Polydopamine-Modified Carboxymethyl Cellulose as Advanced Polysulfide Trapping Binder
Abstract
:1. Introduction
2. Results and Discussion
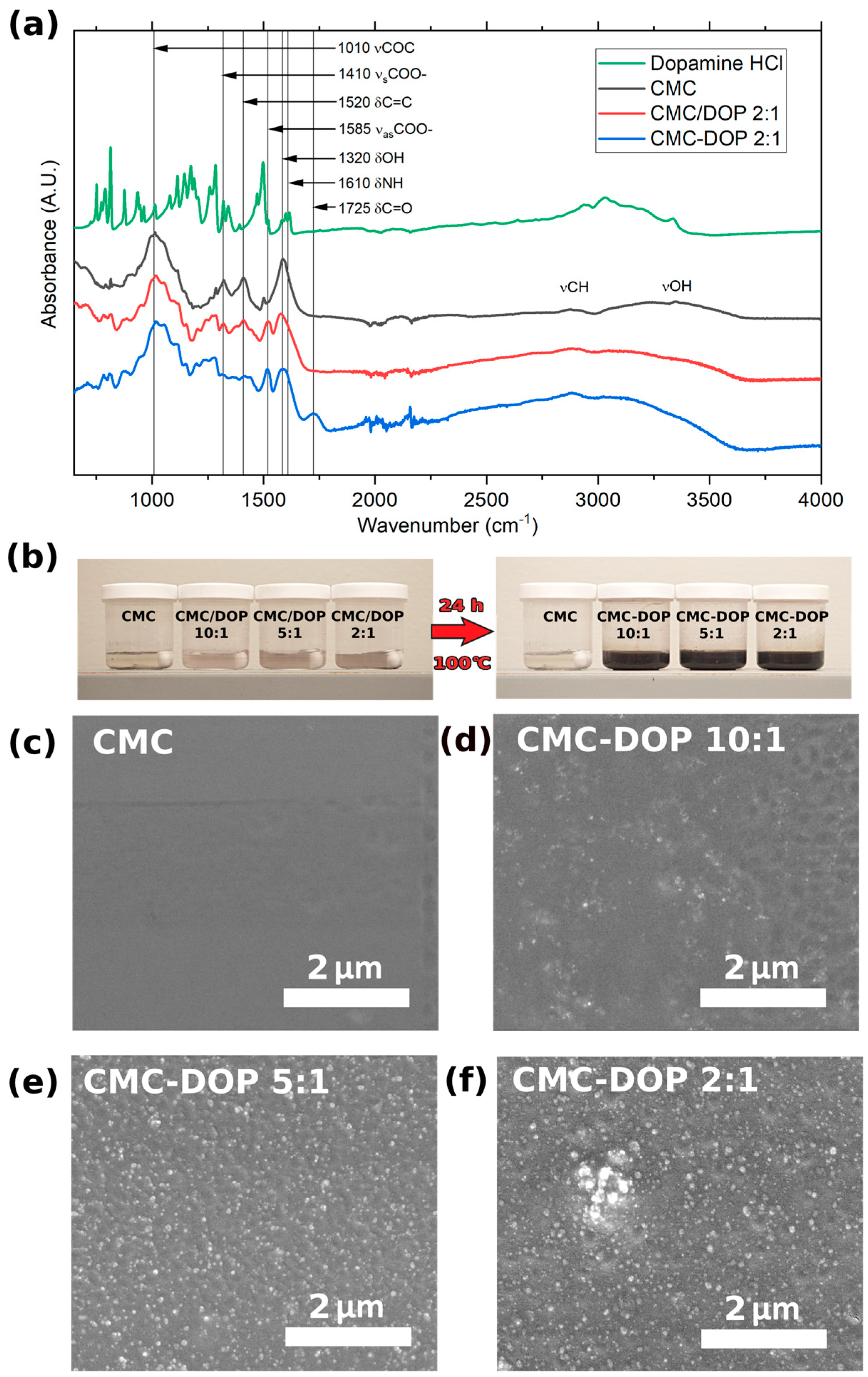
2.1. Synthesis
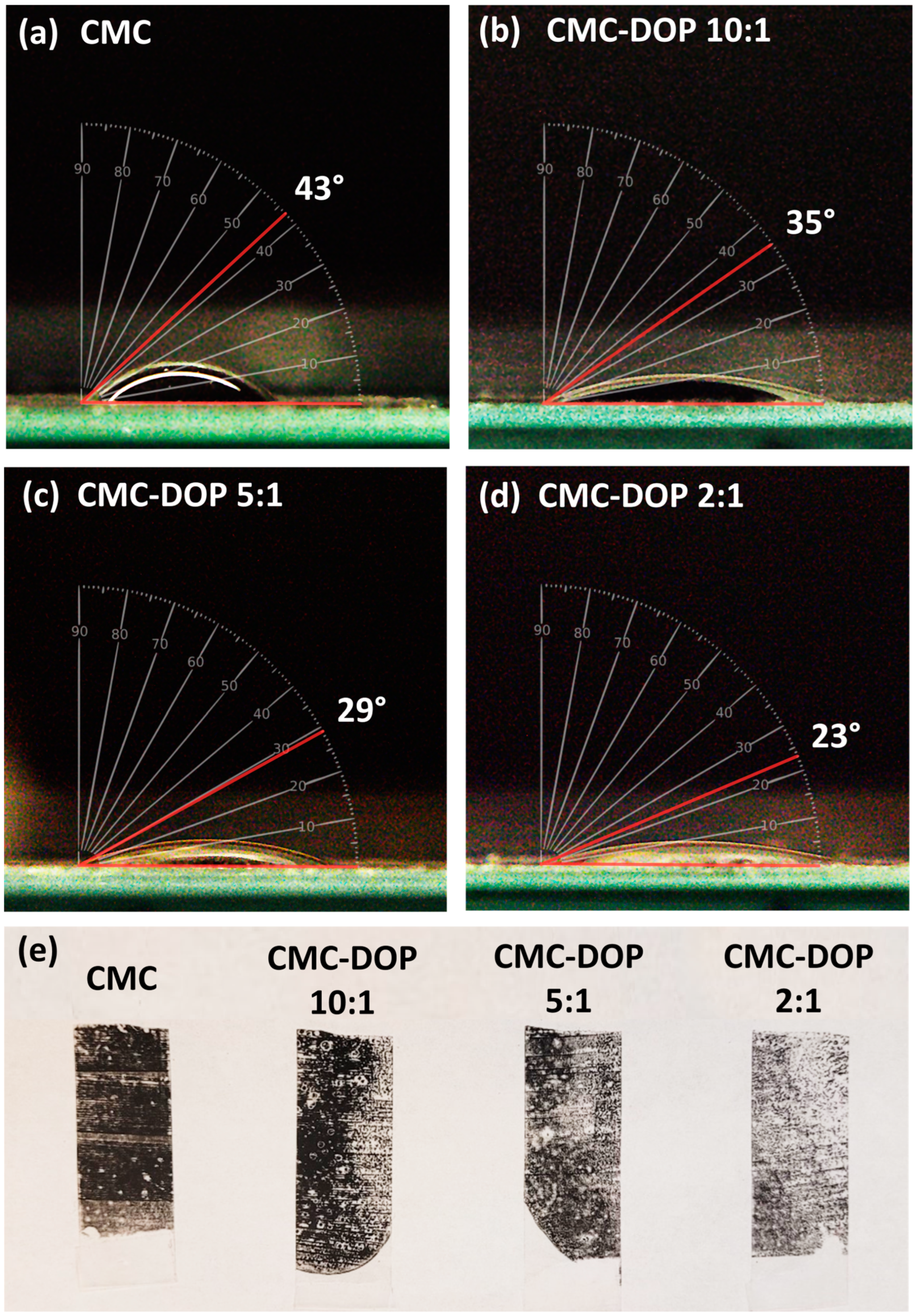
2.2. Electrode Characterizations
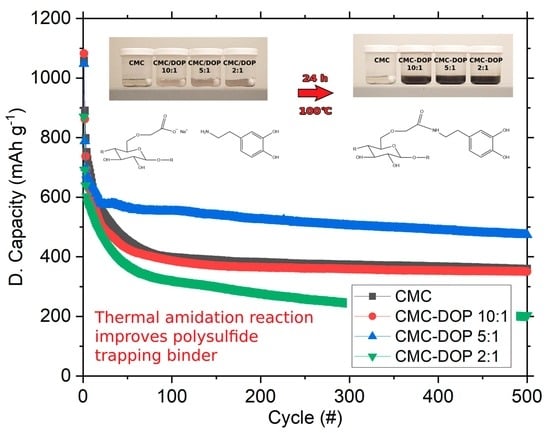
2.3. Electrochemistry
2.4. Post-Mortem Analysis
3. Conclusions
4. Materials and Methods
4.1. Synthesis
4.2. Electrode and Cell Preparation
4.3. Electrochemical Characterizations
4.4. Materials Characterizations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rana, M.; Ahad, S.A.; Li, M.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Review on Areal Capacities and Long-Term Cycling Performances of Lithium Sulfur Battery at High Sulfur Loading. Energy Storage Mater. 2019, 18, 289–310. [Google Scholar] [CrossRef]
- Singh, A.N.; Kim, M.; Meena, A.; Wi, T.; Lee, H.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, 2005605. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Liu, S.; Li, G.-R.; Gao, X.-P. Strategy of Enhancing the Volumetric Energy Density for Lithium–Sulfur Batteries. Adv. Mater. 2021, 33, 2003955. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting through Conversion Reactions. Adv. Mater. 2010, 22, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Q.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Chen, G.; Zhong, B.; Xiang, W.; Zhong, Y. A Review of Cathode Materials in Lithium-Sulfur Batteries. Ionics 2020, 26, 5299–5318. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, J.; Zheng, X.; Wen, J.; Dai, Y.; Wang, Z.; Luo, W.; Huang, Y. Lithium Metal-Based Composite: An Emerging Material for Next-Generation Batteries. Matter 2020, 3, 1009–1030. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.; He, P.; Zhou, H. A Review of Solid-State Lithium-Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy Fuels 2020, 34, 11942–11961. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium Sulfur Batteries, a Mechanistic Review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Huang, J.; Hu, A.; Gong, C.; Yan, C.; Wang, X.; Xiong, J. Strategies toward High-Loading Lithium–Sulfur Battery. Adv. Energy Mater. 2020, 10, 2000082. [Google Scholar] [CrossRef]
- Dysart, A.D.; Cardoza, N.A.; Mitchell, G.; Ortalan, V.; Pol, V.G. Effect of Synthesis Method Using Varying Types of Micropore Level Sulfur Infiltration on Electrochemical Performance in Lithium–Sulfur Batteries. Energy Technol. 2019, 7, 1900194. [Google Scholar] [CrossRef]
- Kim, P.J.; Fontecha, H.D.; Kim, K.; Pol, V.G. Toward High-Performance Lithium-Sulfur Batteries: Upcycling of LDPE Plastic into Sulfonated Carbon Scaffold via Microwave-Promoted Sulfonation. ACS Appl. Mater. Interfaces 2018, 10, 14827–14834. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.N.; Kye, D.K.; Mane, A.U.; Elam, J.W.; Etacheri, V.; Pol, V.G. Blocking Polysulfides in Graphene–Sulfur Cathodes of Lithium–Sulfur Batteries through Atomic Layer Deposition of Alumina. Energy Technol. 2019, 7, 1900621. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Surface Modification of Sulfur Cathodes with PEDOT:PSS Conducting Polymer in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A935–A939. [Google Scholar] [CrossRef]
- Parekh, M.H.; Rao, H.; Jokhakar, D.; Parikh, V.P.; Palanisamy, M.; Pol, V.G. Polysulfide Shuttle Mitigation through a Tailored Separator for Critical Temperature Energy-Dense Lithium-Sulfur Batteries. Sustain. Energy Fuels 2022, 6, 5591–5599. [Google Scholar] [CrossRef]
- Gribble, D.A.; Lin, Z.Y.; Ghosh, S.; Savoie, B.M.; Pol, V.G. Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators. Batteries 2022, 8, 253. [Google Scholar] [CrossRef]
- Wei, Z.; Ren, Y.; Sokolowski, J.; Zhu, X.; Wu, G. Mechanistic Understanding of the Role Separators Playing in Advanced Lithium-sulfur Batteries. InfoMat 2020, 2, 483–508. [Google Scholar] [CrossRef]
- Gribble, D.A.; McCulfor, E.; Li, Z.; Parekh, M.; Pol, V.G. Enhanced Capacity and Thermal Safety of Lithium-Ion Battery Graphite Anodes with Conductive Binder. J. Power Sources 2023, 553, 232204. [Google Scholar] [CrossRef]
- Gribble, D.A.; Li, Z.; Ozdogru, B.; McCulfor, E.; Çapraz, Ö.; Pol, V.G. Mechanistic Elucidation of Electronically Conductive PEDOT:PSS Tailored Binder for a Potassium-Ion Battery Graphite Anode: Electrochemical, Mechanical, and Thermal Safety Aspects. Adv. Energy Mater. 2022, 12, 2103439. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, J.Q.; Peng, H.J.; Titirici, M.M.; Xiang, R.; Chen, R.; Liu, Q.; Zhang, Q. A Review of Functional Binders in Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802107. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.; Gentle, I.R.; Wang, D.W. Carboxymethyl Cellulose Binders Enable High-Rate Capability of Sulfurized Polyacrylonitrile Cathodes for Li-S Batteries. J. Mater. Chem. A 2017, 5, 5460–5465. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, W.; Lu, H.; Jia, M.; Xie, K.; Lai, Y.; Li, J. Water-Soluble Polyacrylic Acid as a Binder for Sulfur Cathode in Lithium-Sulfur Battery. ECS Electrochem. Lett. 2012, 1, A34. [Google Scholar] [CrossRef]
- Pan, J.; Xu, G.; Ding, B.; Chang, Z.; Wang, A.; Dou, H.; Zhang, X. PAA/PEDOT:PSS as a Multifunctional, Water-Soluble Binder to Improve the Capacity and Stability of Lithium-Sulfur Batteries. RSC Adv. 2016, 6, 40650–40655. [Google Scholar] [CrossRef]
- Pang, Z.; Zhang, H.; Ma, Y.; Song, D.; Shi, X.; Zhang, L.; Zhou, Y. Polyglutamic Acid Binder for High-Performance Lithium–Sulfur Batteries. Coatings 2022, 12, 1433. [Google Scholar] [CrossRef]
- Versaci, D.; Nasi, R.; Zubair, U.; Amici, J.; Sgroi, M.; Dumitrescu, M.A.; Francia, C.; Bodoardo, S.; Penazzi, N. New Eco-Friendly Low-Cost Binders for Li-Ion Anodes. J. Solid State Electrochem. 2017, 21, 3429–3435. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, X.; Qian, J.; He, S.; Mao, Y.; Brozena, A.H.; Zhang, Y.; Pollard, T.P.; Borodin, O.A.; Wang, Y.; et al. A Cellulose-Derived Supramolecule for Fast Ion Transport. Sci. Adv. 2022, 8, eadd2031. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.; Zheng, X.; Li, T.; Brozena, A.H.; Mao, Y.; Zhang, Q.; Clifford, B.C.; Rao, J.; Hu, L. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2302098. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.; Pei, A.; Cui, Y. Stabilizing Lithium Metal Anodes by Uniform Li-Ion Flux Distribution in Nanochannel Confinement. J. Am. Chem. Soc. 2016, 138, 15443–15450. [Google Scholar] [CrossRef]
- Hollingshead, S.; Torres, J.E.; Wilker, J.J.; Liu, J.C. Effect of Cross-Linkers on Mussel- and Elastin-Inspired Adhesives on Physiological Substrates. ACS Appl. Bio Mater. 2022, 5, 630–641. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, H.; Bai, Z.; Huang, B.; Su, J.; Chen, G. Durable Polydopamine-Coated Porous Sulfur Core-Shell Cathode for High Performance Lithium-Sulfur Batteries. J. Power Sources 2015, 300, 386–394. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Luchini, A.; Napolitano, A.; Derrico, G.; Vitiello, G.; Szekely, N.; Dischia, M.; Paduano, L. Tris Buffer Modulates Polydopamine Growth, Aggregation, and Paramagnetic Properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Charlot, A.; Fleury, E. Solvent-Free Synthesis of Amidated Carboxymethyl Cellulose Derivatives: Effect on the Thermal Properties. Polymers 2019, 11, 1227. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, L.; Elkolli, M.; Zohra, B. Effect of Acidification on Biological Proprieties of Sodium Carboxymethylcellulose HCl/EtOH. Mater. Biomater. Sci. 2020, 3, 65–70. [Google Scholar]
- Wang, Z.; Huang, T.; Liu, Z.; Yu, A. Dopamine-Modified Carboxymethyl Cellulose as an Improved Aqueous Binder for Silicon Anodes in Lithium-Ion Batteries. Electrochim. Acta 2021, 389, 138806. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine—Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Chhatwal, A.R.; Lomax, H.V.; Blacker, A.J.; Williams, J.M.J.; Marcé, P. Direct Synthesis of Amides from Nonactivated Carboxylic Acids Using Urea as Nitrogen Source and Mg(NO3)2 or Imidazole as Catalysts. Chem. Sci. 2020, 11, 5808–5818. [Google Scholar] [CrossRef]
- Thakur, A.; Ranote, S.; Kumar, D.; Bhardwaj, K.K.; Gupta, R.; Chauhan, G.S. Synthesis of a PEGylated Dopamine Ester with Enhanced Antibacterial and Antifungal Activity. ACS Omega 2018, 3, 7925–7933. [Google Scholar] [CrossRef] [PubMed]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Cellulose at Temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Zheng, W.; Fan, H.; Wang, L.; Jin, Z. Oxidative Self-Polymerization of Dopamine in an Acidic Environment. Langmuir 2015, 31, 11671–11677. [Google Scholar] [CrossRef]
- Egbuhuzor, O.M.; Madufor, I.C.; Nwanonenyi, S.C.; Bokolo, J.O. Adsorption Behavior and Corrosion Rate Model of Sodium Carboxymethyl Cellulose (Na-CMC) Polymer on Aluminium in HCl Solution. Niger. J. Technol. 2020, 39, 369–378. [Google Scholar] [CrossRef]
- Sheng, Y.; Fell, C.R.; Son, Y.K.; Metz, B.M.; Jiang, J.; Church, B.C. Effect of Calendering on Electrode Wettability in Lithium-Ion Batteries. Front. Energy Res. 2014, 2, 56. [Google Scholar] [CrossRef]
- Fu, L.J.; Endo, K.; Sekine, K.; Takamura, T.; Wu, Y.P.; Wu, H.Q. Studies on Capacity Fading Mechanism of Graphite Anode for Li-Ion Battery. J. Power Sources 2006, 162, 663–666. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y. Dissolution and Reprecipitation of Sulfur on Carbon Surface. J. Electron. Mater. 2022, 51, 2926–2932. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid Electrolyte Lithium/Sulfur Battery: Fundamental Chemistry, Problems, and Solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, L.; Shi, S.; Wang, T.; Duan, G.; Liu, X.; Li, Y. Flexible Polydopamine Bioelectronics. Adv. Funct. Mater. 2021, 31, 2103391. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar] [CrossRef]
- Fan, F.Y.; Pan, M.S.; Lau, K.C.; Assary, R.S.; Woodford, W.H.; Curtiss, L.A.; Carter, W.C.; Chiang, Y.-M. Solvent Effects on Polysulfide Redox Kinetics and Ionic Conductivity in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2016, 163, A3111–A3116. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Liu, B.; Li, Y.; Shen, S.; Deng, S.; Lu, C.; Zhang, W.; Xia, Y.; Pan, G.; et al. Electrode Design for Lithium–Sulfur Batteries: Problems and Solutions. Adv. Funct. Mater. 2020, 30, 1910375. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Efficient Electrolytes for Lithium-Metal Batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]





| Before | After | |||||
|---|---|---|---|---|---|---|
| Re (Ω) | Rint (Ω) | Rct (Ω) | Re (Ω) | Rint (Ω) | Rct (Ω) | |
| CMC | 3.00 | 14.37 | 1104 | 7.05 | 2.49 | 14.12 |
| CMC-DOP 10:1 | 2.98 | 34.91 | 693 | 7.01 | 1.30 | 9.56 |
| CMC-DOP 5:1 | 3.94 | 27.99 | 1116 | 3.45 | 0.86 | 4.83 |
| CMC-DOP 2:1 | 2.06 | 28.10 | 800 | 6.15 | 10.41 | 33.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribble, D.A.; Pol, V.G. Polydopamine-Modified Carboxymethyl Cellulose as Advanced Polysulfide Trapping Binder. Batteries 2023, 9, 525. https://doi.org/10.3390/batteries9110525
Gribble DA, Pol VG. Polydopamine-Modified Carboxymethyl Cellulose as Advanced Polysulfide Trapping Binder. Batteries. 2023; 9(11):525. https://doi.org/10.3390/batteries9110525
Chicago/Turabian StyleGribble, Daniel A., and Vilas G. Pol. 2023. "Polydopamine-Modified Carboxymethyl Cellulose as Advanced Polysulfide Trapping Binder" Batteries 9, no. 11: 525. https://doi.org/10.3390/batteries9110525
APA StyleGribble, D. A., & Pol, V. G. (2023). Polydopamine-Modified Carboxymethyl Cellulose as Advanced Polysulfide Trapping Binder. Batteries, 9(11), 525. https://doi.org/10.3390/batteries9110525