Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu9S5 and Sb2S3
2.3. Synthesis of Cu2S@C, Sb2S3@C
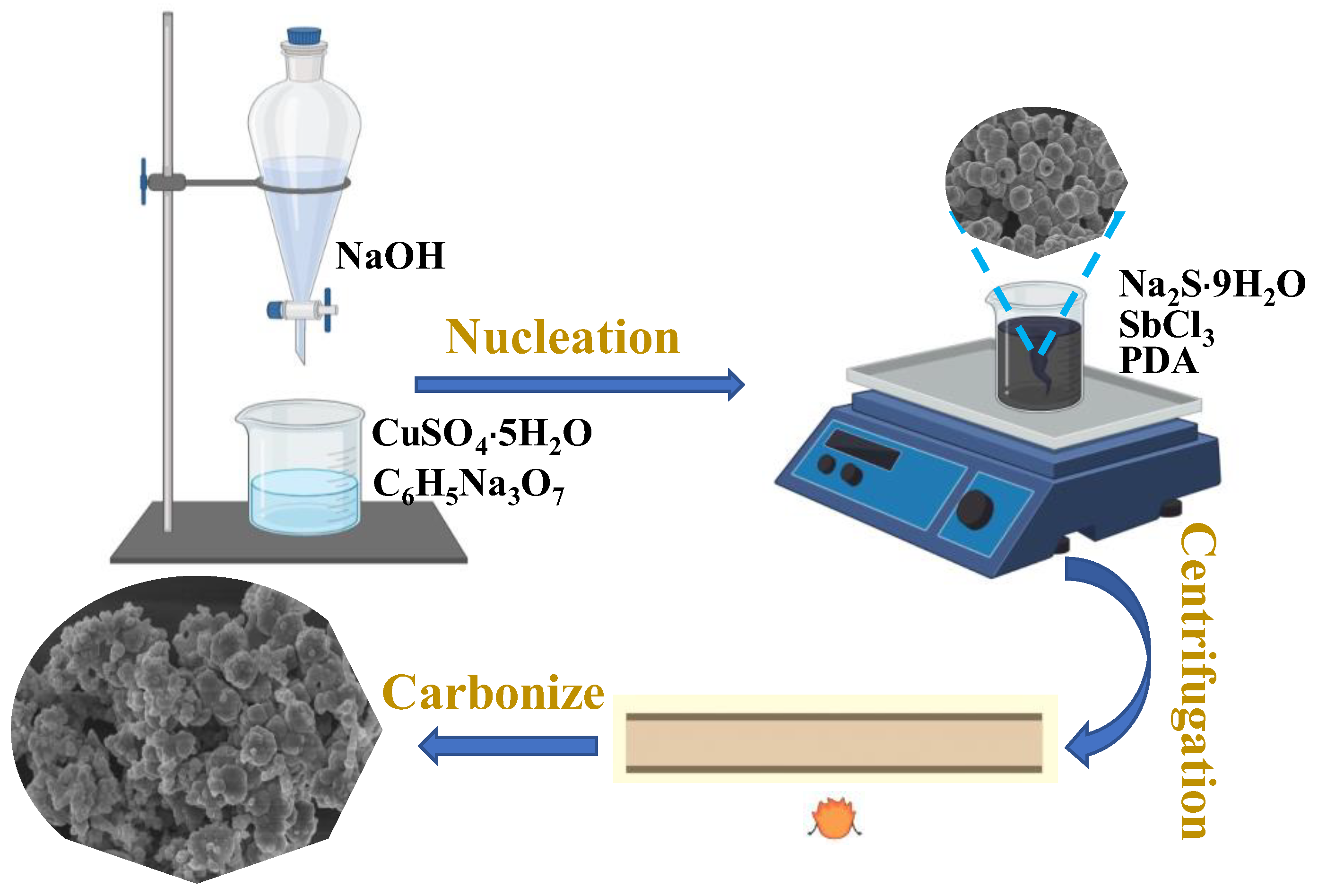
2.4. Synthesis of CuSbSy@C and CuSbSy
3. Results and Discussion
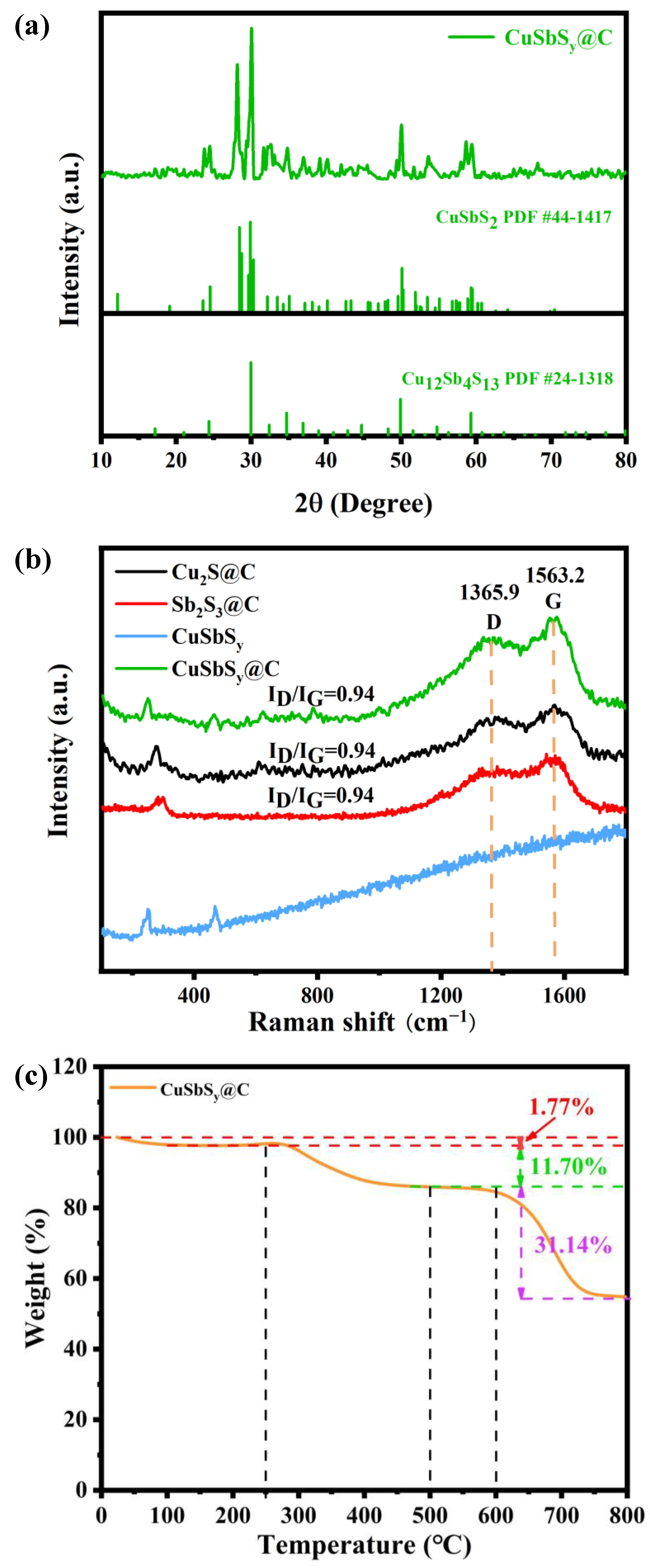
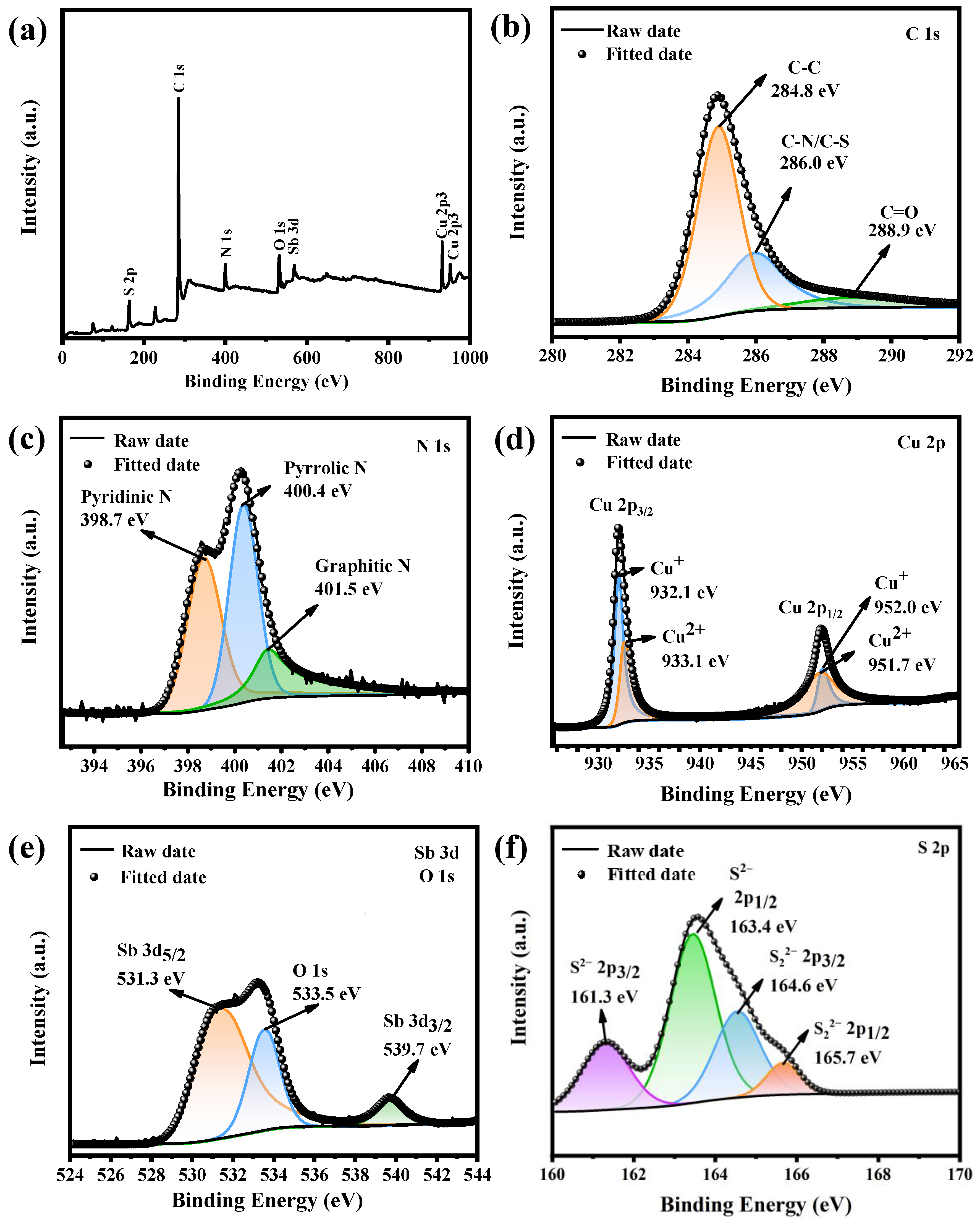
3.1. Material Characterization
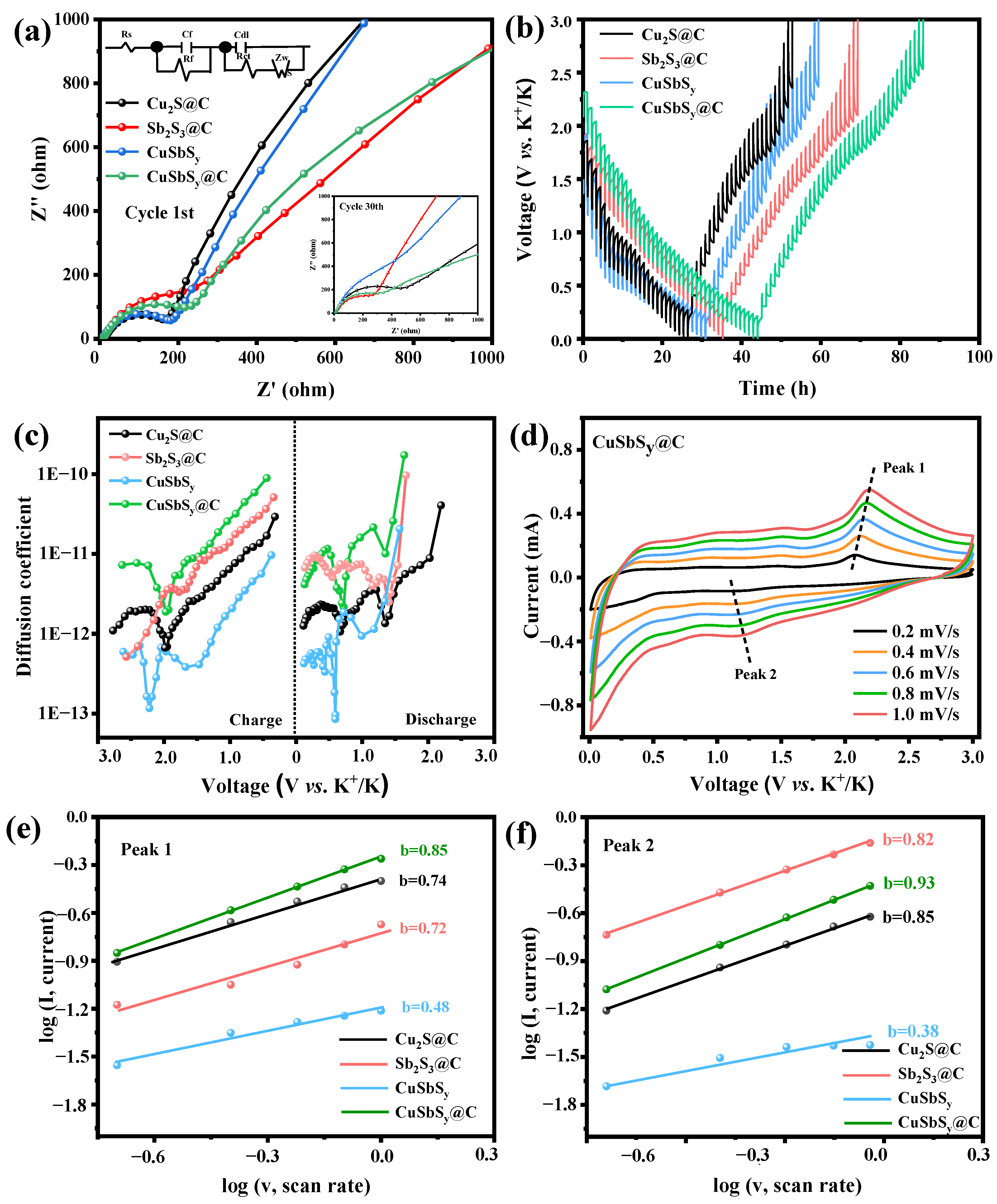
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Yin, J.; Wang, W.; Bayhan, Z.; Alshareef, H.N. Status of Rechargeable Potassium Batteries. Nano Energy 2021, 83, 105792. [Google Scholar] [CrossRef]
- Sha, M.; Liu, L.; Zhao, H.; Lei, Y. Anode Materials for Potassium-Ion Batteries: Current Status and Prospects. Carbon Energy 2020, 2, 350–369. [Google Scholar] [CrossRef]
- Anoopkumar, V.; John, B.; Td, M. Potassium-Ion Batteries: Key to Future Large-Scale Energy Storage? ACS Appl. Energy Mater. 2020, 3, 9478–9492. [Google Scholar]
- Zhang, Q.; Wang, Z.; Zhang, S.; Zhou, T.; Mao, J.; Guo, Z. Cathode Materials for Potassium-Ion Batteries: Current Status and Perspective. Electrochem. Energy Rev. 2018, 1, 625–658. [Google Scholar] [CrossRef]
- Khan, N.; Han, G.; Mazari, S.A. Carbon Nanotubes-Based Anode Materials for Potassium Ion Batteries: A Review. J. Electroanal. Chem. 2022, 907, 116051. [Google Scholar] [CrossRef]
- Hong, Z.; Maleki, H.; Ludwig, T.; Zhen, Y.; Wilhelm, M.; Lee, D.; Kim, K.-H.; Mathur, S. New Insights into Carbon-Based and Mxene Anodes for Na and K-Ion Storage: A Review. J. Energy Chem. 2021, 62, 660–691. [Google Scholar] [CrossRef]
- Ma, L.; Lv, Y.; Wu, J.; Xia, C.; Kang, Q.; Zhang, Y.; Liang, H.; Jin, Z. Recent Advances in Anode Materials for Potassium-Ion Batteries: A Review. Nano Res. 2021, 14, 4442–4470. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Liu, Q.; Wang, J.-Z.; Guo, Z.; Liu, H.-K. Cathode Materials for High-Performance Potassium-Ion Batteries. Cell Rep. Phys. Sci. 2021, 2, 100657. [Google Scholar] [CrossRef]
- Bin, D.-S.; Xu, Y.-S.; Guo, S.-J.; Sun, Y.-G.; Cao, A.-M.; Wan, L.-J. Manipulating Particle Chemistry for Hollow Carbon-Based Nanospheres: Synthesis Strategies, Mechanistic Insights, and Electrochemical Applications. Acc. Chem. Res. 2021, 54, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Huang, K.; Wu, X.; Zhao, W.; Wang, H.; Zhuang, Q.; Ju, Z. Novel Fabrication of N-Doped Hierarchically Porous Carbon with Exceptional Potassium Storage Properties. Carbon 2018, 131, 79–85. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Li, X.; Liu, L.; Yuan, M.; Cao, B.; Li, A.; Chen, X.; Yang, R.; Song, H. Woven Microsphere Architected by Carbon Nanotubes as High-Performance Potassium Ion Batteries Anodes. Chem. Eng. J. 2022, 429, 132272. [Google Scholar] [CrossRef]
- Desai, A.V.; Morris, R.E.; Armstrong, A.R. Advances in Organic Anode Materials for Na-/K-Ion Rechargeable Batteries. ChemSusChem 2020, 13, 4866–4884. [Google Scholar] [CrossRef]
- Wolfson, E.R.; Schkeryantz, L.; Moscarello, E.M.; Fernandez, J.P.; Paszek, J.; Wu, Y.; Hadad, C.M.; McGrier, P.L. Alkynyl-Based Covalent Organic Frameworks as High-Performance Anode Materials for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 41628–41636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, T.; Peng, L.; Yao, W.; Kang, F.; Shu, J.; Yang, C. A Compact Bi2WO6 Microflowers Anode for Potassium-Ion Storage: Taming a Sequential Phase Evolution toward Stable Electrochemical Cycling. Nano Energy 2021, 82, 105784. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, Z.; Fan, L.; Zhang, Q.; Ding, H.; Wang, L.; Yang, H.; Yu, X.; Lu, B. Nature of Bimetallic Oxide Sb2MoO6/rGo Anode for High-Performance Potassium-Ion Batteries. Adv. Sci. Lett. 2019, 6, 1900904. [Google Scholar]
- Chen, X.; Cheng, N.; Zhang, L.; Xiang, G.; Ding, Y.-L.; Liu, Z. Flower-Like Spherical FeCoS2 Coated by Reduced Graphene Oxide as Anode for High Performance Potassium Ion Storage. J. Alloys Compd. 2021, 861, 158458. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, L.; Kong, Z.; Zhai, Y.; Sun, X.; Wang, F.; Jing, Z.; He, X.; Dou, J.; Xu, L. In Situ Growth of CoS2/ZnS Nanoparticles on Graphene Sheets as an Ultralong Cycling Stability. ACS Appl. Mater. Interfaces 2022, 14, 15324–15336. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Su, H.; Jiang, S.; Zhang, J.; Li, D. Hierarchical Bimetallic Selenides CoSe2–MoSe2/rGo for Sodium/Potassium-Ion Batteries Anode: Insights into the Intercalation and Conversion Mechanism. Energy Environ. Mater. 2022, 5, 627–636. [Google Scholar] [CrossRef]
- Chu, J.; Yu, Q.; Yang, D.; Xing, L.; Lao, C.-Y.; Wang, M.; Han, K.; Liu, Z.; Zhang, L.; Du, W.; et al. Thickness-Control of Ultrathin Bimetallic Fe–Mo Selenide@N-Doped Carbon Core/Shell “Nano-Crisps” for High-Performance Potassium-Ion Batteries. Appl. Mater. Today 2018, 13, 344–351. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, Y.; Sun, Z.; Sun, H.; Guo, T.; Zhang, M.; Cui, L.; Jiang, K.; Peng, Y.; Sun, J. Bimetallic Fe-Ni Phosphide Carved Nanoframes toward Efficient Overall Water Splitting and Potassium-Ion Storage. Chem. Eng. J. 2020, 390, 124515. [Google Scholar] [CrossRef]
- Chen, K.-T.; Tuan, H.-Y. Bi–Sb Nanocrystals Embedded in Phosphorus as High-Performance Potassium Ion Battery Electrodes. ACS Nano 2020, 14, 11648–11661. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, C.; Zhao, H.; Lei, Y. Recent Advances in Ferromagnetic Metal Sulfides and Selenides as Anodes for Sodium- and Potassium-Ion Batteries. J. Mater. Chem. A 2021, 9, 9506–9534. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Rehman, Y.; Huang, T.; Zhao, J.; Gu, Q.; Mao, J.; Guo, Z. Phase Engineering of Nickel Sulfides to Boost Sodium- and Potassium-Ion Storage Performance. Adv. Funct. Mater. 2021, 31, 2010832. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, Y.; Li, W.; Fu, Q.; Wu, M.; Manske, E.; Kröger, J.; Lei, Y. Insights into the Crystallinity of Layer-Structured Transition Metal Dichalcogenides on Potassium Ion Battery Performance: A Case Study of Molybdenum Disulfide. Small 2019, 15, 1900497. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yan, C.; Zhao, H.; Lei, Y. Recent Advances in 2D Heterostructures as Advanced Electrode Materials for Potassium-Ion Batteries. Small Struct. 2022, 3, 2100221. [Google Scholar] [CrossRef]
- Liu, Q.; Han, F.; Zhou, J.; Li, Y.; Chen, L.; Zhang, F.; Zhou, D.; Ye, C.; Yang, J.; Wu, X.; et al. Boosting the Potassium-Ion Storage Performance in Soft Carbon Anodes by the Synergistic Effect of Optimized Molten Salt Medium and N/S Dual-Doping. ACS Appl. Mater. Interfaces 2020, 12, 20838–20848. [Google Scholar] [CrossRef]
- Huang, H.; Etogo, C.A.; Chen, C.; Bi, R.; Zhang, L. Realizing Fast Diffusion Kinetics Based on Three-Dimensional Ordered Macroporous Cu9S5@C for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 36982–36991. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Tong, H.; Wu, S.; Wang, D.; Liu, B.; Cheng, L.; Lin, Z.; Hu, L.; Wang, H.; et al. Structural Engineering of Sulfur-Doped Carbon Encapsulated Bismuth Sulfide Core-Shell Structure for Enhanced Potassium Storage Performance. Nano Res. 2021, 14, 3545–3551. [Google Scholar] [CrossRef]
- Yang, S.H.; Park, S.-K.; Park, G.D.; Kim, J.H.; Kang, Y.C. Rational Synthesis of Uniform Yolk–Shell Ni–Fe Bimetallic Sulfide Nanoflakes@Porous Carbon Nanospheres as Advanced Anodes for High-Performance Potassium-/Sodium-Ion Batteries. Chem. Eng. J. 2021, 417, 127963. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Chen, C.; Tian, N.; Zhang, Q.; Zhang, B. Graphene Wrapped Cobalt/Tin Bimetallic Sulfides Composites with Abundant Active Facets and Extended Interlayer Spacing for Stable Sodium/Potassium Storage. Chem. Eng. J. 2022, 442, 136223. [Google Scholar] [CrossRef]
- Wang, J.; Fan, L.; Liu, Z.; Chen, S.; Zhang, Q.; Wang, L.; Yang, H.; Yu, X.; Lu, B. In Situ Alloying Strategy for Exceptional Potassium Ion Batteries. ACS Nano 2019, 13, 3703–3713. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Liu, Y.; Liu, S.; Li, X.; Yang, K.; Li, L. Decoration of Hollow Nitrogen-Doped Carbon Nanofibers with Tapered Rod-Shaped NiCo2S4 as a 3D Structural High-Rate and Long-Lifespan Self-Supported Anode Material for Potassium-Ion Batteries. J. Alloys Compd. 2020, 823, 153631. [Google Scholar] [CrossRef]
- Chang, C.-B.; Chen, K.-T.; Tuan, H.-Y. Large-Scale Synthesis of Few-Layered Copper Antimony Sulfide Nanosheets as Electrode Materials for High-Rate Potassium-Ion Storage. J. Colloid Interface Sci. 2022, 608, 984–994. [Google Scholar] [CrossRef]
- Yang, F.; Gao, H.; Hao, J.; Zhang, S.; Li, P.; Liu, Y.; Chen, J.; Guo, Z. Yolk–Shell Structured FeP@C Nanoboxes as Advanced Anode Materials for Rechargeable Lithium-/Potassium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1808291. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ong, S.J.H.; Yao, Q.; Shi, X.; Hou, K.; Xu, Z.J.; Guan, L. High-Rate and Ultralong Cycle-Life Potassium Ion Batteries Enabled by in Situ Engineering of Yolk–Shell FeS2@C Structure on Graphene Matrix. Adv. Energy Mater. 2018, 8, 1802565. [Google Scholar] [CrossRef]
- Chu, J.; Yu, Q.; Han, K.; Xing, L.; Gu, C.; Li, Y.; Bao, Y.; Wang, W. Yolk–Shell Structured FeS/MoS2@Nitrogen-Doped Carbon Nanocubes with Sufficient Internal Void Space as an Ultrastable Anode for Potassium-Ion Batteries. J. Mater. Chem. A 2020, 8, 23983–23993. [Google Scholar] [CrossRef]
- Wang, W.; Zhi, G.; Liu, L.; Cao, L.; Xie, J.; Sun, L.; Hao, L.; Yao, H. Preparation of Rod-Like CuSbS2 Particles by Soft-Template Synthesis and Electrochemical Performance toward Lithium Storage. J. Nanopart. Res. 2022, 24, 98. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Wang, Y.; Han, M. Hierarchical Hollow CuSbS2 Microspheres with Excellent Photoelectrochemical Properties. Mater. Lett.-X. 2021, 12, 100094. [Google Scholar] [CrossRef]
- Wan, L.; Ma, C.; Hu, K.; Zhou, R.; Mao, X.; Pan, S.; Wong, L.H.; Xu, J. Two-Stage Co-Evaporated CuSbS2 Thin Films for Solar Cells. J. Alloys Compd. 2016, 680, 182–190. [Google Scholar] [CrossRef]
- Smith, J.M.; Wren, J.C.; Odziemkowski, M.; Shoesmith, D.W. The Electrochemical Response of Preoxidized Copper in Aqueous Sulfide Solutions. J. Electrochem. Soc. 2007, 154, C431. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.; Wei, Y.; Xu, B. Achieving Stable and Fast Potassium Storage of Sb2S3@Mxene Anode Via Interfacial Bonding and Electrolyte Chemistry. Chem. Eng. J. 2023, 451, 138891. [Google Scholar] [CrossRef]
- Dutková, E.; Sayagués, M.J.; Fabián, M.; Kováč, J.; Kováč, J.; Baláž, M.; Stahorský, M. Mechanochemical Synthesis of Ternary Chalcogenide Chalcostibite CuSbS2 and Its Characterization. J. Mater. Sci. Mater. Electron. 2021, 32, 22898–22909. [Google Scholar] [CrossRef]
- Yi, Z.; Qian, Y.; Tian, J.; Shen, K.; Lin, N.; Qian, Y. Self-Templating Growth of Sb2Se3@C Microtube: A Convention-Alloying-Type Anode Material for Enhanced K-Ion Batteries. J. Mater. Chem. A 2019, 7, 12283–12291. [Google Scholar] [CrossRef]
- Wu, X.-F.; Li, Z.-J.; Liu, J.-X.; Luo, W.; Gaumet, J.-J.; Mai, L.-Q. Defect Engineering of Hierarchical Porous Carbon Microspheres for Potassium-Ion Storage. Rare Metals 2022, 41, 3446–3455. [Google Scholar] [CrossRef]
- Wang, H.; Xing, Z.; Hu, Z.; Zhang, Y.; Hu, Y.; Sun, Y.; Ju, Z.; Zhuang, Q. Sn-Based Submicron-Particles Encapsulated in Porous Reduced Graphene Oxide Network: Advanced Anodes for High-Rate and Long Life Potassium-Ion Batteries. Appl. Mater. Today 2019, 15, 58–66. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Gong, Z.; Yin, Z.; Guo, X.; Liu, J.; Mao, C.; Zhang, Z.; Li, G. A Robust Strategy for Engineering Fe7S8/C Hybrid Nanocages Reinforced by Defect-Rich MoS2 Nanosheets for Superior Potassium-Ion Storage. ACS Nano 2020, 14, 16046–16056. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, B.; Yu, Q.; Zhang, Y.; Zhang, D.; Li, Y.; Wang, W. Amorphous Cobalt Sulfide/N-Doped Carbon Core/Shell Nanoparticles as an Anode Material for Potassium-Ion Storage. J. Mater. Sci. 2020, 55, 15213–15221. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, J.; Gao, Y.; Gao, J.; Suo, G.; Zuo, P.; Wang, W.; Yin, G. Iron Sulfide/Carbon Hybrid Cluster as an Anode for Potassium-Ion Storage. J. Alloys Compd. 2018, 766, 1086–1091. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Xie, D.; Lin, Z.; Zheng, J.; Zheng, F.; Li, Y.; Liu, Y.; Yang, C.; Liu, M. Chemically Activated Hollow Carbon Nanospheres as a High-Performance Anode Material for Potassium Ion Batteries. J. Mater. Chem. A 2018, 6, 24317–24323. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, Q.B.; Huang, Z.D.; Oh, S.W.; Kim, J.K. SnO2–Graphene–Carbon Nanotube Mixture for Anode Material with Improved Rate Capacities. Carbon 2011, 49, 4524–4534. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Zhang, C. Hierarchical MnMoO4@Nitrogen-Doped Carbon Core-Shell Microspheres for Lithium/Potassium-Ion Batteries. J. Alloys Compd. 2022, 893, 162336. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Q.; Tian, K.-H.; Duan, C.-Q.; Wang, Z.-Y.; Liu, Y.-G. Ultrafine Nano-Scale Cu2Sb Alloy Confined in Three-Dimensional Porous Carbon as an Anode for Sodium-Ion and Potassium-Ion Batteries. Int. J. Miner. Metall. Mater. 2021, 28, 1666–1674. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, S.; Yang, H.; Sheng, B.; Xu, R.; Wang, Q.; Yu, Y. Boosting Potassium Storage Performance of the Cu2S Anode Via Morphology Engineering and Electrolyte Chemistry. ACS Nano 2020, 14, 6024–6033. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zhang, Y.; Li, H.; Zhang, Z.; Li, L.; Yu, Z.; Zhang, W. ZIF-8/ZIF-67-Derived 3D Amorphous Carbon-Encapsulated CoS/Ncnts Supported on CoS-Coated Carbon Nanofibers as an Advanced Potassium-Ion Battery Anode. J. Mater. Chem. A 2019, 7, 5504–5512. [Google Scholar] [CrossRef]
- Merrill, M.D.; Logan, B.E. Electrolyte Effects on Hydrogen Evolution and Solution Resistance in Microbial Electrolysis Cells. J. Power Sources 2009, 191, 203–208. [Google Scholar] [CrossRef]
- Selvi, V.E.; Seenivasan, H.; Rajam, K.S. Electrochemical Corrosion Behavior of Pulse and Dc Electrodeposited Co–P Coatings. Surf. Coat. Technol. 2012, 206, 2199–2206. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, C.; Li, Y.; Zhang, C.; Zhao, H.; Kaiser, U.; Lei, Y. Ultrahigh-Rate and Ultralong-Duration Sodium Storage Enabled by Sodiation-Driven Reconfiguration. Adv. Energy Mater. 2023, 2204324. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, X.; Kang, B.; Wang, H.-E.; Xiong, P.; Chen, Q.; Wei, M.; Li, N.; Qian, Q.; et al. Structural Engineering of Tin Sulfides Anchored on Nitrogen/Phosphorus Dual-Doped Carbon Nanofibres in Sodium/Potassium-Ion Batteries. Carbon 2022, 189, 46–56. [Google Scholar] [CrossRef]
- Yang, G.; Yan, C.; Hu, P.; Fu, Q.; Zhao, H.; Lei, Y. Synthesis of CoSe2 Reinforced Nitrogen-Doped Carbon Composites as Advanced Anodes for Potassium-Ion Batteries. Inorg. Chem. Front. 2022, 9, 3719–3727. [Google Scholar] [CrossRef]
- Tao, L.; Yang, Y.; Wang, H.; Zheng, Y.; Hao, H.; Song, W.; Shi, J.; Huang, M.; Mitlin, D. Sulfur-Nitrogen Rich Carbon as Stable High Capacity Potassium Ion Battery Anode: Performance and Storage Mechanisms. Energy Storage Mater. 2020, 27, 212–225. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Dong, Y.; Yang, G.; Chao, X.; He, S.; Zhao, H.; Fu, Q.; Lei, Y. Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage. Batteries 2023, 9, 238. https://doi.org/10.3390/batteries9050238
Hu P, Dong Y, Yang G, Chao X, He S, Zhao H, Fu Q, Lei Y. Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage. Batteries. 2023; 9(5):238. https://doi.org/10.3390/batteries9050238
Chicago/Turabian StyleHu, Ping, Yulian Dong, Guowei Yang, Xin Chao, Shijiang He, Huaping Zhao, Qun Fu, and Yong Lei. 2023. "Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage" Batteries 9, no. 5: 238. https://doi.org/10.3390/batteries9050238
APA StyleHu, P., Dong, Y., Yang, G., Chao, X., He, S., Zhao, H., Fu, Q., & Lei, Y. (2023). Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage. Batteries, 9(5), 238. https://doi.org/10.3390/batteries9050238






