Abstract
Industrial residues are sources of functional biopolymers with interesting properties for textile applications. This study aims to evaluate the impact of enzymatic pre-treatment on oil yield and phenolic compounds’ content in an aqueous extraction process, as well as the functional properties incorporated into textiles. This research investigated the influence of residue granulometry, biomass percentage, and the application of enzymatic pre-treatment with different enzymes (cellulase, pectinase, xylanase) individually or in combination. Chestnut hedgehog (CH), tobacco plant stems (TPSs), vine shoot trimmings (VSTs), and beer spent grain (BSG) were explored. For textile functionalization, the extracted oils were incorporated into a bio-based formulation and applied on cotton fabric through pad-dry-cure. For CH, the pre-treatment with cellulase and xylanase achieved an oil yield of 149 and 148 mg oil/mL extract, respectively. With the combination of both enzymes, the richest oil in phenolic compounds was extracted: 1967.73 ± 16.86 mg GAE/g biomass. CH and TPS oils presented an antioxidant activity above 60%, and the functionalized textiles also showed the highest antioxidant potential and a UPF of 30. The textiles presented water repellence and washing fastness. This study demonstrates a sustainable oil extraction method and its potential application in the development of functional textiles.
1. Introduction
The textile industry is responsible for 10% of global carbon emissions. In particular, the dyeing and finishing processes are estimated to be responsible for 20% of global clean water pollution mainly due to the harsh fossil-based chemicals conventionally used [1]. Moreover, the overconsumption of natural resources and the reliance on linear models are major environmental concerns [2]. Another global environmental issue is the increasing generation of residues due to population growth, intensive agricultural activities, and industrial development [3]. According to Eurostat statistics, in 2020, the EU generated 2135 million tons of residues, and only 1971 million tons were treated in the EU [4]. Even considering the treatment, the conventional procedures are expensive, demand a high amount of energy, and are associated with environmental impacts, especially greenhouse gas (GHG) emissions [5,6]. Therefore, more sustainable solutions for residue management are needed.
The valorization of residue streams through bioconversion into value-added products for industrial applications is one of the most promising options. Indeed, it also represents a possible response to the textile industry’s demand for new and more sustainable materials. Different industrial residues and by-products are reported as potential sources of valued-added biopolymers with interesting properties for textile applications [7,8,9]. However, only a few examples are reported, and those are restricted to visual or esthetic valorization and coloration. Moreover, those solutions usually fail in industrial scale-up, mainly because the extraction processes are complex, expensive, and apply organic solvents. Natural oils are value-added ingredients for textile applications and are commonly extracted from plants’ leaves, flowers, fruits, seeds, roots, and other parts [10,11]. Some examples of textiles finished with oils extracted from plants are reported. Those include the incorporation of fragrances [12,13], antimicrobials [14], to confer wrinkle recovery properties [15], insect repellence [16], and cosmeto-textiles [17].
The conventional method of oil extraction typically involves mechanical pressing, followed by solvent extraction [18]. However, the environmental and health concerns associated with this approach have driven the investigation into green oil extraction methods. Enzyme-assisted aqueous methods (EAMs) are gaining popularity due to their sustainability and ability to enhance extraction yield while preserving the quality properties of the oil. This method relies on the ability of enzymes such as cellulase, hemicellulase, and pectinase to degrade plant cell walls, facilitating the release of oil bodies [19,20].
Preliminary results were presented in 32nd EUBCE conference [21] and the present paper is presented a more detailed analysis. In this work, enzymatic pre-treatment in a water-based extraction system was evaluated for the extraction of bioactive oils from different industrial residues. Chestnut hedgehogs (CHs), tobacco plant stems (TPSs), vine shoot trimmings (VSTs), and beer spent grain (BSG) were selected for this study. Thanks to the enzymatic pre-treatment, an improvement in oil yields and phenolic compounds’ extraction was achieved. The extracted oils were incorporated into textiles to confer functional properties: antioxidant, ultraviolet (UV) protection, and water repellence.
2. Results
The results of the oils’ characterization, extraction yields, textile coloration, and functional properties are outlined in the following sections.
2.1. Characterization of the Extracted Oils
Oils derived from plants are known to have in their composition pigments and other compounds with staining properties. Since color is an important characteristic that influences the visual appearance of textiles, as well as their commercial and consumer acceptance, the color of the extracted oils was characterized (Table 1).

Table 1.
Colorimetric properties of the oils extracted from the different residues.
As seen in Table 1, oils with different color shades were obtained. According to a* and b* values, the color of all the extracted oils was found in the yellow-red quadrant of CIEL*a*b color space. BSG oil presents higher values for lightness (L*) and the lowest color strength (K/S), which indicates a lighter color. The oils extracted from TPSs and CH showed higher color strength.
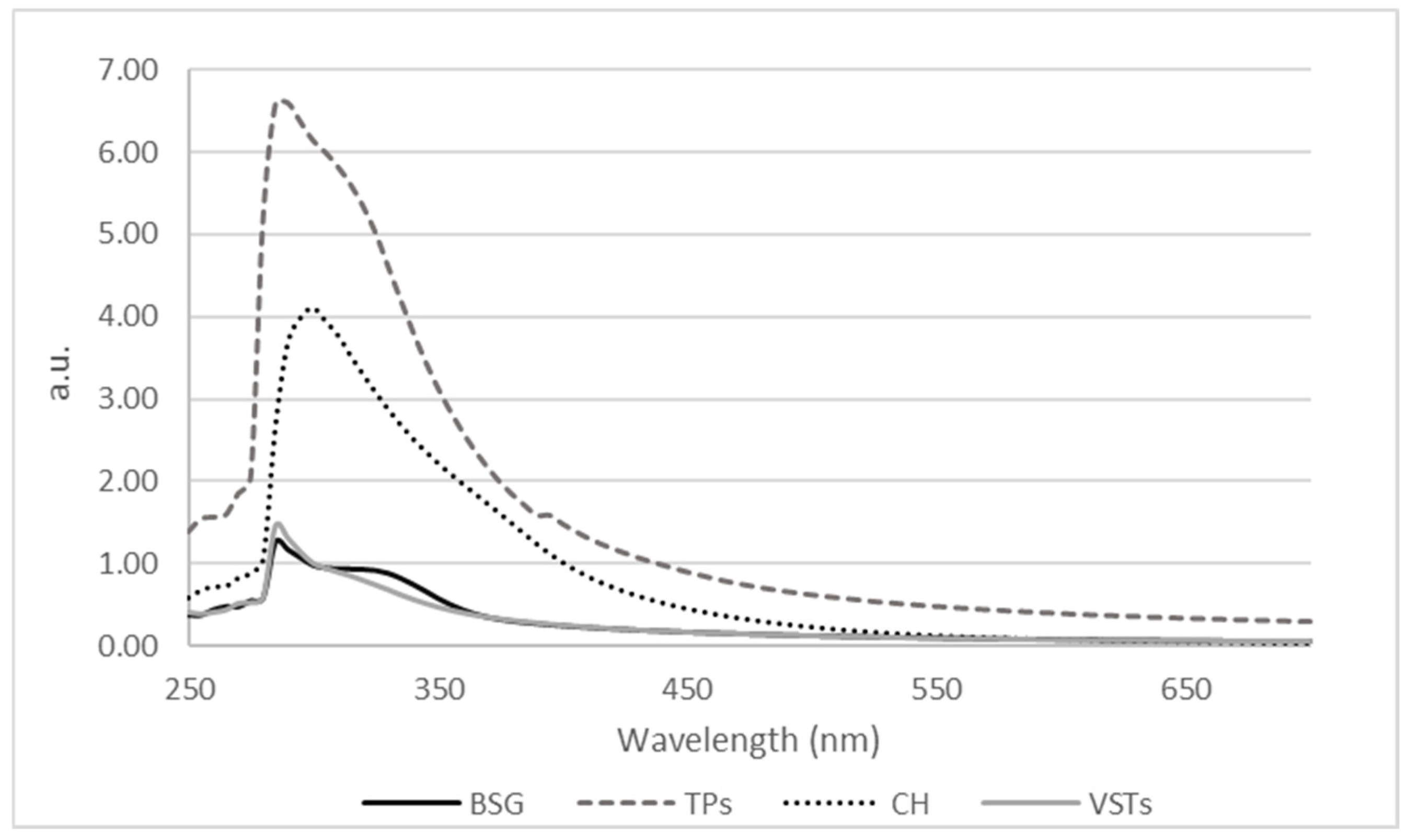
The UV-Vis absorbance spectra of the oils are presented in Figure 1.
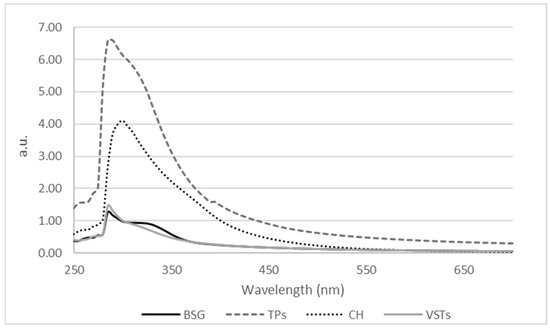
Figure 1.
Absorbance spectra expressed as absorbance units (a.u.) of oils extracted from BSG, TPSs, CH, and VSTs.
The highest peak intensities are seen in TPS (dashed line) and CH (dotted line) oils, suggesting a high concentration of UV-absorbing compounds. This higher absorbance cannot be fully attributed to the darker color of TPSs because compared to the color coordinates data in Table 1, the darker oil was extracted from VSTs. Therefore, the intensity in absorbance cannot be fully attributed to the oils’ color itself but also to the presence of other active compounds that absorb in the peak maximum region. All samples exhibit strong absorbance in the 250–350 nm region, which is typical for oils rich in phenolic compounds such as flavonoids [22] and tocopherols [23]. All the oils showed a clear maximum at 280–300 nm. One study reported that chestnut shell extracts presented an absorption maximum at 270 nm, characteristic of proanthocyanidins [24]. All samples show a linear absorbance in the visible region (400–700 nm), with no significant peaks being detected.
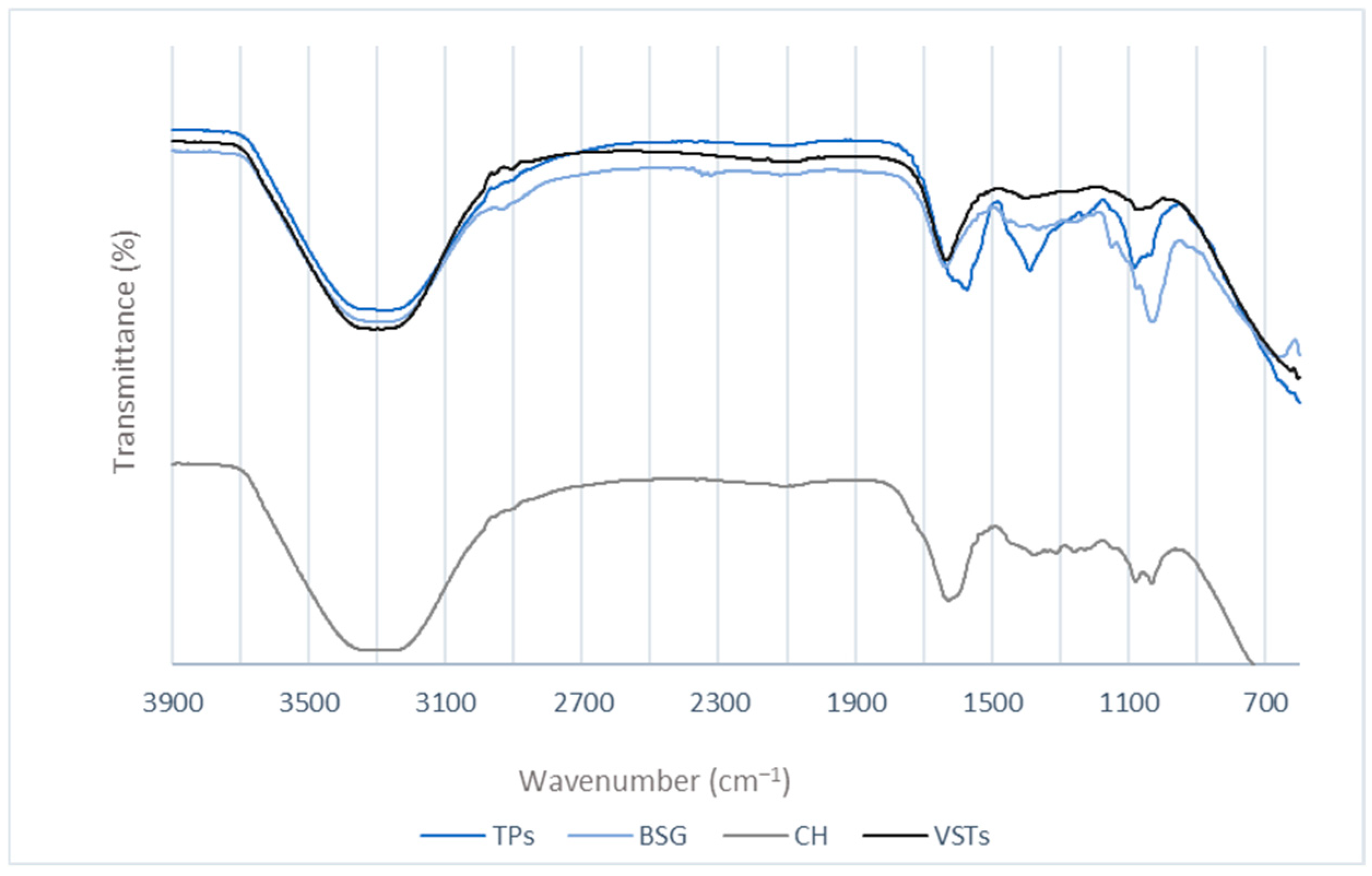
For chemical composition analysis of the oils, the FTIR spectra were recorded (Figure 2).
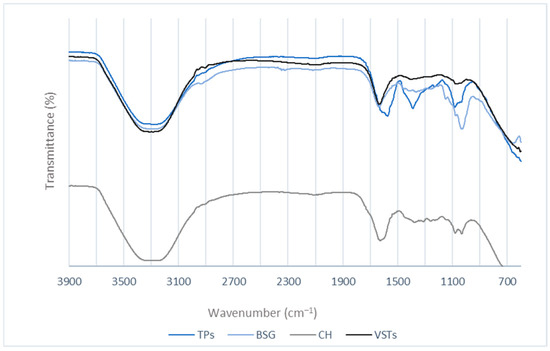
Figure 2.
FTIR spectra for the oils extracted from TPSs, BSG, CH, and VSTs.
A broad peak at 3200 cm−1 in all the oil samples, corresponding to O-H stretching vibrations, indicates the presence of phenols and alcohols [25]. The vibrations in the 2900–2800 cm−1 region (C–H stretching) [26] are characteristic of aliphatic groups [27]. The peaks at 1100–1000 cm−1 are attributed to C–O [28,29]. The peak at around 1600–1500 cm−1 observed for all the oils is recognized as C=C stretches [30]. A differentiated band appears in the TPS oil spectra at 1390 cm−1 that can be attributed to the symmetrical CH3 deformation near 1380 cm−1, also reported in the literature for oregano and thyme essential oils. The stretching vibration and peaks in the 1150–1000 cm−1 region are assigned to the C-O of alcohols [28].
2.2. Effect of Enzymatic Pre-Treatments on Oil Yield and Phenolic Compounds’ Extraction
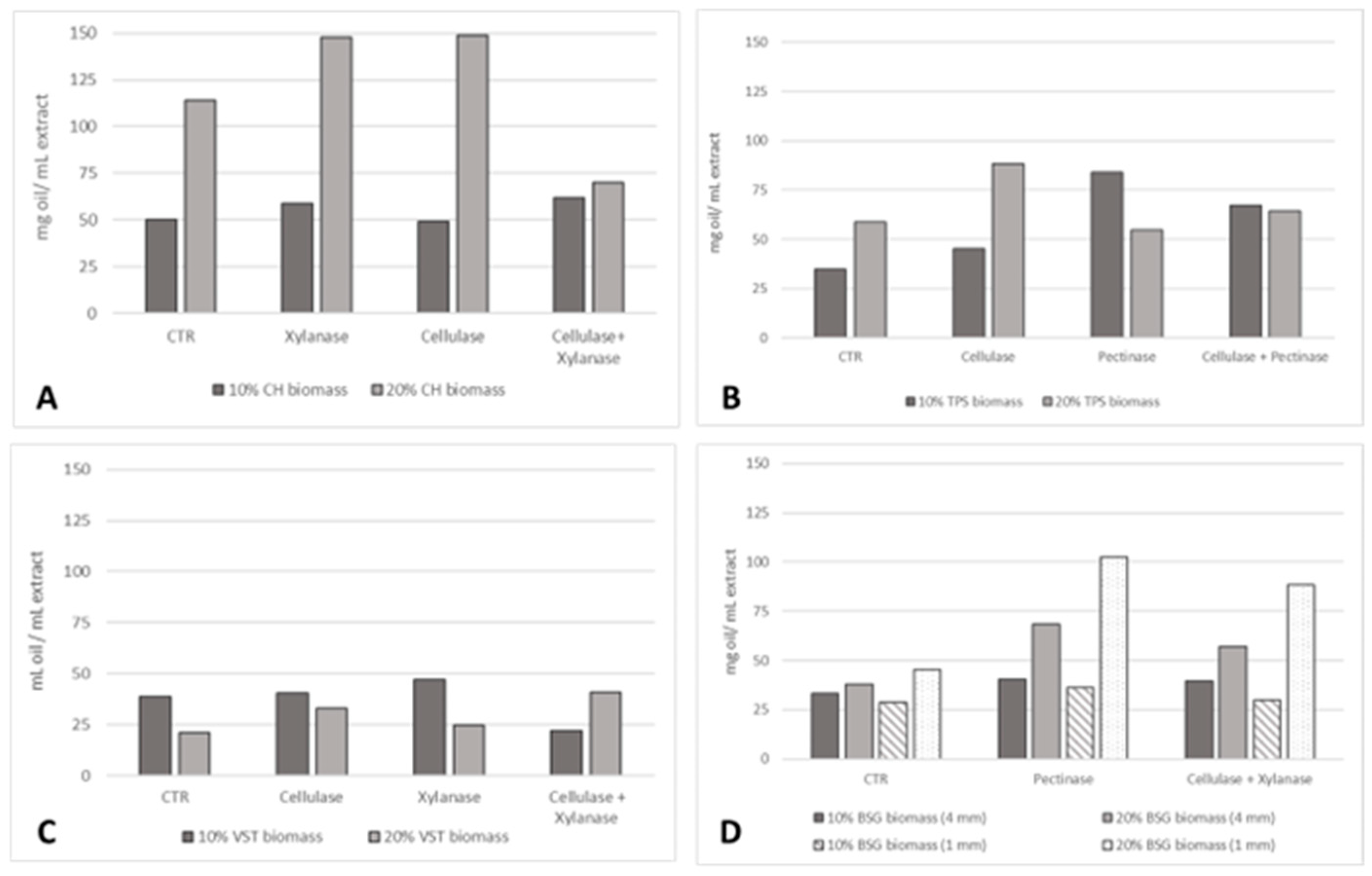
This study explored a sustainable approach to increase oil yield extraction by including an enzymatic pre-treatment in an aqueous extraction process. The influence of biomass percentage and particle sizes was also evaluated. For all the residues, a 30% solids loading was not suitable since the biomass absorbed all the extraction solution and no liquid was recovered to further obtain the oil. The effect of an enzymatic pre-treatment on the extraction yield (mg oil/mL extract) at 10 and 20% residues biomass is presented in Figure 3.
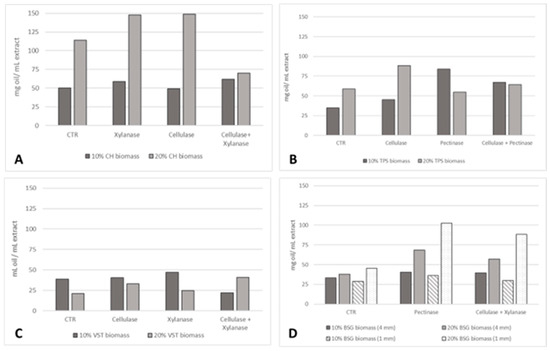
Figure 3.
Oil extraction yield (mg oil/mL extract) applying different enzymatic pre-treatments and residue particle sizes: (A) 4 mm CH; (B) TPSs; (C) 10 mm VSTs; (D) 1 and 4 mm BSG at 10% and 20% biomass.
The highest oil yields were registered for CH, followed by TPSs and BSG, and then VSTs. For CH, the increase in biomass to 20% led to an expected increase in the oil extraction yield. At 20% CH 4 mm, the highest oil yield was promoted by the pre-treatment with cellulase (149 mg oil/mL extract) and xylanase (148 mg oil/mL extract), an increase of 30% compared to the control. At 10% CH biomass, no differences in the pre-treatments were recorded, achieving a similar oil yield, but the combination of xylanase and cellulase was slightly more effective: 62 mg oil/mL extract, a 24% increase. For TPSs, at 10% biomass, the three enzymatic pre-treatments tested resulted in an increase in oil yield: 30% (cellulase), 140% (pectinase), and 92% (cellulase + pectinase). The highest oil extraction yield was reached at 20% biomass with cellulase (88 mg oil/mL extract) and at 10% with pectinase (83 mg oil/mL extract). This higher oil yield at 10% with the pectinase, compared to 20% solids loading, could be due to substrate–enzyme imbalances at the higher solid loading of 20%. Contrary to what was observed for the oils from CH, TPSs, and BSG, in the case of VSTs, 10% biomass and the higher granulometry of 10 mm resulted in a higher oil extraction yield. This is an indication of biomass saturation at 20% biomass. However, the application of the enzymatic pre-treatment with cellulase and xylanase individually and in combination was more beneficial at 20% biomass. For BSG, the highest oil yield was accomplished at 20% biomass (1 mm) thanks to the enzymatic pre-treatment with pectinase (102 mg oil/mL extract) and cellulase in combination with xylanase (88 mg oil/mL extract), corresponding to increases of 257% and 205% compared to the control.
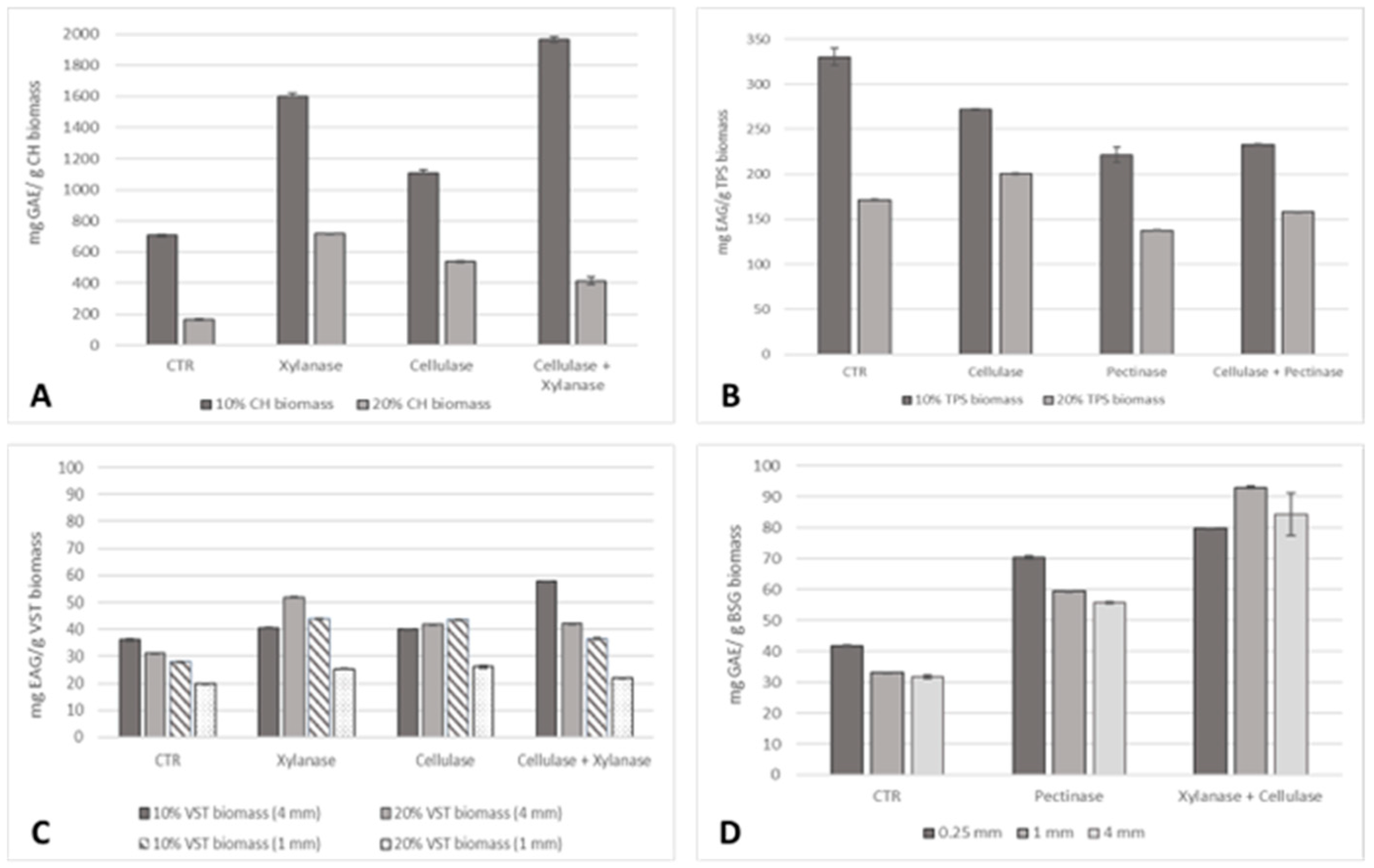
The effect of the enzymatic pre-treatment on the total phenolic content of the extracted oil was also accessed (Figure 4).
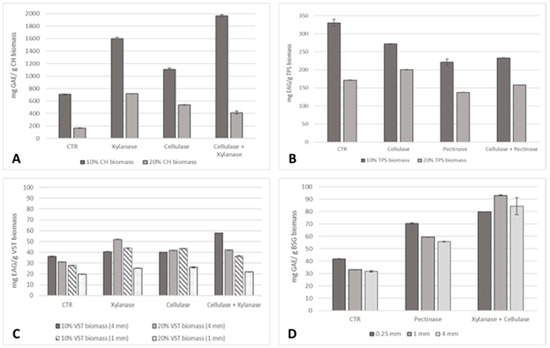
Figure 4.
Total phenolic content (mg GAE/g residue biomass) of the oil samples extracted from (A) 0.25 mm CH, (B) TPSs, (C) 1 and 4 mm VSTs at 10% and 20% biomass, and (D) 0.25, 1, and 4 mm BSG at 10% biomass.
The TPC of CH oil was considerably higher compared to TPSs, VSTs, and BSG for all the extraction conditions. A saturation of biomass was recorded for CH, TPSs, and BSG since the higher values of phenolics corresponded to 10% biomass. For CH, small particle sizes of 0.25 mm at 10% biomass were more advantageous. A high phenolic content of 1968 ± 17 mg GAE/g CH biomass, a 179% increase compared to the control, was achieved by applying the pre-treatment with cellulase and xylanase. For TPSs, only the cellulase pre-treatment at 20% biomass was advantageous to increase oil yield by 16%. For VSTs, the three enzymatic pre-treatments were effective to improve the extraction of phenolic compounds for both 10 and 20% biomass at 1 mm and 4 mm particle sizes. The higher increase corresponds to cellulase + xylanase pre-treatment, reaching 57.79 ± 0.15 mg GAE/g VSTs biomass, a 60% increment at 10% biomass (4 mm). The combination of xylanase + cellulase was also the most effective pre-treatment to achieve BSG oil rich in phenolic compounds. At 10% biomass at 0.25 mm, 70.60 ± 0.22 mg GAE/g BSG biomass (91%) was reached; at 1 mm, 92.92 ± 0.44 mg GAE/g BSG biomass (181%) was reached; and at 4 mm, 84.34 ± 6.82 mg GAE/g BSG biomass (165%) was reached.
2.3. Textile Functional Properties
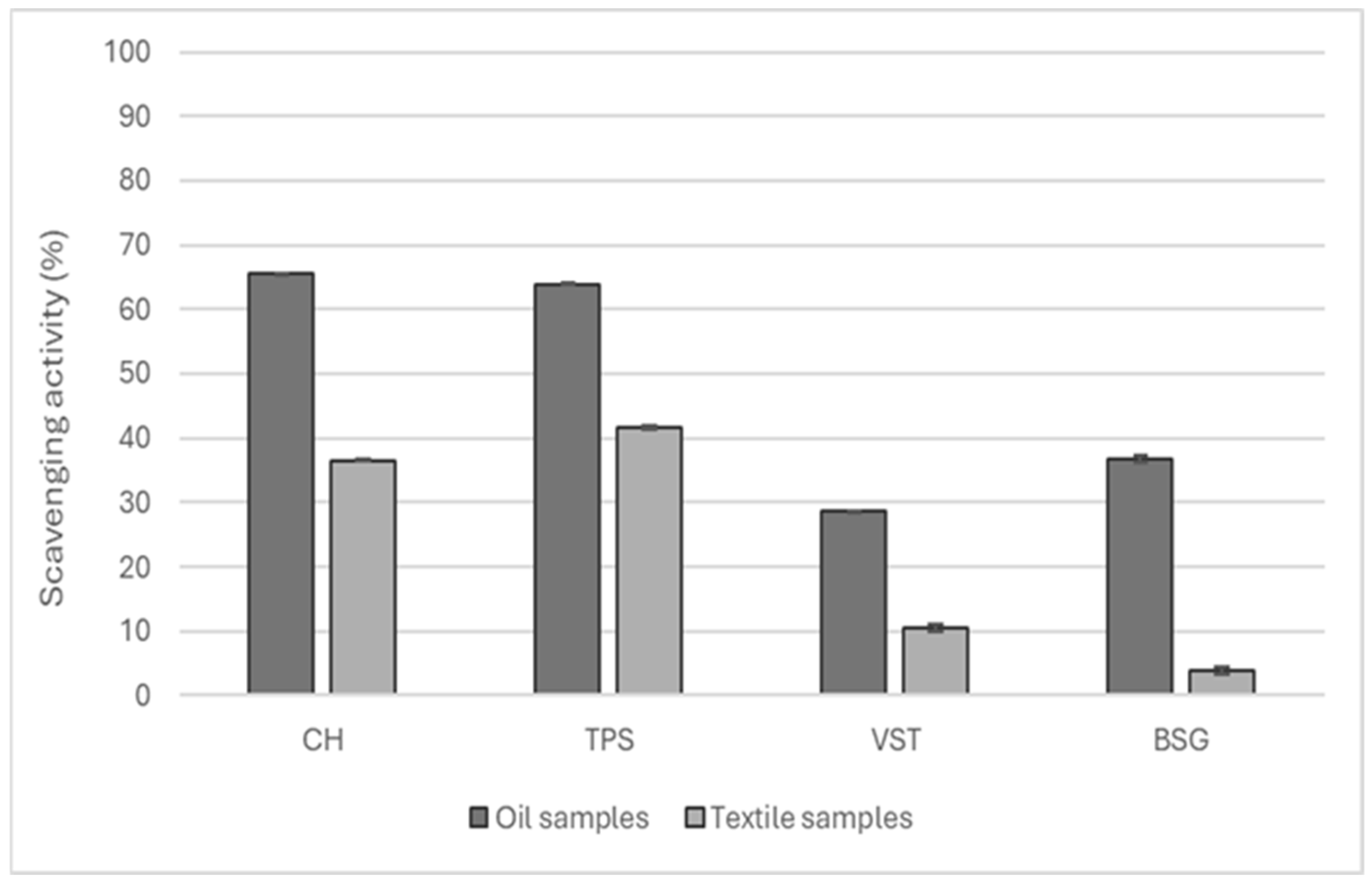
The extracted oils were incorporated in a water-based formulation and impregnated on cotton textiles to add functional properties. The antioxidant potential of the extracted oils and the functionalized textiles was explored, and the results are presented in Figure 5.
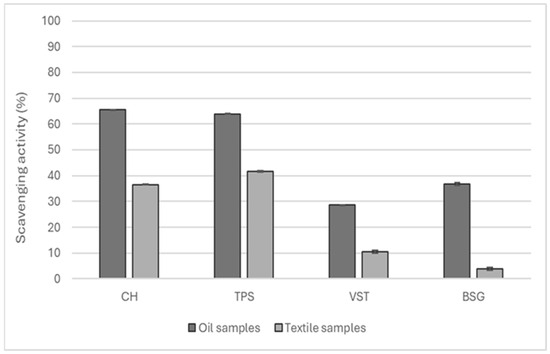
Figure 5.
Antioxidant capacity of the oils extracted from CH, VSTs, TPSs, and BSG and the respective cotton fabrics functionalized.
CH and TPS oil presented an antioxidant potential superior to 60% and, consequently, the textiles functionalized with these oils also achieved higher values of antioxidant activity at around 40%.
In Table 2, the results of the WCA measurement (mean of five measures) on textile samples in which the water drop absorption time was superior to 3 min are summarized.

Table 2.
Water contact angle (WCA) of textile samples functionalized with oils extracted from different residues and enzymatic pre-treatments. The control samples (CTR) are textiles functionalized with the formulations containing oil extracted without the enzymatic pre-treatment.
Theoretically, hydrophobic surfaces have a WCA greater than 90°. Superhydrophobic surfaces exhibit a WCA exceeding 150° [31]. The data in Table 2 show that, overall, the oils extracted by performing the enzymatic pre-treatment exhibited a better water repellence performance. Several textile samples are classified as superhydrophobic. After washing, only the textile sample functionalized with CH (0.25 mm; 20% biomass) extracted by applying the pre-treatment with xylanase and cellulase maintained the hydrophobic characteristics, showing a WCA of 143.33 ± 10.11.
The photoprotective capability of textile materials can be expressed in terms of ultraviolet protection factor (UPF) values. Besides textile structure, composition, and grammage, UV protection is also influenced by textile color. For that, in Table 3, the UPF values and the colorimetric characterization of textiles that achieved an UPF equal or superior to 15 are presented. The original textile (not treated) and the textile sample treated with the functionalization solution without the oil presented an UPF of 3.90 and 3.99, respectively.

Table 3.
UPF and colorimetric characterization of functionalized textile samples with oils extracted from different residues and enzymatic pre-treatments. The controls (CTR) refer to the textiles functionalized with oils extracted without enzymes in the pre-treatment.
According to AS/NZ standard 4399:2017 [32], the UPF protection of textile materials can be classified into three levels: minimum protection (UPF = 15), good protection (UPF = 30), and excellent protection (50, 50+). Data from Table 3 indicate that textiles functionalized with the oils extracted from CH (0.25 mm at 10% and xylanase pre-treatment; 4 mm at 20%, no enzymes—CTR) and TS (10%, CTR) presented the highest UPF protection values, with them being classified as offering good protection. Textiles functionalized with different oils and extraction conditions acquired different color shades. Textiles with more intense colors were functionalized with CH oil (K/S values higher than 3).
3. Discussion
Enzymatic pre-treatment was effective to increase oil yield and phenolic compounds’ extraction. This study was based on the use of enzymes to disrupt the structure and integrity of the cell wall, promoting the oils’ release. The efficiency of the enzymatic pre-treatment was influenced by the type of waste biomass, the percentage added, and particle size. The highest oil yield was achieved with CH, around 150 mg oil/mL extract, at 20% CH biomass, by two enzymatic pre-treatments: the use of cellulase and xylanase individually led to a 30% increase compared to the control (no enzymes added). The combination of the two enzymes was not so effective, perhaps due to competition for active sites. However, for phenolic compounds’ extraction, the pre-treatment with both enzymes was more beneficial at 10% biomass, achieving 1968 ± 17 mg GAE/g biomass. The oil richest in phenolic compounds and antioxidants was obtained from CH. Chestnut hedgehogs (CHs) are rich in phenolic compounds such as flavonoids and tannins that confer antioxidant, anticarcinogenic, and anti-inflammatory properties [33,34]. Kiralan et al. reported that chestnut oil is rich in omega fatty acids such as linoleic and oleic acids and tocopherols. Those constituents are responsible for strong functional activities, but this study did not quantify them [35]. For textile applications, chestnut hedgehogs were applied as part of short fiber non-wovens [36] and composites [37]. One study developed chitosan-based films with the incorporation of chestnut flower essential oil. A 60% DPPH radical scavenging activity was reported for the film with 40 mg/mL oil [38]. A similar antioxidant potential was achieved in our study for CH oil, but this value, as expected, decreased when applied on the cotton textiles since the concentration in the functionalization formulation was only 0.035 mL/mL. For that, in our study, it was expected that increasing the CH oil concentration in the formulation would result in textiles with a higher antioxidant activity being obtained. Also, the films with a chestnut flower shield almost all UV light [38]. In our study, textiles with CH oil also presented the highest UPF value of 30, together with TPS oil. These results are also in line with the UV-VIS spectra of CH and TPS oil, which exhibit the most intense absorbance peaks. The UV-Vis absorbance spectra indicate a maximum absorbance for all oils at around 300 nm. This indicates the presence of conjugated carbon double bonds [39] or aromatic compounds, like phenolic compounds commonly found in plant oils [40,41]. The most intense peaks (higher absorption) correspond to CH and TPS oils, which also presented the highest values for phenolic compounds and antioxidants. Moreover, this can also be related to the deeper or more intense colors of CH and TPS oils, which is in accordance with the highest K/S value recorded in Table 1.
Following CH, TPS oil and the functionalized textiles presented higher values for antioxidant properties and phenolic content. In the literature, the polyphenols content, antioxidants, and antimicrobial properties of tobacco leaves are mostly highlighted [42]. The few articles that have studied tobacco stems mentioned their analgesics, anti-inflammatory and anti-rheumatic benefits [43], and their antioxidant properties [44,45]. Since pectin is a primary constituent of TPSs [46], a high effect of pectinase pre-treatment to promote oil release was recorded, resulting in a 140% superior yield compared to the control at 10% biomass. For phenolic compounds’ extraction, cellulase pre-treatment was the most effective. Several studies report that cellulase improved phenolic compounds’ extraction from the vegetable matrix [47,48,49]. Considering the main components of VSTs, cellulase, and hemicellulose [50], in our study, the application of cellulase and xylanase helps to degrade cell wall polymers and promote the release of oil and phenolic compounds. Despite the main components of BSG being cellulose, hemicellulose, and proteins [50], a high increase in oil yield was also noticed when using pectinase. This can be explained by the fact that the pectinase used in this work promotes the maceration and soft rotting of plant tissue (information from technical sheet from the supplier) and thus the release of oil. In future work, it would be interesting to test the pectinase effect on VSTs and CH. The combination of xylanase and cellulase improved phenolic compounds’ extraction.
When analyzing the impact of particle sizes on oil yield and phenolic compounds’ extraction, particles smaller than 10 mm led to a more efficient extraction, except for oil extraction from VSTs. In the literature, it is explained that larger particles have a smaller surface area, limiting solvent diffusion into their interior and reducing the amount of oil released [51]. However, a 0.25 mm particle size in some conditions led to a lower oil yield. This can be explained by the possible agglomeration of finer particles that reduces the area for solution to flow [52]. Despite noticing an enzyme saturation at 20% biomass in the oil extraction from VSTs and phenolics from CH, TPSs, and BSG, good results were achieved at the lower biomass of 10%, and this did not impart textile functionalization.
Several studies have highlighted the benefits of enzymatic-assisted extraction to enhance oils and functional compounds’ extraction [18,53,54]. Waheed et al. (2020) yielded four times more orange peel essential oil in just half the extraction time with an EAM (lignocellulose-specialized multi-enzymatic complex) compared to conventional hydrodistillation [55]. The extraction of oil from Platycladus orientalis (L.) Franco seed peel waste by enzymatic pre-treatment (cellulase) combined with solvent-free microwave extraction was 24.18% higher than that by the traditional stem distillation method, and the essential oil was richer in polyphenols and flavonoids [56]. The absence of studies in the literature focused on oil extraction from chestnut hedgehogs and the analysis of their functional properties highlights the novelty of this work. Also, enzymatic pre-treatment (hemicellulose/cellulase) microwave-assisted extraction (7.60 ± 0.13 mg/g) achieved a more efficient extraction of essential oil from Cinnamomum burmannii leaves than microwave-assisted extraction (7.25 ± 0.10 mg/g) and hydrodistillation (7.17 ± 0.16 mg/g) [57]. To the best of our knowledge, an EAM was not applied on the residues explored in our study. Indeed, there are no reports of oil extraction from CH, TPSs, or VSTs. Oil from BSG was extracted by Soxhlet with hexane and supercritical carbon dioxide, achieving a phenolic content of 28.3 ± 0.5 and 26.2 ± 0.3 mg GAE/g of the sample, respectively [58]. A higher phenolic content was achieved in our study: 92.92 ± 0.44 mg GAE/g biomass (1 mm at 10% biomass) applying cellulase + xylanase pre-treatment.
Regarding the colorimetric properties, textiles functionalized with different oils exhibited similar color shades. Fabrics treated with CH oil showed a slightly higher K/S value, consistent with the observations for CH oil itself.
The research on textile finishings to confer hydrophobicity have gained attention since the EU’s restriction on PFAs [59]. Flaxseed, castor, olive, and soybean oil were encapsulated on silica nanoparticles and applied on cotton/polyester fabrics to confer water repellence properties’ resistance to washing [60]. In our study, water repellence textiles were developed by the incorporation of oils extracted from CH, TPSs, VSTs, and BSG in a water-based formulation applied by impregnation, a simple and sustainable approach easily applied on industrial textile finishing companies. Washing fastness is a challenge for functionalization (and dyeing) with natural origin compounds. Despite the good results found in terms of maintaining water repellence properties with CH oil after washing, the same was not achieved for the other oils. For that, alternative approaches are needed to maintain the functional properties after washing, such as the encapsulation of the oils, surface treatments, or exploring natural cross-linkers.
The present work highlights valuable insights into functional oils’ extraction from agro-industrial residues by a sustainable approach and further textile applications. This investigation presents a valorization alternative to the non-sustainable traditional incineration of end-of life solutions.
4. Materials and Methods
4.1. Raw Materials and Reagents
Brewer’s spent grain (BSG), vine shoot trimmings (VSTs), and tobacco plant stems (TPSs) were supplied by LETRA (Vila Verde, Portugal), Quinta de Amares (Amares, Portugal), and Cetarsa (Cáceres, Spain), respectively. Chestnut hedgehogs (CHs) were collected from a local producer.
The commercial enzymes used in the textile finishing processes were used in this study. Xylanase (XYLANASE), in powder, was supplied by Sunson Industry Group Co (represented by IMPOCOLOR, Vila Nova de Gaia, Portugal). Pectinase (Newprep 3000 L) and cellulase (NewCell Supreme 40,000 L), both in liquid form, were provided by AQUITEX (Porto, Portugal). The cationic starch was donated by COPAM (Loures, Portugal).
The textile material was a 100% cotton jersey. When the fabric was purchased, it was already desized and bleached. The textile material was washed with 2% phosphate standard detergent to remove dirt, dust, and impurities before proceeding with the functionalization tests.
4.2. Physical Adequation of Residue Biomass
Prior to oil extraction, CH, VST, and BSG residues were dried at 60 °C until a constant weight and <20% moisture were obtained. Then, the residue biomass was grounded to different particle sizes (0.25 mm, 1 mm, 4 mm, and 10 mm) using a Retsch SM 300 cutting mill (Retsch GmbH, Haan, Germany). TPSs were used without any treatment since when they were supplied, they were already dry and grounded. During this study, the residue biomass was stored in a dark environment at room temperature.
4.3. Oils’ Extraction and Recovery
For the enzymatic pre-treatment, residue biomass was added at different solid loadings (10%, 20%, and 30%) to final volume of 150 mL. The enzymatic pre-treatment was conducted in phosphate buffer (Sodium Phosphate Dibasic Heptahydrate 0.01369 M and Sodium Phosphate Monobasic Monohydrate 0.08631 M, adjusted to pH 6). For each extraction condition (particle sizes and solids loading), a control was performed by adding the waste biomass to 150 mL of phosphate buffer (no enzymes were added). For the enzymatic pre-treatments, the enzymes were added at 0.02 mL/mL for cellulase and pectinase and 0.33 mg/mL for xylanase, individually or in mixture. The enzymatic pre-treatments were carried out and the respective controls were used at 50 °C, 20 rpm, for 2 h in a rotator laboratory machine (Mathis Labomat BFA, Oberhasli, Switzerland).
After the enzymatic pre-treatment, the liquid fraction was recovered by centrifugation (2000 rpm for 10 min) at room temperature. The oils were concentrated in a rotary evaporator (RV 3 V, IKA) and stored in sealed vials, protected from light at 0 °C until further use.
4.4. Analytical Methods
Attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR) analysis was performed to characterize the functional groups of the extracted oils. The FTIR spectra of the samples were recorded in the range of 400–4000 cm−1 with a spectral resolution of 2 cm−1.
A UV–Vis spectrometer (UV−2600i, Shimadzu, Duisburg, Germany) was used to record the UV–Vis absorption spectra of the extracted oils in the 250–700 nm scanning range.
Color analysis was conducted in a colorimeter (datacolor, Spectro 750), using the standard illuminant CIE D65 and an observer angle of 10°. Color coordinates from CIEL*a*b* color space, where L* corresponds to lightness; a* corresponds to the transition from green (−a*) to red (+a*); and b* corresponds to the transition from blue (−b*) to yellow (+b*), and K/S (absorption coefficient—K—and scattering coefficient—s) referring to color strength/intensity were accessed.
The total phenolic content (TPC) of the oils was determined by the Follin–Ciocalteu method and expressed in mg equivalent of gallic acid (EAG) per gram of dry residue biomass. The reaction mixture was prepared by mixing 0.5 mL of oil sample (previously diluted) with 1.0 mL of Folin–Ciocalteu’s solution (0.25 mL/mL) and 4.0 mL of NaHCO3 solution (3.8%). The samples reacted for 2 h at room temperature in the dark. After, the absorbance was determined at 765 nm. The control was prepared under the same conditions with distilled water instead of the oil sample. The TPC concentration was then calculated from a gallic acid standard curve (100–900 mg/L µg/mL) in triplicate.
The antioxidant potential of the extracted oils was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. An aliquot (0.15 mL) of each oil sample (previously diluted) was mixed with 6 mL of DPPH stock solution (0.024 mg/mL in ethanol). The control was contained in all the reagents, using ethanol instead of the oil sample. After 30 min in darkness at room temperature, the absorbance of the solution was recorded at 517 nm. The antioxidant activity was determined as its capacity to scavenge the DPPH free radicals, according to the following equation:
Abscontrol refers to the absorbance of the control and Abssample refers to the absorbance of the oil sample.
4.5. Textiles Functionalization
The functionalization solution was prepared under constant magnetic stirring by adding 9 mL of cationic starch water-based stock solution (0.06 g/mL) to 16 mL of H2O, 0.50 mL of Tween 80, and 0.9 mL of the extracted oil. The control solution was prepared without adding the oil. The cotton samples were submerged in the functionalization solution, at room temperature, and submitted to pad-dry-cure, obtaining a wet-pick up superior to 80%. After, the samples were dried at 60 °C for 20 min and cured at 150 °C for 5 min.
4.6. Textile Functional Properties’ Evaluation
The antioxidant potential of the functionalized textiles and the control was evaluated by adding 6 mL of DPPH stock solution (0.024 mg/mL in ethanol) to 100 mg of the textile sample, followed by 120 min of incubation at room temperature. The results were expressed according to Equation (1), where Abscontrol refers to the control sample and Abssample refers to the textiles functionalized with the extracted oils.
The measurement of ultraviolet protection factor (UPF) on the textiles was performed following the standard in Australia/New Zealand A(S/NZ) 4399.
A preliminary repellence test was carried out. For that, 30 µL of distilled water was dropped on the fabrics and the time it started to be absorbed into the fabric surface was recorded. For the fabric samples where the water drop took more than 3 min to be absorbed, the static water contact angle (WCA) between the distilled water drop and the textile sample was measured in a tensiometer (Biolin Scientific, Västra Frölunda, Sweden). In both methods, measurements were repeated on at least five locations for each sample.
The washing fastness was determined via a standard domestic washing process performed at 40 °C for 20 min using 4 g/L of ECE detergent. After the washing process, the textile samples were rinsed with water and dried at room temperature.
5. Conclusions
Our findings demonstrate that enzymatic pre-treatment in a water-based method is a promising biotechnological approach to enhance the yield extraction of oils rich in phenolic compounds. In the literature, several studies report enzymatic-assisted methods for extracting oils and bioactive compounds. However, there are no reports on their application to chestnut hedgehogs, tobacco stems, beer spent grain, or vine shooting trims, nor on the incorporation of these oils to confer functional properties to textiles. In the presented study, for CH, the pre-treatment with cellulase and xylanase, a higher oil yield was achieved: 149 and 148 mg oil/mL extract, respectively. With the combination of both enzymes, the richest oil in the phenolic compounds was extracted: 1967.73 ± 16.86 mg GAE/g biomass. CH and TPS oils presented an antioxidant activity above 60%, and the functionalized textiles also showed the highest antioxidant potential and a UPF of 30. The textiles presented water repellence and washing fastness.
This study introduces an efficient and sustainable approach for oil extraction using a water-based system as an alternative to organic solvents. The extracted oils were incorporated into a water-based formulation and applied to 100% cotton fabric using the pad-dry-cure method, a conventional technique in the textile industry. Enzymes offer high yields and selectivity, making them a cost-effective alternative to traditional organic synthesis while minimizing waste. Their biodegradability and ability to function under mild conditions (neutral pH, ambient temperature, and pressure) reduce energy consumption and the need for specialized equipment. This simplified process lowers investment costs and promotes sustainable, eco-friendly approaches. The methodology presented in this work can be readily adopted by textile finishing companies, not only due to its simplicity and scalability for industrial applications but also because it aligns with REACH regulations, ensuring safety and sustainability through water-based processes.
These findings promote the implementation of circular economy models in the textile industry while boosting industrial symbiosis between the textile and other key industries in Europe.
Author Contributions
Conceptualization, A.M.F.; methodology, A.I.P. and C.R.; validation, A.M.F.; formal analysis, A.M.F.; investigation, A.M.F.; resources, C.J.S.; data curation, A.M.F.; writing—original draft preparation, A.M.F.; writing—review and editing, A.M.F.; visualization, C.J.S.; supervision, C.J.S.; project administration, C.J.S.; funding acquisition, C.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support received from integrated project be@t—Textile Bioeconomy (TC-C12-i01, Sustainable Bioeconomy No. 02/C12-i01.01/2022), promoted by the Recovery and Resilience Plan (RRP), Next Generation EU, for the period 2021–2026.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors kindly thank Cervejaria Letra, Quinta de Amares and Cetarsa for BSG, VSTs, TPSs, and COPAM and IMPOCOLOR for the supply of materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GHG | Greenhouse gas |
| EAMs | Enzyme-assisted aqueous methods |
| CH | Chestnut hedgehog |
| TPSs | Tobacco plant stems |
| BSG | Beer spent grain |
| VSTs | Vine shoot trimmings |
| UV | Ultraviolet |
| ATR-FTIR | Attenuated total reflectance–Fourier transform infrared spectroscopy |
| CIE | Commission Internationale de l’Éclairage |
| L* | Lightness |
| a* | Transition from green (−a*) to red (+a*) |
| b* | Transition from blue (−b*) to yellow (+b*) |
| K/S | Absorption coefficient—K—and scattering coefficient—S |
| TPC | Total phenolic content |
| EAG | Equivalent of gallic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| UPF | Ultraviolet protection factor |
| WCA | Water contact angle |
| a.u. | absorbance units |
References
- European Parliament. The Impact of Textile Production and Waste on the Environment (Infographics). 2020. Available online: https://tinyurl.com/53jt6s6v (accessed on 1 April 2025).
- Arora, N.K.; Mishra, I. Responsible Consumption and Production: A Roadmap to Sustainable Development. Environ. Sustain. 2023, 6, 1–6. [Google Scholar] [CrossRef]
- Peng, X.; Jiang, Y.; Chen, Z.; Osman, A.I.; Farghali, M.; Rooney, D.W.; Yap, P.-S. Recycling Municipal, Agricultural and Industrial Waste into Energy, Fertilizers, Food and Construction Materials, and Economic Feasibility: A Review. Environ. Chem. Lett. 2023, 21, 765–801. [Google Scholar] [CrossRef]
- Eurostat. Waste Statistics. 2024. Available online: https://tinyurl.com/3y3w9pv6 (accessed on 1 April 2025).
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Chen, W.; Yuan, H.; Ma, Y.; Siddiqui, M.A.; Iqbal, A. Assessing Greenhouse Gas Emissions and Energy Efficiency of Four Treatment Methods for Sustainable Food Waste Management. Recycling 2023, 8, 66. [Google Scholar] [CrossRef]
- Gomes, J.M.; Mariz, J.; Rodrigues, C.; Alves, A.L.; Moreira, J.; Vieira, B.; Silva, R.M.; Zille, A.; Silva, C.J. Bioactive Compounds from Banana Leaf Extracts: Influence of Extraction Methodologies and Their Integration into Knitted Hemp Fabrics. Materials 2024, 17, 5884. [Google Scholar] [CrossRef]
- Afonso, T.B.; Bonifácio-Lopes, T.; Costa, E.M.; Pintado, M.E. Phenolic Compounds from By-Products for Functional Textiles. Materials 2023, 16, 7248. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-Industrial By-Products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Tariq, H.; Rehman, A.; Raza, Z.A.; Kishwar, F.; Abid, S. Recent Progress in the Microencapsulation of Essential Oils for Sustainable Functional Textiles. Polym. Bull. 2024, 81, 7585–7629. [Google Scholar] [CrossRef]
- El-Molla, M.M.; El-Ghorab, A.H. Extraction of Eco-Friendly Essential Oils and Their Utilization in Finishing Polyester Fabrics for Fragrant and Medical Textiles. J. Eng. Fiber Fabr. 2022, 17. [Google Scholar] [CrossRef]
- Mehta, S.; MacGillivray, M. Aromatherapy in Textiles: A Systematic Review of Studies Examining Textiles as a Potential Carrier for the Therapeutic Effects of Essential Oils. Textiles 2022, 2, 29–49. [Google Scholar] [CrossRef]
- Zaharia, C.; Diaconu, M.; Muresan, E.I.; Danila, A.; Popescu, A.; Rosu, G. Bioactive Emulsions with Beneficial Antimicrobial Application in Textile Material Production. Cellulose 2020, 27, 9711–9723. [Google Scholar] [CrossRef]
- Stefanovic, B.; Kostic, M.; Bacher, M.; Rosenau, T.; Potthast, A. Vegetable Oils in Textile Finishing Applications: The Action Mode of Wrinkle-Reduction Sprays and Means for Analyzing Their Performance. Text. Res. J. 2014, 84, 449–460. [Google Scholar] [CrossRef]
- Tariq, Z.; Izhar, F.; Malik, M.H.; Oneeb, M.; Anwar, F.; Abbas, M.; Khan, A. Development of Functional Textile via Microencapsulation of Peppermint Oils: A Novel Approach in Textile Finishing. Res. J. Text. Appar. 2024, 28, 337–349. [Google Scholar] [CrossRef]
- Stan, M.S.; Chirila, L.; Popescu, A.; Radulescu, D.M.; Radulescu, D.E.; Dinischiotu, A. Essential Oil Microcapsules Immobilized on Textiles and Certain Induced Effects. Materials 2019, 12, 2029. [Google Scholar] [CrossRef]
- Nde, D.; Foncha, A. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.; de Souza Vandenberghe, L.P.; Soccol, C.R. Oilseed Enzymatic Pretreatment for Efficient Oil Recovery in Biodiesel Production Industry: A Review. Bioenergy Res. 2020, 13, 1016–1030. [Google Scholar] [CrossRef]
- Dunford, N.T. Enzyme-Aided Oil and Oilseed Processing: Opportunities and Challenges. Curr. Opin. Food Sci. 2022, 48, 100943. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Pinheiro, A.I.; Silva, C. Bioeconomy in Textile Industry: Industrial Wastes Valorisation Toward Textile Functionalization. 2024. In Proceedings of the 32nd European Biomass Conference and Exhibition, Marseille, France, 24–27 June 2024. [Google Scholar] [CrossRef]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van der Westhuizen, J.H. Photochemistry of Flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M. Stability of Virgin Olive Oil. 1. Autoxidation Studies. J. Agric. Food Chem. 2002, 50, 716–721. [Google Scholar] [CrossRef]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant Activity and Phenolic Content of Chestnut (Castanea sativa) Shell and Eucalyptus (Eucalyptus globulus) Bark Extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Ogunkanmi, J.O.; Kulla, D.M.; Omisanya, N.O.; Sumaila, M.; Obada, D.O.; Dodoo-Arhin, D. Extraction of Bio-Oil during Pyrolysis of Locally Sourced Palm Kernel Shells: Effect of Process Parameters. Case Stud. Therm. Eng. 2018, 12, 711–716. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J. Comparison of Near-infrared, Fourier Transform-infrared, and Fourier Transform-raman Methods for Determining Olive Pomace Oil Adulteration in Extra Virgin Olive Oil. J. Am. Oil Chem. Soc. 2001, 78, 889–895. [Google Scholar] [CrossRef]
- Wembabazi, E.; Mugisha, P.J.; Ratibu, A.; Wendiro, D.; Kyambadde, J.; Vuzi, P.C. Spectroscopic Analysis of Heterogeneous Biocatalysts for Biodiesel Production from Expired Sunflower Cooking Oil. J. Spectrosc. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Azadeh, E.; Chen, X.; Pizzi, A.; Gérardin, C.; Gérardin, P.; Essawy, H. Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins. J. Renew. Mater. 2022, 10, 3217–3227. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman Fingerprints of Flavonoids—A Review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Rungruangkitkrai, N.; Phromphen, P.; Chartvivatpornchai, N.; Srisa, A.; Laorenza, Y.; Wongphan, P.; Harnkarnsujarit, N. Water Repellent Coating in Textile, Paper and Bioplastic Polymers: A Comprehensive Review. Polymers 2024, 16, 2790. [Google Scholar] [CrossRef]
- AS/NZ Standard 4399:2017; Sun Protective Clothing- Evaluation and Classification. Standards New Zealand: Welington, New Zealand, 2017.
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive Phenolic Components and Potential Health Effects of Chestnut Shell: A Review. J. Food Biochem. 2021, 45, e13696. [Google Scholar] [CrossRef]
- Kiralan, M.; Kiralan, S.S.; Özkan, G.; Karacabey, E. Chestnut (Castanea sativa) Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 199–208. [Google Scholar]
- Ribeiro, J.E.; Rocha, J.; Queijo, L. The Influence of Manufacturing Factors in the Short-Fiber Non-Woven Chestnut Hedgehog Spine-Reinforced Polyester Composite Performance. J. Nat. Fibers 2021, 18, 1307–1319. [Google Scholar] [CrossRef]
- Rocha, J.Q.L. Production of Composite Thermosetting Resin Reinforced with Fibres from Autochthonous Plant, This Case Thorn from Hedgehog of Chestnut. In Proceedings of the In IDEMi 13, Porto, Portugal, 4 June 2013. [Google Scholar]
- Liu, Y.; Zhang, J.; Peng, F.; Niu, K.; Hou, W.; Du, B.; Yang, Y. Development of Chitosan-Based Films Incorporated with Chestnut Flower Essential Oil That Possess Good Anti-Ultraviolet Radiation and Antibacterial Effects for Banana Storage. Coatings 2024, 14, 548. [Google Scholar] [CrossRef]
- Miadonye, A.; Amadu, M.; O’Keefe, T.P. Spectroscopic Determination of the Synergistic Effect of Natural Antioxidants in Bio-Transformer Oils. Results Chem. 2023, 5, 100852. [Google Scholar] [CrossRef]
- Jović, O.; Habinovec, I.; Galić, N.; Andrašec, M. Maceration of Extra Virgin Olive Oil with Common Aromatic Plants Using Ultrasound-Assisted Extraction: An UV-Vis Spectroscopic Investigation. J. Spectrosc. 2018, 2018, 7510647. [Google Scholar] [CrossRef]
- Sadgrove, N.; Padilla-González, G.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Zou, X.; Bk, A.; Rauf, A.; Saeed, M.; Al-Awthan, Y.S.; Al-Duais, M.A.; Bahattab, O.; Hamayoon Khan, M.; Suleria, H.A.R. Screening of Polyphenols in Tobacco (Nicotiana tabacum) and Determination of Their Antioxidant Activity in Different Tobacco Varieties. ACS Omega 2021, 6, 25361–25371. [Google Scholar] [CrossRef]
- Zou, X.; BK, A.; Abu-Izneid, T.; Aziz, A.; Devnath, P.; Rauf, A.; Mitra, S.; Bin Emran, T.; Mujawah, A.A.H.; Lorenzo, J.M.; et al. Current Advances of Functional Phytochemicals in Nicotiana Plant and Related Potential Value of Tobacco Processing Waste: A Review. Biomed. Pharmacother. 2021, 143, 112191. [Google Scholar] [CrossRef]
- Sharma, Y.; Kaur, A.; Bhardwaj, R.; Srivastava, N.; Lal, M.; Madan, S.; Bala, K. Preclinical Assessment of Stem of Nicotiana Tabacum on Excision Wound Model. Bioorg. Chem. 2021, 109, 104731. [Google Scholar] [CrossRef]
- Prommaban, A.; Kheawfu, K.; Chittasupho, C.; Sirilun, S.; Hemsuwimon, K.; Chaiyana, W. Phytochemical, Antioxidant, Antihyaluronidase, Antityrosinase, and Antimicrobial Properties of Nicotiana tabacum L. Leaf Extracts. Evid. Based Complement. Altern. Med. 2022, 1, 5761764. [Google Scholar] [CrossRef]
- Zhao, J.; Ouyang, S.; Qi, H.; Ma, K.; Hu, X.; Wang, G.; Yang, X. Metabolomics and Transcriptomics Uncover the Pectin Hydrolysis during Tobacco Stem Fermentation by Aspergillus niger. J. Clean. Prod. 2024, 442, 141005. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Stanek-Wandzel, N.; Krzyszowska, A.; Zarębska, M.; Gębura, K.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Tomaka, M. Evaluation of Cellulase, Pectinase, and Hemicellulase Effectiveness in Extraction of Phenolic Compounds from Grape Pomace. Int. J. Mol. Sci. 2024, 25, 13538. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Ayuso-Yuste, M.C.; Bernalte, M.J.; Gil, M.V.; Delgado-Adámez, J. Cellulase-Assisted Extraction of Phenolic Compounds from Rice Bran (Oryza sativa L.): Process Optimization and Characterization. J. Food Meas. Charact. 2021, 15, 1719–1726. [Google Scholar] [CrossRef]
- Guimarães, A.; Mota, A.C.; Pereira, A.S.; Fernandes, A.M.; Lopes, M.; Belo, I. Rice Husk, Brewer’s Spent Grain, and Vine Shoot Trimmings as Raw Materials for Sustainable Enzyme Production. Materials 2024, 17, 935. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Mamat, H.; Jusoh, W.M.S.W.; Idham, Z.; Yunus, M.A.C.; Irianto, I. Influence of Particle Size in Supercritical Carbon Dioxide Extraction of Roselle (Hibiscus sabdariffa) on Bioactive Compound Recovery, Extraction Rate, Diffusivity, and Solubility. Sci. Rep. 2023, 13, 10871. [Google Scholar] [CrossRef]
- Ntalikwa, J.W. Solvent Extraction of Jatropha Oil for Biodiesel Production: Effects of Solvent-to-Solid Ratio, Particle Size, Type of Solvent, Extraction Time, and Temperature on Oil Yield. J. Renew. Energy 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.; Mousavi Khaneghah, A. Recent Advances in Orange Oil Extraction: An Opportunity for the Valorisation of Orange Peel Waste a Review. Int. J. Food Sci. Technol. 2019, 54, 925–932. [Google Scholar] [CrossRef]
- Vovk, H.; Karnpakdee, K.; Ludwig, R.; Nosenko, T. Enzymatic Pretreatment of Plant Cells for Oil Extraction. Food Technol. Biotechnol. 2023, 61, 160–178. [Google Scholar] [CrossRef]
- Waheed, A.; Akram, S.; Ashraf, R.; Mushtaq, M.; Adnan, A. Kinetic Model and Optimization for Enzyme-assisted Hydrodistillation of D-limonene-rich Essential Oil from Orange Peel. Flavour Fragr. J. 2020, 35, 561–569. [Google Scholar] [CrossRef]
- Wei, L.; Pu, D.; Mi, S.; Yang, H.; Wei, L.; Lu, Q.; Liu, M.; Zu, Y. Essential Oil Extraction and Evaluation from the Fresh Platycladus orientalis (L.) Franco Seed Peel Waste by an Environment-Friendly Method. Sustain. Chem. Pharm. 2022, 29, 100771. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Cui, G.; Wei, M.; Zou, Z.; Ni, H. Efficient Extraction of Essential Oil from Cinnamomum burmannii Leaves Using Enzymolysis Pretreatment and Followed by Microwave-Assisted Method. LWT 2021, 147, 111497. [Google Scholar] [CrossRef]
- Ferrentino, G.; Ndayishimiye, J.; Haman, N.; Scampicchio, M. Functional Activity of Oils from Brewer’s Spent Grain Extracted by Supercritical Carbon Dioxide. Food Bioprocess Technol. 2019, 12, 789–798. [Google Scholar] [CrossRef]
- Tagliaro, I.; Mariani, M.; Akbari, R.; Contardi, M.; Summa, M.; Saliu, F.; Nisticò, R.; Antonini, C. PFAS-Free Superhydrophobic Chitosan Coating for Fabrics. Carbohydr. Polym. 2024, 333, 121981. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; Sedik, A.; Mosaad, M.M.; Othman, H.A. Natural Oils\Silicon Based Materials for Imparting New Properties to Cellulose Based Fabrics. Results Chem. 2023, 5, 100812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).