Graphite Separation from Lithium-Ion Battery Black Mass Using Froth Flotation and Quality Evaluation for Reuse as a Secondary Raw Material Including Non-Battery Applications
Abstract
1. Introduction
2. Materials and Methods
- NMC (Nickel–Manganese–Cobalt Oxide) cathode material + graphite anode material with Copper (Cu) and Aluminum (Al) as conductive foils, whereas two different black mass batches used (denoted as NMC 1 and NMC 2).
- LFP (Lithium–Iron–Phosphate) cathode material + graphite anode material with Cu and Al as conductive foils (denoted as LFP).
- LIBs from power tools, pedelecs, and e-bikes, as well as a mixture of NMC and LCO (Lithium–Cobalt–Oxide), cathode materials + graphite anode material with Cu and Al as conductive foils (denoted as PSP).
- LIBs from mobile phones and laptops, as well as a mixture of NMC and LCO, cathode materials + graphite anode material with Cu and Al as conductive foils (denoted as HL).
2.1. Material Characterization
2.1.1. Particle Size Distribution (PSD)
2.1.2. Chemical Composition and Density
2.2. Froth Flotation for Graphite Separation
3. Results
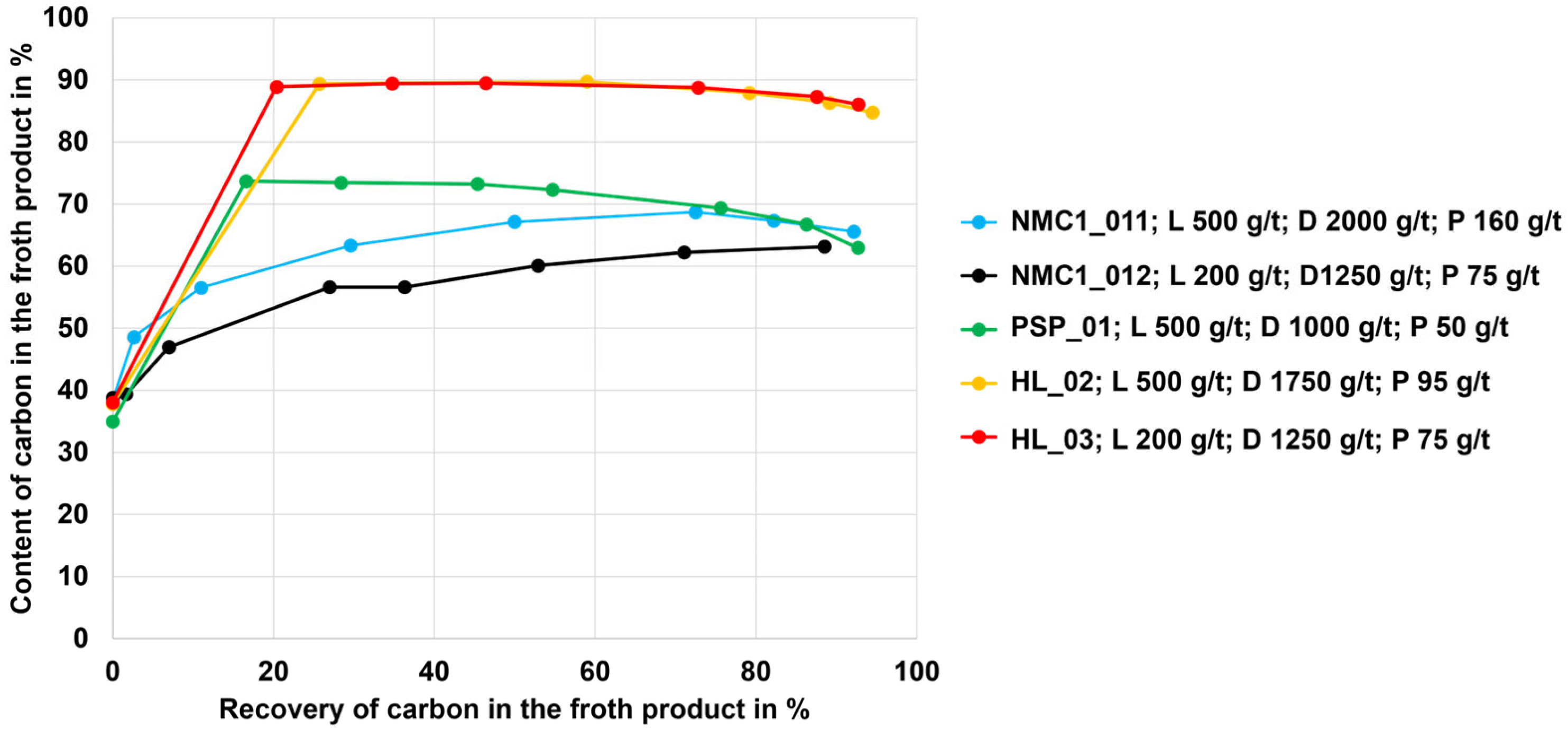
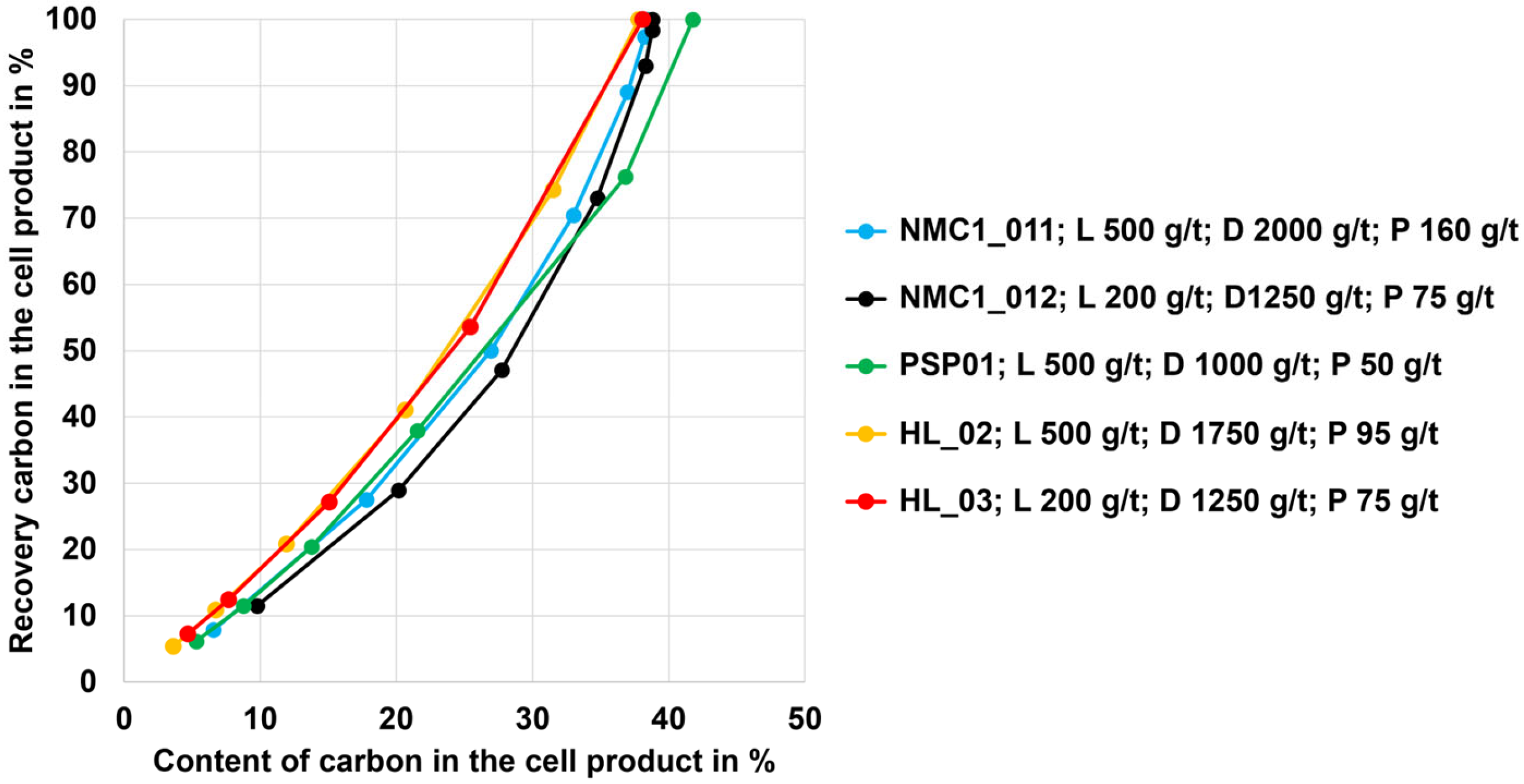
3.1. Flotation Results for the NMC, PSP, and LFP Black Mass
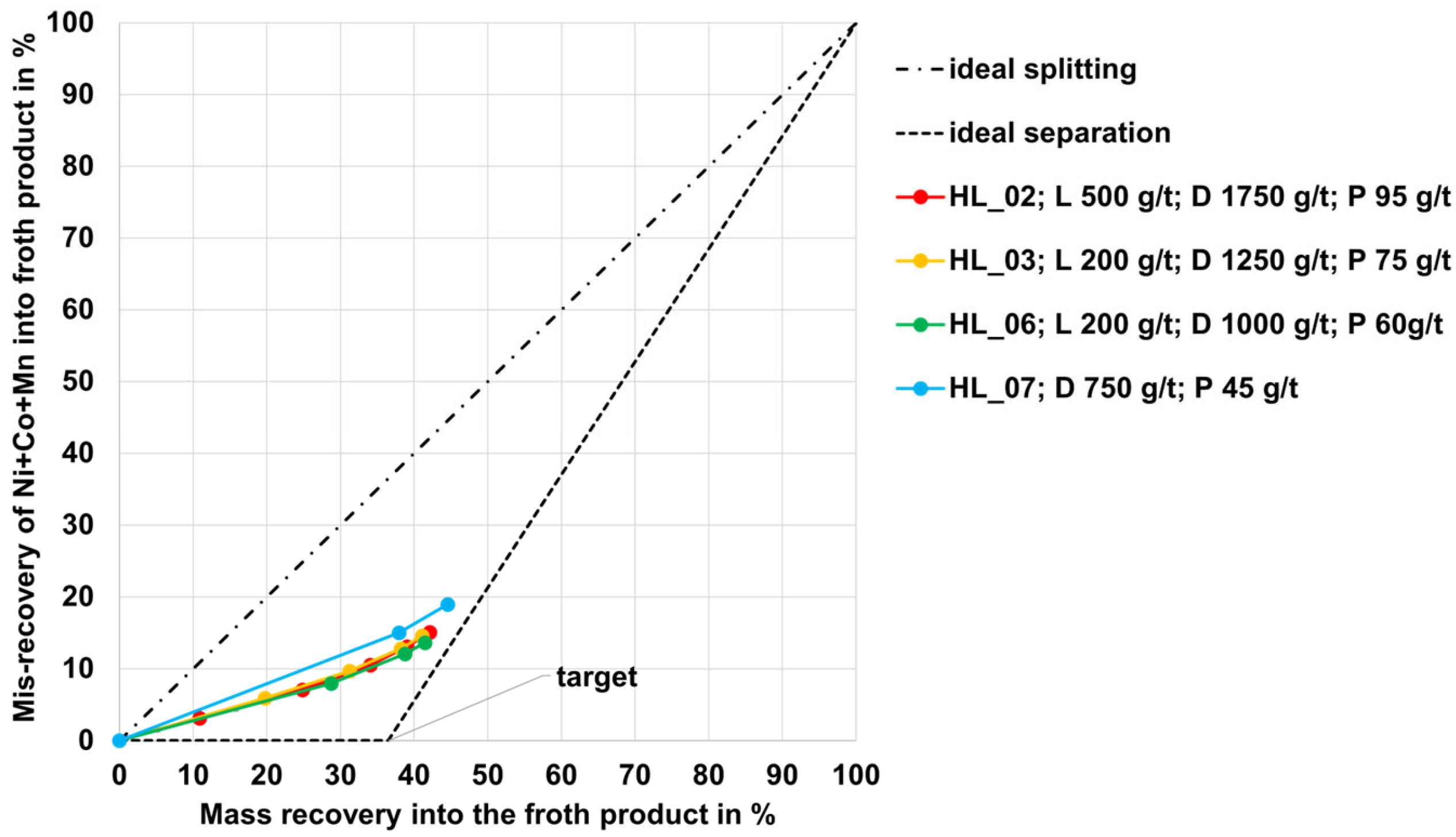
3.2. Flotation Results for the HL Black Mass
3.3. Evaluation of the Flotation Graphite as a Secondary Raw Material for Refractory Production
4. Discussion
4.1. Comparison of Black Mass Types According to the Carbon Content
4.2. Use of Flotation Graphite as a Secondary Carbon Carrier in Other Energy-Intensive Industries
5. Conclusions
- Black mass containing NMC and LCO cathode materials could be treated using froth flotation with graphite recovery rates in the range between 90 and 95%; the low-carbon (cell) products showed final carbon contents between 4 and 10 wt.% (as such, the goal to derive a low-carbon product was reached).
- The currently investigated black mass containing the LFP cathode material did not disperse well in water and therefore showed no selectivity with regard to graphite separation via froth flotation (dispersion may be different with other LFP samples).
- For each of the different black mass samples investigated, a separate flotation scheme including a reagent regime must be developed. There is likely an influence of the conditions during the thermal LIB pre-processing step or another influence of the different cell geometries on the separation efficiency (this hypothesis needs to be investigated more intensively). The black mass sample HL (mixture of NMC and LCO cathode and graphite anode material) and the NMC black mass samples showed the highest selectivity (carbon-enriched product with 8–10 wt.% of undesired metals Co, Cu, Ni, Mn, and Al from the black mass). The PSP black mass (also a mixture of NMC and LCO cathode and graphite anode material) showed less selectivity with ~30 wt.% undesired metal contents in the carbon-enriched product.
- A flotation scheme was elaborated, comprising rougher and scavenger steps, and the separation of carbon-enriched products was achieved. Additionally, the flotation concept allows for a certain flexibility in terms of required product quality, i.e., it is possible to end the flotation process at an earlier stage (i.e., at a lower number of scavenger stages) to end with a higher carbon content in the cell product (if carbon is required as a reducing agent for a subsequent pyrometallurgical treatment to recover metals from the black mass).
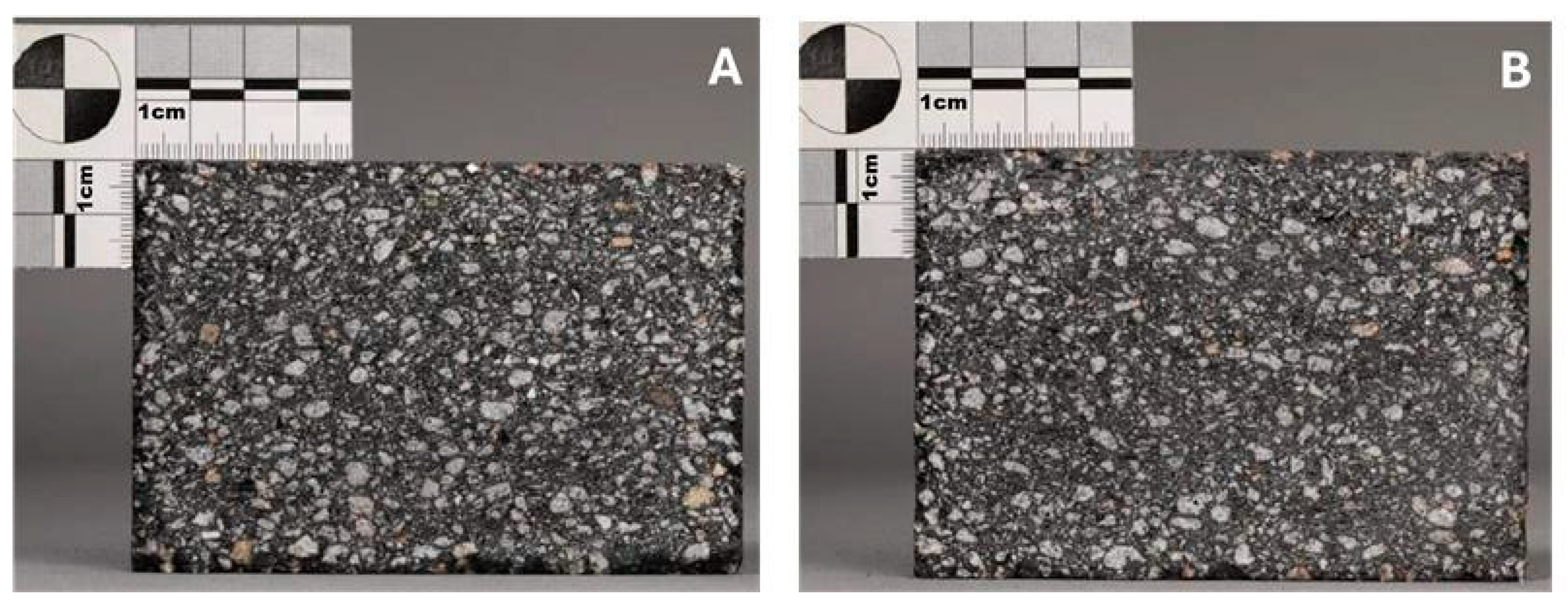
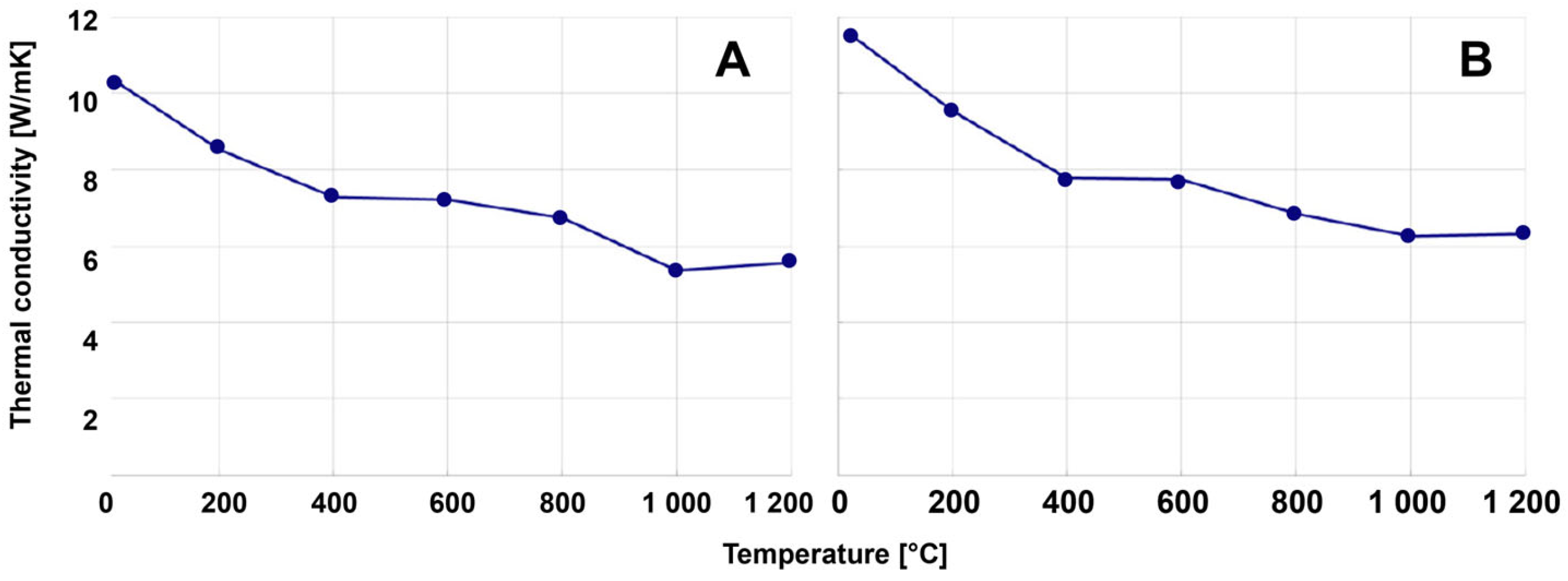
- The carbon-enriched product was used as a secondary carbon source for a refractory material (magnesia carbon bricks), whereas an analysis of the brick chemistry, as well as thermo-mechanic properties in terms of density, porosity, cold crushing strength, hot modulus of rupture (the maximum bending stress that can be applied to a material before it breaks), or thermal conductivity, showed no negative influence on brick quality.
- It could be demonstrated that flotation graphite can be used as a secondary source for non-battery applications, representing a valuable positive example that contributes to a more complete closure of the battery life cycle and thus to a more circular economy.
- Upscaling of the currently used froth flotation (process sequence and flotation reagents) to obtain more reproducible data about the expected graphite product quality including impurities (tramp elements) present in the flotation graphite.
- Elaboration of a comprehensive connection between operating conditions of the thermal spent LIB pre-processing step and carbon separation efficiencies obtained via froth flotation.
- In case the flotation graphite is considered to be used for open-loop recycling practices in industrial sectors other than battery production, different application-oriented scenarios must be comprehensively investigated; in case the flotation graphite is used as carbon carrier in an EAF for crude steelmaking, slag foaming efficiency, as well as the transfer of tramp elements into the metal melt, should be comprehensively investigated; in case the flotation graphite is used for refractory production (magnesia-carbon bricks), the high-temperature behavior of the bricks with the flotation graphite should be investigated in long-term trials.
- In-depth discussion on the economic viability of open-loop recycling approaches compared to other graphite recovery methods.
- Comparison of the environmental impact of graphite flotation and subsequent graphite recycling versus primary graphite production to gain added value with regards to circular economy contributions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Test No. | Flotation Stage | Reagent | Test No. | Flotation Stage | Reagent | |
|---|---|---|---|---|---|---|
| Type and Dosage in g/t | Type and Dosage in g/t | |||||
| NMC_1_05 | Ro * | K 250; M 150 | PSP_02 | Ro | L 500; D 500; P 30 | |
| Scav1 | K 150; M 50 | Scav1 | D 500; P 15 | |||
| Total | K 400; M 200 | Total | L 500; D 1000; P 45 | |||
| NMC_1_06 | Ro | K 800; M 150 | PSP_03 | Ro | D 500; P 10 | |
| Scav1 | K 150 | Scav1 | D 250; P 10 | |||
| Total | K 950; M 150 | Scav2 | D 250; P 10 | |||
| NMC_1_07 | Ro | K 250; M 250 | Scav3 | D 250; P 10 | ||
| Scav1 | K 150; M 50 | Total | D 1250; P 40 | |||
| Total | K 400; M 300 | HL_01 | Ro | L 500; D 800; M 100 | ||
| NMC_1_11 | Ro | L 500; D 500; P 30 | Scav1 | D 500; M 100 | ||
| Scav1 | D 250; P 15 | Scav2 | D 500; M 100 | |||
| Scav2 | D 250; P 15 | Scav3 | D 500; M 100 | |||
| Scav3 | D 250; P 15 | Scav4 | D 500; M 100 | |||
| Scav4 | D 250; P 15 | Total | L 500; D 2800; M 500 | |||
| Scav5 | D 250; P 20 | HL_02 | Ro | L 500; D 500; P 30 | ||
| Total | L 500; D 1750; P 110 | Scav1 | D 250; P 15 | |||
| NMC_1_12 | Ro | L 200; D 500; P 30 | Scav2 | D 250; P 15 | ||
| Scav1 | D 250; P 15 | Scav3 | D 250; P 15 | |||
| Scav2 | D 250; P 15 | Scav4 | D 500; P 20 | |||
| Scav3 | D 250; P 15 | Total | L 500; D 1750; P 95 | |||
| Scav4 | D 250; P 15 | HL_03 | Ro | L 200; D 500; P 30 | ||
| Scav5 | D 300; P 20 | Scav1 | D 250; P 15 | |||
| Total | L 200; D 1800; P 110 | Scav2 | D 250; P 15 | |||
| NMC_1_13 | Ro | L 1000; D 1300; P 30 | Scav3 | D 250; P 15 | ||
| Scav1 | D 250; P 15 | Total | L 200; D 1250; P 75 | |||
| Scav2 | D 250; P 15 | HL_06 | Ro | L 200; D 500; P 30 | ||
| Scav3 | D 250; P 15 | Scav1 | D 250; P 15 | |||
| Total | L 1000; D 2050; P 75 | Scav2 | D 250; P 15 | |||
| NMC_2_17 | Ro | L 200; D 500; P 30 | Total | L 200; D 1000; P 60 | ||
| Scav1 | D 500; P 15 | HL_07 | Ro | D 500; P 30 | ||
| Scav2 | D 250; P 15 | Scav1 | D 250; P 15 | |||
| Scav3 | D 500; P 15 | Total | D 750; P 45 | |||
| Total | L 200; D 1750; P 75 | |||||
| PSP_01 | Ro | L 500; D 100; P 10 | ||||
| Scav1 | D 100; P 10 | |||||
| Scav2 | D 100; P 10 | |||||
| Scav3 | D 100; P 10 | |||||
| Scav4 | D 100; P 10 | |||||
| Total | L 500; D 600; P 50 |
References
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success Story of graphite as a Lithium-Ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Larcher, D.; Beattie, S.; Morcrette, M.; Jumas, K.E.; Tarascon, J. Recent findings and prospects in the field of pure metals as negative electrodes for Li-ion batteries. J. Mater. Chem. 2007, 17, 3759–3772. [Google Scholar] [CrossRef]
- European Carbon and Graphite Association. Graphite in Batteries. Available online: https://ecga.net/wp-content/uploads/2023/02/Graphite-in-batteries_Infosheet_final.pdf (accessed on 16 September 2024).
- European Commission. Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials (COM(2023) 160 Final); European Commission: Brussels, Belgium, 2023. [Google Scholar]
- European Union. Regulation (EU) 2023/1542 Concerning Batteries and Waste Batteries. 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R1542 (accessed on 5 December 2024).
- Han, S.-J.; Xu, L.; Chen, C.; Wang, Z.-Y.; Fu, M.-L.; Yuan, B. Recovery of graphite from spent lithium-ion batteries and its wastewater treatment application: A review. J. Sep. Purif. Technol. 2024, 330, 125289. [Google Scholar] [CrossRef]
- Yildiz, T.; Wiechers, P.; Nirschl, H.; Gleiß, M. Direct recycling of carbon black and graphite from an aqueous anode slurry of lithium-ion batteries by centrifugal fractionation. Next Energy 2023, 2, 100082. [Google Scholar] [CrossRef]
- Ahuis, M.; Aluzoun, A.; Keppeler, M.; Melzig, S.; Kwade, A. Direct recycling of lithium-ion battery production scrap—Solvent-based recovery and reuse of anode and cathode coating materials. J. Power Sources 2024, 593, 233995. [Google Scholar] [CrossRef]
- Zhao, L.; Xin-yu, Z.; Yi-ye, L.; Hao, J.; Yan-qin, H.; Ming-xin, X.; Qiang, L. Recovery of electrode materials from a spent Lithium-Ion Battery through a pyrolysis-coupled mechanical milling method. Energy Fuels 2024, 38, 9280–9319. [Google Scholar] [CrossRef]
- Rong, T.; Yuan, Y.; Yang, H.; Yu, H.; Zuo, H.; Wang, J.; Xue, Q. Investigation of the enrichment-purification process and electrochemical performance of kish graphite in dust from blast furnace tapping yard. J. Waste Manag. 2024, 175, 121–132. [Google Scholar] [CrossRef]
- Remus, R.; Aguado Monsonet, M.A.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Iron and Steel Production; European Commission, Joint Research Centre: Brussels, Belgium, 2013. [Google Scholar]
- Jia, P.; Sun, J.; Li, S.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. A feasible recycling route for spent graphite: Microwave-assisted puffing with Fe2O3 loading to construct high-performance anode for lithium-ion batteries. Mater. Today Sustain. 2024, 25, 100620. [Google Scholar] [CrossRef]
- Mancini, M.; Hoffmann, M.F.; Martin, J.; Weirather-Kostner, D.; Axmann, P.; Wohlfahrt-Mehrens, M. A proof-of-concept of direct recycling of anode and cathode active materials: From spent batteries to performance in new Li-ion cells. J. Power Sources 2024, 595, 233997. [Google Scholar] [CrossRef]
- Subramanyan, K.; Jyothilakshmi, S.; Ulaganathan, M.; Lee, Y.-S.; Aravindan, V. An efficient upcycling of graphite anode and separator for Na-ion Batteries via solvent-co-intercalation process. Carbon 2024, 216, 118525. [Google Scholar] [CrossRef]
- Milian, Y.e.; Jamett, N.; Cruz, C.; Herrera-Leon, S.; Chacana-Olivares, J. A comprehensive review of emerging technologies for recycling spent lithium-ion batteries. Sci. Total Environ. 2024, 910, 165843. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Savihirta, H.; Wilson, B.P.; Lundström, M.; Serna-Guerrero, R. A study on recovery strategies of graphite from mixed lithium-ion battery chemistries using froth flotation. Waste Manag. 2024, 180, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Olutogun, M.; Vanderbruggen, A.; Frey, C.; Rudolph, M.; Bresser, D.; Passerini, S. Recycled graphite for more sustainable lithium-ion batteries. Carbon Energy 2024, 6, e483. [Google Scholar] [CrossRef]
- Drzymała, J.; Swatek, A. Mineral Processing: Foundations of Theory and Practice of Mineralogy, 1st ed.; Wroclaw University of Technology: Wroclaw, Poland, 2008. [Google Scholar]
- Wills, B.A.; Finch, J.A. Chapter 12—Froth Flotation. In Wills’ Mineral Processing Technology, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 265–380. [Google Scholar]
- Chehreh Chelgani, S.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A review of graphite beneficiation techniques. Miner. Process. Extr. Metall. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Vasumathi, N.; Sarjekar, A.; Chandrayan, H.; Chennakesavulu, K.; Reddy, G.; Ramanjaneya, V.K. A Mini Review on Flotation Techniques and Reagents Used in Graphite Beneficiation. Int. J. Chem. Eng. 2023, 2023, 1007689. [Google Scholar] [CrossRef]
- Andreola, F.; Castellini, E.; Ferreira, J.M.F.; Olhero, S.; Romagnoli, M. Effect of sodium hexametaphosphate and ageing on the rheological behaviour of kaolin dispersions. Appl. Clay Sci. 2006, 31, 56–64. [Google Scholar] [CrossRef]
- Arnberger, A.; Coskun, E.; Rutrecht, B. Recycling von Lithium-Ionen Batterien. In Recycling und Rohstoffe, 11th ed.; Thiel, S., Thomé-Kozmiensky, E., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 583–599. [Google Scholar]
- Salces, A.M.; Bremerstein, I.; Rudolph, M.; Vanderbruggen, A. Joint recovery of graphite and lithium metal oxides from spent lithium-ion batteries using froth flotation and investigation on process water re-use. Miner. Eng. 2022, 184, 107670. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Hayagan, N.; Bachmann, K.; Ferreira, A.; Werner, D.; Horn, D.; Peuker, U. Lithium-Ion Battery Recycling—Influence of Recycling Processes on Component Liberation and Flotation Separation Efficiency. ACS EST Eng. 2022, 2, 2130–2141. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhu, J.; Min, F.; Chen, J.; Shi, O. Effect of inorganic cations on enhancing graphite/kerosene adsorption and reducing carbon emission in graphite flotation. Fuel 2022, 314, 122740. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Saito, K.; Bruckard, W.; Menacho, J.; Hoadley, A. Flotation behavior of the most common electrode materials in lithium-ion batteries. Sep. Purif. Technol. 2022, 301, 121885. [Google Scholar] [CrossRef]
- Nazari, S.; Zhou, S.; Hassanzadeh, A.; Li, J.; He, Y.; Bu, X.; Kowalczuk, P.B. Influence of operating parameters on nanobubble-assisted flotation of graphite. J. Mat. Res. Technol. 2022, 20, 3891–3904. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the use of alternative carbon sources in EAF steelmaking. Metals 2022, 11, 222. [Google Scholar] [CrossRef]
- Zulhan, Z. Der Einfluss unterschiedlicher Kohlenstoffträger auf die Schaumschlackenbildung im Elektrolichtbogenofen. In Berichte aus dem Institut fuer Eisenhuettenkunde (German Document); Bleck, W., Krupp, U., Muenstermann, S., Senk, D., Eds.; Shaker Verlag: Aachen, Germany, 2006; Volume 8. [Google Scholar]
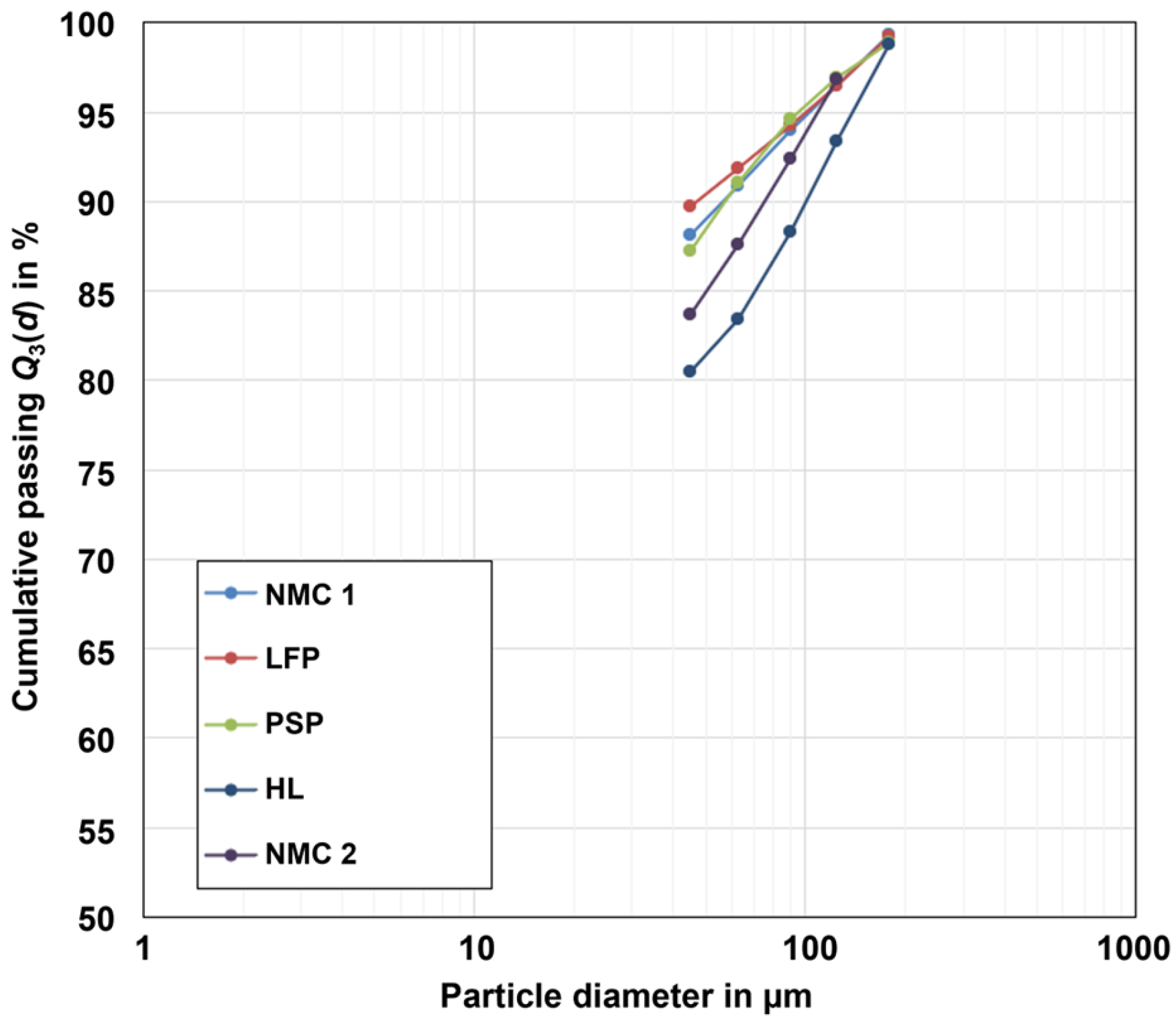

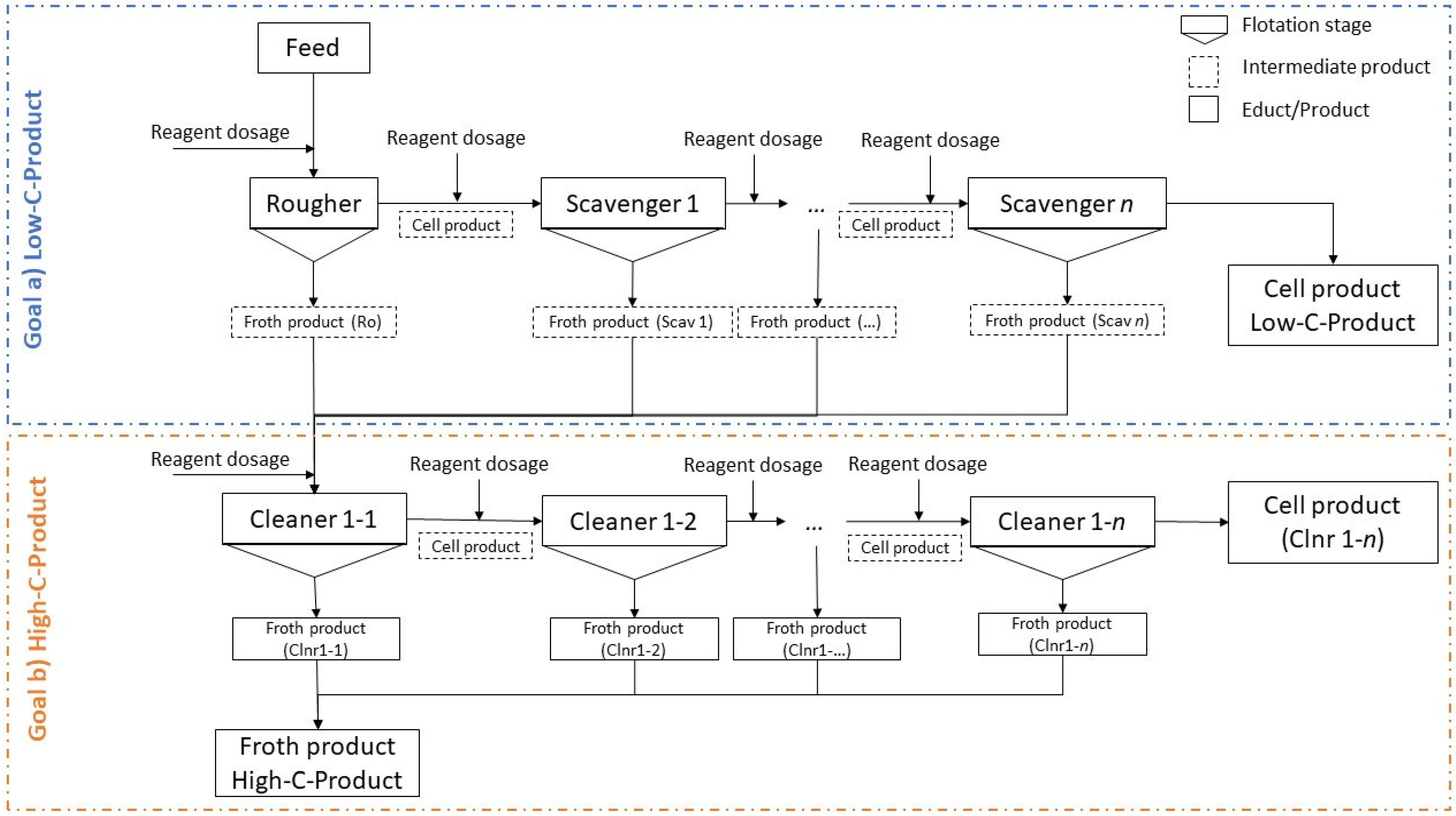
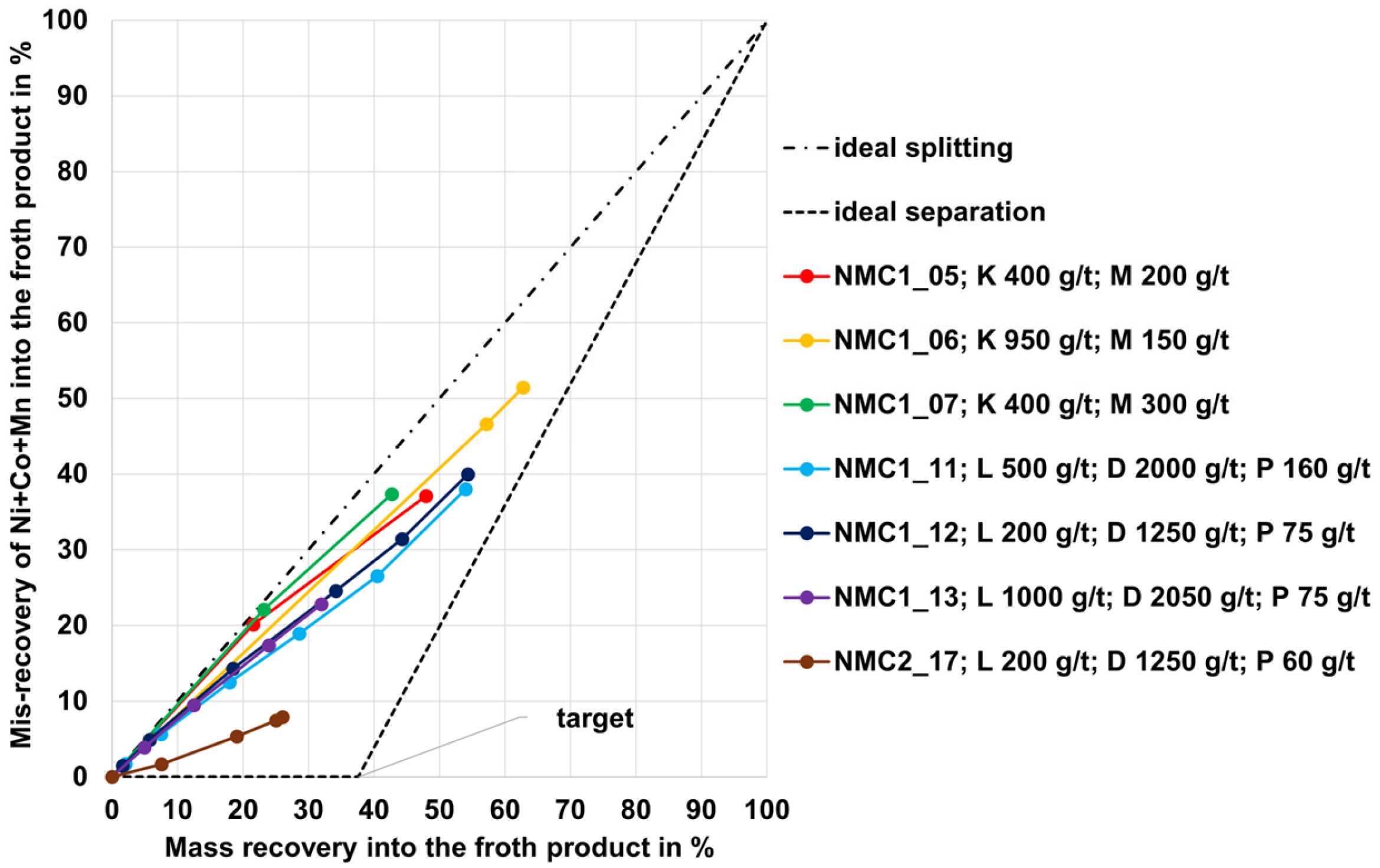
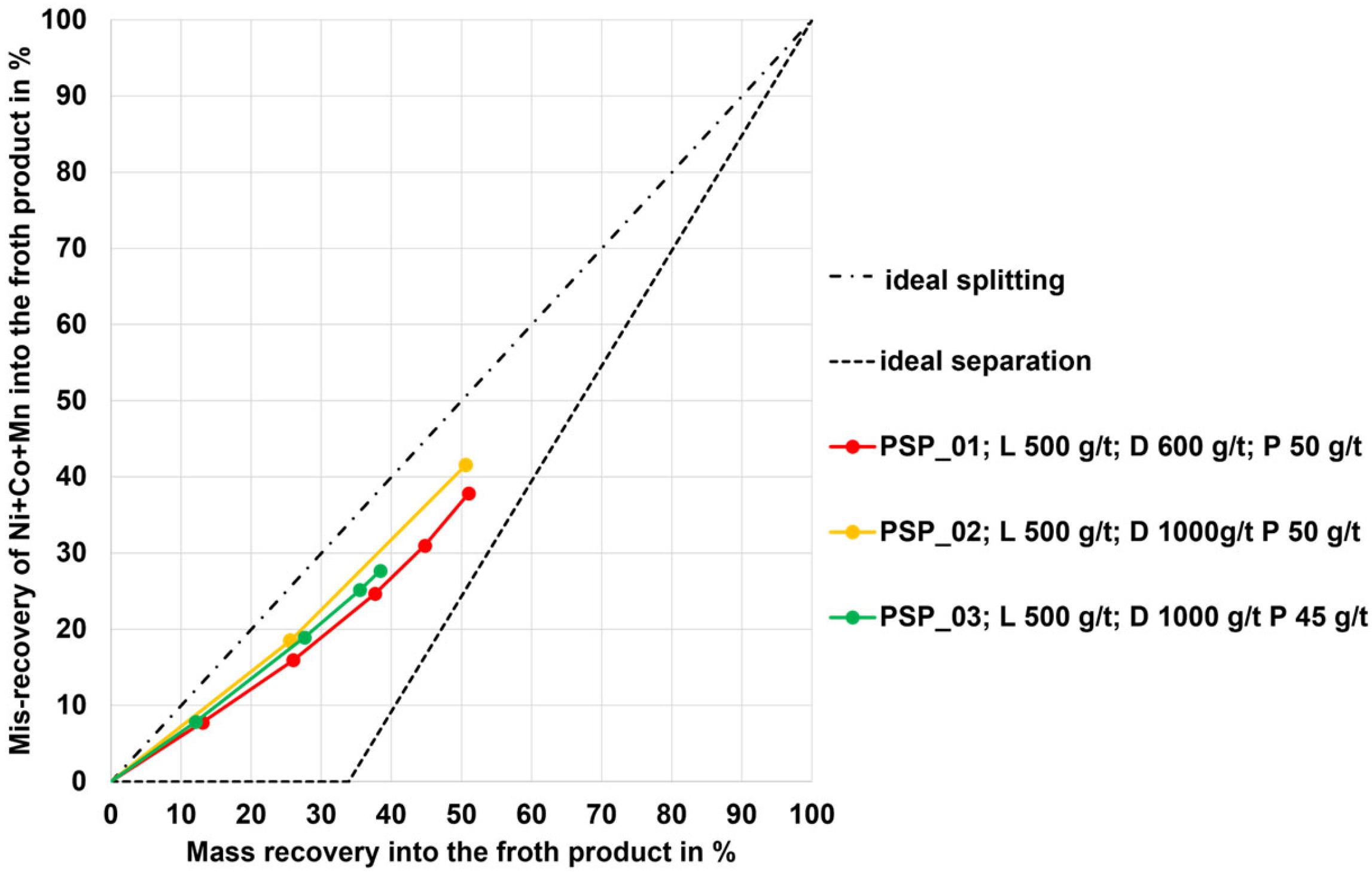

| Property | Unit | NMC 1 | NMC 2 | LFP | PSP | HL |
|---|---|---|---|---|---|---|
| Nickel + Cobalt + Manganese (Ni+Co+Mn) | wt.% | 38.0 | 28.4 | 0.6 | 35.5 | 41.0 |
| Iron + Phosphorus (Fe+P) | wt.% | 1.8 | 1.7 | 33.3 | 3.9 | 1.3 |
| Aluminum + Copper (Al+Cu) | wt.% | 8.3 | 19.3 | 8.5 | 9.8 | 10.4 |
| Carbon | wt.% | 37.6 | n.a. * | 32.6 | 33.9 | 36.5 |
| Density | g/cm3 | 3.1 | n.a. * | 2.8 | 3.1 | 3.4 |
| Flotation Test | Froth Product | Cell Product | ||||
|---|---|---|---|---|---|---|
| C wt.% | Ni+Co+Mn wt.% | Al+Cu wt.% | C wt.% | Ni+Co+Mn wt.% | Al+Cu wt.% | |
| NMC 1_11 1 | 65.6 | 27.8 | 3.5 | 6.6 | 53.3 | 16.9 |
| NMC 1_12 1 | 63.2 | 29.6 | 4.0 | 9.8 | 52.9 | 17.4 |
| NMC2_17 2 | n.a. | 8.7 | 5.2 | n.a. | 35.4 | 29.6 |
| PSP_02 3 | 63.0 | 26.8 | 3.3 | 5.3 | 46.8 | 17.2 |
| Flotation Test | Froth Product | Cell Product | ||||
|---|---|---|---|---|---|---|
| C wt.% | Ni+Co+Mn wt.% | Al+Cu wt.% | C wt.% | Ni+Co+Mn wt.% | Al+Cu wt.% | |
| HL_02 | 84.8 | 14.7 | 3.6 | 3.6 | 60.3 | 22.1 |
| HL_03 | 86.0 | 14.4 | 3.6 | 4.7. | 59.0 | 21.0 |
| Stage-Product | Reagent Dosage g t−1 | Mass Recovery | Carbon Grade | Carbon Recovery | ||
|---|---|---|---|---|---|---|
| L | D | P | % | wt.% | % | |
| Ro-Feed | 200 | 100 | 37.9 | 100 | ||
| Ro-Scav2-Froth | 1000 | 60 | 41.3 | 86.3 | 94.1 | |
| Scav2-Cell | 58.7 | 3.8 | 5.9 | |||
| Clnr1-Feed | 100 | 41.3 | 86.3 | 94.1 | ||
| Clnr1-Froth1 | 125 | 7.5 | 15 | 94.2 | 37.3 | |
| Clnr1-Froth2 | 100 | 7.5 | 25.5 | 94.0 | 63.3 | |
| Clnr1-Froth3 | 100 | 7.5 | 31.7 | 93.4 | 78.3 | |
| Clnr1-Froth4 | 100 | 7.5 | 36.7 | 91.9 | 89.0 | |
| Clnr1-Cell | 4.64 | 42.3 | 5.2 | |||
| Parameter | Standard MgO-C Brick | MgO-C Brick with 1 wt.% Flotation Graphite |
|---|---|---|
| Before coking at 1500 °C | ||
| Bulk density [g/cm3] | 3.16 | 3.17 |
| Apparent porosity [%vol] | 5.7 | 5.9 |
| CCS [MPa] | 69 | 59 |
| HMOR 1400 °C [MPa] | 7.8 | 6.9 |
| HMOR 1500 °C [MPa] | 5.1 | 5.0 |
| After coking at 1500 °C | ||
| Bulk density [g/cm3] | 3.12 | 3.13 |
| Apparent porosity [%vol] | 10.0% | 9.9 |
| CCS [MPa] | 38% | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rieger, J.; Stuhr, S.; Rutrecht, B.; Morgenbesser, S.; Nigl, T.; Arnberger, A.; Kunanz, H.; Lesiak, S. Graphite Separation from Lithium-Ion Battery Black Mass Using Froth Flotation and Quality Evaluation for Reuse as a Secondary Raw Material Including Non-Battery Applications. Recycling 2025, 10, 75. https://doi.org/10.3390/recycling10020075
Rieger J, Stuhr S, Rutrecht B, Morgenbesser S, Nigl T, Arnberger A, Kunanz H, Lesiak S. Graphite Separation from Lithium-Ion Battery Black Mass Using Froth Flotation and Quality Evaluation for Reuse as a Secondary Raw Material Including Non-Battery Applications. Recycling. 2025; 10(2):75. https://doi.org/10.3390/recycling10020075
Chicago/Turabian StyleRieger, Johannes, Stephan Stuhr, Bettina Rutrecht, Stefan Morgenbesser, Thomas Nigl, Astrid Arnberger, Hartwig Kunanz, and Stefanie Lesiak. 2025. "Graphite Separation from Lithium-Ion Battery Black Mass Using Froth Flotation and Quality Evaluation for Reuse as a Secondary Raw Material Including Non-Battery Applications" Recycling 10, no. 2: 75. https://doi.org/10.3390/recycling10020075
APA StyleRieger, J., Stuhr, S., Rutrecht, B., Morgenbesser, S., Nigl, T., Arnberger, A., Kunanz, H., & Lesiak, S. (2025). Graphite Separation from Lithium-Ion Battery Black Mass Using Froth Flotation and Quality Evaluation for Reuse as a Secondary Raw Material Including Non-Battery Applications. Recycling, 10(2), 75. https://doi.org/10.3390/recycling10020075







