Abstract
Solid sodium ion batteries (SIBs) show a significant amount of potential for development as energy storage systems; therefore, there is an urgent need to explore an efficient solid electrolyte for SIBs. Na3Zr2Si2PO12 (NZSP) is regarded as one of the most potential solid-state electrolytes (SSE) for SIBs, with good thermal stability and mechanical properties. However, NZSP has low room temperature ionic conductivity and large interfacial impedance. F−doped NZSP has a larger grain size and density, which is beneficial for acquiring higher ionic conductivity, and the composite system prepared with epoxy can further improve density and inhibit Na dendrite growth. The composite system exhibits an outstanding Na+ conductivity of 0.67 mS cm−1 at room temperature and an ionic mobility number of 0.79. It also has a wider electrochemical stability window and cycling stability.
1. Introduction
Energy storage systems (ESSs) are gradually becoming essential and important in people’s daily lives, as these can provide us with convenience in many aspects [1,2,3,4]. As a hopeful substitute for lithium-ion batteries (LIBs), SIBs have caught the attention of a number of researchers recently because of their rich sodium resources, low prices and excellent sustainability. However, because sodium is more reactive than lithium, it is more likely to form dendrites in the conventional liquid electrolyte battery system, posing serious efficiency problems and safety hazards. Hence, research on solid or quasi-solid sodium ion batteries is of great importance for improving battery efficiency and safety [5,6,7,8,9,10,11,12,13,14,15,16].
To date, different types of sodium materials, such as Na-β″-Al2O3, sulfides, polymers, and Na superionic conductor (NASICON) have been reported for use as sodium ion solid-state electrolytes. As a solid electrolyte, Na-β″-Al2O3 is now successfully used in Na-S batteries; however, it is sensitive to moisture [17] and has a high preparation temperature, which poses some limitations to its production applications. Most sulfide-based solid electrolytes are limited in their application due to their instability in air [18] and narrow electrochemical stability window [19], despite their high ionic conductivity and good ductility. The polymer solid electrolyte is flexible, and the contact between it and the electrode is flexible, which makes it malleable and easy to process and shape. However, the ionic conductivity and ion transfer number dose not meet the imposed requirements when using at room temperature. To achieve ionic conductivity for battery applications, a temperature of 60 °C or higher is required. This temperature approaches the melting point of anode, Na (97 °C), which can cause safety problems. By comparing with the sodium-ion solid electrolyte above, a significant amount of attention has been devoted to NASICON (Na1+xZr2SixP3−xO12) because of its high ionic conductivity, wide window of electrochemical stability and stability in air. Hong [20] and Goodenough [21] were the first to study Na1+xZr2SixP3−xO12. The highest ionic conductivity was 10−4 S cm−1 (x = 2) at room temperature [20,22]. It has also been further improved by doping modifications of NASICON. The rare earth element La-doped Na3Zr2Si2PO12 has an ionic conductivity comparable with that of ionic liquid electrolytes (10−1 S cm−1) [23].
In recent years, several studies have reported anion-assisted ways to increase the room temperature’s ionic conductivity [23,24,25]. Lu et al. modified the Li6.25Ga0.25La3Zr2O12 crystals via F−assisted synthesis to generate smoother and quicker diffusion channels for Li+, thus improving the ionic conductivity [23]. Li et al. reported that anion-substituted Li3xLa2/3−xTiO3 has a higher ionic conductivity [24]. Goodenough reports that the substitution of F for OH, which allows Li2(OH)0.9F0.1Cl to have an improved Li+ diffusion path [26]. Although anion-assisted NZSPs have high ionic conductivity, they still do not provide effective inhibition of dendrite growth, resulting in uneven Na plating or streaking, leading to poor cell performance. This is because voids still exist on the surface of the SSE, leading to the uneven deposition of Na on the electrode surface and promoting the growth of dendrites. Here, we investigated F−-assisted NZSP solid electrolyte materials. It was found that the grain size and densities of NZSP increased with the introduction of F. However, it is still not enough to inhibit the growth of Na dendrites. Then, we prepared a composite system by depositing epoxy into the pores of NZSPF via vacuum adsorption. The composite system greatly modified the cycling performance of the cell with almost no decrease in ionic conductivity and no increase in impedance. The ionic conductivity of epoxy-NZSPF0.7 is 0.67 mS cm−1 (NZSPF0.7 is 0.95 mS cm−1). In addition, epoxy-NZSPF0.7 has a wider electrochemical stability window. Finally, we also assembled a Na|epoxy-NZSPF0.7|Na3V2(PO4)3 quasi-solid SIB, with good cycling performance at 40 °C.
2. Materials and Methods
2.1. Synthesis of NZSPFx Solid Electrolyte
Traditional solid-phase reactions were adopted to synthesize F−-assisted NZSPFx materials. Na2CO3, ZrO2, SiO2, and NH4H2PO4 were weighed according to certain stoichiometric ratios and then x mol of NaF (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0) was added separately. The final results were labeled NZSPFx. Balls, materials and ethanol were added to the ball mill tank in the ratio of 32:5:8. The milling was then carried out with a planetary ball mill at 400 rpm for 10 h. The 12 h drying of precursors was carried out under vacuum at 80 °C; then, the mixtures were transferred to a muffle furnace for 12 h preheating at 900 °C, followed by 3 h sintering at 1050 °C. The sintered powders were further ball-milled at 200 rpm for 10 h to obtain a uniform powder size. During the ball-milling process, a 5% mass fraction of PVA solution was added to act as a binder. The pressing of powers into pellets (diameter 16 mm, thickness 1 mm) was carried out at 120 MPa. Finally, the 4 h sintering of pellets was carried out at 800 °C to remove the PVA solution. After cooling to room temperature, the 24 h sintering of pellets was performed at 1100 °C to obtain NZSPFx solid electrolyte.
2.2. Synthesis of Epoxy-NZSPF0.7 Solid Electrolyte
The DGEBA (diglycidyl ether of bisphenol-A) and PACM (4,4’-diaminodicyclohexylmethane) were dissolved in THF in stoichiometric ratios to obtain 1 mol L−1 solutions, respectively. Then, the two solutions were mixed in a volume ratio of 2:1 (DGEBA:PACM) and stirred for 1 h. The sintered NZSPF ceramic sheets in 2.1 were completely immersed in the mixed solution, kept in a vacuum environment for 20 min and cycled three times. The sintering of pellets was carried out at 150 °C for 24 h to obtain an epoxy-NZSPFx solid electrolyte.
2.3. Characterization and Measurements
A Bruker D8 Advance X-ray diffractometer configured with Cu Kα radiation in the 2θ scope of 10–60° was adopted to collect the X-ray diffraction (XRD) patterns, and the collection of data was carried out at 5° min−1. The chemical component in an Escalab 250Xi instrument from Thermo Scientific configured with an Al Kα micro-focused X-ray source was demonstrated via X-ray photoelectron spectroscopy (XPS) tests. The collection of Fourier transform infrared (FTIR) spectra were carried out on a Nicoletis 10 spectrometer from Thermo Scientific. The spectrum was from 4000 cm−1 to 400 cm−1. The microstructures of SSE were studied using scanning electron microscopy (SEM) (QUNATA-FEG). The measurement of thermostability was carried out via thermogravimetric analysis (TGA) on a Netzch STA449F3 analyzer under N2 conditions at a heating rate of 10 °C/min from 25 to 700 °C.
A CHI660E (ChenHua) electrochemical workstation with an AC amplitude of 10 Mv and a frequency scope from 10 Hz to 106 Hz was adopted to make electrochemical impedance spectroscopy (EIS) tests at room temperature. A combination of direct-current (DC) polarization and alternating-current (AC) impedance was used for the ion transference number test. A CR2032 cell case was chosen to assemble a Na/NZSPFx/Na symmetric battery. AN AC impedance test is performed on the battery before the DC polarization test; then, a small bias voltage (5 mV) is added to the battery for the DC polarization test, and after the planned current of the battery is stabilized, the battery is then tested for AC impedance again. The electrochemical stability windows of NZSPF0.7 and epoxy-NZSPF0.7 solid electrolytes were evaluated with cyclic voltammetry (CV) and linear sweep voltammetry (LSV). The tests were performed on the Au|NZSPF0.7|Na and Au|epoxy-NZSPF0.7|Na cells and the scanning rate was 0.2 mV s−1 at room temperature. The electrochemical stability test was carried out to test the interfacial stability and Na stripping–plating behavior. The testing of assembled Na|NZSPFx|Na and Na|epoxy-NZSPF0.7|Na symmetric cells was carried out on a Blue Power Test System CT2001A with a constant current density of 0.1 mA cm−1 at 40 °C.
The assembly of the full cell was carried out in an argon atmosphere glove box using Na metal as the anode, NZSPF0.7 and epoxy-NZSPF0.7 as the solid electrolyte, and Na3V2(PO4)3 as the cathode using a 2032 cell case. For the preparation of the cathode electrode sheet, Na3V2(PO4)3, the mixture of acetylene black carbon and PVDF was carried out in NMP with a mass ratio of 7:2:1. Using Al foil as a fluid collector, the coasting of the slurry was carried out on the surface of Al foil, followed by 12 h OF drying under vacuum at 80 °C to eliminate the NMP solvent. The Na metal, solid electrolyte and cathode pole piece were assembled in the order of Na|epoxy-NZSPF0.7|Na3V2(PO4)3. The addition of 10 μL liquid electrolyte (ethylene carbonate (EC) as the solvent and NaClO4 as the salt) was conducted between the solid electrolyte and the pole pieces to wet the contact surface. The constant current charge/discharge test of the batteries was performed on the LAND CT2001A test system at 40 °C to evaluate the long-cycle performance.
3. Results and Discussion
To investigate the influence of adding F−, we used XRD to measure the lattice structure of samples with different fluorine contents. According to Figure S1, the main diffraction peaks of NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0) are consistent with the monoclinic NZSP structure [27]. This phenomenon proves that the introduction of F− did not destroy the crystal structure. However, with the increase in NaF content, a ZrO2 secondary phase was detected. This may be due to the reaction of Si4+/P5+ with F during the process of high-temperature sintering, as it leads to a reduction in Si4+/P5+ content and, finally, the deposition of the ZrO2 secondary phase. In addition, no fluorine-related phases were observed in the XRD patterns of all samples, which could be due to F occupying positions in the lattice dot matrix or high-temperature volatilization.
These featured peaks of P-O and Si-O groups (Figure S2) were analyzed with FTIR spectroscopy. All samples displayed the same stretching or bending vibration pattern. The peaks at 598 and 1128 cm−1 were ascribed to P-O stretching in tetrahedral PO43− units [25,27], while the peaks at 501 and 863 cm−1 were ascribed to the Si-O stretching in tetrahedral SiO44− units [25,28]. These outcomes definitively prove that SiO44− and PO43− units are in the NZSPFx crystal structure. These peaks are shifted to varying degrees, presumably due to F occupying the position of the O element in the NZSPFx lattice, causing the conformity of positive and negative centers in the lattice [26].
To further investigate the elemental composition of NZSPFx, XPS was used to characterize NZSP, NZSPF0.7. Figure S3a shows the full XPS spectra of NZSP and NZSPF0.7. Different peak areas corresponding to Na, Zr, Si, P, O are obviously found, which proves that the major components of the ceramics is NZSPF0.7. High-resolution XPS (Figure S3b) is adopted to detect the F 1s peak, which proves the successful doping of the F element. The binding energy at 684 eV corresponds to the F-Si/P bond [29,30], showing that the F− takes up the O2− place in the NZSPFx lattice. The intensities of the P 2p and Si 2p peaks reduce slightly under the help of F− (Figure S3c,d), indicating a slight decrease in the concentration of P5+ and Si4+ because of the reaction of P5+/Si4+ with F and the volatilization of the results at high temperatures. In addition, a slight shift in P 2p and Si 2p to a higher binding energy was observed in NZSPF0.7 by comparing with the undoped sample, and the presence of P/Si-F bonds [31] can explain this shift, which also proves that F− takes up part of the O2− sites in the NZSPF0.7 lattice.
Although the doping of NaF can reduce the resistance of NZSP (Figure S5) and improve the ionic conductivity (Figure S6), the cycling stability is deemed unsatisfactory (Figure S7). This is because the NZSPF0.7 grain boundaries are not dense, which also leads to the ability of growing and forming Na dendrites in the crevices (Figure S9). Therefore, it is necessary to modify NZSPF0.7 to strengthen the interface between the SSE and the electrode, and to stop the development of Na dendrites.
Figure 1a shows the FTIR spectra of the epoxy, NZSPF0.7, and epoxy-NZSPF0.7 composite system. The FTIR spectra of epoxy-NZSPF0.7 do not show new peaks, only a simple superposition of the two monomers. This indicates that no new substances are produced in the composite system and no reaction occurs between the two monomers. Figure 1b shows the TGA curves of epoxy, NZSPF0.7, and epoxy-NZSPF0.7. It can be seen that in the tested temperature range, NZSPF0.7 does not undergo mass loss and is thermally stable, while the mass loss of epoxy starts to occur at 300 °C and almost completely decomposes after reaching 600 °C. This coincides with the weight loss range of the composite system, and it can be determined that the mass loss of the composite system is caused by epoxy. This indicates that epoxy was successfully filled into the voids of NZSPF0.7, further improving the densities of the ceramics (Figure S10).
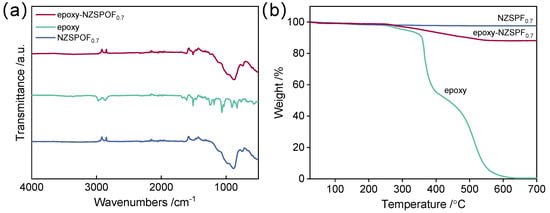
Figure 1.
(a) FTIR spectra and (b) TGA analysis for NZSPF0.7, epoxy and epoxy−NZSPF0.7.
To observe the surface difference between NZSPF0.7 and epoxy-NZSPF0.7, and the distribution of epoxy resin in NZSPF0.7, SEM and EDS tests were performed on both NZSPF0.7 (Figure S4) and epoxy-NZSPF0.7 (Figure 2). Figure S4 shows the SEM image of NZSPF0.7. The SEM images show that with the growth in NaF content, the size of NZSPFx grains gradually grows larger. However, F promotes grain growth, reduces grain boundary concentration and increases ceramic density to a certain extent, which can efficiently decrease the grain boundary concentration, increase the ionic conductivity and reduce the grain boundary resistance [32,33]. There are still a large number of interstices in the middle of the ceramic after high-temperature sintering, and the existence of these interstices leads to uneven deposition of dendrites here and reduces the electrochemical performance. Figure 2 shows the surface of epoxy-NZSPF0.7 composite solid electrolyte after polishing; compared with NZSPF0.7, the gap in the composite system is significantly reduced, which is a good verification of the increased densities. The denseness of epoxy-NZSPF0.7 is 97.6% and NZSPF0.7 is 93.4%, while that of un-assisted NZSP is only 79.8%. This shows that the filling of epoxy can raise the densities of the composite system because the prior condition for a solid electrolyte to have a high ionic conductivity is a high density [34,35]. Additionally, Figure 2c displays the EDS discussion on the epoxy-NZSPF0.7 ceramic sample. Because epoxy contains a large number of C elements, by observing the distribution of C elements, we can find that epoxy is evenly distributed in the composite system. The maps also confirm that O, P, Si, F, Na and Zr factors generally show an even homogeneous distribution in the composite system, and the content of the F element is essentially the same as that of NASPF0.7.
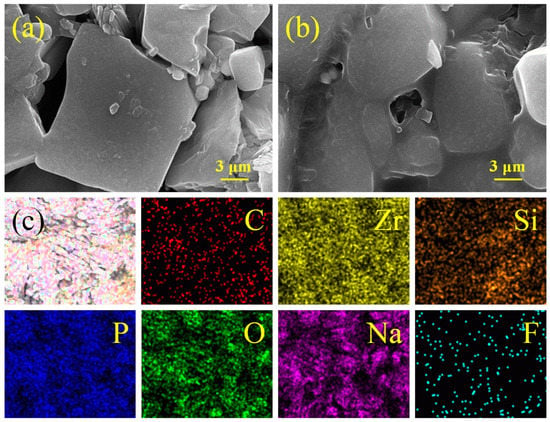
Figure 2.
SEM image of (a) NZSPF0.7 and (b) epoxy-NZSPF0.7; (c) EDS mapping of epoxy-NZSPF0.7.
The electrical conductivities of the solid electrolyte were investigated with EIS. Figure 3a displays the impedance spectra of NZSPF0.7 and epoxy-NZSPF0.7. According to the Nyquist curve, the resistance of NZSPF0.7 and epoxy-NZSPF0.7 are calculated as 370 Ω and 420 Ω. The overall conductivity measures from the EIS are 0.95 and 0.67 mS cm−1. The impedance of epoxy-NZSPF0.7 becomes larger and the ionic conductivity decreases are due to the fact that the epoxy impedance of the pure phase is extremely large; therefore, filling the gap to NZSPF0.7 results in a growth in the impedance and a decline in the ionic conductivity of epoxy-NZSPF0.7.
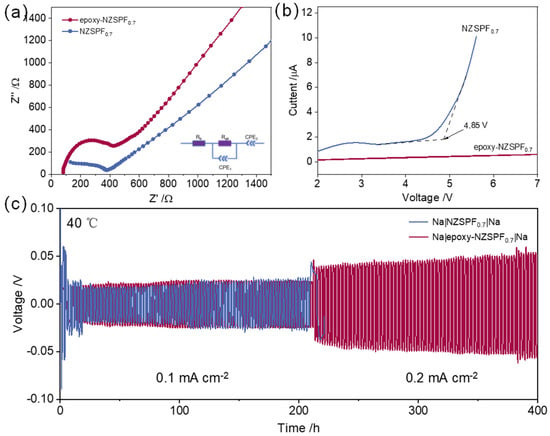
Figure 3.
(a) EIS measurements at room temperature; (b) LSV of the Au|NZSPF0.7|Na and Au|epoxy−NZSPF0.7|Na cells at room temperature; (c) variable current cycling of Na|NZSPF0.7|Na and Na|epoxy−NZSPF0.7|Na symmetric cells (the current density is 0.1 mA cm−2 and becomes 0.2 mA cm−2 after 200 h).
To verify the conduction behavior of sodium ions, the sodium ion transfer number (tNa+) was determined. The tNa+ (40 °C) of NZSPF0.7 was 0.84, while that of epoxy-NZSPF0.7 was 0.79. This proves that the filling of epoxy does not significantly affect the tNa+ and Na+ is still transported by NZSPF0.7 as a transport channel rather than epoxy.
In addition to having a high ionic conductivity, a wide-range electrochemical stability window is also a basic necessity for SSE in actual use. The electrochemical stability windows of NZSPF0.7 and epoxy-NZSPF0.7 is assessed via LSV with Au|NZSPF0.7|Na and Au|epoxy-NZSPF0.7|Na batteries with a scan rate of 0.2 mV s−1 at 30 °C (Figure 3b). The electrochemical stability window of the NZSPF0.7 is 4.85 V, while the current intensity of epoxy-NZSPF0.7 remains constant in the range of 2–7 V. This indicates that the electrochemical steadiness window of theepoxy-NZSPF0.7 up to 7 V. It is expected that epoxy-NZSPF0.7 can be used as a high-voltage sodium battery. The electrochemical properties of NZSPF0.7, NZSPF0.7 and epoxy-NZSPF0.7 are listed in Table 1.

Table 1.
Electrochemical properties of NZSPF0, NZSPF0.7 and epoxy-NZSPF0.7.
Figure 3c shows that epoxy-NZSPF0.7 can be cycled steadily for above 200 h (100 cycles) at a current density of 0.1 mA cm−2 with no fluctuation in potential, and remains stable for nearly 200 h when the current density grows to 0.2 mA cm−2. This indicates that epoxy-NZSPF0.7 can promote the even deposition of Na+ and stop the Na dendrites from developing. In contrast, NZSPF0.7 could only keep steady at 0.1 mA cm−2 and immediately short-circuited at 0.2 mA cm−2. It can be noted that NZSPF0.7 needs 5–10 h to adapt to the electrode with significant potential fluctuations, while epoxy-NZSPF0.7 hardly needs this process. Compared to this, epoxy-NZSPF0.7 requires a shorter activation time and does not show significant short-circuiting after increasing the current, indicating that it has higher electrode stability.
Finally, Na|NZSPF0.7|Na3V2(PO4)3 and Na|epoxy-NZSPF0.7|Na3V2(PO4)3 solid SIBs were assembled. Figure 4 shows the performances of batteries. The polarization of NZSPF0.7 is bigger with the gradual current rate growth (Figure 4a), while the charge/discharge curve of epoxy-NZSPF0.7 is smooth and the polarization is basically unchanged (Figure 4b). The discharge specific capacities of epoxy-NZSPF0.7 are 96.9, 80.1, 68.9 and 48.9 mAh g−1 at 0.1C, 0.5C, 1C and 2C. In the case of the reversion of the current density to 1C and 0.5C, the given capacities are 60.8 and 70.5 mAh g−1 (Figure 4c). This shows that when the current returns to the same rates, it has excellent reversibility and steadiness, although the reversible capacity is slightly reduced. Figure 4d shows the cycling performance of cells assembled with two different electrolytes at a current rate of 0.1C at 40 °C. The discharge-specific capacity remains 93.7 mAh g−1 after 150 cycles, which is 95.8% capacity retention of initial capacity (97.8 mAh g−1). The mean coulombic efficiency is close to 99%, which is only 79.1% for Na3V2(PO4)3|NZSPF0.7|Na battery.
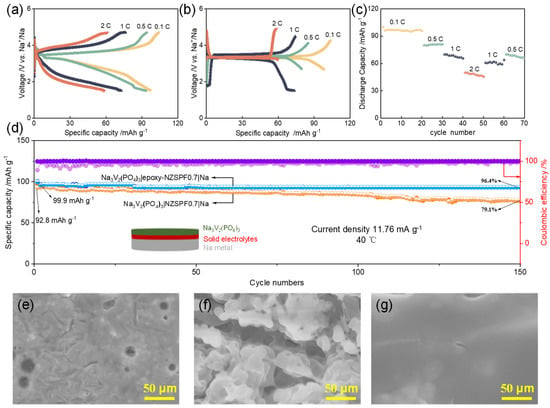
Figure 4.
(a) Charging–discharging profiles of Na3V2(PO4)3|NZSPF0.7|Na at different ates; (b,c) charging–discharging profiles of Na3V2(PO4)3|epoxy-NZSPF0.7|Na at different rates; (d) cycling performance of the Na3V2(PO4)3|epoxy−NZSPF0.7|Na battery at a current density of 11.76 mA g−1; (e) SEM image of sodium metal surface before cycling; SEM image of sodium metal surface of (f) Na3V2(PO4)3|NZSPF0.7|Na battery and (g) Na3V2(PO4)3|epoxy−NZSPF0.7|Na battery after cycling.
Figure 4e–g shows the SEM images of the Na electrode surface. Obviously, after 150 cycles, Na dendrites were formed at the boundaries and voids of the NZSPF0.7 solid electrolyte (Figure 4f), resulting in inhomogeneous Na plating/striping with a corresponding aggravation of the cell behavior [30]. However, the Na electrode surface of Na3V2(PO4)3|epoxy-NZSPF0.7|Na battery was clean and smooth (Figure 4g), and almost no Na deposition was found, indicating that the epoxy-filled dielectric material can effectively prevent the formation of dendrites and enhance the battery cycling behavior.
4. Conclusions and Outlook
In conclusion, the grain boundary concentration of NZSP was effectively reduced by introducing NaF into the NZSP solid electrolyte and a denser NZSPF0.7 with improved ionic conductivity was obtained (0.98 mS cm−1); however, NZSPF0.7 did not stop the development of sodium dendrites well and had poor interfacial properties. For this reason, a composite solid electrolyte epoxy-NZSPF0.7 was prepared via simply vacuum adsorption and by filling the interstitial space of NZSPF0.7 with epoxy and curing it. Epoxy-NZSPF0.7 combines the great ionic conductivity of inorganic electrolytes as well as the good interfacial contact from organic polymer electrolytes, with high ionic conductivity (0.67 mS cm−1) as well as excellent interfacial performance and cycling behavior (the initial capacity of cells is 97.9 mAh g−1 with a 96.4% retention rate after 150 turns). According to the above-presented results, this study may offer a novel idea for research concerning solid electrolytes for sodium-ion batteries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries9060331/s1. Figure S1. (a) XRD patterns of NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0); Figure S2. FTIR spectras of NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0); Figure S3. XPS spectra of (a) survey spectra, (b) F 1s, (c) P 2p and (d) Si 2p of NZSPF0.7; Figure S4. SEM images of NZSPFx ceramic pellets, (a) x = 0, (b) x = 0.1, (c) x = 0.3, (d) x = 0.5, (e) x = 0.7, and (f) x = 1.0; (g,h) the corresponding elemental mapping in the square of (e) image; (h) densities and relative densities of NZSPFx; Figure S5. EIS measurements performed of NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0) solid electrolytes; Figure S6. Ion conductivity (red) and ion transfer number (blue) of NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0) solid electrolytes; Figure S7. cycling performance of Na3V2(PO4)3|NZSPF0.7|Na battery; Figure S8. Arrhenius plots of NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, and 1.0) solid electrolytes; Figure S9. SEM images of the sodium metal surface of cell Na3V2(PO4)3|NZSPF0.7|Na (a) before and (b) after cycling; Figure S10. The densities of NZSPF0.7 and epoxy- NZSPF0.7; Figure S11. Variable current cycling of Na|NZSPF0.7|Na symmetric cells (the current density is 0.1 mA cm−2 and becomes 0.2 mA cm−2 after 200 h); Figure S12. Variable current cycling of Na|epoxy-NZSPF0.7|Na symmetric cells (the current density is 0.1 mA cm−2 and becomes 0.2 mA cm−2 after 200 h); Table S1. Chemical composition for NZSPFx (x = 0, 0.1, 0.3, 0.5, 0.7, 1.0).
Author Contributions
Y.F.: conceptualization, data analysis, and writing of the original draft. D.L.: experimental execution, data analysis and discussion. Y.S.: experimental execution and discussion. G.Z.: writing-review and editing. H.G.: research design, funding supporting and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support provided the Key National Natural Science Foundation of Yunnan Province (2019FY003023).
Institutional Review Board Statement
Not applicable, as studies on humans and animals are not involved.
Informed Consent Statement
Not applicable, as studies on humans are not involved.
Data Availability Statement
The data are not publicly available due to the data required to reproduce these findings forming part of an ongoing study.
Acknowledgments
The authors acknowledge financial support provided by the National Natural Science Foundation of China (No. 52064049), the National Natural Science Foundation of Yunnan Province (202301AS070040), Key Laboratory of Solid-State Ions for Green Energy of Yunnan University, the Electron Microscope Center of Yunnan University for the support of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 7411, 294–303. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 23, 11503–11618. [Google Scholar] [CrossRef]
- Yoo, H.D.; Liang, Y.L.; Li, Y.F.; Yao, Y. High areal capacity hybrid magnesium-lithium-ion battery with 99.9% coulombic efficiency for large-scale energy storage. ACS Appl. Mater. Interfaces 2015, 12, 7001–7007. [Google Scholar] [CrossRef]
- Hao, F.; Liang, Y.L.; Zhang, Y.; Chen, Z.Y.; Zhang, J.B.; Ai, Q.; Guo, H.; Fan, Z.; Lou, J.; Yao, Y. High-energy all-solid-state organic-lithium batteries based on ceramic electrolytes. ACS Energy Lett. 2021, 1, 201–207. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on sodium-ion batteries. Chem. Rev. 2014, 23, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Che, H.Y.; Chen, S.L.; Xie, Y.Y.; Wang, H.; Amine, K.; Liao, X.Z.; Ma, Z.F. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 2017, 5, 1075–1101. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A Review. Chem. Rev. 2017, 15, 10403–10473. [Google Scholar] [CrossRef]
- Cohn, A.P.; Muralidharan, N.; Carter, R.; Share, K.; Pint, C.L. Anode-free sodium battery through in situ Plating of sodium metal. Nano Lett. 2017, 2, 1296–1301. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.H.; Hitz, G.T.; McOwen, D.W.; Li, Y.J.; Xu, S.M.; Wen, Y.; Zhang, L.; Wang, C.W.; Pastel, G.; et al. Three-dimensional bilayer garnet solid electrolyte based high energy density lithium metal-sulfur batteries. Energy Environ. Sci. 2017, 7, 1568–1575. [Google Scholar] [CrossRef]
- Li, H.S.; Ding, Y.; Ha, H.; Shi, Y.; Peng, L.L.; Zhang, X.G.; Ellison, C.J.; Yu, G.H. An all-stretchable-component sodium-ion full battery. Adv. Mater. 2017, 29, 1700898. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Lushington, A.; Sun, Q.; Yadegari, H.; Wang, B.Q.; Xiao, W.; Li, R.Y.; Sun, X.L. Superior stable and long life sodium metal anodes achieved by atomic layer dposition. Adv. Mater. 2017, 29, 1606663. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Liu, B.H.; Liang, M.F.; Lv, Z.L.; Adair, K.R.; Sun, X.L. Few-layer MoSe2 nanosheets with expanded (002) planes confined in hollow carbon nanospheres for ultrahigh-performance Na-ion batteries. Adv. Funct. Mater. 2018, 28, 1707480. [Google Scholar] [CrossRef]
- Zhao, C.L.; Liu, L.L.; Qi, X.G.; Lu, Y.X.; Wu, F.X.; Zhao, J.M.; Yu, Y.; Hu, Y.S.; Chen, L.Q. Solid-state sodium batteries. Adv. Energy Mater. 2018, 17, 1601196. [Google Scholar] [CrossRef]
- Kwak, H.; Lyoo, J.; Park, J.; Han, Y.; Asakura, R.; Remhof, A.; Battaglia, C.; Kim, H.; Hong, S.T.; Jung, Y.S. Na2ZrCl6 enabling highly stable 3 V all-solid-state Na-ion batteries. Energy Storage Mater. 2021, 37, 47–54. [Google Scholar] [CrossRef]
- He, X.Z.; Ji, X.; Zhang, B.; Rodrigo, N.D.; Hou, S.; Gaskell, H.; Deng, T.; Wan, H.L.; Liu, S.F.; Xu, J.J.; et al. Tuning interface lithiophobicity for lithium metal solid-state batteries. ACS Energy Lett. 2022, 7, 131–139. [Google Scholar] [CrossRef]
- Bay, M.C.; Grissa, R.; Egorov, K.V.; Asakura, R.; Batrtaglia, C. Low Na-β′′-alumina electrolyte/cathode interfacial resistance enabled by a hydroborate electrolyte opening up new cell architecture designs for all-solid-state sodium batteries. Mater. Futures 2022, 1, 031001. [Google Scholar] [CrossRef]
- Will, F.G. Effect of water on beta alumina conductivity. J. Electrochem. Soc. 1976, 6, 834–836. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, K.; Mi, J.L.; Lu, L.; Zhao, L.R.; Wang, L.M.; Li, Y.M.; Zeng, H. Na3PSe4: A novel chalcogenide solid electrolyte with high Ionic conductivity. Adv. Energy Mater. 2015, 5, 1501294. [Google Scholar] [CrossRef]
- Chi, X.W.; Liang, Y.L.; Hao, F.; Zhang, Y.; Whiteley, J.; Dong, H.; Hu, P.; Lee, S.; Yao, Y. Tailored organic electrode material compatible with sulfide electrolyte for stable all-solid-state sodium batteries. Angew. Chem. Int. Edit. 2018, 10, 2630–2634. [Google Scholar] [CrossRef]
- Hong, H.Y.P. Crystal-structures and crystal-chemistry in system Na1+XZr2SiXP3−XO12. Mater. Res. Bull. 1976, 2, 173–182. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion yransport in skeleton structures. Mater. Res. Bull. 1976, 2, 203–220. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.F.; Wang, S.M.; Liu, D.L.; Mei, Z.Y.; An, Q.; Jiang, J.W.; Guo, H. Enhanced ionic conductivity of a Na3Zr2Si2PO12 solid electrolyte with Na2SiO3 obtained by liquid phase sintering for solid-state Na+ batteries. Nanoscale 2022, 14, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Meng, X.Y.; Alonso, J.A.; Fernandez-Diaz, M.T.; Sun, C.W. Effects of fluorine doping on structural and electrochemical properties of Li6.25Ga0.25La3Zr2O12 as electrolytes for solid-state lithium batteries. ACS Appl. Mater. Interfaces 2019, 2, 2042–2049. [Google Scholar] [CrossRef]
- Li, J.X.; Wen, Z.Y.; Xu, X.X.; Zhu, X.J. Lithium-ion conduction in the anion substituted La2/3−xLi3x−yTiO3−yFy electrolyte with perovskite-type structure. Solid State Ionics 2005, 29–30, 2269–2273. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhou, W.D.; Xin, S.; Li, S.; Zhu, J.L.; Lu, X.J.; Cui, Z.M.; Jia, Q.X.; Zhou, J.S.; Zhao, Y.S.; et al. Fluorine-doped antiperovskite electrolyte for all-solid-state lithium-ion batteries. Angew. Chem. Int. Edit. 2016, 34, 9965–9968. [Google Scholar] [CrossRef]
- He, S.N.; Xu, Y.L.; Chen, Y.J.; Ma, X.N. Enhanced ionic conductivity of an F−-assisted Na3Zr2Si2PO12solid electrolyte for solid-state sodium batteries. J. Mater. Chem. A. 2020, 25, 12594–12602. [Google Scholar] [CrossRef]
- Liu, C.; Wen, Z.Y.; Rui, K. High ion conductivity in garnet-type F-doped Li7La3Zr2O12. Int. J. Inorg. Mater. 2015, 9, 995–1000. [Google Scholar]
- Song, S.F.; Duong, H.M.; Korsunsky, A.M.; Hu, N.; Lu, L. A Na+ superionic conductor for room-temperature sodium batteries. Sci. Rep. 2016, 6, 32330. [Google Scholar] [CrossRef]
- Lee, S.H. Surface properties of fluoroethylene carbonate-derived solid electrolyte interface on graphite negative electrode by narrow-range cycling in cell formation process. Appl. Surf. Sci. 2014, 322, 64–70. [Google Scholar] [CrossRef]
- Kanezashi, M.; Matsutani, T.; Wakihara, T.; Nagasawa, H.; Okubo, T.; Tsuru, T. Preparation and has permeation properties of fluorine-silica membranes with controlled amorphous silica structures: Effect of fluorine source and calcination temperature on network size. ACS Appl. Mater. Interfaces 2017, 29, 24625–24633. [Google Scholar] [CrossRef]
- Dalavi, S.; Guduru, P.; Lucht, B.L. Performance enhancing electrolyte additives for lithium ion batteries with silicon anodes. J. Electrochem. Soc. 2012, 5, A642–A646. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Gurniak, E.; Jones, B.H.; Wheeler, D.R.; Rodriguez, M.A.; McDaniel, A.H. Scaling effects in sodium zirconium silicate phosphate (Na1+xZr2SixP3−xO12) ion-conducting thin films. J. Am. Ceram. Soc. 2016, 8, 2729–2736. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.J.; Liu, Q.; Mei, Z.Y.; Yang, L.; Duan, L.Y.; Guo, H. An asymmetric bilayer polymer-ceramic solid electrolyte for high-performance sodium metal batteries. J. Enerdy Chem. 2022, 74, 18–25. [Google Scholar] [CrossRef]
- Xu, X.X.; Wen, Z.Y.; Yang, X.L.; Chen, L.D. Dense nanostructured solid electrolyte with high Li-ion conductivity by spark plasma sintering technique. Mater. Res. Bull. 2008, 8–9, 2334–2341. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Figueiredo, F.M.; Marques, F.M.B.; Franco, J.I. Influence of microstructure on the electrical properties of NASICON materials. Solid State Ionics 2001, 1–2, 173–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).