Molybdenum Nitride and Oxide Quantum Dot @ Nitrogen-Doped Graphene Nanocomposite Material for Rechargeable Lithium Ion Batteries
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Samples
2.2. Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
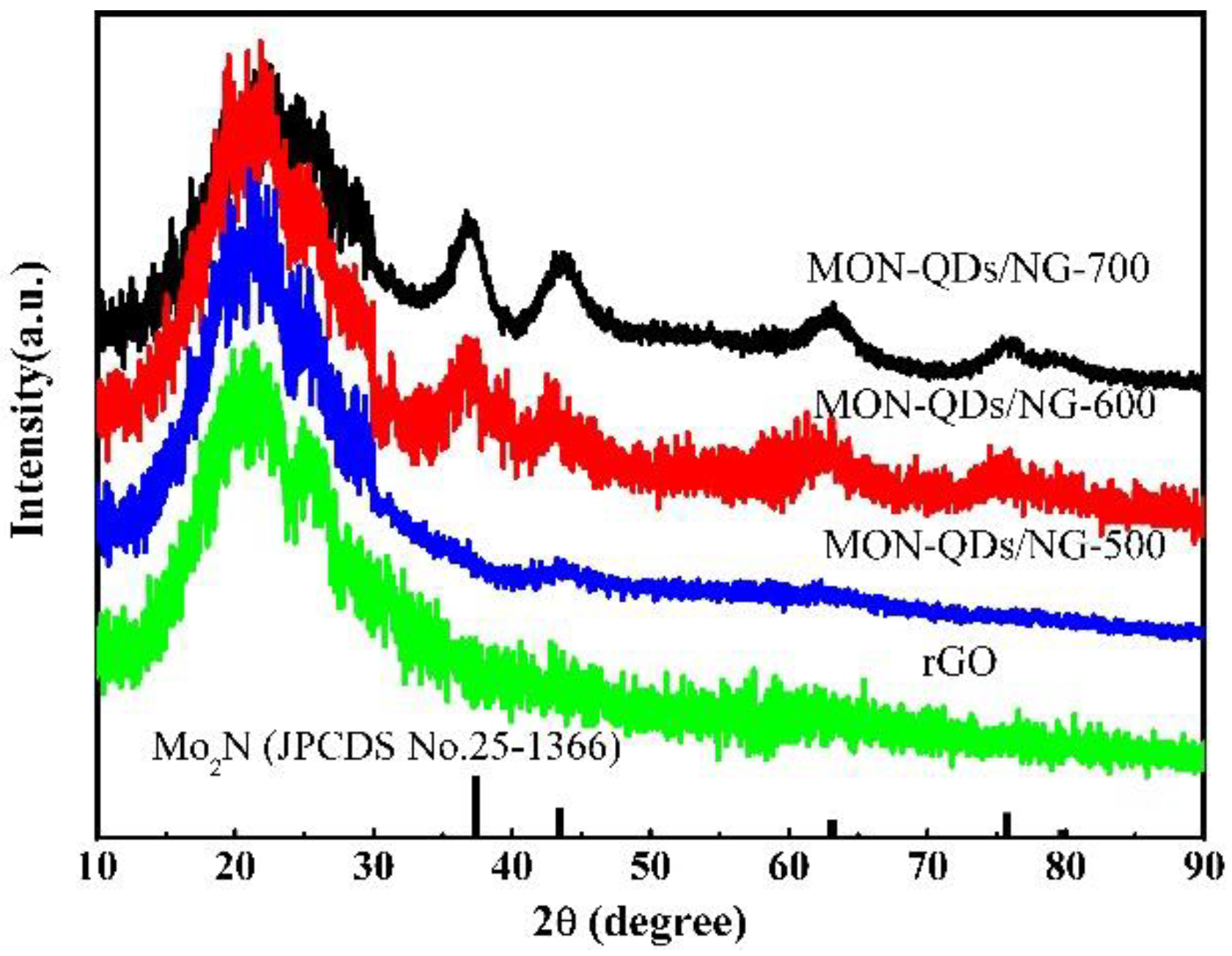
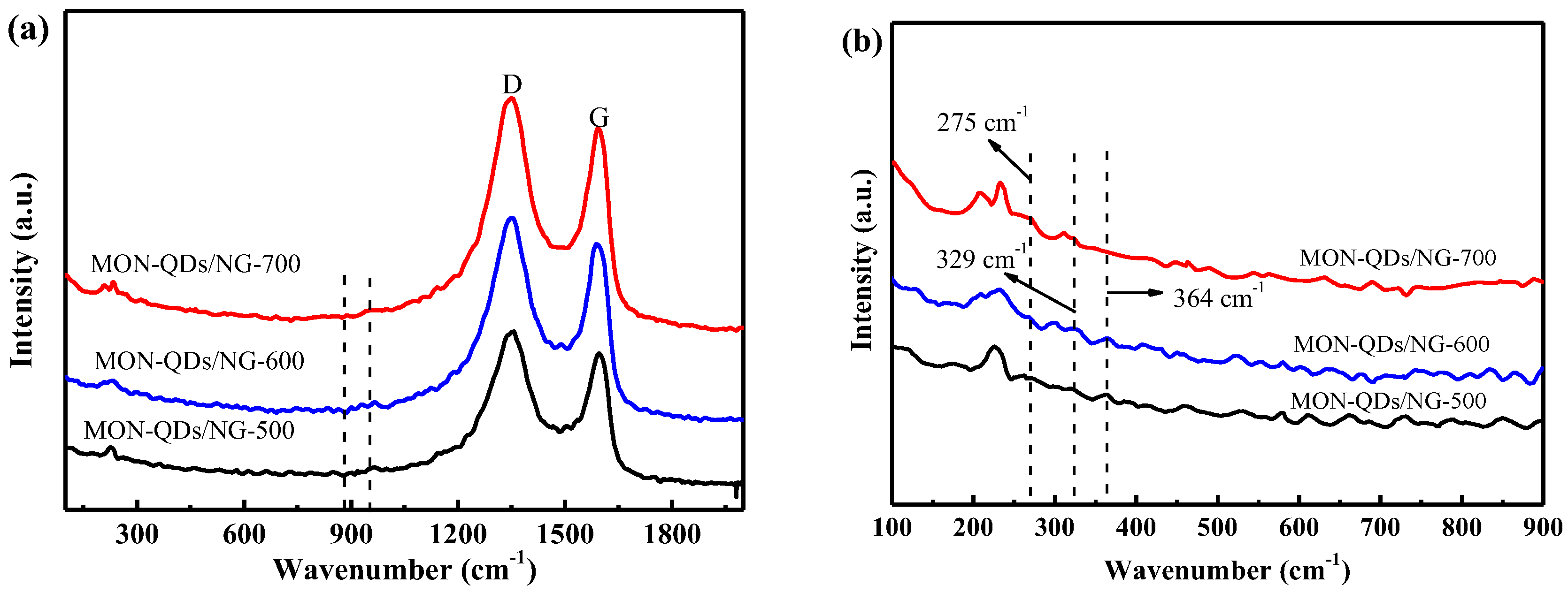
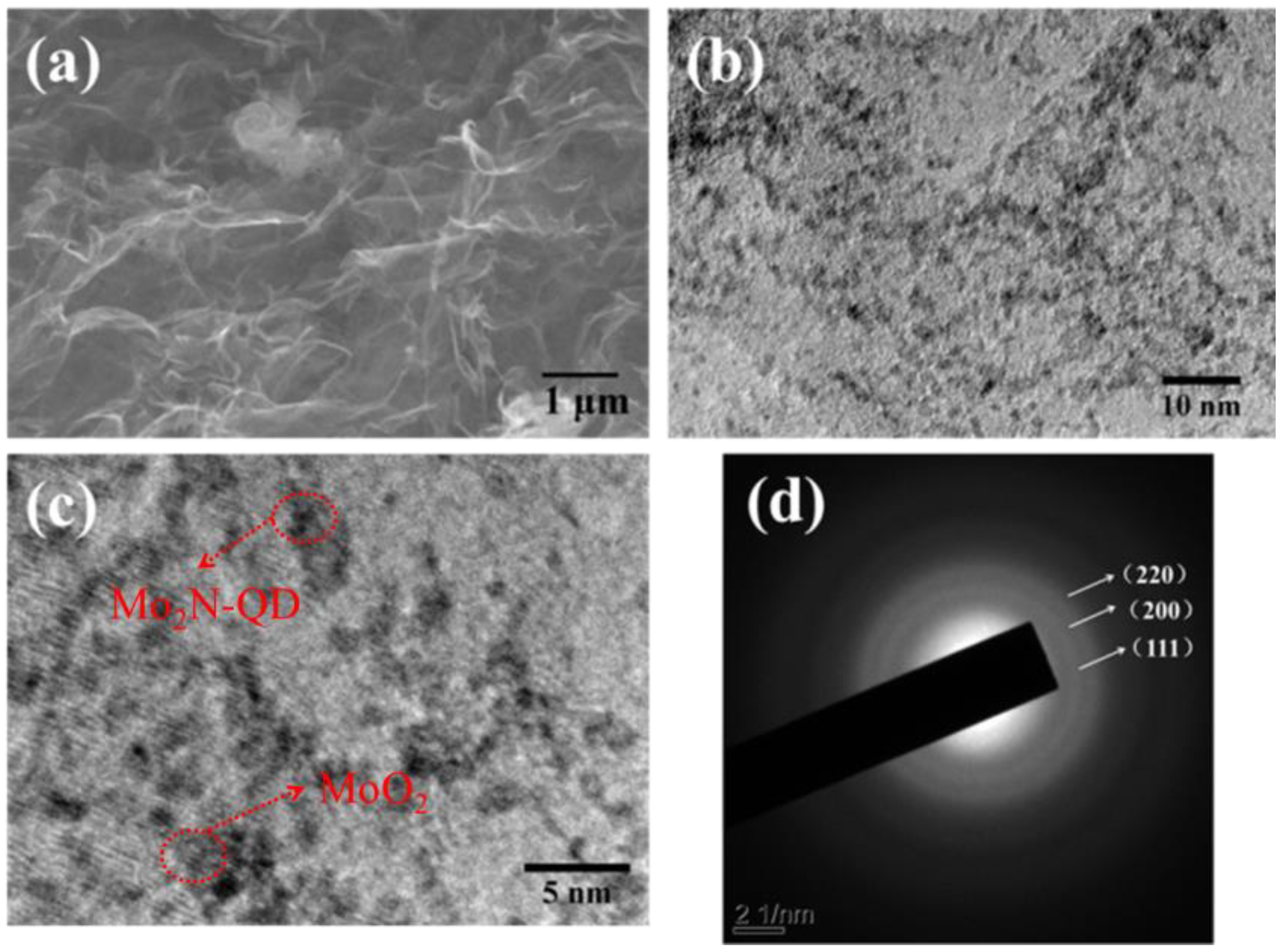
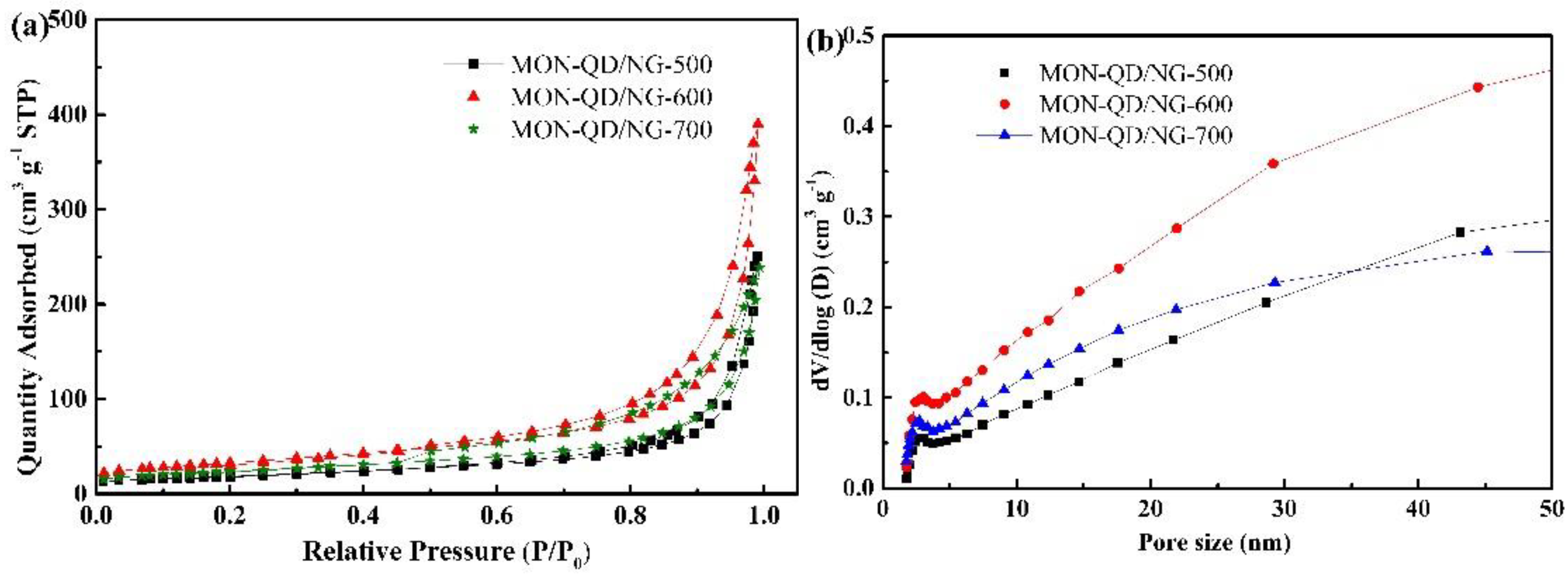
3.1. Structural and Characterization
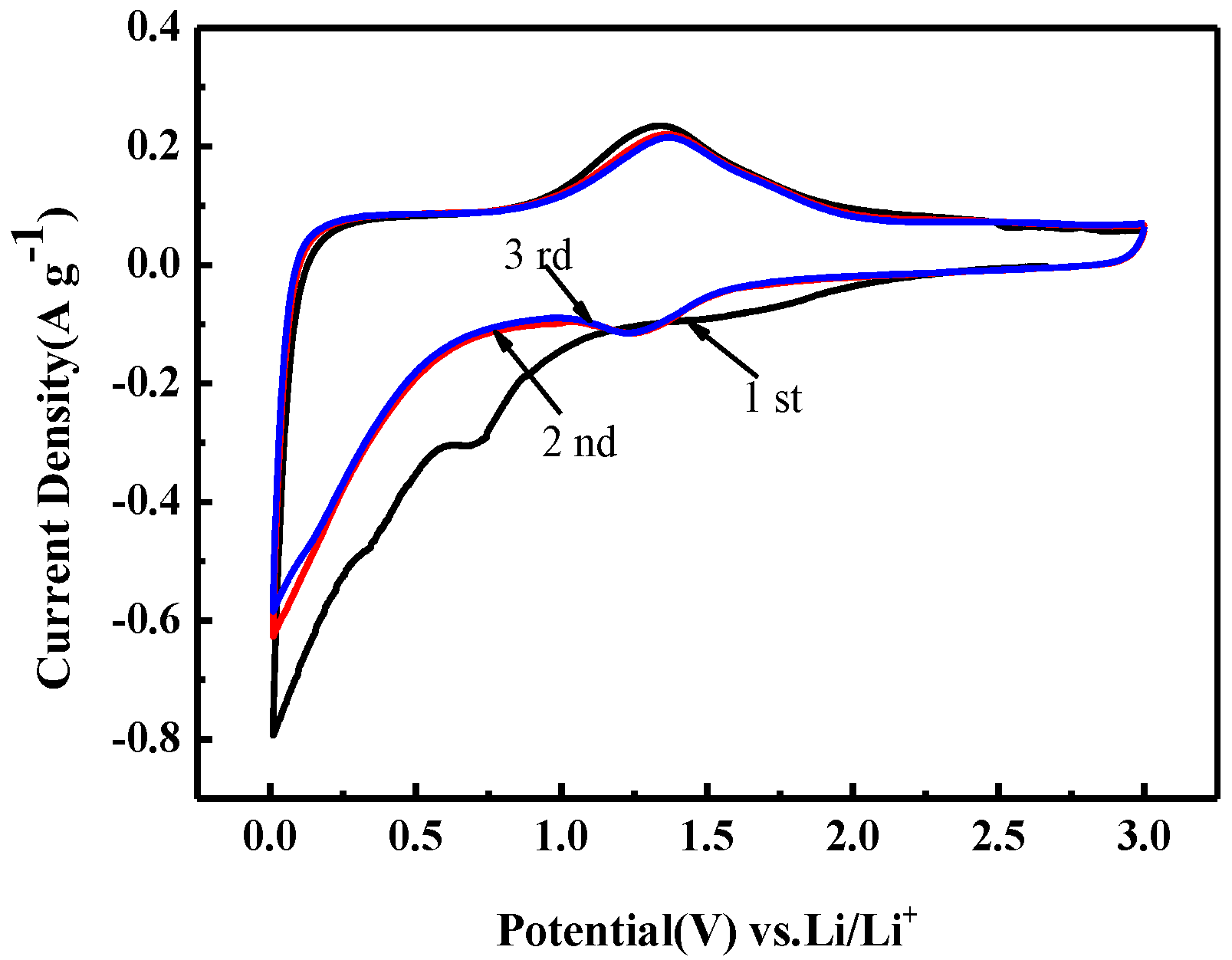
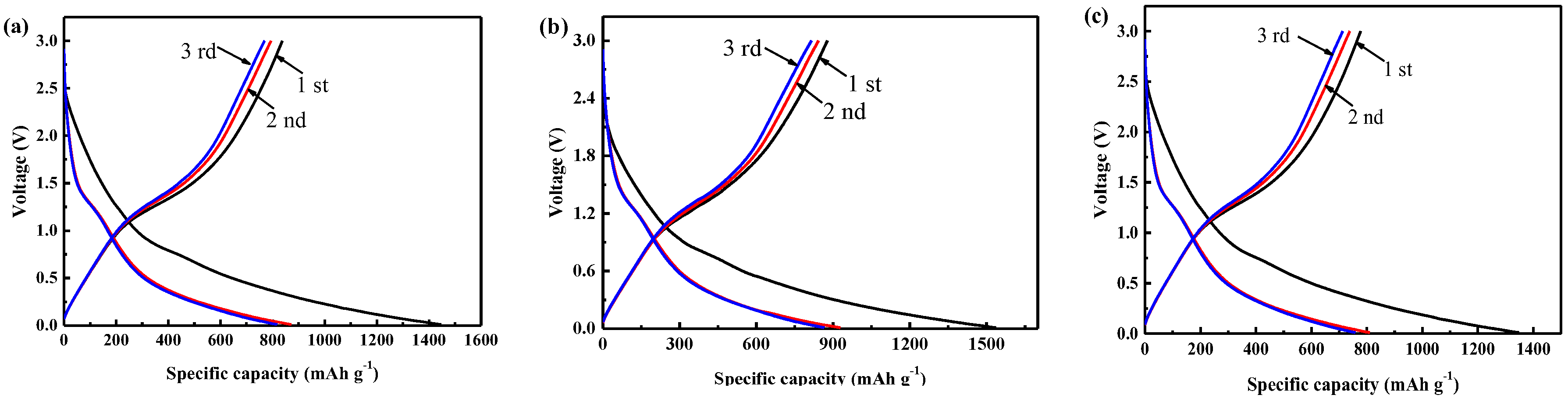
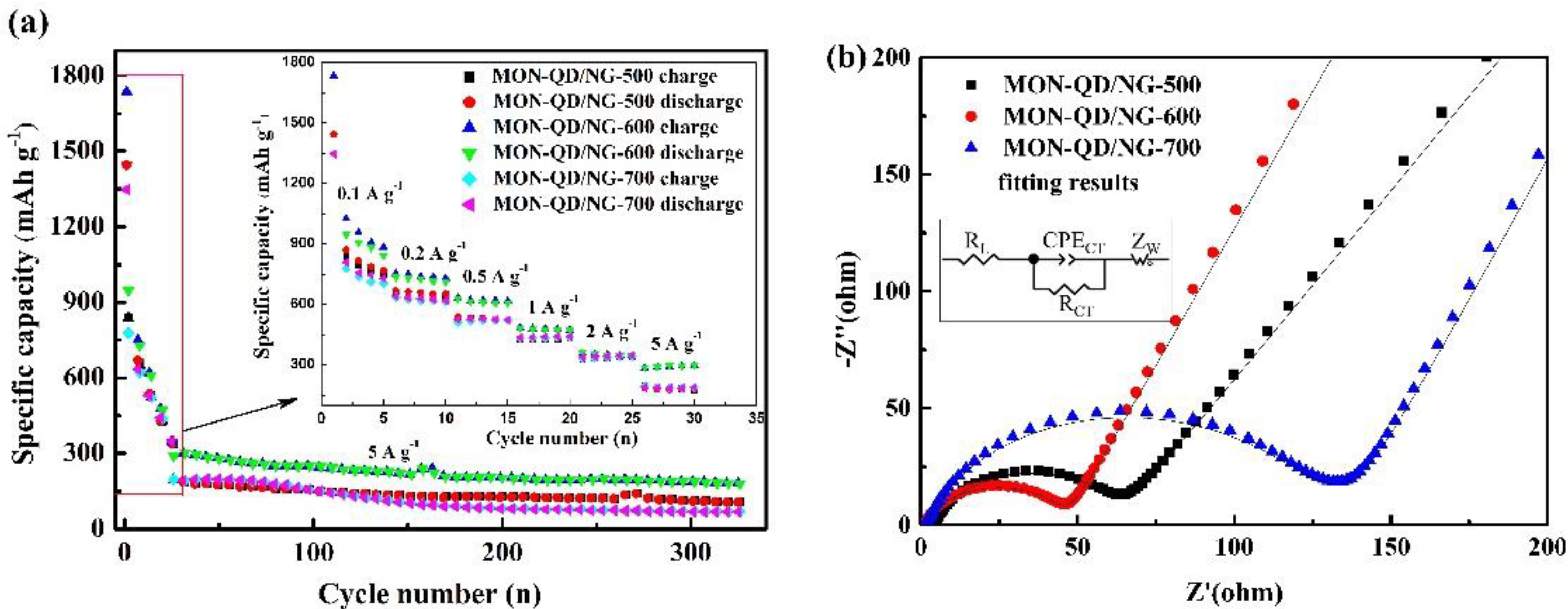
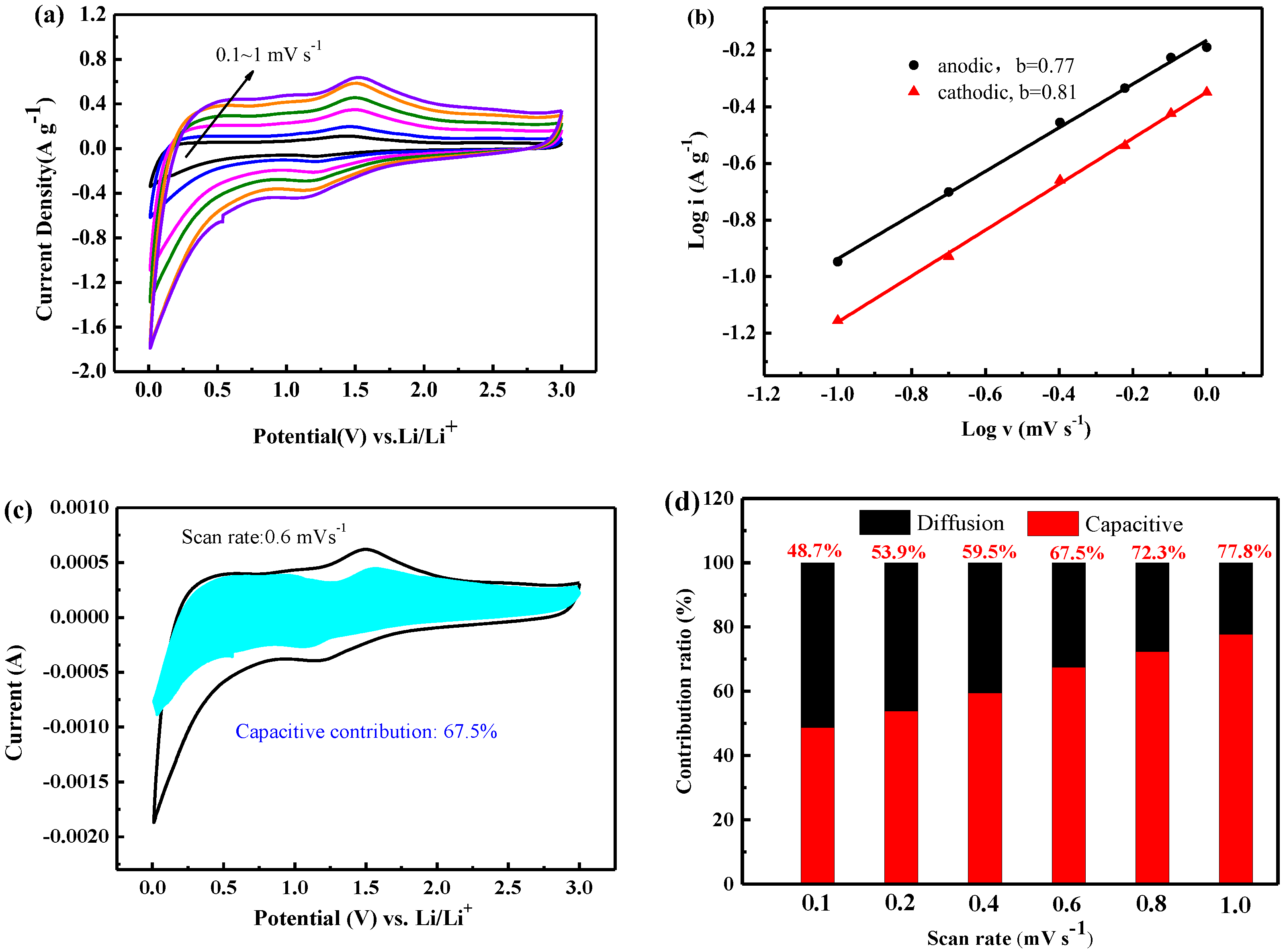
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Zhang, K.; Pan, H.; Wang, L.; Wang, D.; Dai, W.; Qin, H.; Li, G.; Zhang, J. 2D molybdenum nitride nanosheets as anode materials for improved lithium storage. Nanoscale 2018, 10, 18936–18941. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.K.; Sen, U.K.; Choudhury, D.; Mitra, S.; Sarkar, S.K. Atomic Layer Deposited Molybdenum Nitride Thin Film: A Promising Anode Material for Li Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 6606–6615. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-C.; Lee, K.-H.; Lee, Y.-W.; Kim, S.-J.; Kim, D.-M.; Kim, M.-C.; Park, K.-W. Mesoporous molybdenum nitride nanobelts as an anode with improved electrochemical properties in lithium ion batteries. J. Power Sources 2014, 269, 534–541. [Google Scholar] [CrossRef]
- Joshi, S.; Qi, W.; Puntambekar, A.; Chakrapani, V. Facile Synthesis of Large Area Two Dimensional Layers of Transition Metal Nitride and Their Use as Insertion Electrodes. ACS Energy Lett. 2018, 2, 1257–1262. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering Empty Space between Si Nanoparticles for Lithium-Ion Battery Anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef]
- Dang, W.; Wang, W.; Yang, Y.; Wang, Y.; Huang, J.; Fang, X.; Wu, L.P.; Rong, Z.H.; Chen, X.; Li, X.; et al. One-step hydrothermal synthesis of 2D WO3 nanoplates@ graphene nanocomposite with superior anode performance for lithium ion battery. Electrochim. Acta 2019, 313, 99–108. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Fang, L.; Yang, J.; Wu, K.; Wang, Y. Hierarchical Molybdenum Nitride Nanochexes by a Textured Self-Assembly in Gas-Solid Phase for the Enhanced Application in Lithium Ion Batteries. ACS Nano 2015, 9, 6817–6825. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Zhu, Y.; Shen, Y.; Xie, A. In-Situ Synthesis of Petal-Like MoO2@MoN/NF Heterojunction As Both an Advanced Binder-Free Anode and an Electrocatalyst for Lithium Ion Batteries and Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 9153–9163. [Google Scholar] [CrossRef]
- Li, L.; Jia, X.N.; Zhang, Y.; Qiu, T.Y.; Hong, W.W.; Jiang, Y.L.; Zou, G.Q.; Hou, H.S.; Chen, X.C.; Ji, X.B. Li4Ti5O12 quantum dot decorated carbon frameworks from carbon dots for fast lithium ion storage. Mat. Chem. Front. 2019, 3, 1761–1767. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, G.; Zhang, K.; Zhang, L.; Han, P.; Dong, S. Molybdenum nitride/nitrogen-doped graphene hybrid material for lithium storage in lithium ion batteries. Electrochim. Acta 2014, 150, 15–22. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Zhang, X.D.; He, W.; Xu, G.G.; Ren, M.M.; Liu, J.H.; Yang, X.N.; Wang, F. In situ fabrication of Na3V2(PO4)(3) quantum dots in hard carbon nanosheets by using lignocelluloses for sodium ion batteries. J. Mater. Sci. Technol. 2019, 35, 2396–2403. [Google Scholar] [CrossRef]
- Pan, X.; Xi, B.; Lu, H.; Zhang, Z.; An, X.; Liu, J.; Feng, J.; Xiong, S. Molybdenum Oxynitride Atomic Nanoclusters Bonded in Nanosheets of N-Doped Carbon Hierarchical Microspheres for Efficient Sodium Storage. Nanomicro Lett. 2022, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, V.; Peter, I.J. Graphene Quantum Dots Supported Mo3S4 as a Promising Candidate for Pt-Free Counter Electrode in Dye-Sensitized Solar Cell and Supercapacitor Applications. ECS J. Solid State Sci. Technol. 2021, 10, 091002. [Google Scholar] [CrossRef]
- Kumar, R.; Bhuvana, T.; Sharma, A. Ammonolysis synthesis of nickel molybdenum nitride nanostructures for high-performance asymmetric supercapacitors. New J. Chem. 2020, 44, 14067–14074. [Google Scholar] [CrossRef]
- He, T.; Zhang, W.; Manasa, P.; Ran, F. Quantum dots of molybdenum nitride embedded in continuously distributed polyaniline as novel electrode material for supercapacitor. J. Alloys Compd. 2020, 812, 152138. [Google Scholar] [CrossRef]
- Borah, D.J.; Mostako, A.T.T.; Borgogoi, A.T.; Saikia, P.K.; Malakar, A. Modified top-down approach for synthesis of molybdenum oxide quantum dots: Sonication induced chemical etching of thin films. RSC Adv. 2020, 10, 3105–3114. [Google Scholar] [CrossRef]
- Zheng, C.; Luo, N.; Huang, S.; Wu, W.; Huang, H.; Wei, M. Nanocomposite of Mo2N Quantum Dots@MoO3@Nitrogen-Doped Carbon as a High-Performance Anode for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 10198–10206. [Google Scholar] [CrossRef]
- Liu, J.; Tang, S.; Lu, Y.; Cai, G.; Liang, S.; Wang, W.; Chen, X. Synthesis of Mo2N nanolayer coated MoO2 hollow nanostructures as high-performance anode materials for lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2691–2697. [Google Scholar] [CrossRef]
- Afanasiev, P. New single source route to the molybdenum nitride Mo(2)N. Inorg. Chem. 2002, 41, 5317–5319. [Google Scholar] [CrossRef]
- Akhmetzyanova, U.; Pelíšková, L.; Skuhrovcová, L.; Tišler, Z.; Opanasenko, M. Molybdenum Nitrides, Carbides and Phosphides as Highly Efficient Catalysts for the (hydro)Deoxygenation Reaction. ChemistrySelect 2019, 4, 8453. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Li, L.; Zhang, L.; Fang, H.; Song, Y.; Li, X. Preparation and lithium storage properties of Mo2N quantum dots@nitrogen-doped graphene composite. CIESC J. 2020, 71, 5854–5862. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Alexander, S.; Zhengzong, S.; Alexander, S.; Alemany, L.B.; Wei, L.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, M.; Chen, W.; Zeng, L.; Wei, M. An in situ formed Se/CMK-3 composite for rechargeable lithium-ion batteries with long-term cycling performance. J. Mater. Chem. A 2016, 4, 13646–13651. [Google Scholar] [CrossRef]
- Liu, X.B.; Amiinu, I.S.; Liu, S.J.; Pu, Z.H.; Li, W.Q.; Ye, B.; Tan, D.M.; Mu, S.C. H2O2-Assisted Synthesis of Porous N-Doped Graphene/Molybdenum Nitride Composites with Boosted Oxygen Reduction Reaction. Adv. Mater. Interfaces 2017, 4, 8. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, J.; Li, Y.; Lee, K.-T.; Liu, C.; Lin, H.; Cheng, Q.; Lan, Q.; Wu, L.; Tang, S.; et al. In situ SiO2 etching strategy to prepare rice husk-derived porous carbons for supercapacitor application. J. Taiwan Inst. Chem. Eng. 2017, 81, 383–390. [Google Scholar] [CrossRef]
- Maldonado, S.; Morin, S.; Stevenson, K.J. Structure, composition, and chemical reactivity of carbon nanotubes by selective nitrogen doping. Carbon 2006, 44, 1429–1437. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Ma, Q.; Zhang, Q.; Bai, H.; Yi, W.; Liu, J.; Han, J.; Xi, G. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 14903. [Google Scholar] [CrossRef]
- Peng, T.; Tan, Z.; Zhang, M.; Li, L.; Wang, Y.; Guan, L.; Tan, X.; Pan, L.; Fang, H.; Wu, M. Facile and cost-effective manipulation of hierarchical carbon nanosheets for pseudocapacitive lithium/potassium storage. Carbon 2020, 165, 296–305. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, J.; Ruan, Y.; Ao, X.; Ostrikov, K.; Zhang, W.; Lu, J.; Li, Y.Y. Construction of MoO2 Quantum Dot-Graphene and MoS2 Nanoparticle-Graphene Nanoarchitectures toward Ultrahigh Lithium Storage Capability. ACS Appl. Mater. Interfaces 2017, 9, 28441–28450. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, J.; Rui, X.; Cao, X.; Chen, C.; Zhang, H.; Hng, H.H.; Yan, Q. Controlled synthesis of carbon-coated cobalt sulfide nanostructures in oil phase with enhanced li storage performances. ACS Appl Mater. Interfaces 2012, 4, 2999–3006. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au–Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Lu, X.; Qi, F.; Liang, Y.; Trukhanov, A.; Wu, Y.; Sun, Z.; Lu, X. Fully Active Bimetallic Phosphide Zn0.5Ge0.5P: A Novel High-Performance Anode for Na-Ion Batteries Coupled with Diglyme-Based Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 31803–31813. [Google Scholar] [CrossRef]
- Ali, Y.; Lee, S. An integrated experimental and modeling study of the effect of solid electrolyte interphase formation and Cu dissolution on CuCo2O4-based Li-ion batteries. Int. J. Energy Res. 2021, 46, 3017–3033. [Google Scholar] [CrossRef]
- Wang, F.-M.; Rick, J. Synergy of Nyquist and Bode electrochemical impedance spectroscopy studies to commercial type lithium ion batteries. Solid State Ion. 2014, 268, 31–34. [Google Scholar] [CrossRef]
- Ogihara, N.; Kawauchi, S.; Okuda, C.; Itou, Y.; Takeuchi, Y.; Ukyo, Y. Theoretical and Experimental Analysis of Porous Electrodes for Lithium-Ion Batteries by Electrochemical Impedance Spectroscopy Using a Symmetric Cell. J. Electrochem. Soc. 2012, 159, A1034–A1039. [Google Scholar] [CrossRef]
- Rui, X.H.; Li, C.; Liu, J.; Cheng, T.; Chen, C.H. The Li3V2(PO4)(3)/C composites with high-rate capability prepared by a maltose-based sot-gel route. Electrochim. Acta 2010, 55, 6761–6767. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Yang, L.; Zhou, K.; Wang, Y.; Sun, D.; Tang, Y.; Wang, H. Engineering hierarchical structure and surface of Na4MnV(PO4)3 for ultrafast sodium storage by a scalable ball milling approach. Nano Energy 2022, 99, 107396. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Li, H.; Lang, J.; Lei, S.; Chen, J.; Wang, K.; Liu, L.; Zhang, T.; Liu, W.; Yan, X. A High-Performance Sodium-Ion Hybrid Capacitor Constructed by Metal-Organic Framework-Derived Anode and Cathode Materials. Adv. Funct. Mater. 2018, 28, 1800757. [Google Scholar] [CrossRef]
- Das, S.R.; Majumder, S.B.; Katiyar, R.S. Kinetic analysis of the Li+ ion intercalation behavior of solution derived nano-crystalline lithium manganate thin films. J. Power Sources 2005, 139, 261–268. [Google Scholar] [CrossRef]
- Veronica, A.; Jérémy, C.; Lowe, M.A.; Jong Woung, K.; Pierre-Louis, T.; Tolbert, S.H.; Abruña, H.D.; Patrice, S.; Bruce, D. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518. [Google Scholar]
- Zhu, T.; Wang, Y.; Li, Y.; Cai, R.; Zhang, J.; Yu, C.; Wu, J.; Cui, J.; Zhang, Y.; Ajayan, P.M.; et al. CoO Quantum Dots Anchored on Reduced Graphene Oxide Aerogels for Lithium-Ion Storage. ACS Appl. Nano Mater. 2020, 3, 10369–10379. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Guo, X.; Zhang, J.; Liu, P.; Yan, J. Porous Mo2N nano-column array thin film electrode for lithium ion storage. Results Phys. 2019, 15, 102715. [Google Scholar] [CrossRef]
- Ji, W.; Shen, R.; Yang, R.; Yu, G.; Guo, X.; Peng, L.; Ding, W. Partially nitrided molybdenum trioxide with promoted performance as an anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 699–704. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, H.; Wei, Z.; Han, B.H. Graphene-molybdenum oxynitride porous material with improved cyclic stability and rate capability for rechargeable lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 16898–16906. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhao, T.; Chen, R.; Fang, H.; Yang, Y.; Cao, Y.; Zhang, L. Molybdenum Nitride and Oxide Quantum Dot @ Nitrogen-Doped Graphene Nanocomposite Material for Rechargeable Lithium Ion Batteries. Batteries 2023, 9, 32. https://doi.org/10.3390/batteries9010032
Wang L, Zhao T, Chen R, Fang H, Yang Y, Cao Y, Zhang L. Molybdenum Nitride and Oxide Quantum Dot @ Nitrogen-Doped Graphene Nanocomposite Material for Rechargeable Lithium Ion Batteries. Batteries. 2023; 9(1):32. https://doi.org/10.3390/batteries9010032
Chicago/Turabian StyleWang, Lixia, Taibao Zhao, Ruiping Chen, Hua Fang, Yihao Yang, Yang Cao, and Linsen Zhang. 2023. "Molybdenum Nitride and Oxide Quantum Dot @ Nitrogen-Doped Graphene Nanocomposite Material for Rechargeable Lithium Ion Batteries" Batteries 9, no. 1: 32. https://doi.org/10.3390/batteries9010032
APA StyleWang, L., Zhao, T., Chen, R., Fang, H., Yang, Y., Cao, Y., & Zhang, L. (2023). Molybdenum Nitride and Oxide Quantum Dot @ Nitrogen-Doped Graphene Nanocomposite Material for Rechargeable Lithium Ion Batteries. Batteries, 9(1), 32. https://doi.org/10.3390/batteries9010032





