Recent Progress in Flame-Retardant Polymer Electrolytes for Solid-State Lithium Metal Batteries
Abstract
:1. Introduction
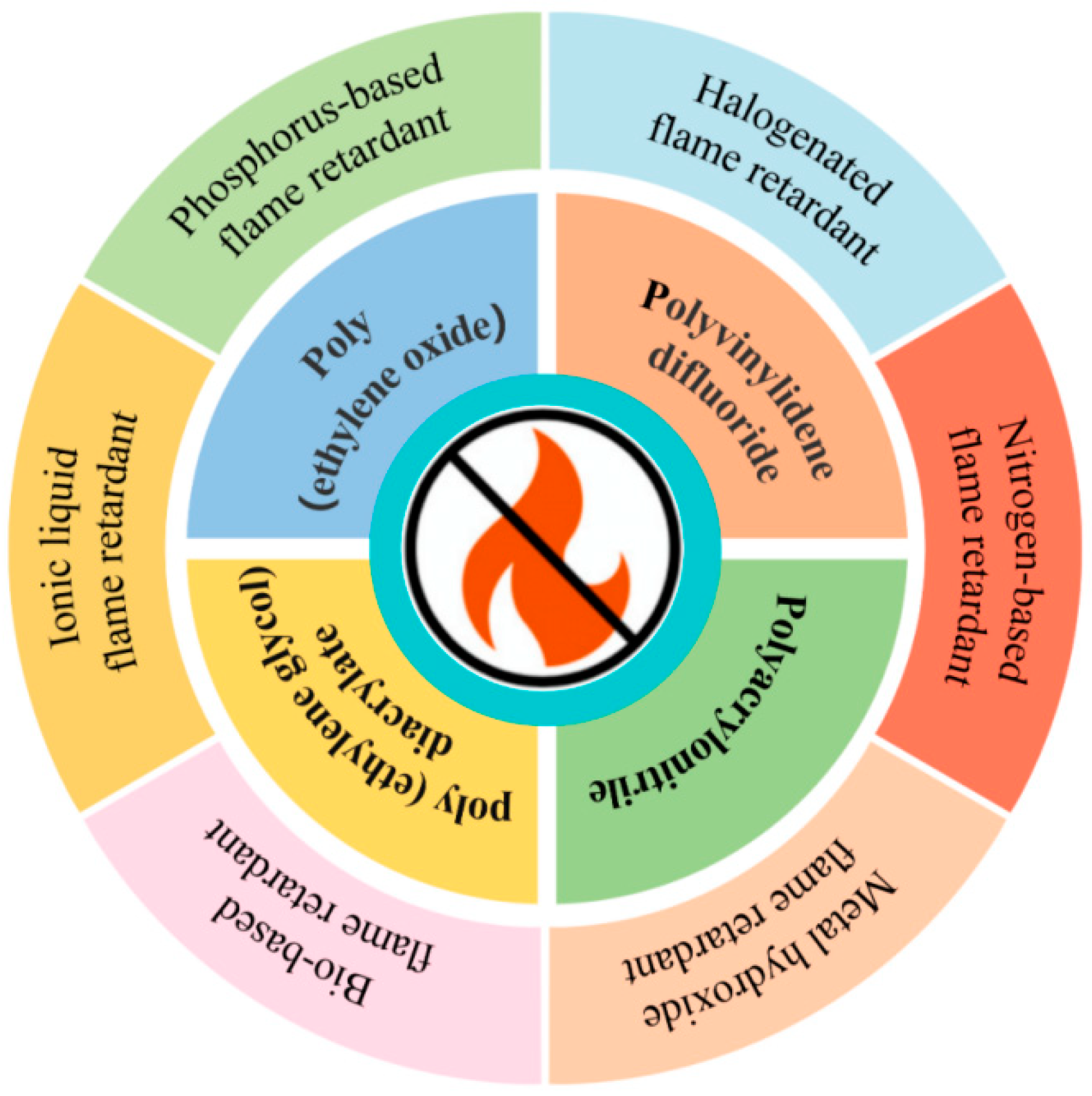
2. PEO-Based Flame-Retardant Polymer Electrolyte
2.1. Phosphonium Flame Retardant
2.2. Metal Hydroxide Flame Retardant
2.3. Nitrogenous Flame Retardants
2.4. Bio-Based Flame Retardant
2.5. Halogen Flame Retardants
3. PVDF-Based Flame-Retardant Solid Polymer Electrolyte
3.1. Halide Flame Retardant
3.2. Phosphonium Flame Retardant
4. PAN-/PUA-Based Flame-Retardant Solid Polymer Electrolyte
4.1. Nitrogenous Flame Retardants
4.2. Phosphonium Flame Retardant
5. PEGDA-Based Flame-Retardant Solid Polymer Electrolyte
5.1. Phosphonium Flame Retardant
5.2. Ionic Liquid Flame Retardant
6. Summary and Prospects
- At present, the main flame retardants are phosphorus flame retardants, metal hydroxide flame retardants, nitrogen flame retardants, halogen flame retardants, bio-based flame retardants, and ionic liquid flame retardants. However, halogen flame retardants will produce harmful halogen gas and corrosive harmful hydrogen halide gas when burned and when encountering water. Therefore, it is necessary to explore environmentally friendly phosphorus-based and nitrogen-based flame retardants. Designing flame retardants that combine P and N elements will construct more efficient and environmentally friendly polymer electrolytes.
- Although adding flame retardants can greatly improve the flame retardancy of solid polymer electrolytes, excessive addition affects ionic conductivity, interface stability, and mechanical properties. For instance, phosphorus introduced into the polymer skeleton will achieve compatibility and enhance the flame retardancy of the polymer. In the future, discovering polymers with good compatibility with flame retardants is in need of attention. The relationship between the content of flame retardants and the ionic conductivity of polymer electrolytes also needs consideration.
- Adding flame retardants to increase flame retardancy will inevitably increase battery costs. To meet the needs of commercial large-scale production, it is necessary to reduce the cost of flame-retardant electrolytes. It is possible to consider adding multiple flame retardants utilizing the synergistic effect between different flame retardants to achieve better flame retardancy. Developing simple and low-cost flame-retardant solid polymer electrolytes is also a development direction for Li-metal batteries.
- Polymer electrolytes have the advantages of high flexibility, low interfacial impedance, good film-forming properties, and low cost, but their low ionic conductivity seriously hinders their application in solid-state batteries. Adding an appropriate amount of lithium salt can improve ionic conductivity. So, exploring new types of lithium salt and constructing cross-linked network copolymer structures can improve ionic conductivity. The film-making process of polymer electrolytes needs innovation to reduce the thickness and improve ion conductivity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, B.; Li, Z. Towards world’s low carbon development: The role of clean energy. Energy 2022, 307, 118160. [Google Scholar] [CrossRef]
- Wang, T. The Current Situation and Prospect of Lithium Batteries for New Energy Vehicles. J. Phys. Conf. Ser. 2021, 2014, 012015. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Z.; Yuan, Y.; Zheng, X.; Park, S.; Wei, S.; Li, L.; Ma, Y.; Liu, S.; Chen, J.; et al. Ultrafast Electrical Pulse Synthesis of Highly Active Electrocatalysts for Beyond-Industrial-Level Hydrogen Gas Batteries. Adv. Mater. 2023, 35, 2300502. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Z.; Sun, J.; Luo, R.; Xu, K.; Si, M.; Kang, J.; Yuan, Y.; Liu, S.; Ahmad, T.; et al. Constructing robust heterostructured interface for anode-free zinc batteries with ultrahigh capacities. Nat. Commun. 2023, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, C.-Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.-Q.; Yu, D.; Liu, Y.; Titirici, M.-M.; Chueh, Y.-L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Wang, X.; Zhou, A.a.; Yang, Z. Research progress and application prospect of solid-state electrolytes in commercial lithium-ion power batteries. Energy Storage Mater. 2021, 35, 70–87. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Eshetu, G.G.; Mathiron, D.; Ribière, P.; Armand, M.; Laruelle, S. Thermal behaviour of the lithiated-graphite/electrolyte interface through GC/MS analysis. Electrochim. Acta 2012, 83, 402–409. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Kerzhner-Haller, I.; Anani, A.; Howard, J.N. Thermal Stability Studies of Binder Materials in Anodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 4470. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Arreaga-Salas, D.E.; Sra, A.K.; Roodenko, K.; Chabal, Y.J.; Hinkle, C.L. Progression of Solid Electrolyte Interphase Formation on Hydrogenated Amorphous Silicon Anodes for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 9072–9077. [Google Scholar] [CrossRef]
- Maurya, D.K.; Dhanusuraman, R.; Guo, Z.; Angaiah, S. Composite polymer electrolytes: Progress, challenges, and future outlook for sodium-ion batteries. Adv. Compos. Hybrid Mater. 2022, 5, 2651–2674. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.A.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Chao, L. Three–dimensional fiber network reinforced polymer electrolyte for dendrite–free all–solid–state lithium metal batteries. Energy Storage Mater. 2021, 41, 631–641. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y. Porous membrane host-derived in-situ polymer electrolytes with double-stabilized electrode interface enable long cycling lithium metal batteries. Chem. Eng. J. 2022, 433, 134471. [Google Scholar] [CrossRef]
- Bai, L.; Ghiassinejad, S.; Brassinne, J.; Fu, Y.; Wang, J.; Yang, H.; Vlad, A.; Minoia, A.; Lazzaroni, R.; Gohy, J.-F. High Salt-Content Plasticized Flame-Retardant Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 44844–44859. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Wang, Y.-Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, G.; Deng, J. Comparable Investigation of Phosphorus-Based Flame Retardant Electrolytes on LiFePO4 Cathodes. J. Electrochem. Soc. 2022, 169, 050532. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Huang, X.; Shi, Z.; Wang, Z.; Liu, Z.; Hu, R.; Liu, J.; Zhu, M. A flexible composite solid electrolyte with a highly stable interphase for dendrite-free and durable all-solid-state lithium metal batteries. J. Mater. Chem. A 2020, 8, 18043–18054. [Google Scholar] [CrossRef]
- Bevington, C.; Williams, A.J.; Guider, C.; Baker, N.C.; Meyer, B.; Babich, M.A.; Robinson, S.; Jones, A.; Phillips, K.A. Development of a Flame Retardant and an Organohalogen Flame Retardant Chemical Inventory. Sci. Data 2022, 9, 295. [Google Scholar] [CrossRef]
- Lyu, P.; Hou, Y.; Hu, J.; Liu, Y.; Zhao, L.; Feng, C.; Ma, Y.; Wang, Q.; Zhang, R.; Huang, W.; et al. Composites Filled with Metal Organic Frameworks and Their Derivatives: Recent Developments in Flame Retardants. Polymers 2022, 14, 5279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, P.; Li, S.; Wang, X.; Li, F.; Ma, J.; Chai, J.; Zhang, J.; Xu, G.; Huang, Z.; et al. A Flame Retardant Ionic Conductor Additive for Safety-Reinforced Liquid Electrolyte of Lithium Batteries. J. Electrochem. Soc. 2017, 164, A1559. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Yan, S.-S.; Li, J.; Dong, H.; Zhou, P.; Wan, L.; Chen, X.-X.; Zhang, W.-L.; Xia, Y.-C.; Wang, P.-C.; et al. Lithium Bromide-Induced Organic-Rich Cathode/Electrolyte Interphase for High-Voltage and Flame-Retardant All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 24469–24479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, L.; Wu, X.; Wang, J.; Li, Q.; Pan, K.; He, J. Research Progress and Application of PEO-Based Solid State Polymer Composite Electrolytes. Front. Energy Res. 2021, 9, 726738. [Google Scholar] [CrossRef]
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.A.; et al. 3D Asymmetric Bilayer Garnet-Hybridized High-Energy-Density Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 751–760. [Google Scholar] [CrossRef]
- Han, L.; Liao, C.; Mu, X.; Wu, N.; Xu, Z.; Wang, J.; Song, L.; Kan, Y.; Hu, Y. Flame-Retardant ADP/PEO Solid Polymer Electrolyte for Dendrite-Free and Long-Life Lithium Battery by Generating Al, P-rich SEI Layer. Nano Lett. 2021, 21, 4447–4453. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Gao, S.; Cai, S.; Wang, Q.; Liu, J.; Liu, Z. A novel polyphosphonate flame-retardant additive towards safety-reinforced all-solid-state polymer electrolyte. Mater. Chem. Phys. 2020, 239, 122014. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Chen, S.; Xu, Z.; Wang, J.; Nuli, Y.; Guo, Y.; Liang, C. Inherently flame-retardant solid polymer electrolyte for safety-enhanced lithium metal battery. Chem. Eng. J. 2021, 410, 128415. [Google Scholar] [CrossRef]
- Sain, M.; Park, S.H.; Suhara, F.; Law, S. Flame retardant and mechanical properties of natural fibre–PP composites containing magnesium hydroxide. Polym. Degrad. Stab. 2004, 83, 363–367. [Google Scholar] [CrossRef]
- Haurie, L.; Fernández, A.I.; Velasco, J.I.; Chimenos, J.M.; Lopez Cuesta, J.-M.; Espiell, F. Thermal stability and flame retardancy of LDPE/EVA blends filled with synthetic hydromagnesite/aluminium hydroxide/montmorillonite and magnesium hydroxide/aluminium hydroxide/montmorillonite mixtures. Polym. Degrad. Stab. 2007, 92, 1082–1087. [Google Scholar] [CrossRef]
- Sheng, O.; Jin, C.; Luo, J.; Yuan, H.; Huang, H.; Gan, Y.; Zhang, J.; Xia, Y.; Liang, C.; Zhang, W.; et al. Mg2B2O5 Nanowire Enabled Multifunctional Solid-State Electrolytes with High Ionic Conductivity, Excellent Mechanical Properties, and Flame-Retardant Performance. Nano Lett. 2018, 18, 3104–3112. [Google Scholar] [CrossRef]
- Pongsuk, P.; Pumchusak, J. Effect of Ultrasonication on the Morphology, Mechanical Property, Ionic Conductivity, and Flame Retardancy of PEO-LiCF3SO3-Halloysite Nanotube Composites for Use as Solid Polymer Electrolyte. Polymers 2022, 14, 3710. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yu, D.; Zhou, J.; Li, S.; Gao, X.; Han, Y.; Qi, P.; Feng, X.; Wang, B. Inorganic and organic hybrid solid electrolytes for lithium-ion batteries. CrystEngComm 2016, 18, 4236–4258. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Liu, J.; Miller, J.D. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries. Nano Energy 2017, 31, 478–485. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Liu, T.; Wang, Y.; Yu, J.; Hu, Z. Molecular composite electrolytes of polybenzimidazole/polyethylene oxide with enhanced safety and comprehensive performance for all-solid-state lithium ion batteries. Polymer 2022, 239, 124450. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Taib, M.N.A.M.; Antov, P.; Savov, V.; Fatriasari, W.; Madyaratri, E.W.; Wirawan, R.; Osvaldová, L.M.; Hua, L.S.; Ghani, M.A.A.; Al Edrus, S.S.A.O.; et al. Current progress of biopolymer-based flame retardant. Polym. Degrad. Stab. 2022, 205, 110153. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, Z.; Wang, D.; Yuan, H.; Zhang, H.; Tan, Y. A flame retarded polymer-based composite solid electrolyte improved by natural polysaccharides. Compos. Commun. 2021, 26, 100774. [Google Scholar] [CrossRef]
- Sakai, S.; Takagi, Y.; Yamada, Y.; Yamaguchi, T.; Kawakami, K. Reinforcement of porous alginate scaffolds by incorporating electrospun fibres. Biomed. Mater. 2008, 3, 034102. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, S.-Y.; Zhang, M.; Zeng, H.-Y.; Wu, K.; Tian, X.-Y.; Chen, C.-R.; Pan, Y. Fabrication of green alginate-based and layered double hydroxides flame retardant for enhancing the fire retardancy properties of polypropylene. Carbohydr. Polym. 2020, 234, 115891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, T.; Wang, S.; Wang, C.; Li, D.; Xia, Y. Alginate Fiber-Grafted Polyetheramine-Driven High Ion-Conductive and Flame-Retardant Separator and Solid Polymer Electrolyte for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 56780–56789. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.; Dahoe, A.E. On the performance and mechanism of brominated and halogen free flame retardants in formulations of glass fibre reinforced poly(butylene terephthalate). Polym. Degrad. Stab. 2014, 104, 71–86. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Cui, Y.; Wan, J.; Ye, Y.; Liu, K.; Chou, L.-Y.; Cui, Y. A Fireproof, Lightweight, Polymer–Polymer Solid-State Electrolyte for Safe Lithium Batteries. Nano Lett. 2020, 20, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.-D.; Li, R.-X.; Feng, S.-H.; Liu, G.-Y.; Zhao, J.-Q. Flame retardancy and its mechanism of polymers flame retarded by DBDPE/Sb2O3. J. Cent. South Univ. Technol. 2008, 15, 64–68. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.-Y.; Liu, X.-M.; Tang, J.-H.; Li, Z.-M. Flame-retardant and mechanical properties of high-density rigid polyurethane foams filled with decabrominated dipheny ethane and expandable graphite. J. Appl. Polym. Sci. 2009, 111, 2372–2380. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Sasikumar, M.; Raja, M.; Krishna, R.H.; Jagadeesan, A.; Sivakumar, P.; Rajendran, S. Influence of Hydrothermally Synthesized Cubic-Structured BaTiO3 Ceramic Fillers on Ionic Conductivity, Mechanical Integrity, and Thermal Behavior of P(VDF–HFP)/PVAc-Based Composite Solid Polymer Electrolytes for Lithium-Ion Batteries. J. Phys. Chem. C 2018, 122, 25741–25752. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Y.; Yao, P.; Ding, Z.; Tang, Q.; Wu, J.; Ye, Z.; Huang, K.; Liu, X. Hybridizing poly(vinylidene fluoride-co-hexafluoropropylene) with Li6.5La3Zr1.5Ta0.5O12 as a lithium-ion electrolyte for solid state lithium metal batteries. Chem. Eng. J. 2019, 367, 230–238. [Google Scholar] [CrossRef]
- Sun, Y.; Zhan, X.; Hu, J.; Wang, Y.; Gao, S.; Shen, Y.; Cheng, Y.-T. Improving Ionic Conductivity with Bimodal-Sized Li7La3Zr2O12 Fillers for Composite Polymer Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 12467–12475. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Xu, Z.; Zhou, S.; Zhao, Z.; Zeng, K.; Xiao, M.; Meng, Y.; Xu, Y. Nonflammable highly-fluorinated polymer electrolytes with enhanced interfacial compatibility for dendrite-free lithium metal batteries. J. Power Sources 2021, 510, 230411. [Google Scholar] [CrossRef]
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Sun, W.; Zheng, C.; Xie, K. Flame retardant and stable Li1.5Al0.5Ge1.5(PO4)3-supported ionic liquid gel polymer electrolytes for high safety rechargeable solid-state lithium metal batteries. J. Phys. Chem. C 2018, 122, 10334–10342. [Google Scholar] [CrossRef]
- Mi, J.; Ma, J.; Chen, L.; Lai, C.; Yang, K.; Biao, J.; Xia, H.; Song, X.; Lv, W.; Zhong, G.; et al. Topology crafting of polyvinylidene difluoride electrolyte creates ultra-long cycling high-voltage lithium metal solid-state batteries. Energy Storage Mater. 2022, 48, 375–383. [Google Scholar] [CrossRef]
- Han, L.; Liao, C.; Liu, Y.; Yu, H.; Zhang, S.; Zhu, Y.; Li, Z.; Li, X.; Kan, Y.; Hu, Y. Non-flammable sandwich-structured TPU gel polymer electrolyte without flame retardant addition for high performance lithium ion batteries. Energy Storage Mater. 2022, 52, 562–572. [Google Scholar] [CrossRef]
- Lin, W.; Liu, J.; Xue, L.; Li, Y.; Yu, H.; Xiong, Y.; Chen, D.; Ciucci, F.; Yu, J. Nonflammable, robust and flexible electrolytes enabled by phosphate coupled polymer–polymer for Li-metal batteries. J. Colloid Interface Sci. 2022, 621, 222–231. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Gotoh, D.; Egashira, M.; Morita, M. Alkylphosphate-based nonflammable gel electrolyte for LiMn2O4 positive electrode in lithium-ion battery. J. Power Sources 2008, 185, 1425–1428. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Niida, Y.; Egashira, M.; Morita, M. Nonflammable gel electrolyte containing alkyl phosphate for rechargeable lithium batteries. J. Power Sources 2006, 163, 238–242. [Google Scholar] [CrossRef]
- Morita, M.; Niida, Y.; Yoshimoto, N.; Adachi, K. Polymeric gel electrolyte containing alkyl phosphate for lithium-ion batteries. J. Power Sources 2005, 146, 427–430. [Google Scholar] [CrossRef]
- Wang, X.; Yasukawa, E.; Kasuya, S. Nonflammable Trimethyl Phosphate Solvent-Containing Electrolytes for Lithium-Ion Batteries: I. Fundamental Properties. J. Electrochem. Soc. 2001, 148, A1058. [Google Scholar] [CrossRef]
- Xu, K.; Ding, M.S.; Zhang, S.; Allen, J.L.; Jow, T.R. An Attempt to Formulate Nonflammable Lithium Ion Electrolytes with Alkyl Phosphates and Phosphazenes. J. Electrochem. Soc. 2002, 149, A622. [Google Scholar] [CrossRef]
- Lv, Q.; Song, Y.; Wang, B.; Wang, S.; Wu, B.; Jing, Y.; Ren, H.; Yang, S.; Wang, L.; Xiao, L.; et al. Bifunctional flame retardant solid-state electrolyte toward safe Li metal batteries. J. Energy Chem. 2023, 81, 613–622. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, Z.; Chen, Z.; Li, X.; Chen, A.; Li, P.; Wang, Y.; Zhi, C. Bifunctional separators design for safe lithium-ion batteries: Suppressed lithium dendrites and fire retardance. Nano Energy 2022, 97, 107204. [Google Scholar] [CrossRef]
- Shen, Z.; Zhong, J.; Jiang, S.; Xie, W.; Zhan, S.; Lin, K.; Zeng, L.; Hu, H.; Lin, G.; Lin, Y.; et al. Polyacrylonitrile Porous Membrane-Based Gel Polymer Electrolyte by In Situ Free-Radical Polymerization for Stable Li Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 41022–41036. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Croce, F.; Brown, S.D.; Greenbaum, S.G.; Slane, S.M.; Salomon, M. Lithium-7 NMR and ionic conductivity studies of gel electrolytes based on polyacrylonitrile. Chem. Mater. 1993, 5, 1268–1272. [Google Scholar] [CrossRef]
- Zhou, D.; He, Y.-B.; Liu, R.; Liu, M.; Du, H.; Li, B.; Cai, Q.; Yang, Q.-H.; Kang, F. In Situ Synthesis of a Hierarchical All-Solid-State Electrolyte Based on Nitrile Materials for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1500353. [Google Scholar] [CrossRef]
- Zhou, D.; Fan, L.-Z.; Fan, H.; Shi, Q. Electrochemical performance of trimethylolpropane trimethylacrylate-based gel polymer electrolyte prepared by in situ thermal polymerization. Electrochim. Acta 2013, 89, 334–338. [Google Scholar] [CrossRef]
- Lv, P.; Xie, S.; Sun, Q.; Chen, X.; He, Y. Flame-Retardant Solid Polymer Electrolyte Based on Phosphorus-Containing Polyurethane Acrylate/Succinonitrile for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 7199–7209. [Google Scholar] [CrossRef]
- Nearingburg, B.; Elias, A.L. Photopolymerizable sulfonated poly(ethylene glycol) proton exchange membranes for microfluidic and fuel cell applications. J. Membr. Sci. 2012, 389, 148–154. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release. Biomater. Sci. 2016, 4, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Li, M.; Li, G.; Li, B.; Zhang, S.; Ming, H.; Qiu, J.; Chen, J.; Zhao, P. A new composite gel polymer electrolyte based on matrix of PEGDA with high ionic conductivity for lithium-ion batteries. Electrochim. Acta 2020, 354, 136622. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, G.; Chen, D.; Chen, J.; Qi, P.; Sun, J.; Gu, X.; Zhang, S. Constructing flame-retardant gel polymer electrolytes via multiscale free radical annihilating agents for Ni-rich lithium batteries. Energy Storage Mater. 2022, 50, 495–504. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, J.; Li, H.; Gu, X.; Fei, B.; Zhang, S. A green way to simultaneously enhance the mechanical, flame retardant and anti-ultraviolet aging properties of polylactide composites by the incorporation of tannic acid derivatives. Polym. Degrad. Stab. 2022, 196, 109831. [Google Scholar] [CrossRef]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Tannic acid, a promising anti-photoaging agent: Evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP-1 expression in L929 fibroblasts exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Yang, J.; Sun, W.; Ban, Q.; Gai, L.; Gong, Y.; Xu, Z.; Liu, L. Flame-Retardant, Highly Conductive, and Low-Temperature-Resistant Organic Gel Electrolyte for High-Performance All-Solid Supercapacitors. ChemSusChem 2021, 14, 2056–2066. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.M.; Zhao, J.Q.; Huang, J.Y. Synthesis and properties of a modified unsaturated polyester resin with phosphorus-containing pendant groups. Polym. Bull. 2013, 70, 1097–1111. [Google Scholar] [CrossRef]
- Sen, S.; Goodwin, S.E.; Barbará, P.V.; Rance, G.A.; Wales, D.; Cameron, J.M.; Sans, V.; Mamlouk, M.; Scott, K.; Walsh, D.A. Gel–Polymer Electrolytes Based on Poly(Ionic Liquid)/Ionic Liquid Networks. ACS Appl. Polym. Mater. 2021, 3, 200–208. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, X.; Dong, B.; Sun, N.; Zheng, L. Poly(ionic liquid) hydrogels exhibiting superior mechanical and electrochemical properties as flexible electrolytes. J. Mater. Chem. A 2016, 4, 1112–1118. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. AlChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Liang, L.; Yuan, W.; Chen, X.; Liao, H. Flexible, nonflammable, highly conductive and high-safety double cross-linked poly(ionic liquid) as quasi-solid electrolyte for high performance lithium-ion batteries. Chem. Eng. J. 2021, 421, 130000. [Google Scholar] [CrossRef]
- Chen, X.; Liang, L.; Hu, W.; Liao, H.; Zhang, Y. POSS hybrid poly(ionic liquid) ionogel solid electrolyte for flexible lithium batteries. J. Power Sources 2022, 542, 231766. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhao, F.; Zhou, X.; Ding, Y.; Yu, G. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater. 2018, 15, 46–52. [Google Scholar] [CrossRef]
- He, R.; Kyu, T. Effect of Plasticization on Ionic Conductivity Enhancement in Relation to Glass Transition Temperature of Crosslinked Polymer Electrolyte Membranes. Macromolecules 2016, 49, 5637–5648. [Google Scholar] [CrossRef]
- Chen, H.; He, P.; Li, M.; Wen, Y.; Wang, Y.; Qiu, J.; Cao, G.; Zhao, P.; Zhang, S.; Ming, H. Enabling Stable Cycling of 4.6 V High-Voltage LiCoO2 with an In Situ-Modified PEGDA-Based Quasi-Solid Electrolyte. ACS Appl. Energy Mater. 2022, 5, 5170–5181. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, H.; Gim, J.; Ngo, A.T.; Ma, Z.-F.; Chen, Z. Identifying Active Sites for Parasitic Reactions at the Cathode–Electrolyte Interface. J. Phys. Chem. Lett. 2019, 10, 589–594. [Google Scholar] [CrossRef]

















| Electrolyte Type | Flame Retardant Type | Flame Retardant | Ionic Conductivity | Coulombic Efficiency | Li+ Transfer Number |
|---|---|---|---|---|---|
| PEO | Phosphonium flame retardant | ADP [29] | 3.7 × 10−5 S cm−1 (30 °C) | 99.95% after 1000 cycles at 1 C | - |
| PBMP [30] | 1.25 × 10−5 S cm−1 | ~100% after 100 cycles at 0.02 C | - | ||
| BEPA/OPTTA [31] | 0.28 × 10−3 S cm−1 (25 °C) | - | - | ||
| Metal hydroxide flame retardant | Mg2B2O5 [34] | 1.53 × 10−4 S cm−1 (40 °C) | ~ 100% after 100 cycles at 1.0 C and 50 °C | 0.44 | |
| HNT [35] | 1.1×10−4 S cm−1 | - | - | ||
| Nitrogenous flame retardants | PBI [38] | 1.8 × 10−4 S cm−1 (30 °C) | ~ 100% after 100 cycles at 0.2 C and 60 °C | - | |
| Bio-based flame retardant | AF-PEA [44] | 1.8 × 10−3S cm−1 (25 °C) | > 98.5% after 1500 cycles at 2 C and 80 °C | 0.58 | |
| Halogen flame retardant | DBDPE [47] | 6.7 × 10−6S cm−1 (30 °C) | ~100% after 300 cycles at 0.5 C and 60 °C | - | |
| PVDF | Halide flame retardant | ED [55] | 4.41 × 10−3 S cm−1 (30 °C) | ~100% after 1000 cycles at 1 C and 30 °C | 0.51 |
| Phosphonium flame retardant | LAGP [56] | 0.76 × 10−3 S cm−1 (30 °C) | ~ 100% after 50 cycles at 0.05 C | 0.54 | |
| LLPO [57] | 4.84 × 10−4 S cm−1 (25 °C) | ~100% after 1000 cycles at 1 C | 0.47 | ||
| TMP [59] | 1.86 × 10−4 S cm−1 (30 °C) | >98.8% after 300 cycles at 1 C | 0.42 | ||
| HAP [65] | 7.4 × 10−4 S cm−1 | ~99.3% after 600 cycles at 0.5 C | 0.41 | ||
| PAN/PUA | Nitrogenous flame retardant | SN [70] | 2.32 × 10 −3 S cm−1 (25 °C) | 99.9% at 0.1 C | 0.57 |
| Phosphonium flame retardant | DOPO [72] | 2.66 × 10−4 S cm−1 (RT) | ~100% after 100 cycles at 0.2 C | 0.55 | |
| PEGDA | Phosphonium flame retardant | HCCP [76] | 9.86 × 10−4 S cm−1 (30 °C) | ~100% after 400 cycles at 1 C | 0.54 |
| DOPO [80] | 4 × 10−3S cm−1 (20 °C) | - | - | ||
| Ionic liquid flame retardant | VEIM-TFSI [85] | 1.03 × 10−3 S cm−1 (RT) | 97.8% after 200 cycles at 0.1 C | 0.47 | |
| BMIm [TFSI [86] | 2.5 × 10−3 S cm−1 (RT) | ~98% after 200 cycles at 0.2 C | 0.51 | ||
| IL-CN [89] | 4.97 × 10−3 S cm−1 (RT) | ~99% after 115 cycles at 0.5 C | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Xu, X.; Luo, X.; Ji, S.; Zhao, J.; Liu, J.; Huo, Y. Recent Progress in Flame-Retardant Polymer Electrolytes for Solid-State Lithium Metal Batteries. Batteries 2023, 9, 439. https://doi.org/10.3390/batteries9090439
Liao Y, Xu X, Luo X, Ji S, Zhao J, Liu J, Huo Y. Recent Progress in Flame-Retardant Polymer Electrolytes for Solid-State Lithium Metal Batteries. Batteries. 2023; 9(9):439. https://doi.org/10.3390/batteries9090439
Chicago/Turabian StyleLiao, Yubin, Xijun Xu, Xiongwei Luo, Shaomin Ji, Jingwei Zhao, Jun Liu, and Yanping Huo. 2023. "Recent Progress in Flame-Retardant Polymer Electrolytes for Solid-State Lithium Metal Batteries" Batteries 9, no. 9: 439. https://doi.org/10.3390/batteries9090439
APA StyleLiao, Y., Xu, X., Luo, X., Ji, S., Zhao, J., Liu, J., & Huo, Y. (2023). Recent Progress in Flame-Retardant Polymer Electrolytes for Solid-State Lithium Metal Batteries. Batteries, 9(9), 439. https://doi.org/10.3390/batteries9090439