Valorization of Underused Biomass of Acacia dealbata and Acacia melanoxylon Through Vermicomposting as an Alternative Substrate for Cucumber Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Experimental Design
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Effect of Acacia Vermicompost on Cucumber Germination
3.2. Effect of Acacia Vermicompost on Early Growth of Cucumber
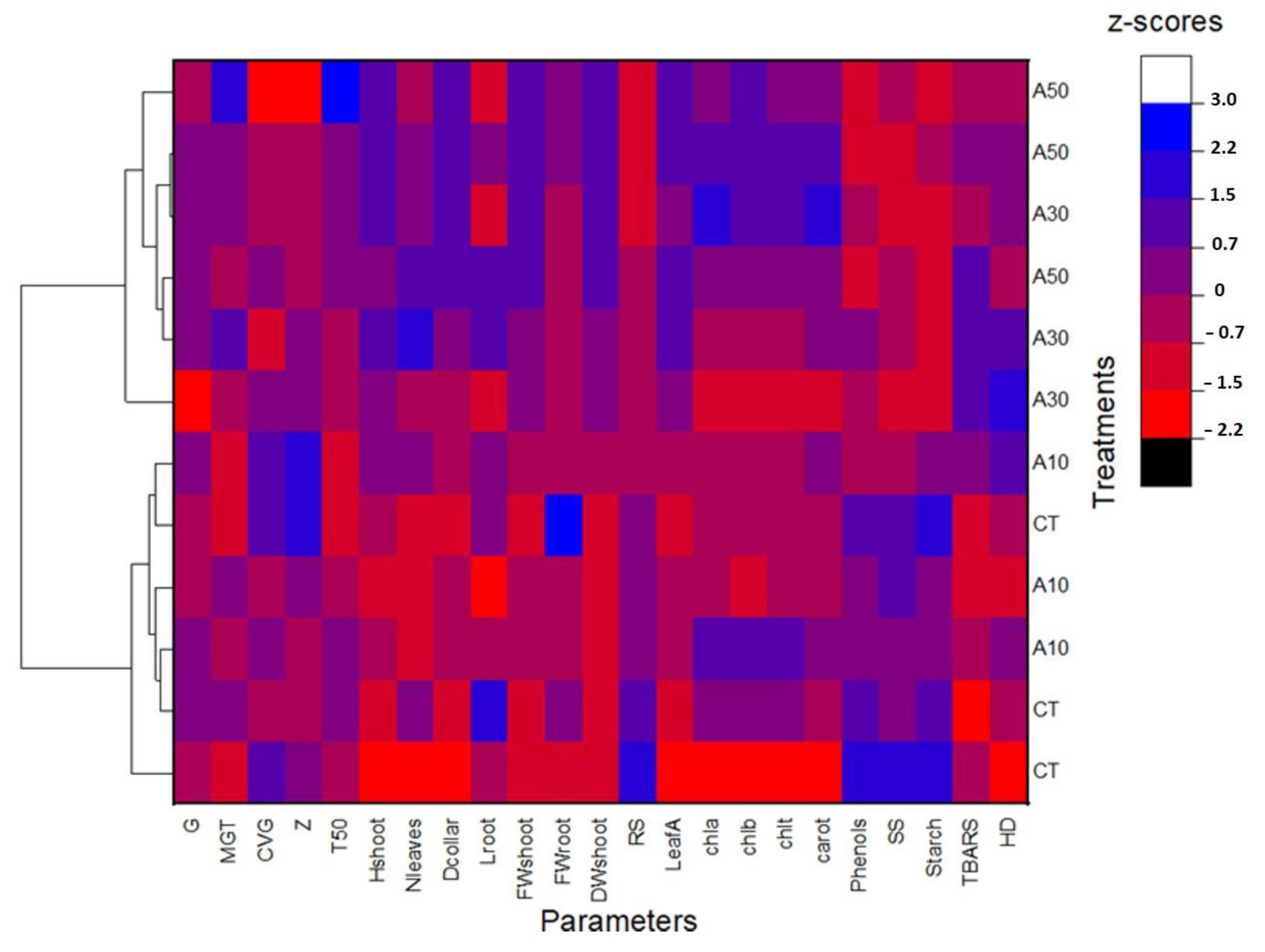
3.3. Heatmap with Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| IAPS | Invasive alien plant species. |
| TBARS | Total thiobarbituric acid-reactive substances. |
| DM | Dry matter. |
| FW | Fresh weight. |
| EC | Electrical conductivity. |
| OM | Organic matter. |
| G | Germination percentage. |
| MGT | Mean germination time. |
| CVG | Coefficient of velocity of germination. |
| T50 | Time of 50% of germination. |
| Z | Synchrony of germination. |
| SD | Standard deviation. |
| CT | Control treatment. |
| A10 | Substrate with 10% of Acacia vermicompost. |
| A30 | Substrate with 30% of Acacia vermicompost. |
| A50 | Substrate with 50% of Acacia vermicompost. |
References
- Bacher, S.; Galil, B.S.; Nuñez, M.A.; Ansong, M.; Cassey, P.; Dehnen-Schmutz, K.; Fayvush, G.; Hiremath, A.J.; Ikegami, M.; Martinou, A.F.; et al. Impacts of invasive alien species on nature, nature’s contributions to people, and good quality of life. In Thematic Assessment Report on Invasive Alien Species and their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Roy, H.E., Pauchard, A., Stoett, P., Truong, T.R., Eds.; IPBES Secretariat: Bonn, Germany, 2023; pp. 397–559. [Google Scholar] [CrossRef]
- Marchante, E.; Gouveia, A.C.; Brundu, G.; Marchante, H. Australian Acacia species in Europe. In Wattles—Australian Acacia Species Around the World; Richardson, D.M., Le Roux, J.J., Marchante, E., Eds.; CABI: Wallingford, UK, 2023; pp. 148–166. [Google Scholar] [CrossRef]
- Marchante, E.; Skulska, I.; Colaço, C.; Ulm, F.; Gonzalez, L.; Duarte, L.; Neves, S.; Gonçalves, C.; Maggiolli, S.; Dias, J.; et al. Management of invasive Australian Acacia species in the iberian peninsula. In Wattles—Australian Acacia Species Around The World; Richardson, D.M., Le Roux, J.J., Marchante, E., Eds.; CABI: Wallingford, UK, 2023; pp. 438–454. [Google Scholar] [CrossRef]
- Colaço, M.C.; Sequeira, A.C.; Skulska, I. Genus Acacia in mainland Portugal: Knowledge and experience of stakeholders in their management. Land 2023, 12, 2026. [Google Scholar] [CrossRef]
- Arán, D.; García-Duro, J.; Cruz, O.; Casal, M.; Reyes, O. Understanding biological characteristics of Acacia melanoxylon in relation to fire to implement control measurements. Ann. For. Sci. 2017, 74, 61. [Google Scholar] [CrossRef]
- Passos, I.; Marchante, H.; Pinho, R.; Marchante, E. What we don’t seed: The role of long-lived seed banks as hidden legacies of invasive plants. Plant Ecol. 2017, 218, 1313–1324. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Rodríguez, J.; González, L.; Lorenzo, P. Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann. For. Sci. 2017, 74, 55. [Google Scholar] [CrossRef]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; González, L. Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Guedes, L.M.; Villaseñor-Parada, C.; González, L.; Hernández, V. Allelopathic effect of the invasive Acacia dealbata Link (Fabaceae) on two native plant species in south-central Chile. Gayana Bot. 2015, 72, 231–239. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Reigosa, M.J. Allelopathic potential of Acacia melanoxylon on the germination and root growth of native species. Weed Biol. Manag. 2011, 11, 18–28. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Riveiro, S.F.; Cruz, Ó.; Reyes, O. Are the invasive Acacia melanoxylon and Eucalyptus globulus drivers of other species invasion? Testing their allelochemical effects on germination. New Forests 2024, 55, 751–767. [Google Scholar] [CrossRef]
- Míguez, C.; Cancela, A.; Álvarez, X.; Sánchez, A. The reuse of bio-waste from the invasive species Tradescantia fluminensis as a source of phenolic compounds. J. Clean. Prod. 2022, 336, 130293. [Google Scholar] [CrossRef]
- Rawat, Y.S.; Singh, G.S.; Tekleyohannes, A.T. Optimizing the benefits of invasive alien plants biomass in South Africa. Sustainability 2024, 16, 1876. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Tran, T.V.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.-V.N. Invasive plants as biosorbents for environmental remediation: A review. Environ. Chem. Lett. 2022, 20, 1421–1451. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Morais, M.C. Repurposing waste from aggressive Acacia invaders to promote its management in large invaded areas in Southwestern Europe. Plants 2024, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.; Khwairakpam, M. Feasibility of vermicomposting for the management of terrestrial weed Ageratum conyzoides using earthworm species Eisenia fetida. Environ. Technol. Innov. 2020, 18, 100696. [Google Scholar] [CrossRef]
- Pandit, L.; Sethi, D.; Pattanayak, S.K.; Nayak, Y. Bioconversion of lignocellulosic organic wastes into nutrient rich vermicompost by Eudrilus eugeniae. Bioresour. Technol. Rep. 2020, 12, 100580. [Google Scholar] [CrossRef]
- Oyege, I.; Balaji Bhaskar, M.S. Effects of vermicompost on soil and plant health and promoting sustainable agriculture. Soil Syst. 2023, 7, 101. [Google Scholar] [CrossRef]
- Poornima, S.; Dadi, M.; Subash, S.; Manikandan, S.; Karthik, V.; Deena, S.R.; Balachandar, R.; Kumaran, S.K.N.; Subbaiya, R. Review on advances in toxic pollutants remediation by solid waste composting and vermicomposting. Sci. Afr. 2024, 23, e02100. [Google Scholar] [CrossRef]
- Rehman, S.u.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing plant growth and combating abiotic and biotic stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, T.; Abbasi, S.A. Vermicomposting eliminates the toxicity of Lantana (Lantana camara) and turns it into a plant friendly organic fertilizer. J. Hazard. Mater. 2015, 298, 46–57. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, T.; Abbasi, S.A. Vermicomposting transforms allelopathic parthenium into a benign organic fertilizer. J. Environ. Manag. 2016, 180, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Clause, J.; Barot, S.; Forey, E. Earthworms promote greater richness and abundance in the emergence of plant species across a grassland-forest ecotone. J. Plant Ecol. 2016, 30, 454–1465. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V.K. Vermicomposting—An effective tool for the management of invasive weed Parthenium hysterophorus. Bioresour Technol. 2011, 102, 5891-5. [Google Scholar] [CrossRef]
- Yadav, R.H. Assessment of different organic supplements for degradation of Parthenium hysterophorus by vermitechnology. J Environ Health Sci Eng. 2015, 13, 44. [Google Scholar] [CrossRef]
- Ameta, S.K.; Ameta, R.; Soni, D.; Ameta, S.C. Vermicomposting of Parthenium hysterophorus with different organic wastes and activators. Academia Arena 2016, 8, 34–38. [Google Scholar] [CrossRef]
- Singh, C.K.; Kumar, A. Vermicomposting of terrestrial weeds Lantana camara L. and Parthenium hysterophorus L.: Agriculture solid waste. Ecol. Quest. 2017, 28, 63–69. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Conversion of a toxic weed into vermicompost by Eisenia fetida: Nutrient content and earthworm fecundity. Bioresour. Technol. Rep. 2020, 11, 100530. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Management of invasive weed Parthenium hysterophorus through vermicomposting using a polyculture of Eisenia fetida and Eudrilus eugeniae. Environ. Sci. Pollut. Res. Int. 2021, 28, 29710–29719. [Google Scholar] [CrossRef]
- Gusain, R.; Suthar, S. Vermicomposting of invasive weed Ageratum conyzoids: Assessment of nutrient mineralization, enzymatic activities, and microbial properties. Bioresour. Technol. 2020, 312, 123537. [Google Scholar] [CrossRef]
- Wijeysingha, I.S.; Fernando, K.M.C. Bioconversion of perennial weeds of Chromolaena odorata (L.) R.M. King & H. Rob., Sida rhombifolia L., and Lantana camara L. by vermicomposting process. J. Agric. Sci. 2021, 15, 52–68. [Google Scholar] [CrossRef]
- Ganguly, R.K.; Al-Helal, M.A.; Chakraborty, S.K. Management of invasive weed Chromolaena odorata (Siam weed) through vermicomposting: An eco-approach utilizing organic biomass valorization. Environ. Technol. Innov. 2022, 28, 102952. [Google Scholar] [CrossRef]
- Suthar, S.; Sharma, P. Vermicomposting of toxic weed—Lantana camara biomass: Chemical and microbial properties changes and assessment of toxicity of end product using seed bioassay. Ecotoxicol. Environ. Saf. 2013, 95, 179–187. [Google Scholar] [CrossRef]
- Chaudhuri, P.S.; Debnath, S. Physico-chemical changes during vermicomposting of a terrestrial weed, Mikania micrantha and leaf litters of Acacia auriculiformis and Bambusa polymorpha mixed with cowdung. J. Environ. Biol. 2020, 41, 178–185. [Google Scholar] [CrossRef]
- Kauser, H.; Khwairakpam, M. Fate of invasive weed Mikania micrantha Kunth using vermitechnology employing three monoculture of earthworm species. Bioresour. Technol. Rep. 2021, 16, 100827. [Google Scholar] [CrossRef]
- Gupta, R.; Mutiyar, P.K.; Rawat, N.K.; Saini, M.S.; Garg, V.K. Development of a water hyacinth based vermireactor using an epigeic earthworm Eisenia fetida. Bioresour. Technol. 2007, 98, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Ankaram, S.; Laxmi, M.; Rao, K.R. Management of water hyacinth (Eichhornia crassipes), an aquatic weed waste, by vermicomposting technology. Int. J. Environ. Technol. Manage. 2012, 15, 195–207. [Google Scholar] [CrossRef]
- Deka, A.; Deka, S.; Baruah, C.K. Vermicomposting of water hyacinth Eichhornia crassipes (Mart. Solms) employing indigenous earthworm species. Int. J. Adv. Chem. Eng. Biol. Sci. 2014, 1, 89–92. [Google Scholar]
- Bernal, D.A.; Lastiri Hernández, M.A.H.; Osben, H.R.B.; Ramos, S.M.C.; Mora, M. Vermicompost as an alternative of management for water hyacinth. Rev. Int. Contam. Ambie. 2016, 32, 425–433. [Google Scholar] [CrossRef]
- Pottipati, S.; Jat, N.; Kalamdhad, A.S. Bioconversion of Eichhornia crassipes into vermicompost on a large scale through improving operational aspects of in-vessel biodegradation process: Microbial dynamics. Bioresour. Technol. 2023, 374, 128767. [Google Scholar] [CrossRef]
- Suthar, S.; Pandey, B.; Gusain, R.; Gaur, R.Z.; Kumar, K. Nutrient changes and biodynamics of Eisenia fetida during vermicomposting of water lettuce (Pistia sp.) biomass: A noxious weed of aquatic system. Environ. Sci. Pollut. Res. 2017, 24, 199–207. [Google Scholar] [CrossRef]
- Ganeshkumar, T.; Premalatha, M.; Gajalakshmi, S.; Abbasi, S.A. A new process for the rapid and direct vermicomposting of the aquatic weed salvinia (Salvinia molesta). Bioresour. Bioprocess. 2014, 1, 26. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, T.; Abbasi, S.A. Generation of highly potent organic fertilizer from pernicious aquatic weed Salvinia molesta. Environ. Sci. Pollut. Res. 2018, 25, 4989–5002. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.; Hussain, N.; Gajalakshmi, S.; Abbasi, S.A. Effect of vermicast generated from an allelopathic weed lantana (Lantana camara) on seed germination, plant growth, and yield of cluster bean (Cyamopsis tetragonoloba). Environ. Sci. Pollut. Res. 2014, 21, 12539–12548. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef]
- Hirschler, O.; Osterburg, B.; Weimar, H.; Glasenapp, S.; Ohmes, M.-F. Peat Replacement in Horticultural Growing Media: Availability of Bio-Based Alternative Materials: Thünen Working Paper 190; Thünen Working Paper; Johann Heinrich von Thünen Institute: Braunschweig, Germany, 2022. [Google Scholar] [CrossRef]
- Alami, E.; Karimi, M.; Chalavi, V. Investigation of compost and vermicompost of water hyacinth as growing media for lily (Longiflorum × Asiatic). Int. J. Hortic. Sci. Technol. 2021, 8, 271–280. [Google Scholar] [CrossRef]
- Quintela-Sabarís, C.; Mendes, L.A.; Domínguez, J. Vermicomposting as a sustainable option for managing biomass of the invasive tree Acacia dealbata Link. Sustainability 2022, 14, 13828. [Google Scholar] [CrossRef]
- Coutinho, J.; Sá, C.; Trindade, A. Manual de Colheita de Amostras e de Dados no Campo: Sistema de Monitorização Nacional do solo; DGADR: Lisboa, Portugal, 2024; p. 50. [Google Scholar]
- Huang, K.; Li, F.; Li, J.; Helard, D.; Hirooka, K. Rapid vermicomposting of fresh fruit and vegetable wastes using earthworm Eisenia foetida. J. Jpn. Soc. Civil Eng. Ser. G (Environ. Res.) 2012, 68, 113–120. [Google Scholar] [CrossRef]
- Morais, M.C.; Nascimento-Gonçalves, E.; Azevedo, T.; Lopes, H.; Coimbra, A.M.; Sousa, J.R.; Roboredo, M.; Oliveira, P. Avaliação da fitotoxicidade em resíduos de Acacia dealbata e Robinia pseudoacacia vermicompostados. Presented at the VIII Jornadas de Engenharia Agronómica, Vila Real, 20 November 2024. [Google Scholar]
- Ballesta, J.; García-Navarro, F.J.; García-Giménez, R.; Trujillo-Gonzalez, J.M.; Iñigo, V.; Asensio, C. Agroecological analysis of cucumber (Cucumis sativus L.) crops in orchards in a Mediterranean environment. J. Agric. Crops 2018, 4, 16–28. [Google Scholar]
- Chen, T.-W.; Gomez Pineda, I.M.; Brand, A.M.; Stützel, H. Determining ion toxicity in cucumber under salinity stress. Agronomy 2020, 10, 677. [Google Scholar] [CrossRef]
- Brandenberger, L.; Shrefler, J.; Rebek, E.; Damicone, J. Cucumber Production; HLA-6023; Oklahoma Cooperative Extension Service: Stillwater, OK, USA; Oklahoma State University: Stillwater, OK, USA, 2021. [Google Scholar]
- Ranal, M.A.; de Santana, D.G. How and why to measure the germination process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Ranal, M.A.; de Santana, D.G.; Ferreira, W.R.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Rev. Bras. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef]
- Sestak, Z.; Catsky, J.; Jarvis, P.G. Plant Photosynthesis Production, Manual of Methods; Junk Publishers: The Hague, The Netherlands, 1971; p. 818. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagentes. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Oliveira, D.A.; Villar, J.J.; Oliveira, R.A.; Costa, F.G. Produção de mudas de pimentão e alface em diferentes combinações de substrato. Rev. Verde Agroecol. Desenvolv. Sustent. 2008, 3, 133–137. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Edwards, C.; Subler, S.; Metzger, J. Earthworm-processed organic wastes as components of horticultural potting media for growing marigold and vegetable seedlings. Compost Sci. Util. 2000, 8, 215–223. [Google Scholar] [CrossRef]
- Bachman, G.R.; Metzger, J.D. Growth of bedding plants in commercial potting substrate amended with vermicompost. Bioresour. Technol. 2008, 99, 3155–3161. [Google Scholar] [CrossRef]
- Sedera, F.A.A.; Eid, S.M.; Badr, L.A.; Emam, M.S.A.; Hawash, A.M.H. Using organic amendments to improve productivity of cucumber plants grown under sandy substrate culture. Ann. Agric. Sci. 2015, 53, 679–692. [Google Scholar] [CrossRef]
- Sopheak, T.; Panida, D.; Chaowanee, L.; Sararat, M. Vermicompost efficacy in improvement of cucumber (Cucumis sativus L.) productivity, soil nutrients, and bacterial population under greenhouse condition. Asia Pac. J. Sci. Technol. 2021, 27, APST-27-02-04. [Google Scholar] [CrossRef]
- Atmaca, L.; Tüzel, Y.; Öztekin, G.B. Influences of vermicompost as a seeding growth medium on organic greenhouse cucumber production. Acta Hortic. 2014, 1041, 37–46. [Google Scholar] [CrossRef]
- Jankauskienė, J.; Laužikė, K.; Kavaliauskaitė, D. Effects of vermicompost on quality and physiological parameters of cucumber (Cucumis sativus L.) seedlings and plant productivity. Horticulturae 2022, 8, 1009. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J.; Jamroz, E.; Bekier, J. Compost from willow biomass (Salix viminalis L.) as a horticultural substrate alternative to peat in the production of vegetable transplants. Sci. Rep. 2022, 12, 17617. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Singh, P.C.; Gupta, M.; Sinha, A.; Vaish, A.; Shukla, A.; Singh, N.; Tewari, S.K. Influence of earthworm culture on fertilization potential and biological activities of vermicomposts prepared from different plant wastes. J. Plant Nutr. Soil Sci. 2011, 174, 420–429. [Google Scholar] [CrossRef]
- Calderon, E.; Mortley, D.G. Vermicompost soil amendment influences yield, growth responses and nutritional value of Kale (Brassica oleracea Acephala group), Radish (Raphanus sativus) and Tomato (Solanum lycopersicum L). J. Soil Sci. Environ. Manage. 2021, 12, 86–93. [Google Scholar] [CrossRef]



| Units | Acacia Vermicompost | Commercial Substrate | |
|---|---|---|---|
| DM | % | 22.5 | 41.5 |
| pH (1:5) | 7.3 | 5.8 | |
| EC (1:5) | dS m−1 | 1.61 | 0.77 |
| OM | g kg−1 DM | 798 | 749 |
| C/N | 14.70 | 56.52 | |
| N-NH4+ | mg kg−1 FW | 27.72 | 7.62 |
| N-NO3− | mg kg−1 FW | 423 | 145 |
| N | g kg−1 DM | 31.49 | 7.69 |
| P | g kg−1 DM | 1.38 | 1.37 |
| K | g kg−1 DM | 15.9 | 4.27 |
| Ca | g kg−1 DM | 18.8 | 17.3 |
| Mg | g kg−1 DM | 3.60 | 1.69 |
| S | g kg−1 DM | 2.02 | 1.69 |
| B | mg kg−1 DM | 16.5 | 10.7 |
| Fe | mg kg−1 DM | 985 | 5051 |
| Cu | mg kg−1 DM | 6.63 | 14.06 |
| Zn | mg kg−1 DM | 40.9 | 43.0 |
| Mn | mg kg−1 DM | 327 | 164 |
| Ni | mg kg−1 DM | 0.58 | 5.53 |
| Cd | mg kg−1 DM | 0.04 | 0.09 |
| Pb | mg kg−1 DM | 1.36 | 7.38 |
| Cr | mg kg−1 DM | 1.41 | 11.37 |
| Hg | ug kg−1 DM | 14.4 | 15.4 |
| Parameter | Formula |
|---|---|
| Germination percentage (G, %) | |
| Mean germination time (MGT, day) | |
| Coefficient of velocity of germination (CVG, %) | |
| Time of 50% of germination (T50, day) | |
| Synchrony of germination (Z) | , being C = |
| G (%) | MGT (Day) | CVG (%) | Z | T50 (Day) | |
|---|---|---|---|---|---|
| CT | 93.3 ± 5.8 | 4.3 ± 0.2 | 23.4 ± 1.1 | 0.63 ± 0.14 | 3.6 ± 0.07 |
| A10 | 96.7 ± 5.8 | 4.3 ± 0.2 | 23.4 ± 1.0 | 0.64 ± 0.14 | 3.6 ± 0.07 |
| A30 | 93.3 ± 11.5 | 4.5 ± 0.2 | 22.3 ± 1.1 | 0.56 ± 0.06 | 3.7 ± 0.04 |
| A50 | 96.7 ± 5.8 | 4.5 ± 0.2 | 22.1 ± 1.1 | 0.43 ± 0.13 | 3.9 ± 0.26 |
| p-value | 0.900 | 0.379 | 0.384 | 0.256 | 0.242 |
| CT | A10 | A30 | A50 | |
|---|---|---|---|---|
| Shoot height (cm) | 4.54 ± 0.91 c | 5.62 ± 1.05 b | 6.81 ± 0.71 a | 6.98 ± 1.18 a |
| Number of leaves | 3.8 ± 0.5 a | 3.9 ± 0.5 a | 4.0 ± 0.5 a | 4.0 ± 0.4 a |
| Root collar diameter (mm) | 2.33 ± 0.34 d | 2.69 ± 0.42 c | 3.13 ± 0.47 b | 3.41 ± 0.46 a |
| Height/root collar diameter | 1.95 ± 0.33 a | 2.13 ± 0.49 a | 2.23 ± 0.41 a | 2.06 ± 0.37 a |
| Root length (cm) | 5.67 ± 1.18 a | 5.35 ± 1.11 a | 5.40 ± 0.85 a | 5.61 ± 1.07 a |
| Shoot fresh weight (g) | 0.62 ± 0.17 d | 0.84 ± 0.24 c | 1.28 ± 0.30 b | 1.47 ± 0.38 a |
| Root fresh weight (g) | 0.77 ± 0.52 a | 0.55 ± 0.22 a | 0.59 ± 0.23 a | 0.65 ± 0.29 a |
| Shoot dry weight (g) | 0.046± 0.016 b | 0.051 ± 0.016 b | 0.085 ± 0.018 a | 0.092 ± 0.025 a |
| Root/shoot ratio | 1.26 ± 0.28 a | 0.96 ± 0.19 b | 0.80 ± 0.17 c | 0.81 ± 0.17 c |
| Leaf area (cm2) | 8.92 ± 2.65 c | 13.90 ± 3.95 b | 20.26 ± 4.81 a | 22.51 ± 6.53 a |
| CT | A10 | A30 | A50 | |
|---|---|---|---|---|
| Chlorophyll a (mg g−1 DW) | 3.60 ± 0.90 a | 4.31 ± 0.68 a | 4.30 ± 1.33 a | 4.76 ± 0.33 a |
| Chlorophyll b (mg g−1 DW) | 1.91 ± 0.47 a | 2.07 ± 0.46 a | 1.99 ± 0.46 a | 2.31 ± 0.19 a |
| Chlorophyll total (mg g−1 DW) | 5.49 ± 1.36 a | 6.35 ± 1.12 a | 6.16 ± 1.77 a | 7.03 ± 0.47 a |
| Carotenoids (mg g−1 DW) | 0.73 ± 0.14 a | 0.93 ± 0.07 a | 0.97 ± 0.27 a | 1.00 ± 0.06 a |
| Phenols (mg g−1 DW) | 6.99 ± 0.25 a | 6.12 ± 0.27 b | 5.79 ± 0.40 bc | 5.17 ± 0.17 c |
| Soluble sugars (mg g−1 DW) | 5.29 ± 0.67 a | 4.57 ± 0.54 ab | 3.24 ± 0.49 b | 3.45 ± 0.40 b |
| Starch (mg g−1 DW) | 115.14 ± 4.11 a | 85.96 ± 4.33 b | 53.93 ± 1.72 c | 58.46 ± 3.08 c |
| TBARS (mg g−1 DW) | 0.033 ± 0.001 a | 0.035 ± 0.002 a | 0.038 ± 0.003 a | 0.038 ± 0.003 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, M.C.; Nascimento-Gonçalves, E.; Azevedo, T.; Lopes, H.; Ferreira, H.; Coimbra, A.M.; Gonçalves, B.; Sousa, J.R.; Roboredo, M.; Oliveira, P.A. Valorization of Underused Biomass of Acacia dealbata and Acacia melanoxylon Through Vermicomposting as an Alternative Substrate for Cucumber Production. Recycling 2025, 10, 120. https://doi.org/10.3390/recycling10030120
Morais MC, Nascimento-Gonçalves E, Azevedo T, Lopes H, Ferreira H, Coimbra AM, Gonçalves B, Sousa JR, Roboredo M, Oliveira PA. Valorization of Underused Biomass of Acacia dealbata and Acacia melanoxylon Through Vermicomposting as an Alternative Substrate for Cucumber Production. Recycling. 2025; 10(3):120. https://doi.org/10.3390/recycling10030120
Chicago/Turabian StyleMorais, Maria C., Elisabete Nascimento-Gonçalves, Tiago Azevedo, Henda Lopes, Helena Ferreira, Ana M. Coimbra, Berta Gonçalves, João R. Sousa, Marta Roboredo, and Paula A. Oliveira. 2025. "Valorization of Underused Biomass of Acacia dealbata and Acacia melanoxylon Through Vermicomposting as an Alternative Substrate for Cucumber Production" Recycling 10, no. 3: 120. https://doi.org/10.3390/recycling10030120
APA StyleMorais, M. C., Nascimento-Gonçalves, E., Azevedo, T., Lopes, H., Ferreira, H., Coimbra, A. M., Gonçalves, B., Sousa, J. R., Roboredo, M., & Oliveira, P. A. (2025). Valorization of Underused Biomass of Acacia dealbata and Acacia melanoxylon Through Vermicomposting as an Alternative Substrate for Cucumber Production. Recycling, 10(3), 120. https://doi.org/10.3390/recycling10030120










