for high quality blast furnace injection fuel production: Physicochemical characteristics and gasification kinetics analysis. Int. J. Miner. Mater Met. 2024, 31, 268–281. [Google Scholar] [CrossRef]
- Abhi, T.D.; MacDermid-Watts, K.; Salaudeen, S.A. Challenges and Opportunities of Agricultural Biomass as a Replacement for PCI Coal in the Ironmaking Blast Furnace: A Review. J. Sustain. Met. 2023, 9, 927–949. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.L.; Wu, S.X.; Wu, J.L.; Xu, K.; Liu, J.W.; Wang, G. Feasibility analysis of biomass hydrochar blended coal injection for blast furnace. Sustainability 2022, 14, 10885. [Google Scholar] [CrossRef]
- Yin, C.Y.; El Harbawi, M.; Jiang, Z.T. Life Cycle Assessment of Production of Hydrochar via Hydrothermal Carbonization of Date Palm Fronds Biomass. Materials 2023, 16, 6653. [Google Scholar] [CrossRef]
- Sobek, S.; Tran, Q.K.; Junga, R.; Werle, S. Hydrothermal carbonization of the waste straw: A study of the biomass transient heating behavior and solid products combustion kinetics. Fuel 2022, 314, 122725. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z. Hydrothermal carbonization of typical components of municipal solid waste for deriving hydrochars and their combustion behavior. Bioresour. Technol. 2017, 243, 539–547. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhang, J.L.; Shao, J.G.; Liu, Z.J.; Zhang, G.H.; Xu, T.; Guo, J.; Wang, H.Y.; Xu, R.S.; Lin, H. Thermal behavior and kinetic analysis of co-combustion of waste biomass/low rank coal blends. Energy Convers. Manag. 2016, 124, 414–426. [Google Scholar] [CrossRef]
- Wang, S.S.; Zou, C.; Wang, C.; Luo, C.; Yang, H.P.; Pu Yang Luo, J.H.; Peng, C.; Wang, C.; Li, Z.C. Influence of the synergistic effects between coal and hemicellulose/cellulose/lignin on the co-combustion of coal and lignocellulosic biomass. Fuel 2022, 311, 122585. [Google Scholar] [CrossRef]
- Wang, P.; Wang, G.; Zhang, J.; Lee, J.Y.; Li, Y.; Wang, C. Co-combustion characteristics and kinetic study of anthracite coal and palm kernel shell char. Appl. Therm. Eng. 2018, 143, 736–745. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zahid, I.; Danial, A.; Minaret, J.; Cao, Y.J.; Dutta, A. Hydrothermal carbonization of miscanthus: Processing, properties, and synergistic Co-combustion with lignite. Energy 2021, 225, 120200. [Google Scholar] [CrossRef]
- Liu, Z.G.; Quek, A.; Hoekman, S.K.; Srinivasan, M.P.; Balasubramanian, R. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012, 123, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Xia, W.J.; Xu, T.; Wang, H. Thermal behaviors and harmful volatile constituents released from asphalt components at high temperature. J. Hazard. Mater. 2019, 373, 741–752. [Google Scholar] [CrossRef]
- Mo, W.L.; Wu, Z.F.; He, X.Q.; Qiang, W.J.; Wei, B.; Wei, X.Y.; Wu, Y.L.; Fan, X.; Ma, F.Y. Functional group characteristics and pyrolysis/combustion performance of fly ashes from Karamay oily sludge based on FT-IR and TG-DTG analyses. Fuel 2021, 296, 120669. [Google Scholar] [CrossRef]
- Zhou, C.C.; Liu, G.J.; Cheng, S.W.; Fang, T.; Lam, P.K.S. Thermochemical and trace element behavior of coal gangue, agricultural biomass and their blends during co-combustion. Bioresour. Technol. 2014, 166, 243–251. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A.; Dutta, A. Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Yang, T.H.; Du, C.Z.; Li, B.S.; Liu, Z.; Kai, X.P. Influence of alkali and alkaline earth metals on the hydrothermal liquefaction of lignocellulosic model compounds. Renew. Energy 2022, 188, 1038–1048. [Google Scholar] [CrossRef]
- Tymoszuk, M.; Mroczek, K.; Kalisz, S.; Kubiczek, H. An investigation of biomass grindability. Energy 2019, 183, 116–126. [Google Scholar] [CrossRef]
- Tremel, A.; Becherer, D.; Fendt, S.; Gaderer, M. Spliethoff, H. Performance of entrained flow and fluidised bed biomass gasifiers on different scales. Energy Convers. Manag. 2013, 69, 95–106. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Li, T.; Su, B.X.; Wang, G.W.; Liang WZhang, C.L.; Ren, K. Optimization of pulverized coal injection into blast furnace. Iron Steel 2022, 57, 22–31. [Google Scholar]
- Zhang, X.F.; Li, Y.L.; Zhang, X.W.; Ma, P.Y.; Xing, X.J. Co-combustion of municipal solid waste and hydrochars under non-isothermal conditions: Thermal behaviors, gaseous emissions and kinetic analyses by TGA–FTIR. Energy 2023, 265, 126373. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Sun, H.; Yin, H.L.; Xu, F.X. Evaluation of fuel properties and combustion behaviour of hydrochar derived from hydrothermal carbonisation of agricultural wastes. J. Energy Inst. 2023, 108, 101209. [Google Scholar] [CrossRef]
- Lin, Y.S.; Ma, X.Q.; Peng, X.W.; Hu, S.C.; Yu, Z.S.; Fang, S.W. Effect of hydrothermal carbonization temperature on combustion behavior of hydrochar fuel from paper sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Zhu, G.K.; Yang, L.; Gao, Y.; Xu, J.Y.; Chen, H.J.; Zhu, Y.Z.; Wang, Y.F.; Liao, C.H.; Lu, C.; Zhu, C. Characterization and pelletization of cotton stalk hydrochar from HTC and combustion kinetics of hydrochar pellets by TGA. Fuel 2019, 244, 479–491. [Google Scholar] [CrossRef]
- Liang, W.; Jiang, C.H.; Wang, G.W.; Ning, X.J.; Zhang, J.L. Research on the co-combustion characteristics and kinetics of agricultural waste hydrochar and anthracite. Renew. Energy 2022, 194, 1119–1130. [Google Scholar] [CrossRef]
- Ma, Q.L.; Han, L.J.; Huang, G.Q. Potential of water-washing of rape straw on thermal properties and interactions during co-combustion with bituminous coal. Bioresour. Technol. 2017, 234, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.J.; Wang, S.Z.; Kuang, C.; Tang, X.Y. Insight into the kinetic analysis of catalytic combustion for biomass after alkaline metals loaded pretreatment. Fuel 2017, 203, 501–513. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Gajek, M.; Nowińska, K.; Nowak, W. Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 2020, 261, 116421. [Google Scholar] [CrossRef]
- Su, B.X.; Wang, G.W.; Li, R.G.; Xu, K. Co-Combustion Behavior of Paper Sludge Hydrochar and Pulverized Coal: Low Rank Coal and Its Product by Hydrothermal Carbonization. Energies 2022, 15, 5619. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Zhu, J.W.; Sun, W.; Xu, Y.N.; Wu, S.H. Co-combustion and ash characteristics of Zhundong coal with rice husk hydrochar prepared by the hydrothermal carbonization technology for co-combustion. IET Renew. Power Gener. 2022, 16, 329–338. [Google Scholar] [CrossRef]
- Farrow, T.S.; Sun, C.G.; Snape, C.E. Impact of biomass char on coal char burn-out under air and oxy-fuel conditions. Fuel 2013, 114, 128–134. [Google Scholar] [CrossRef]
- Qiao, L.; Mu, X.G.; Deng, C.B.; Wang, X.F.; Wang, Y.S. Experimental Study on Catalytic Action of Intrinsic Metals in Coal Spontaneous Combustion. ACS Omega 2023, 8, 13680–13689. [Google Scholar] [CrossRef]
- Mundike, J.; Collard, F.X.; Görgens, J.F. Co-combustion characteristics of coal with invasive alien plant chars prepared by torrefaction or slow pyrolysis. Fuel 2018, 225, 62–70. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Y.Q.; Wang, Z.Q.; Huang, J.J.; Fang, Y.T. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char. Bioresour. Technol. 2014, 173, 11–20. [Google Scholar] [CrossRef] [PubMed]
- GB/T 32151.5-2015; Requirements of the Greenhouse Gas Emission Accounting and Reporting—Part 5: Iron and Steel Production Enterprise. Carbon Management: Beijing, China, 2015.
- GB/T 212-2008; Proximate Analysis of Coal. China National Coal Standardization Technology Committee: Beijing, China, 2008.
- GB/T 34534-2017; Coke—Determination of Ash Composition—X-Ray Fluorescence Spectrometric Method. China National Coal Standardization Technology Committee: Beijing, China, 2017.
- GB/T 18511-2017; Determination of Ignition Temperature of Coal. China National Coal Standardization Technology Committee: Beijing, China, 2017.
- AQ 1045-2007; Criterion of Explosive Identification of Coal Dust. Chongqing Branch of Coal Science Research Institute: Chongqing, China, 2007.
- Xu, Y.G.; Zhang, C.; Xia Ji Duan, Y.H.; Yin, J.J.; Chen, G. Experimental study on the comprehensive behavior of combustion for blended coals. Chem. Eng. 2010, 5, 435–440. [Google Scholar] [CrossRef]
- Essenhigh, R.H.; Misra, M.K.; Shaw, D.W. Ignition of coal particles: A review. Combust. Flame 1989, 77, 3–30. [Google Scholar] [CrossRef]
- Jiang, X.M.; Li, J.B.; Qiu, J.R. Study on combustion characteristics of micro-pulverized. Coal. Proc. CSEE 2000, 20, 71–76. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).