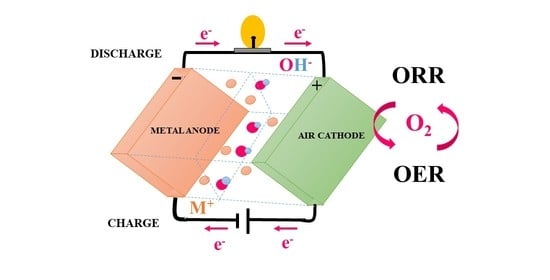
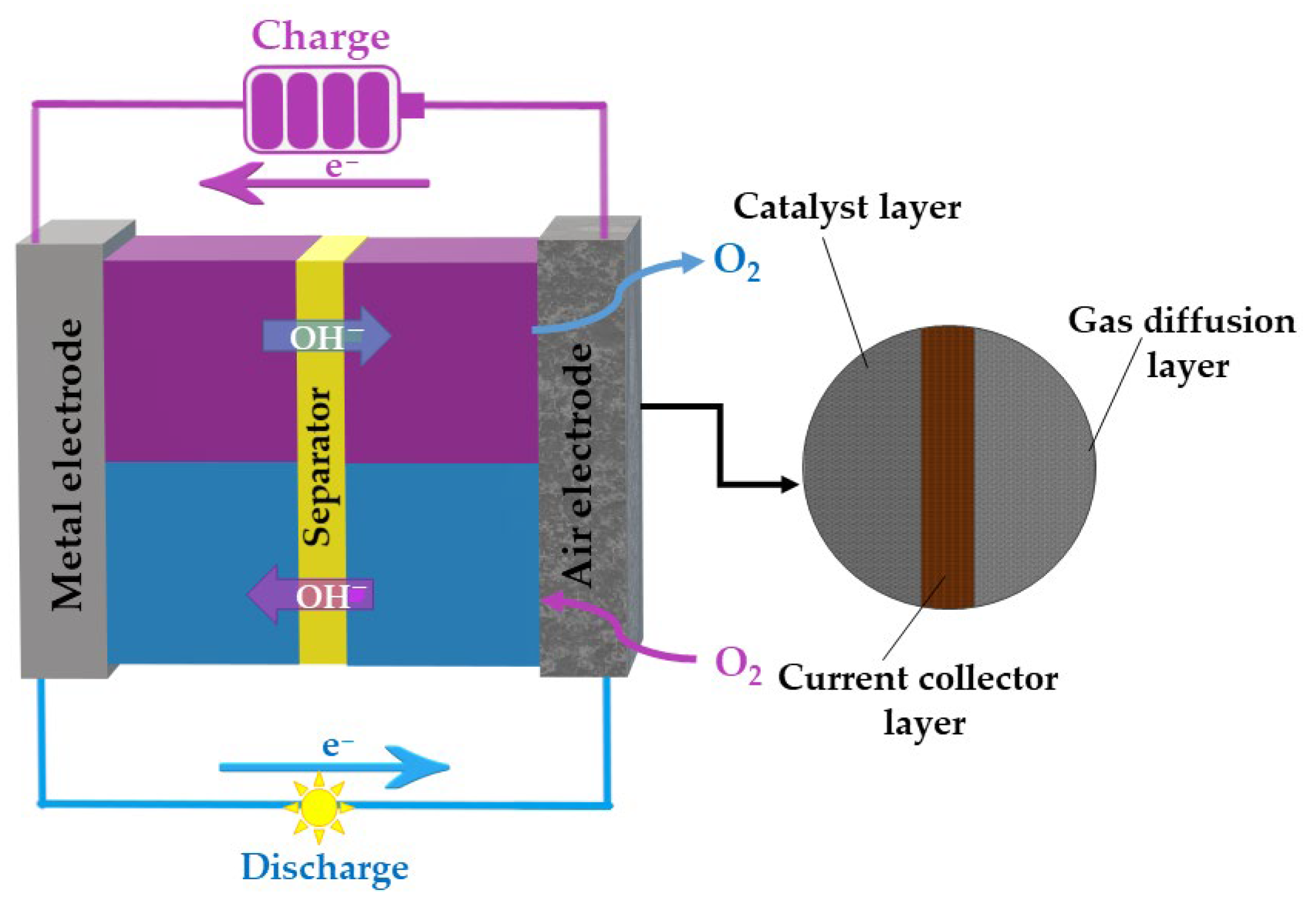
Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries
Abstract
1. Introduction
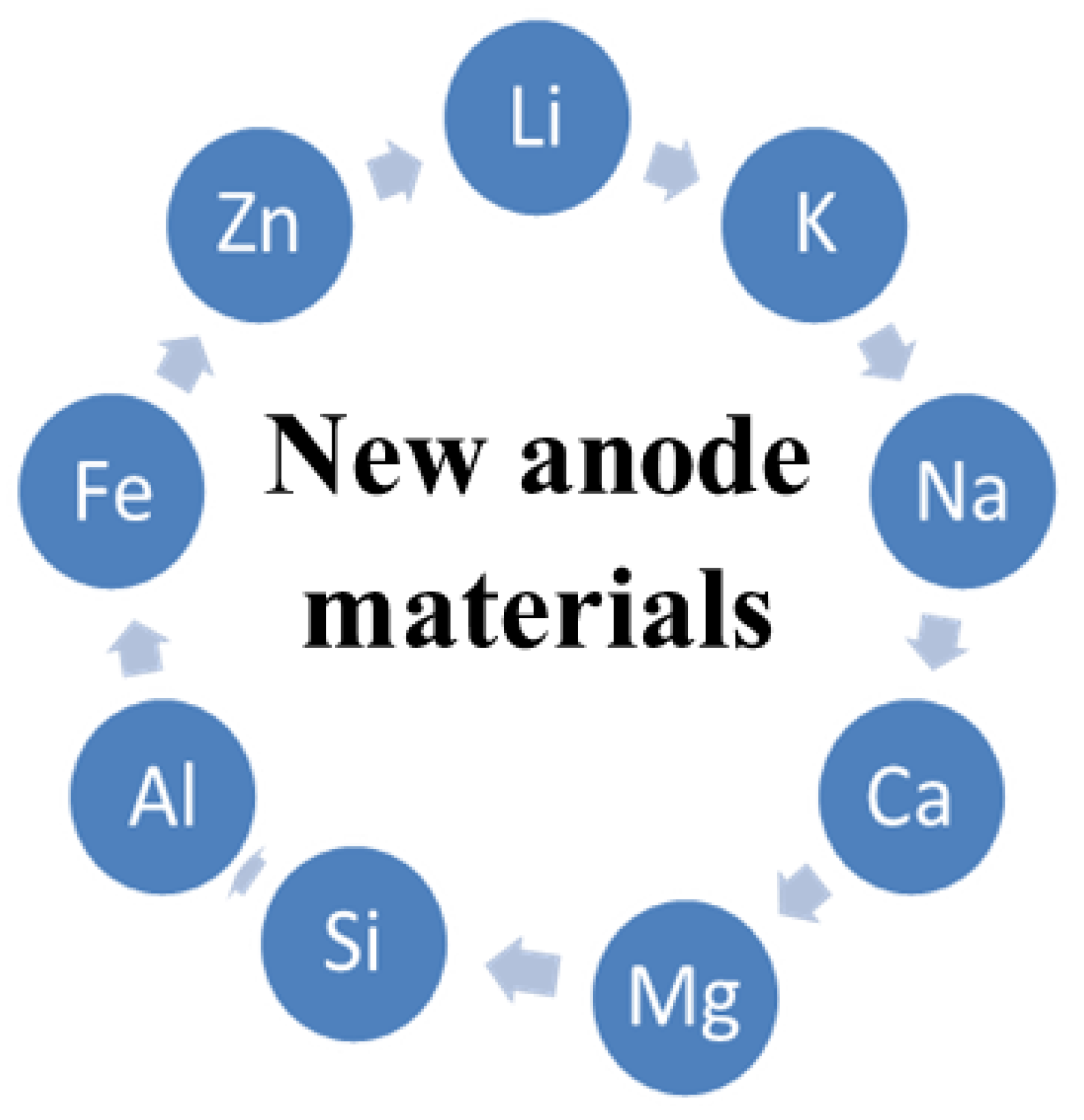
2. Anode Materials for MABs
| Anode Material | EOCV (V) vs. RHE | Durability | Pmax (mW cm−2) | Specific Energy (Wh Kg−1) | Ref. |
|---|---|---|---|---|---|
| Li | 2.9 | Up to 700 cycles | 2 | 3458 | [17] |
| Zn | 1.7 | 500 h at −10 and 25 °C, 150 h at 40 °C under charging at 100 mA cm−2 | 10 to 435 | 1218 | [25] |
| Al | 2.7 | Long | 315 | 8100 | [26] |
| Na | 2.4 | Holds 90% of its cell capacity after 300 cycles | 39 | 1600 | [26] |
| Mg | 2.9 | - | 112.4 | 6800 | [27] |
| Fe | 1.3 | More than 10,000 charge/discharge cycles | - | 913 | [28] |
| Si | 2.2 | Short | 0.3 | 8470 | [29] |
| Ca | 3.1 | Long | - | 250 | [21,22] |
| K | - | Long | - | 935 | [30] |
3. Mechanism of OER and ORR in Alkaline Media
4. ORR Catalysts
4.1. The ORR Catalysts for Zn–Air Batteries
4.2. ORR Catalysts for Aluminium–air Batteries
5. Bifunctional ORR/OER Catalysts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, L.; Noor, T.; Iqbal, N. An overview of metal-air batteries, current progress, and future perspectives. J. Energy Storage 2022, 56, 106075. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal-Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Li, L.; Chang, Z.-W.; Zhang, X.B. Recent Progress on the Development of Metal-Air Batteries. Adv. Sustain. Syst. 2017, 1, 1700036r. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Zou, P.; Sun, B.; Tao, S. Historical development and novel concepts on electrolytes for aqueous rechargeable batteries. Energy Environ. Sci. 2022, 15, 1805–1839. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent progress of metal-air batteries—A mini review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Cohn, G.; Starosvetsky, D.; Hagiwara, R.; Macdonald, D.D.D.; Ein-Eli, Y. Silicon-air batteries. Electrochem. Commun. 2009, 11, 1916–1918. [Google Scholar] [CrossRef]
- Das, S.K.; Mahapatra, S.; Lahan, H. Aluminium-ion batteries: Developments and challenges. J. Mater. Chem. A 2017, 5, 6347–6367. [Google Scholar] [CrossRef]
- Dewi, E.L.; Oyaizu, K.; Nishide, H.; Tsuchida, E. Cationic polysulfonium membrane as separator in Zn-air cell. J. Power Sources 2003, 115, 149–152. [Google Scholar] [CrossRef]
- Saputra, H.; Othman, R.; Sutjipto, A.G.E.; Muhida, R. MCM-41 as a new separator material for electrochemical cell: Application in Zn-air system. J. Memb. Sci. 2011, 367, 152–157. [Google Scholar] [CrossRef]
- Gu, Z.; Xin, X.; Xu, Z.; He, J.; Wu, J.; Sun, Y.; Yao, X. Garnet Electrolyte-Based Integrated Architecture for High-Performance All-Solid-State Lithium-Oxygen Batteries. Adv. Funct. Mater. 2023, 2301583. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, N.; Zhao, X.; Wang, C.; Zhang, T.; Xu, S.; Guo, X. Combination of Li-rich layered-oxide with O2 cathodes for high-energy Li-ion/Li-O2 hybrid batteries. Appl. Phys. Lett. 2022, 120, 193901. [Google Scholar] [CrossRef]
- Yi, X.; Liu, X.; Qin, B.; Zhao, X.; Leong, K.W.; Pan, W.; Jiang, K.; Ma, S.; Hao, Z.; Leung, D.Y.C.; et al. High-energy composite cathode for solid-state lithium-oxygen battery boosted by ultrafine carbon nanotube catalysts and amorphous lithium peroxide. Mater. Today Chem. 2023, 29, 101430. [Google Scholar] [CrossRef]
- Tufail, M.K.; Zhai, P.; Jia, M.; Zhao, N.; Guo, X. Design of Solid Electrolytes with Fast Ion Transport: Computation-Driven and Practical Approaches. Energy Mater. Adv. 2023, 4, 1–35. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Segre, C.U.; Pour, G.S.; Vazquez, M.; Benard, L. Aqueous air cathodes and catalysts for metal-air batteries. Curr. Opin. Electrochem. 2023, 38, 101246. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. High Energy Density Metal-Air Batteries: A Review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Salado, M.; Lizundia, E. Advances, challenges, and environmental impacts in metal–air battery electrolytes. Mater. Today Energy 2022, 28, 101064. [Google Scholar] [CrossRef]
- Sun, J.; Wang, N.; Qiu, Z.; Xing, L.; Du, L. Recent Progress of Non-Noble Metal Catalysts for Oxygen Electrode in Zn-Air Batteries: A Mini Review. Catalysts 2022, 12, 843. [Google Scholar] [CrossRef]
- Hosseini, S.; Chiu, C.Y.; Pourzolfaghar, H.; Su, C.A.; Li, Y.Y. Techno-economically feasible beverage can as superior anode in rechargeable Al-air batteries. Sustain. Mater. Technol. 2023, 35, e00560. [Google Scholar] [CrossRef]
- Chawla, N.; Safa, M. Sodium batteries: A review on sodium-sulfur and sodium-air batteries. Electronics 2019, 8, 1201. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Z.; Chen, J. Magnesium-air batteries: From principle to application. Mater. Horiz. 2014, 1, 196–206. [Google Scholar] [CrossRef]
- Weinrich, H.; Durmus, Y.E.; Tempel, H.; Kungl, H.; Eichel, R.-A. Silicon and Iron as Resource-Efficient Anode Materials for Ambient-Temperature Metal-Air Batteries: A Review. Materials 2019, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, S.; Luo, Z.; Li, S.; Zhao, X.; Fang, Q.; Wei, X.; Wang, H.; Wang, C.; Zhu, Z.; et al. Synergies of Ru/Co Nanoparticles and Co Single Atoms Active Sites toward Efficient Electrocatalysis of Oxygen Reduction Reaction for Zn-Air Battery. Adv. Funct. Mater. 2023, 463, 142184. [Google Scholar] [CrossRef]
- Niu, W.J.; Yan, Y.Y.; Li, R.J.; Zhao, W.W.; Chen, J.L.; Liu, M.J.; Gu, B.; Liu, W.W.; Chueh, Y.L. Identifying the impact of Fe nanoparticles encapsulated by nitrogen-doped carbon to Fe single atom sites for boosting oxygen reduction reaction toward Zn-air batteries. Chem. Eng. J. 2023, 456, 140858. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Tian, M.; Liu, X.; Zhang, F.; Zhang, J.; Wang, K.; Gao, S. Engineering the electronic structure of Fe-N/C catalyst via fluorine self-doping for enhanced oxygen reduction reaction in liquid and all-solid-state Zn-air batteries. Electrochim. Acta 2023, 443, 141907. [Google Scholar] [CrossRef]
- Li, J.; Zou, S.; Huang, J.; Wu, X.; Lu, Y.; Liu, X.; Song, B.; Dong, D. Mn-N-P doped carbon spheres as an efficient oxygen reduction catalyst for high performance Zn-Air batteries. Chinese Chem. Lett. 2023, 34, 107222. [Google Scholar] [CrossRef]
- Wang, R.; Meng, Z.; Yan, X.; Tian, T.; Lei, M.; Pashameah, R.A.; Abo-Dief, H.M.; Algadi, H.; Huang, N.; Guo, Z.; et al. Tellurium intervened Fe-N codoped carbon for improved oxygen reduction reaction and high-performance Zn-air batteries. J. Mater. Sci. Technol. 2023, 137, 215–222. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Alfaruqi, M.H.; Kwak, W.-J.; Kim, J.; Hwang, J.-Y. Initial investigation and evaluation of potassium metal as an anode for rechargeable potassium batteries. J. Mater. Chem. A 2020, 8, 16718–16737. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Feng, C.; Faheem, M.B.; Fu, J.; Xiao, Y.; Li, C.; Li, Y. Fe-Based Electrocatalysts for Oxygen Evolution Reaction: Progress and Perspectives. ACS Catal. 2020, 10, 4019–4047. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Huang, B.; Wang, P.; Pei, Y. Hydroxyl group modification improves the electrocatalytic ORR and OER activity of graphene supported single and bi-metal atomic catalysts (Ni, Co, and Fe). J. Mater. Chem. A 2019, 7, 24583–24593. [Google Scholar] [CrossRef]
- Kundu, M.K.; Mishra, R.; Bhowmik, T.; Kanrar, S.; Barman, S. Three-dimensional hierarchically porous iridium oxide-nitrogen doped carbon hybrid: An efficient bifunctional catalyst for oxygen evolution and hydrogen evolution reaction in acid. Int. J. Hydrogen Energy 2020, 45, 6036–6046. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Šljukić, B. Efficient bifunctional cerium-zeolite electrocatalysts for oxygen evolution and oxygen reduction reactions in alkaline media. Synth. Met. 2023, 292, 117231. [Google Scholar] [CrossRef]
- Środa, B.; Dymerska, A.G.; Zielińska, B.; Mijowska, E. Promotion of MXene (Ti3C2Tx) as a robust electrocatalyst for oxygen evolution reaction via active sites of ZIF-67—In situ mechanism investigations. Int. J. Hydrogen Energy 2023, 48, 18696–18707. [Google Scholar] [CrossRef]
- Zhang, X.P.; Wang, H.Y.; Zheng, H.; Zhang, W.; Cao, R. O–O bond formation mechanisms during the oxygen evolution reaction over synthetic molecular catalysts. Chin. J. Catal. 2021, 42, 1253–1268. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. ChemInform Abstract: Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. ChemInform 2013, 44, 2013–2036. [Google Scholar] [CrossRef]
- Mudassar, M.; Noor, T.; Iqbal, N. Materials Science & Engineering B Advances in MXenes synthesis and MXenes derived electrocatalysts for oxygen electrode in metal-air batteries: A review. Mater. Sci. Eng. B 2023, 292, 116400. [Google Scholar] [CrossRef]
- Markovic, N.M.; Schmidt, T.J.; Stamenkovic, V.; Ross, P.N. Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Kong, J.; Cheng, W. Recent advances in the rational design of electrocatalysts towards the oxygen reduction reaction. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 951–969. [Google Scholar] [CrossRef]
- Tu, F.D.; Wu, Z.Y.; Guo, P.; Shen, L.X.; Zhang, Z.Y.; Dai, Y.K.; Ma, M.; Liu, J.; Xu, B.; Zhang, Y.L.; et al. Fe-N-C catalysts decorated with oxygen vacancies-rich CeOx to increase oxygen reduction performance for Zn-air batteries. J. Colloid Interface Sci. 2023, 637, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, W.; Duan, C.; Li, J.; Ding, S.; Zhang, L.; Zhu, J.; Ni, Y. Cellulose nanofibers carbon aerogel based single-cobalt-atom catalyst for high-efficiency oxygen reduction and Zn-air battery. J. Colloid Interface Sci. 2023, 629, 778–785. [Google Scholar] [CrossRef] [PubMed]
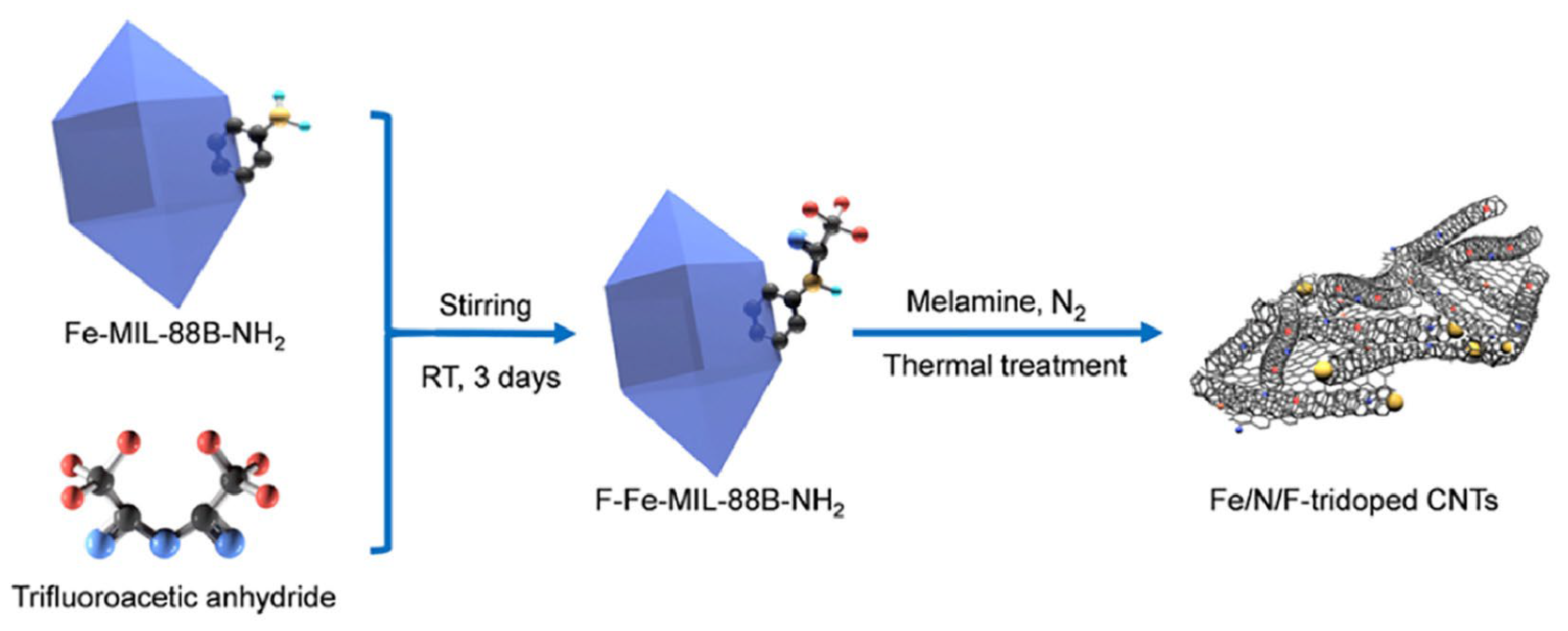
- Yu, A.; Long, W.; Zhu, L.; Zhao, Y.; Peng, P.; Li, F.-F. Transformation of postsynthesized F-MOF to Fe/N/F-tridoped carbon nanotubes as oxygen reduction catalysts for high power density Zn-air batteries. Chin. Chem. Lett. 2023, 34, 107860. [Google Scholar] [CrossRef]
- Zhou, G.; Yan, X.; Zhang, T.; Wang, K.; Zhang, J.; Guo, J. MOFs-derived hierarchical porous carbon supported Co@NC nanocapsules for pH universal oxygen reduction reaction and Zn-air batteries. Appl. Surf. Sci. 2023, 621, 156906. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, Z.; Li, B.; Li, Y.; Jin, C.; Xiang, T.; Wang, W.; Wu, Z.; Xue, H.; Cao, Y.; et al. Unveiling the inter-molecular charge transfer mechanism of N-doped graphene/carbon nanotubes heterostructure toward oxygen reduction process for Zn-air battery. Appl. Surf. Sci. 2023, 614, 156096. [Google Scholar] [CrossRef]
- Chang, H.; Guo, Y.-F.; Liu, X.; Wang, P.-F.; Xie, Y.; Yi, T.-F. Dual MOF-derived Fe/N/P-tridoped carbon nanotube as high-performance oxygen reduction catalysts for Zn-air batteries. Appl. Catal. B Environ. 2023, 327, 122469. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Chen, Z.; Chi, Y.; Sun, J.; Yuan, D.; Zhang, L. Hierarchically porous Co@N-doped carbon fiber assembled by MOF-derived hollow polyhedrons enables effective electronic/mass transport: An advanced 1D oxygen reduction catalyst for Zn-air battery. J. Energy Chem. 2023, 76, 117–126. [Google Scholar] [CrossRef]
- Zhao, Q.; Tan, X.; Liu, T.; Hou, S.; Ni, W.; Huang, H.; Zhang, J.; Yang, Z.; Li, D.; Hu, H.; et al. Engineering adjacent N, P and S active sites on hierarchical porous carbon nanoshells for superior oxygen reduction reaction and rechargeable Zn-air batteries. J. Colloid Interface Sci. 2023, 633, 1022–1032. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Cheng, R.; Li, K.; Li, Z.; Jiang, M.; Wang, F.; Yang, Z.; Zhao, T.; Meng, P.; Fu, C. Rational design of boron-nitrogen coordinated active sites towards oxygen reduction reaction in aluminum-air batteries with robust integrated air cathode. J. Power Sources 2023, 556, 232476. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Son, H.; Li, O.L. Plasma-engineered cobalt nanoparticle encapsulated N-doped graphene nanoplatelets as high-performance oxygen reduction reaction electrocatalysts for aluminum–air batteries. Catal. Today 2023, 420, 114025. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Cheng, R.; Xue, Q.; Wang, F.; Yang, Z.; Meng, P.; Jiang, M.; Zhang, J.; Fu, C. Enhanced electronic interaction between iron phthalocyanine and cobalt single atoms promoting oxygen reduction in alkaline and neutral aluminum-air batteries. Chem. Eng. J. 2022, 450, 138213. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.; Lu, D. Synthesis of cobalt-anchored N-doped carbon as an oxygen reduction reaction catalyst for aluminum-air batteries. Appl. Catal. A Gen. 2022, 646, 118847. [Google Scholar] [CrossRef]
- Tang, W.; Li, B.; Teng, K.; Wang, X.; Liu, R.; Wu, M.; Zhang, L.; Ren, P.; Zhang, J.; Feng, M. Advanced noble-metal-free bifunctional electrocatalysts for metal-air batteries. J. Mater. 2022, 8, 454–474. [Google Scholar] [CrossRef]
- Yu, T.; Xu, H.; Jin, Z.; Zhang, Y.; Qiu, H.J. Noble metal-free high-entropy oxide/Co-N-C bifunctional electrocatalyst enables highly reversible and durable Zn-air batteries. Appl. Surf. Sci. 2023, 610, 155624. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, Z.; Ge, X.; Yang, S.; Peng, Y.; Kang, Z.; Liu, Z.; Lee, J.Y.; Zhao, D. A metal-free ORR/OER bifunctional electrocatalyst derived from metal-organic frameworks for rechargeable Zn-Air batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, R.; Zhao, S.; Ma, W.; Zhang, X.; Song, Y.; Ma, C.; Shi, J. B, N, F tri-doped lignin-derived carbon nanofibers as an efficient metal-free bifunctional electrocatalyst for ORR and OER in rechargeable liquid/solid-state Zn-air batteries. Appl. Surf. Sci. 2022, 598, 153891. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Xu, H.; Zhou, Y.; Chen, X.; An, Z.; Chen, Y.; Chen, P. Tuning active sites for highly efficient bifunctional oxygen electrocatalysts of rechargeable Zn-air battery. J. Colloid Interface Sci. 2023, 640, 549–557. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, D.E.; Lee, S.Y.; Park, C.B.; Kim, C.K. Cobalt nanoparticles-encapsulated holey nitrogen-doped carbon nanotubes for stable and efficient oxygen reduction and evolution reactions in rechargeable Zn-air batteries. Appl. Catal. B Environ. 2023, 325, 122386. [Google Scholar] [CrossRef]
- Lan, T.; Du, H.; Li, Y.; Qu, K.; Zhao, J.; Zhang, X.; Dong, Y.; Zhang, Y.; Zhang, X.; Zhang, D. One-pot synthesis of NiFe-MOF/NiFe2O4 hollow spheres and their application as bifunctional ORR/OER electrocatalysts in Zn-air batteries. J. Alloys Compd. 2023, 943, 169144. [Google Scholar] [CrossRef]
- Bo, L.; Shi, W.; Nian, F.; Hu, Y.; Pu, L.; Li, P.; Zhang, Z.; Tong, J. Interface engineering of Co3S4@Co3O4/N, S-doped carbon core@shell nanostructures serve as an excellent bifunctional ORR/OER electrocatalyst for rechargeable Zn-air battery. Sep. Purif. Technol. 2023, 307, 122536. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Etesami, M.; Theerthagiri, J.; Choi, M.Y.; Wannapaiboon, S.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Tailoring the MOF structure via ligand optimisation afforded a dandelion flower like CoS/Co-Nx/CoNi/NiS catalyst to enhance the ORR/OER in Zn-air batteries. Nanoscale 2022, 14, 17908–17920. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Guo, S.; Zhang, Z.; Zeng, X.; Dong, P.; Li, M.; Xiao, J.; Zhang, C.; Hu, J.; et al. FeZrRu Trimetallic bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Electrochim. Acta 2023, 437, 141502. [Google Scholar] [CrossRef]
- García-Rodríguez, M.; Flores-Lasluisa, J.X.; Cazorla-Amorós, D.; Morallón, E. Metal oxide Perovskite-Carbon composites as electrocatalysts for Zn-air batteries. Optimisation of ball-milling mixing parameters. J. Colloid Interface Sci. 2023, 630, 269–280. [Google Scholar] [CrossRef] [PubMed]




| ORR Air Cathode | EOCV (V) vs. RHE | E1/2 (V) vs. RHE | Durability | Pmax (mW cm−2) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|
| RuCo@Co-N-C | 1.45 | 0.90 | Long-term cyclic stability for 100 h. | 154.3 at ~0.69 V and ~225 mA cm−2 | 767.4 | [25] |
| Pt/C + RuO2 | 1.39 | / | / | 119.8 at ~0.57 V and ~210 mA cm−2 | 717.0 | [25] |
| Fe SAs/NPs@NC + IrO2 | 1.446 | / | Excellent durability of 1000 cycles over 183 h. | 107.9 at ~0.83 V and ~130 mA cm−2 | 734.5 at 10 mA cm−2 | [26] |
| Pt/C + IrO2 | 1.477 | / | Low durability for 90 h. | 92.9 at ~0.80 V and ~115 mA cm−2 | 658.3 at 10 mA cm−2 | [26] |
| Fe-FeNC | 1.49 | 0.82 | Long-term cyclic durability for 120 h. | 141 at ~0.63 V and 222 mA cm−2 | 760 at 10 mA cm−2 | [27] |
| 0.05 Mn-N-P-C | 1.45 | 0.82 | The ZAB mechanically recharged multiple times within 80 h without the degradation of voltage. | 133 at ~0.71 V and ~188 mA cm−2 | 830 at 5 mA cm−2 | [28] |
| Te/Fe-N-C | 1.49 | 0.88 | ΔE = 22 mV (50,000 s). | 250 at ~0.71 V and ~350 mA cm−2 | 770 at 20 mA cm−2 | [29] |
| FeNC-Ce-950 | 1.480 | 0.921 | The ZAB’s charge/discharge voltages with FeNC-Ce-950 were high for about 11 h. | 175 at ~0.69 V and 255 mA cm−2 | 757 at 10 mA cm−2 | [43] |
| Co SAs/NCNA | 1.49 | 0.98 | / | 206 at ~0.59 V and ~350 mA cm−2 | 769 | [44] |
| FeNFC800 | 1.56 | 0.829 | Galvanostatic discharge curve at 10 mA cm−2 for 20,000 s. | 196 at ~0.78 V and ~250 mA cm−2 | / | [45] |
| 0.4Co@NC-900 | 1.50 | 0.91 | Excellent stability after long-term charge–discharge cycling tests over 130 h. | 203 at ~0.62 V and 325 mA cm−2 | 792 | [46] |
| N-G/CNTs-900 | 1.45 | 0.838 | Two N-G/CNTs-900-based ZABs were connected in series with an electronic light display screen for more than 24 h. | 133.6 at ~0.61 V and 220.10 mA cm−2 | 707 at 10 mA cm−2 | [47] |
| P-Fe-N-CNTs | 1.498 | 0.8843 | The durability of the ZAB was satisfactory, and the voltage retention rate was 95.1% after 144 h. | 145 at ~0.64 V and ~225 mA cm−2 | 885 at 10 mA cm−2 | [48] |
| Co@N-HPCFs | / | 0.831 | The excellent electrochemical stability of the Co@N-HPCF-800-based ZAB was observed in a long-term test with 600 cycles (200 h) at 2 mA cm−2. | 136.2 at ~0.53 V and ~255 mA cm−2 | 723 at 5 mA cm−2 | [49] |
| NPS-HPCNs | 1.479 | 0.86 | The 200 h long-term cycling test of the NPS-HPCN-based battery at a current density of 10 mA cm−2 showed reinforced charge and discharge potentials. | 206 at ~0.59 V and ~350 mA cm−2 | / | [50] |
| ORR Air Cathode | EOCV (V) vs. RHE | E1/2 (V) vs. RHE | Durability | Pmax (mW cm−2) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|
| Co@N/GNP | 1.40 | 0.98 | The durability of this AAB was investigated at 25 mA cm−2, and it was noticed that the Co@N/GNP cathode showed voltage degradation at 29,460 s. | 143.04 at 0.74 V and 191.83 mA cm−2 | / | [53] |
| Integrated B–N-G | / | 0.868 | High durability of AAB construction. | 152.4 at ~0.81 V and ~188 mA cm−2 | / | [52] |
| Slurry-cast B–N-G | / | / | / | 137.1 at ~0.78 V and ~175 mA cm−2 | / | [52] |
| Pt/C | / | 0.854 | / | 136.2 at ~0.64 V and ~212 mA cm−2 | / | [52] |
| FePc@Co-SAs/PCNF | 0.87 | / | 196.36 at ~0.79 V and ~250 mA cm−2 | / | [54] | |
| Co-N-C | 1.7 | 0.838 | After 20000 s, retained 95% of the performance. | 148 at ~0.99 V and ~150 mA cm−2 | 1148 at 50 mA cm−2 | [55] |
| Bifunctional Air Cathode | EOCV (V) vs. RHE | E1/2 (V) vs. RHE | η10 (V) vs. RHE | Charge and Discharge Voltage (V) | Durability | Pmax (mW cm−2) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BNPC-1100 1 | / | 0.793 | 1.38 | 2.19 and 1.16 V after 100 h of the test. | The ZAB did not show significant performance loss at a charge–discharge current density of 2 mA cm−2 for 100 h. | / | / | [58] |
| BNPC-1000 | / | 0.749 | 1.41 | The discharging voltage was 1.12 V after 100 h of the test. | The ZAB deteriorated during the 100 h cycling test. | // | / | [58] |
| BNF-LCF 2 | 1.536 | / | 1.542 | 2.07 and 1.10 V. | The ZAB showed a stable charge–discharge performance after 600 cycles. | 99.4 at ~0.57 V and ~175 mA cm−2 | 791.5 at 10 mA cm−2 | [59] |
| Pt/C + RuO2 | 1.507 | / | / | 2.17 and 1.10 V. | The ZAB’s charge–discharge performance rapidly dropped after the 300th cycle. | 68.3 at ~0.53 V and ~130 mA cm−2 | 720 at 10 mA cm−2 | [59] |
| BM_30_350_O2 3 | / | / | 1.60 | 2.03 and 1.26 V. | The ZAB’s efficiency reduced to 55% after 30 h. | 34.6 at 0.52 V and 67.1 mA cm−2 | 764 at 5 mA cm−2;738 at 10 mA cm−2 | [66] |
| Pt/C | / | / | / | / | The ZAB’s efficiency reduced to 52% after 30 h of continuous charge–discharge cycling. | 69.4 at 0.52 V and 110.1 mA cm−2 | 741 at 5 mA cm−2;737 at 10 mA cm−2 | [66] |
| Co@H-NCNT 4 | 1.520 | / | 1.544 | / | The ZAB showed good cycle stability for 300 cycles (100 h). | 207 at ~0.69 V and ~300 mA cm−2 | 879.7 | [61] |
| Ni@H-NCNT | / | / | / | / | / | 145.7 at ~0.61 V and ~240 mA cm−2 | [61] | |
| Fe@H-NCNT | / | / | / | / | / | 178.8 at ~0.67 V and ~265 mA cm−2 | [61] | |
| Pt/C-IrO2 | / | / | / | / | / | 166.1 at ~0.64 V and ~260 mA cm−2 | 786.8 | [61] |
| Co3S4@Co3O4/NSC-260–8 | 1.420 | 0.822 | 1.512 | 2.073 and 1.134 V. | The ZAB showed robust stability and no obvious decrease in the voltage for 200 h of charge–discharge cycles. | 122 at 0.45 V and 272 mA cm−2 | 885 at 20 mA cm−2 | [63] |
| NiFe-MOF/NiFe2O4 | 1.397 | / | 1.502 | 1.96 and 1.13 V. | / | 158.4 at 0.64 V and 246.1 mA cm−2 | 700 at 2 mA cm−2 | [62] |
| Co9S8/Co–Nx/CoNi/Ni3S2@CNS-4 | 1.59 | 0.860 | 1.580 | / | / | 206.9 at 0.64 V and 325 mA cm−2, | 801 at 10 mA cm−2 | [64] |
| FeZrRu/C | 1.46 | 0.912 | 1.650 | / | The ZAB showed stable discharge and charge potential for more than 10 h. | 221.34 at ~0.63 V and ~350 mA cm−2 | / | [65] |
| Fe3O4/CoO@CF | 1.46 | 0.83 | 1.600 | The ZAB showed long-term stability in charge/discharge performance. | 137 at ~0.68 V and ~200 mA cm−2 | 740 at 5 mA cm−2 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikić, J.; Nastasić, A.; Martins, M.; Sequeira, C.A.C.; Šljukić, B. Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries. Batteries 2023, 9, 394. https://doi.org/10.3390/batteries9080394
Milikić J, Nastasić A, Martins M, Sequeira CAC, Šljukić B. Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries. Batteries. 2023; 9(8):394. https://doi.org/10.3390/batteries9080394
Chicago/Turabian StyleMilikić, Jadranka, Ana Nastasić, Marta Martins, César A. C. Sequeira, and Biljana Šljukić. 2023. "Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries" Batteries 9, no. 8: 394. https://doi.org/10.3390/batteries9080394
APA StyleMilikić, J., Nastasić, A., Martins, M., Sequeira, C. A. C., & Šljukić, B. (2023). Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries. Batteries, 9(8), 394. https://doi.org/10.3390/batteries9080394