Ultra-Thick Cathodes for High-Energy Lithium-Ion Batteries Based on Aluminium Foams—Microstructural Evolution during Densification and Its Impact on the Electrochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
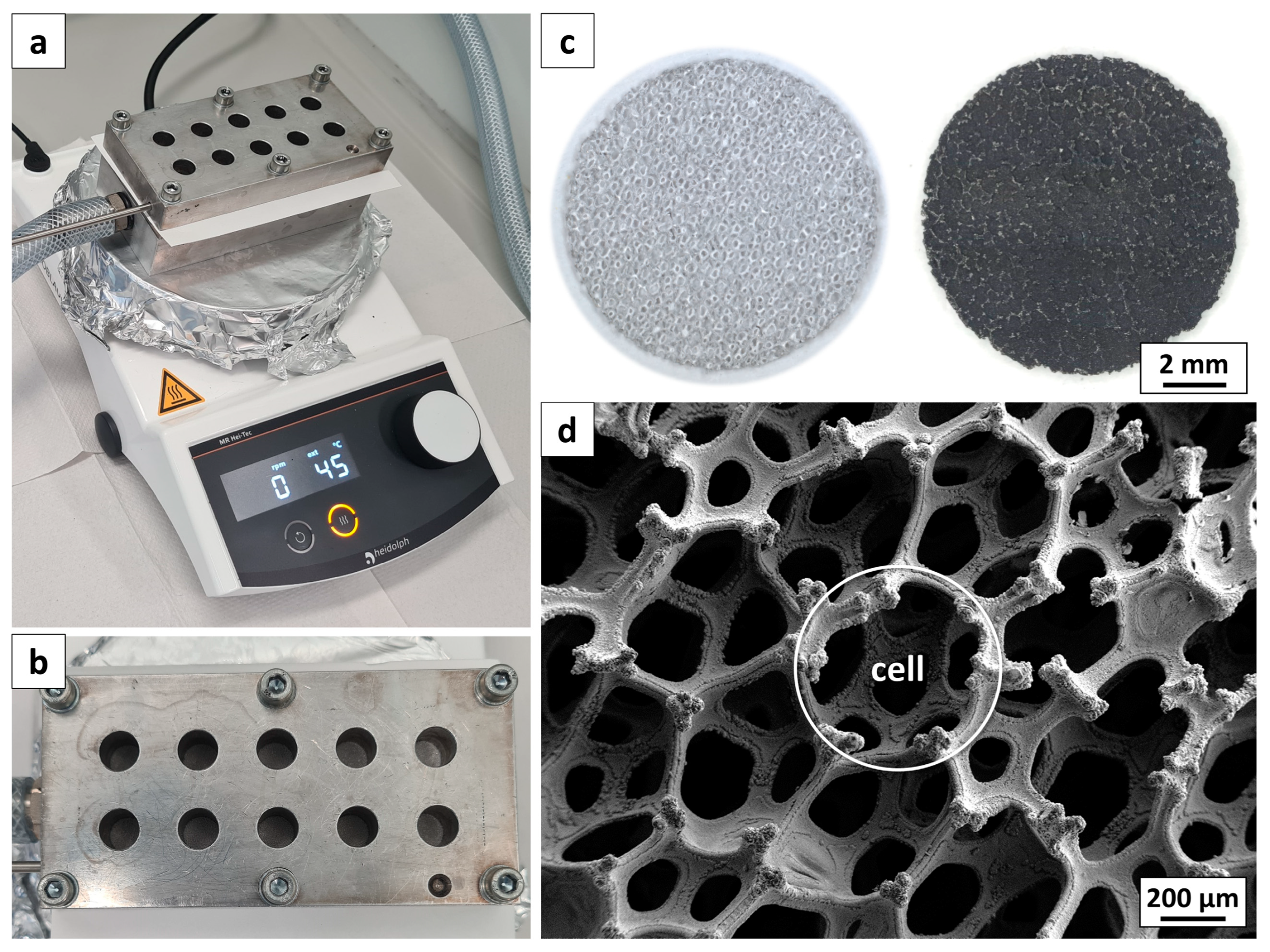
2.2. Electrode Preparation
2.3. Microstructure Analysis
2.4. Electrochemical Characterization
3. Results and Discussion
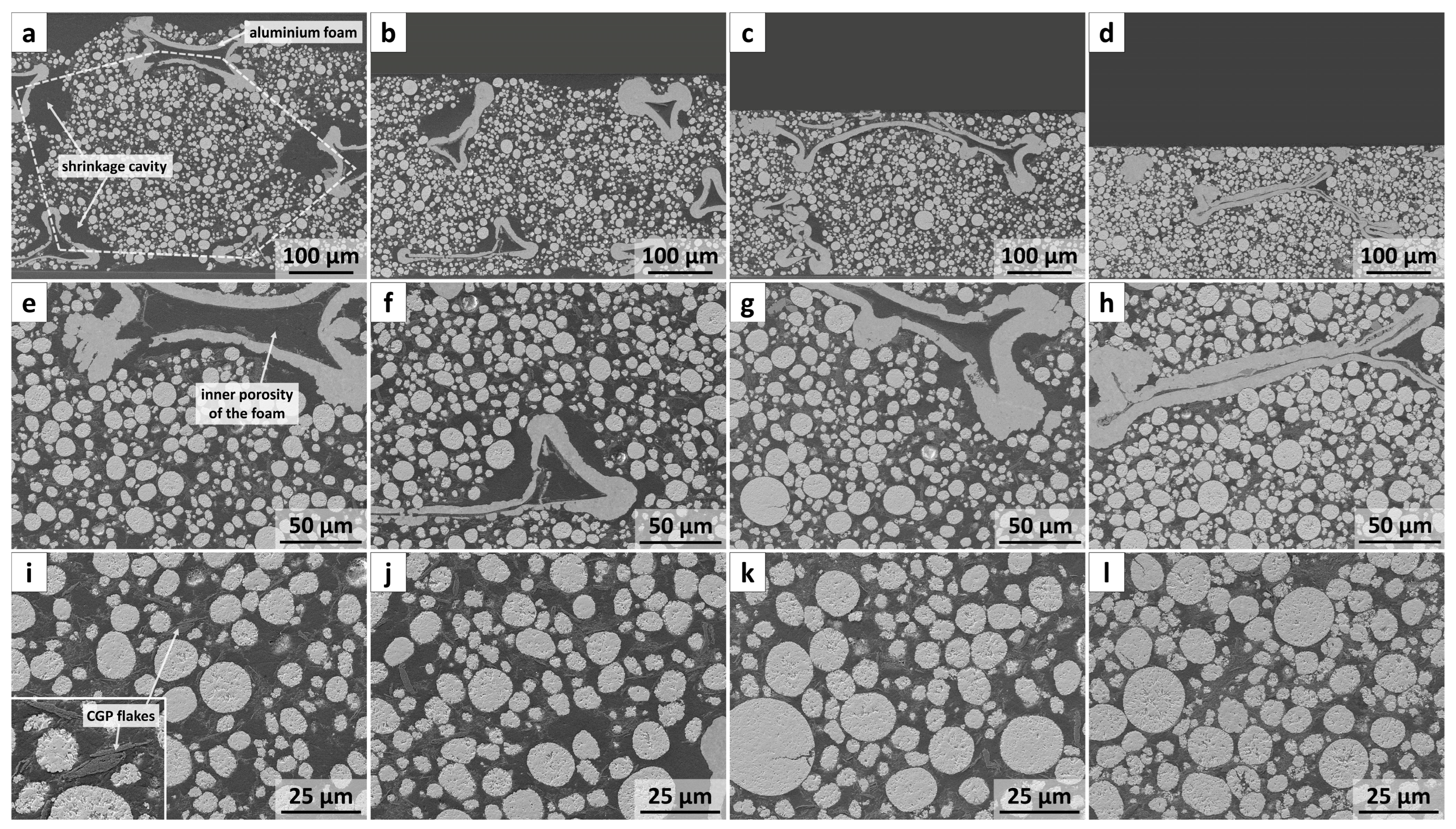
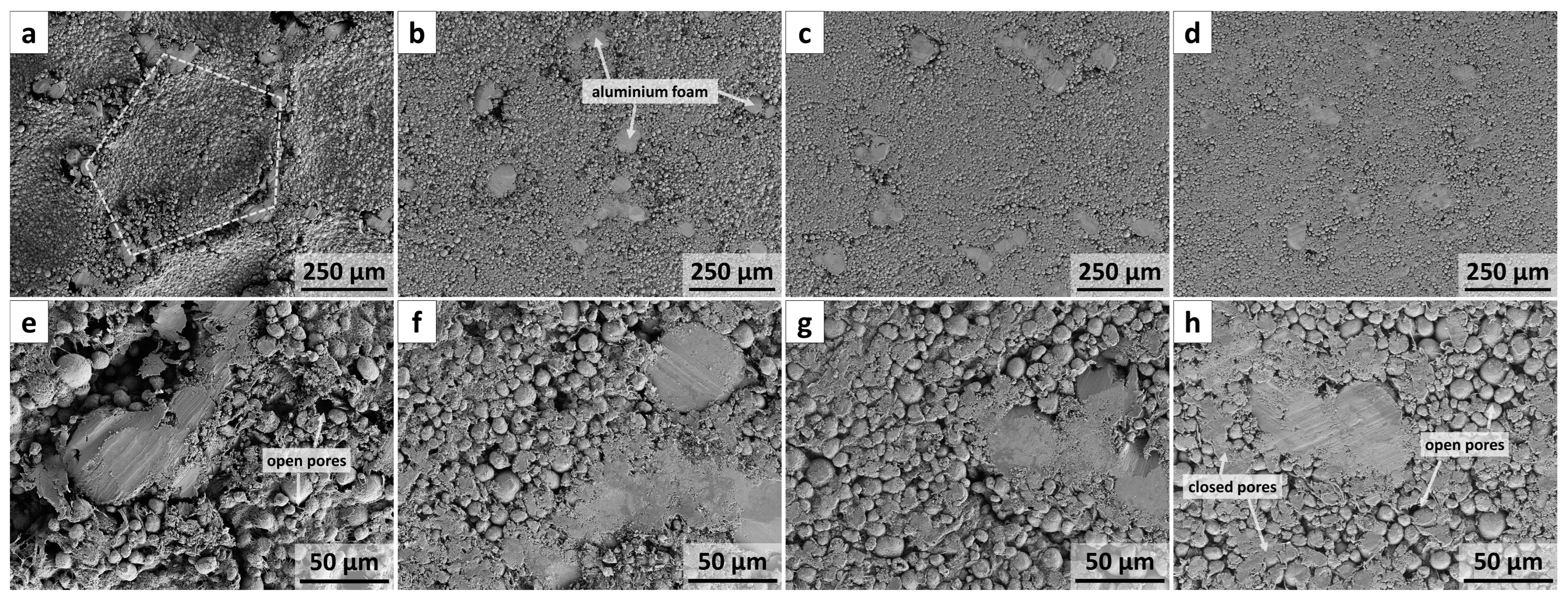
3.1. Microstructure Investigation
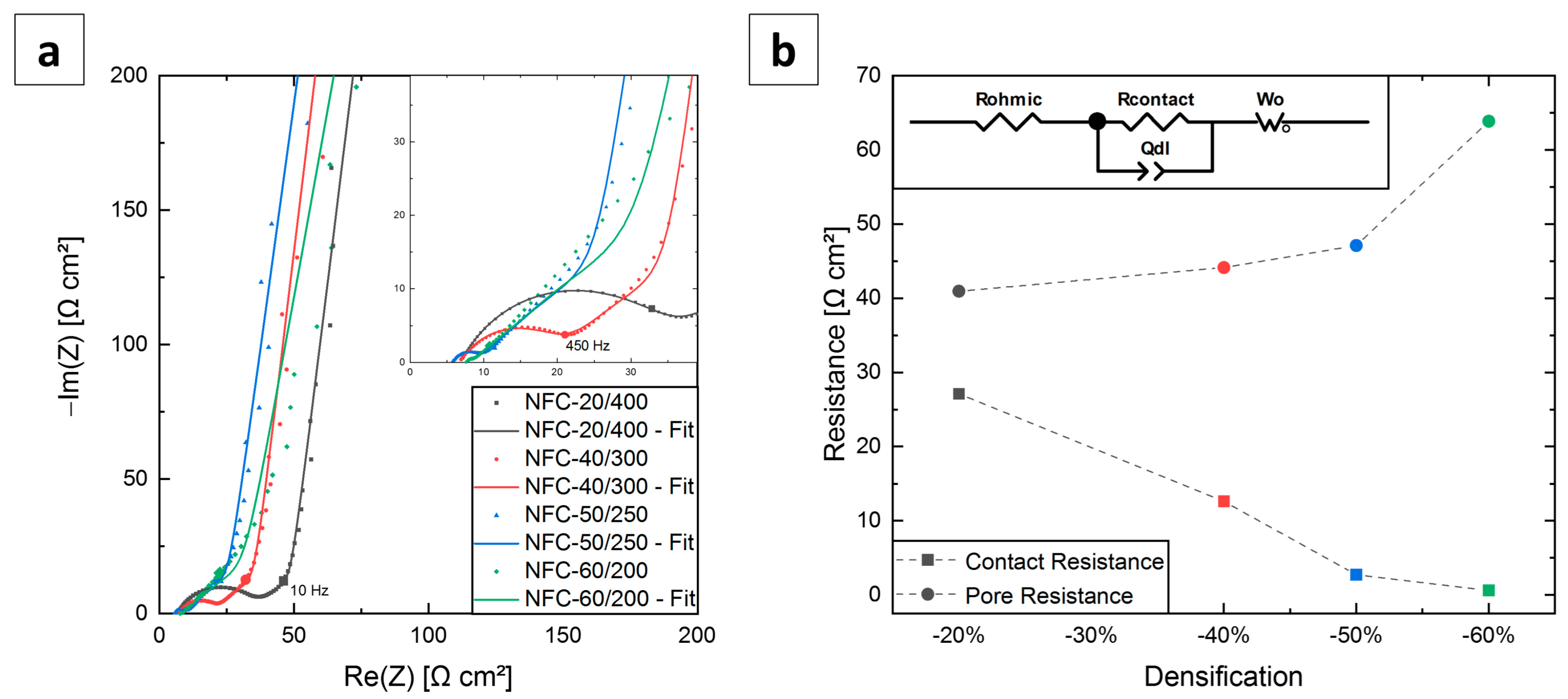
3.2. Electrochemical Impedance Spectroscopy
3.3. C-Rate Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. Weinheim. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.; Edström, K.; Ayerbe, E.; Berecibar, M.; Bhowmik, A.; Castelli, I.E.; Clark, S.; Dominko, R.; Erakca, M.; Franco, A.A.; et al. Rechargeable Batteries of the Future—The State of the Art from a BATTERY 2030+ Perspective. Adv. Energy Mater. 2022, 12, 2102904. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2016, 163, A138–A149. [Google Scholar] [CrossRef]
- Xu, M.; Reichman, B.; Wang, X. Modeling the effect of electrode thickness on the performance of lithium-ion batteries with experimental validation. Energy 2019, 186, 115864. [Google Scholar] [CrossRef]
- Yu, S.; Kim, S.; Kim, T.Y.; Nam, J.H.; Cho, W.I. Model Prediction and Experiments for the Electrode Design Optimization of LiFePO4/Graphite Electrodes in High Capacity Lithium-ion Batteries. B. Kor. Chem. Soc. 2013, 34, 79–88. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L.; Daniel, C.; Kalnaus, S.; Li, J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J. Appl. Electrochem. 2017, 47, 405–415. [Google Scholar] [CrossRef]
- Issatayev, N.; Nuspeissova, A.; Kalimuldina, G.; Bakenov, Z. Three-dimensional foam-type current collectors for rechargeable batteries: A short review. J. Power Sources Adv. 2021, 10, 100065. [Google Scholar] [CrossRef]
- Abe, H.; Kubota, M.; Nemoto, M.; Masuda, Y.; Tanaka, Y.; Munakata, H.; Kanamura, K. High-capacity thick cathode with a porous aluminum current collector for lithium secondary batteries. J. Power Sources 2016, 334, 78–85. [Google Scholar] [CrossRef]
- Jin, S.; Jiang, Y.; Ji, H.; Yu, Y. Advanced 3D Current Collectors for Lithium-Based Batteries. Adv. Mater. Weinheim. 2018, 30, 1802014. [Google Scholar] [CrossRef]
- Yue, Y.; Liang, H. 3D Current Collectors for Lithium-Ion Batteries: A Topical Review. Small Methods 2018, 2, 1800056. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Khau, B.V.; Davies, G.; Hertzberg, B.; Steingart, D.A.; Arias, A.C. A High Areal Capacity Flexible Lithium-Ion Battery with a Strain-Compliant Design. Adv. Energy Mater. 2015, 5, 1401389. [Google Scholar] [CrossRef]
- Shaijumon, M.M.; Perre, E.; Daffos, B.; Taberna, P.-L.; Tarascon, J.-M.; Simon, P. Nanoarchitectured 3D cathodes for Li-ion microbatteries. Adv. Mater. Weinheim. 2010, 22, 4978–4981. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, D.; Ji, S.; Huo, Y.; Liu, J. FeF 3 @C nanotube arrays grown on carbon fabric as a free-standing cathode for lithium-ion batteries. Mater. Chem. Front. 2022, 6, 3512–3521. [Google Scholar] [CrossRef]
- Poetz, S.; Fuchsbichler, B.; Schmuck, M.; Koller, S. Development of a 3d current collector for the positive electrode in lithium-ion batteries. J Appl. Electrochem. 2014, 44, 989–994. [Google Scholar] [CrossRef]
- Wang, J.S.; Liu, P.; Sherman, E.; Verbrugge, M.; Tataria, H. Formulation and characterization of ultra-thick electrodes for high energy lithium-ion batteries employing tailored metal foams. J. Power Sources 2011, 196, 8714–8718. [Google Scholar] [CrossRef]
- Fritsch, M.; Standke, G.; Heubner, C.; Langklotz, U.; Michaelis, A. 3D-cathode design with foam-like aluminum current collector for high energy density lithium-ion batteries. J. Energy Storage 2018, 16, 125–132. [Google Scholar] [CrossRef]
- Yang, G.F.; Song, K.Y.; Joo, S.K. A metal foam as a current collector for high power and high capacity lithium iron phosphate batteries. J. Mater. Chem. A 2014, 2, 19648–19652. [Google Scholar] [CrossRef]
- Song, K.Y.; Joo, S.K. High electrochemical performance bendable Li secondary batteries based on a three-dimensional metal foam-type current collector. Mater. Res. Bull. 2017, 94, 328–334. [Google Scholar] [CrossRef]
- Yang, G.-F.; Song, K.-Y.; Joo, S.-K. Ultra-thick Li-ion battery electrodes using different cell size of metal foam current collectors. RSC Adv. 2015, 5, 16702–16706. [Google Scholar] [CrossRef]
- Feng, H.; Chen, Y.; Wang, Y. Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors. Batteries 2019, 5, 21. [Google Scholar] [CrossRef]
- Noelle, D.J.; Wang, M.; Qiao, Y. Improved safety and mechanical characterizations of thick lithium-ion battery electrodes structured with porous metal current collectors. J. Power Sources 2018, 399, 125–132. [Google Scholar] [CrossRef]
- Yang, G.F.; Song, J.S.; Kim, H.Y.; Joo, S.K. Metal Foam as Positive Electrode Current Collector for LiFePO 4 -Based Li-Ion Battery. Jpn. J. Appl. Phys. 2013, 52, 10MB13. [Google Scholar] [CrossRef]
- Song, K.Y.; Jang, G.S.; Tao, J.; Lee, J.H.; Joo, S.K. Effects of Electrode Thickness on Three-Dimensional NiCrAl Metal Foam Cathode for Lithium Ion Battery. J. Nanosci. Nanotechnol. 2018, 18, 992–998. [Google Scholar] [CrossRef]
- Song, K.Y.; Jang, G.S.; Tao, J.; Lee, J.H.; Joo, S.K. Influence of Conductive Carbon Content Using a Three-Dimensional Foam-Type Current Collector for Lithium Ion Battery. J. Electrochem. Soc. 2016, 163, A2981–A2987. [Google Scholar] [CrossRef]
- Schmidt, D.; Kamlah, M.; Knoblauch, V. Highly densified NCM-cathodes for high energy Li-ion batteries: Microstructural evolution during densification and its influence on the performance of the electrodes. J. Energy Storage 2018, 17, 213–223. [Google Scholar] [CrossRef]
- Ademmer, M.; Prifling, B.; Weller, M.; Hilger, A.; Osenberg, M.; Manke, I.; Knoblauch, V.; Schmidt, V. Investigating the influence of the calendering process on the 3D microstructure of single-layer and two-layer cathodes in lithium-ion batteries using synchrotron tomography. J. Power Sources 2022, 548, 231960. [Google Scholar] [CrossRef]
- Choi, J.; Son, B.; Ryou, M.-H.; Kim, S.H.; Ko, J.M.; Lee, Y.M. Effect of LiCoO2 Cathode Density and Thickness on Electrochemical Performance of Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2013, 4, 27–33. [Google Scholar] [CrossRef]
- Yang, G.-F.; Joo, S.-K. Calendering effect on the electrochemical performances of the thick Li-ion battery electrodes using a three dimensional Ni alloy foam current collector. Electrochim. Acta 2015, 170, 263–268. [Google Scholar] [CrossRef]
- Hafner, C.; Bernthaler, T.; Knoblauch, V.; Schneider, G. The Materialographic Preparation and Microstructure Characterization of Lithium Ion Accumulators. Pract. Metallogr. 2012, 49, 75–85. [Google Scholar] [CrossRef]
- Weisenberger, C.; Guth, G.; Bernthaler, T.; Knoblauch, V. New Quality Evaluation Approaches for Lithium Ion Batteries Using the Interference Layer Metallography in Combination with Quantitative Structural Analysis. Pract. Metallogr. 2014, 51, 5–31. [Google Scholar] [CrossRef]
- Bolsinger, M.; Weller, M.; Ruck, S.; Kaya, P.; Riegel, H.; Knoblauch, V. Selective surface treatment by means of IR-laser—A new approach to enhance the rate capability of cathodes for Li-ion batteries. Electrochim. Acta 2020, 330, 135163. [Google Scholar] [CrossRef]
- Ogihara, N.; Kawauchi, S.; Okuda, C.; Itou, Y.; Takeuchi, Y.; Ukyo, Y. Theoretical and Experimental Analysis of Porous Electrodes for Lithium-Ion Batteries by Electrochemical Impedance Spectroscopy Using a Symmetric Cell. J. Electrochem. Soc. 2012, 159, A1034–A1039. [Google Scholar] [CrossRef]
- Landesfeind, J.; Pritzl, D.; Gasteiger, H.A. An Analysis Protocol for Three-Electrode Li-Ion Battery Impedance Spectra: Part I. Analysis of a High-Voltage Positive Electrode. J. Electrochem. Soc. 2017, 164, A1773–A1783. [Google Scholar] [CrossRef]
- Pritzl, D.; Landesfeind, J.; Solchenbach, S.; Gasteiger, H.A. An Analysis Protocol for Three-Electrode Li-Ion Battery Impedance Spectra: Part II. Analysis of a Graphite Anode Cycled vs. LNMO. J. Electrochem. Soc. 2018, 165, A2145–A2153. [Google Scholar] [CrossRef]
- Carbonari, G.; Müller, V.; Scurtu, R.-G.; Memm, M.; Hoffmann, A.; Wohlfahrt-Mehrens, M. Communication—Edge Quality Contribution on the Electrical Impedance of Lithium-Ion Batteries Electrodes. J. Electrochem. Soc. 2020, 167, 080504. [Google Scholar] [CrossRef]
- Gaberscek, M.; Moskon, J.; Erjavec, B.; Dominko, R.; Jamnik, J. The Importance of Interphase Contacts in Li Ion Electrodes: The Meaning of the High-Frequency Impedance Arc. Electrochem. Solid State Lett. 2008, 11, A170. [Google Scholar] [CrossRef]
- Raccichini, R.; Furness, L.; Dibden, J.W.; Owen, J.R.; García-Araez, N. Impedance Characterization of the Transport Properties of Electrolytes Contained within Porous Electrodes and Separators Useful for Li-S Batteries. J. Electrochem. Soc. 2018, 165, A2741–A2749. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373–A1387. [Google Scholar] [CrossRef]
- Cronau, M.; Paulus, A.; Pescara, L.P.; Kroll, M.; Renz, D.; Mekontso, J.A.; Marx, A.; Roling, B. What Limits the Rate Capability of Ultrathick Composite Electrodes in Lithium-Ion Batteries? A Case Study on the Thickness-Dependent Impedance of LiCoO2 Cathodes. Batter. Supercaps 2022, 5, e202200194. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.-C.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef]
- Zheng, H.; Tan, L.; Liu, G.; Song, X.; Battaglia, V.S. Calendering effects on the physical and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 cathode. J. Power Sources 2012, 208, 52–57. [Google Scholar] [CrossRef]
- Kremer, L.S.; Hoffmann, A.; Danner, T.; Hein, S.; Prifling, B.; Westhoff, D.; Dreer, C.; Latz, A.; Schmidt, V.; Wohlfahrt-Mehrens, M. Manufacturing Process for Improved Ultra-Thick Cathodes in High-Energy Lithium-Ion Batteries. Energy Technol. 2020, 8, 1900167. [Google Scholar] [CrossRef]
- Hoffmann, A.; Heider, E.A.; Dreer, C.; Pfeifer, C.; Wohlfahrt-Mehrens, M. Influence of the Mixing and Dispersing Process on the Slurry Properties and the Microstructure and Performance of Ultrathick Cathodes for Lithium-Ion Batteries. Energy Technol. 2022, 11, 2200484. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, J.; Fan, S.; Xing, X.; Deng, L.; Gong, Y. SiOxCy Microspheres with Homogeneous Atom Distribution for a High-Performance Li-Ion Battery. Nano Lett. 2022, 22, 9559–9565. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Wang, B. Preparation and Electrochemical Performance of LiFePO4-based Electrode Using Three-Dimensional Porous Current Collector. Int. J.Electrochem. Sci. 2012, 7, 8753–8760. [Google Scholar]
- Zhang, H.; Yu, X.; Braun, P.V. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 2011, 6, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Nurpeissova, A.; Adi, A.; Aishova, A.; Mukanova, A.; Kim, S.-S.; Bakenov, Z. Synergistic effect of 3D current collector structure and Ni inactive matrix on the electrochemical performances of Sn-based anodes for lithium-ion batteries. Mater. Today Energy 2020, 16, 100397. [Google Scholar] [CrossRef]
- Ovejas, V.J.; Cuadras, A. State of charge dependency of the overvoltage generated in commercial Li-ion cells. J. Power Sources 2019, 418, 176–185. [Google Scholar] [CrossRef]
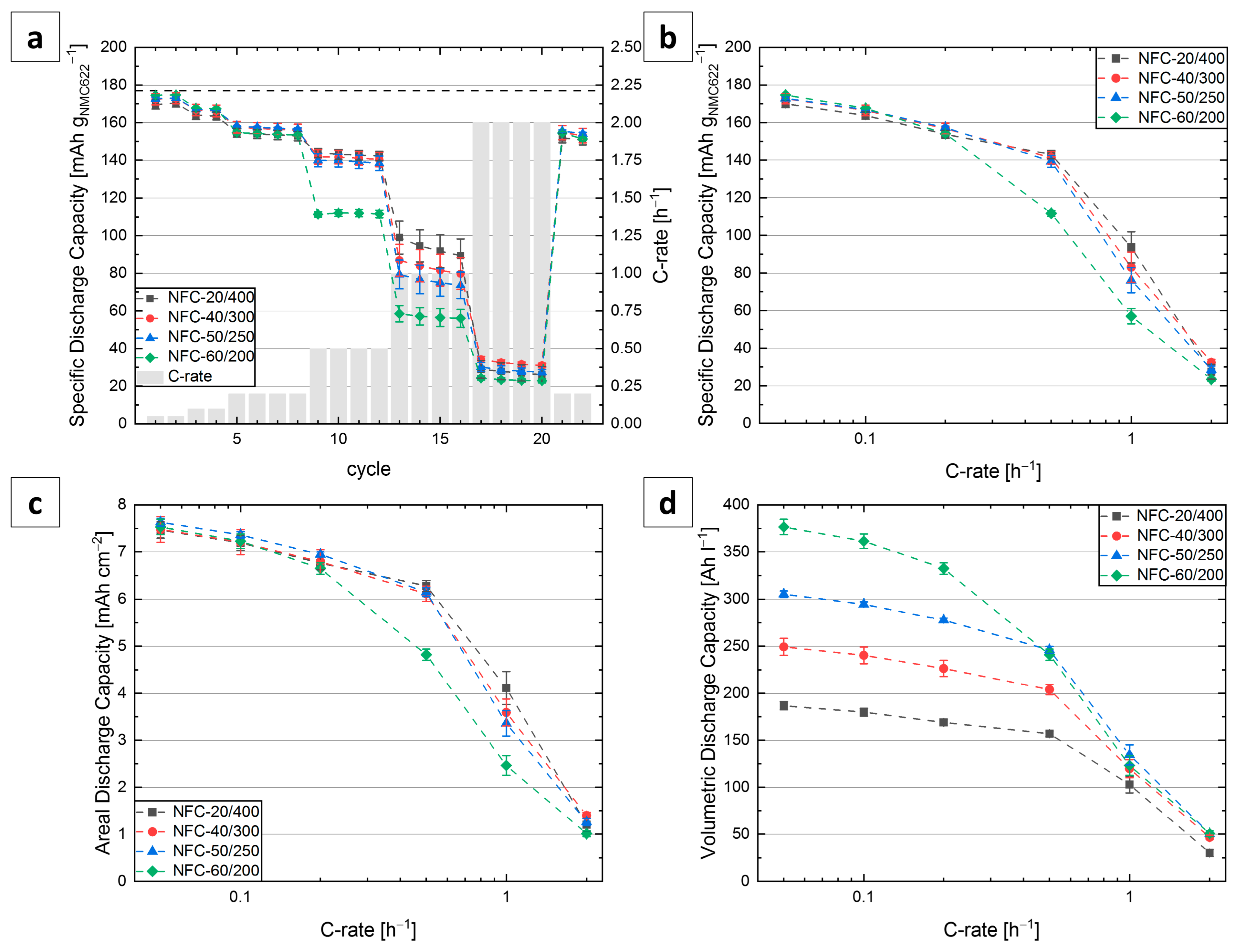

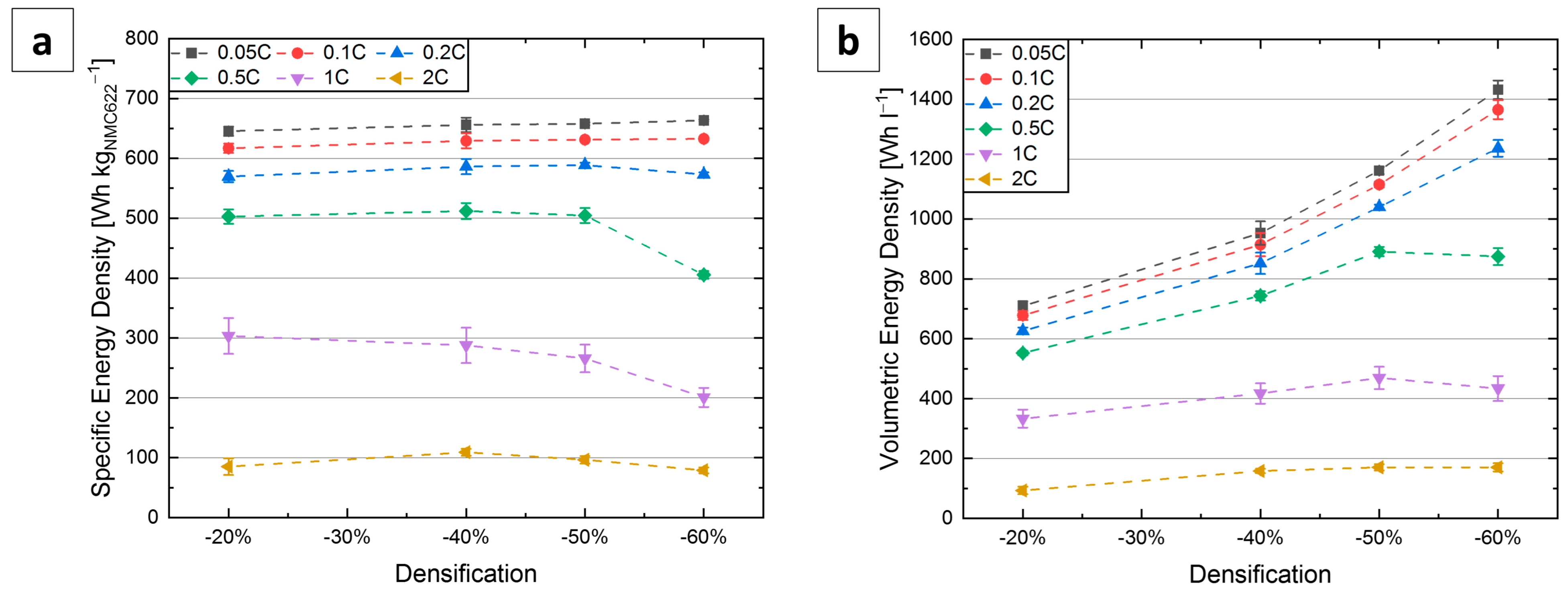
| Notation | Degree of Densification [%] | Thickness t [µm] | Electrode Porosity εelectrode [vol.-%] | Electrode Density ρelectrode [g cm−3] | NMC622 Loading [mg cm−2] |
|---|---|---|---|---|---|
| NFC-20/400 | 20 | 400 | 65 | 1.37 | 44.0 |
| NFC-40/300 | 40 | 300 | 53 | 1.82 | 43.3 |
| NFC-50/250 | 50 | 250 | 44 | 2.20 | 44.2 |
| NFC-60/200 | 60 | 200 | 30 | 2.71 | 43.1 |
| Notation | Rohmic [Ω cm2] | Rcontact [Ω cm2] | Rpore [Ω cm2] |
|---|---|---|---|
| NFC-20/400 | 6.8 | 27.1 | 40.9 |
| NFC-40/300 | 6.7 | 12.6 | 44.1 |
| NFC-50/250 | 5.8 | 2.7 | 47.1 |
| NFC-60/200 | 7.5 | 0.6 | 63.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oehm, J.; Kamlah, M.; Knoblauch, V. Ultra-Thick Cathodes for High-Energy Lithium-Ion Batteries Based on Aluminium Foams—Microstructural Evolution during Densification and Its Impact on the Electrochemical Properties. Batteries 2023, 9, 303. https://doi.org/10.3390/batteries9060303
Oehm J, Kamlah M, Knoblauch V. Ultra-Thick Cathodes for High-Energy Lithium-Ion Batteries Based on Aluminium Foams—Microstructural Evolution during Densification and Its Impact on the Electrochemical Properties. Batteries. 2023; 9(6):303. https://doi.org/10.3390/batteries9060303
Chicago/Turabian StyleOehm, Jonas, Marc Kamlah, and Volker Knoblauch. 2023. "Ultra-Thick Cathodes for High-Energy Lithium-Ion Batteries Based on Aluminium Foams—Microstructural Evolution during Densification and Its Impact on the Electrochemical Properties" Batteries 9, no. 6: 303. https://doi.org/10.3390/batteries9060303
APA StyleOehm, J., Kamlah, M., & Knoblauch, V. (2023). Ultra-Thick Cathodes for High-Energy Lithium-Ion Batteries Based on Aluminium Foams—Microstructural Evolution during Densification and Its Impact on the Electrochemical Properties. Batteries, 9(6), 303. https://doi.org/10.3390/batteries9060303


.png)




