Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion
Abstract
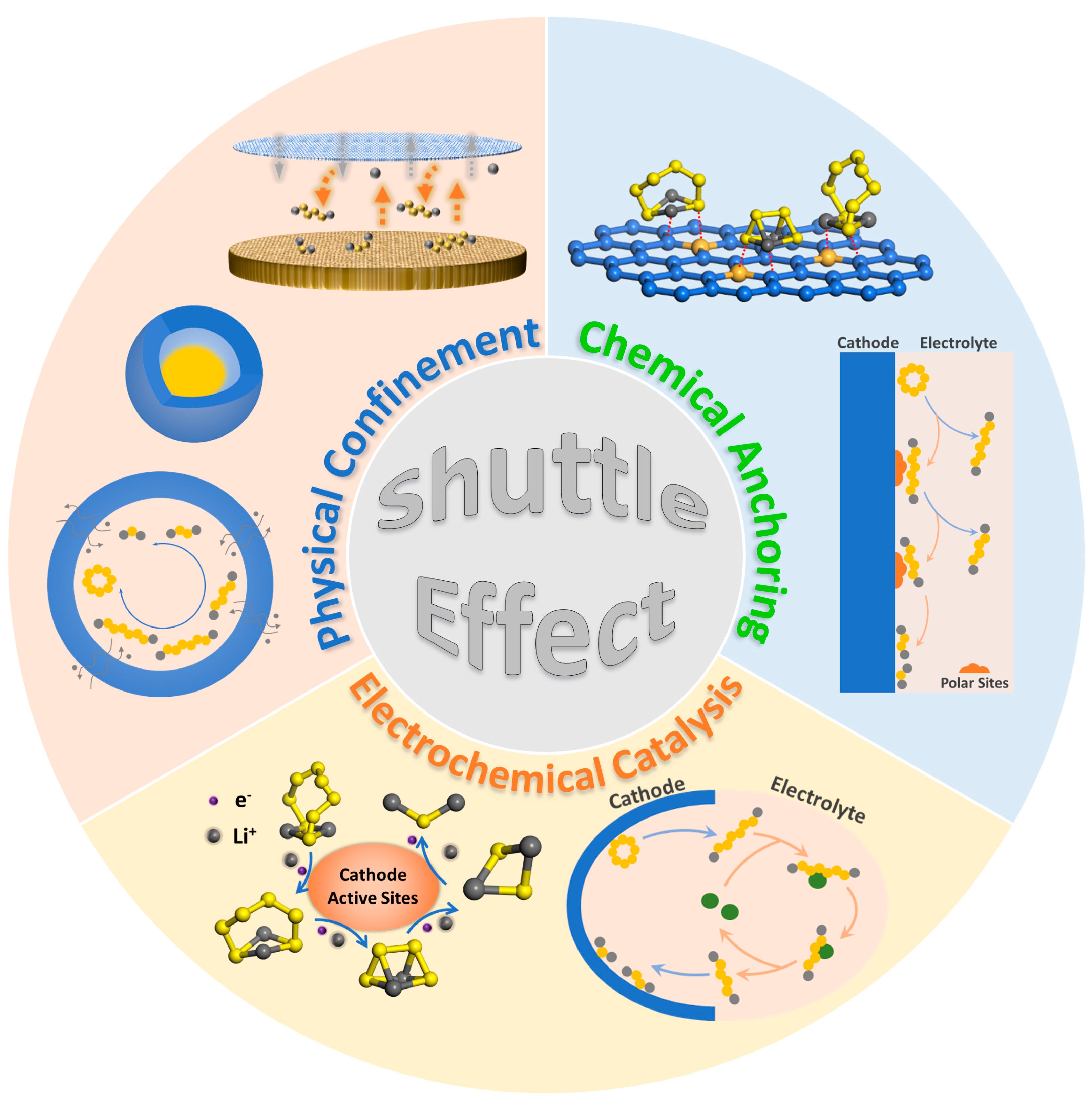
1. Introduction
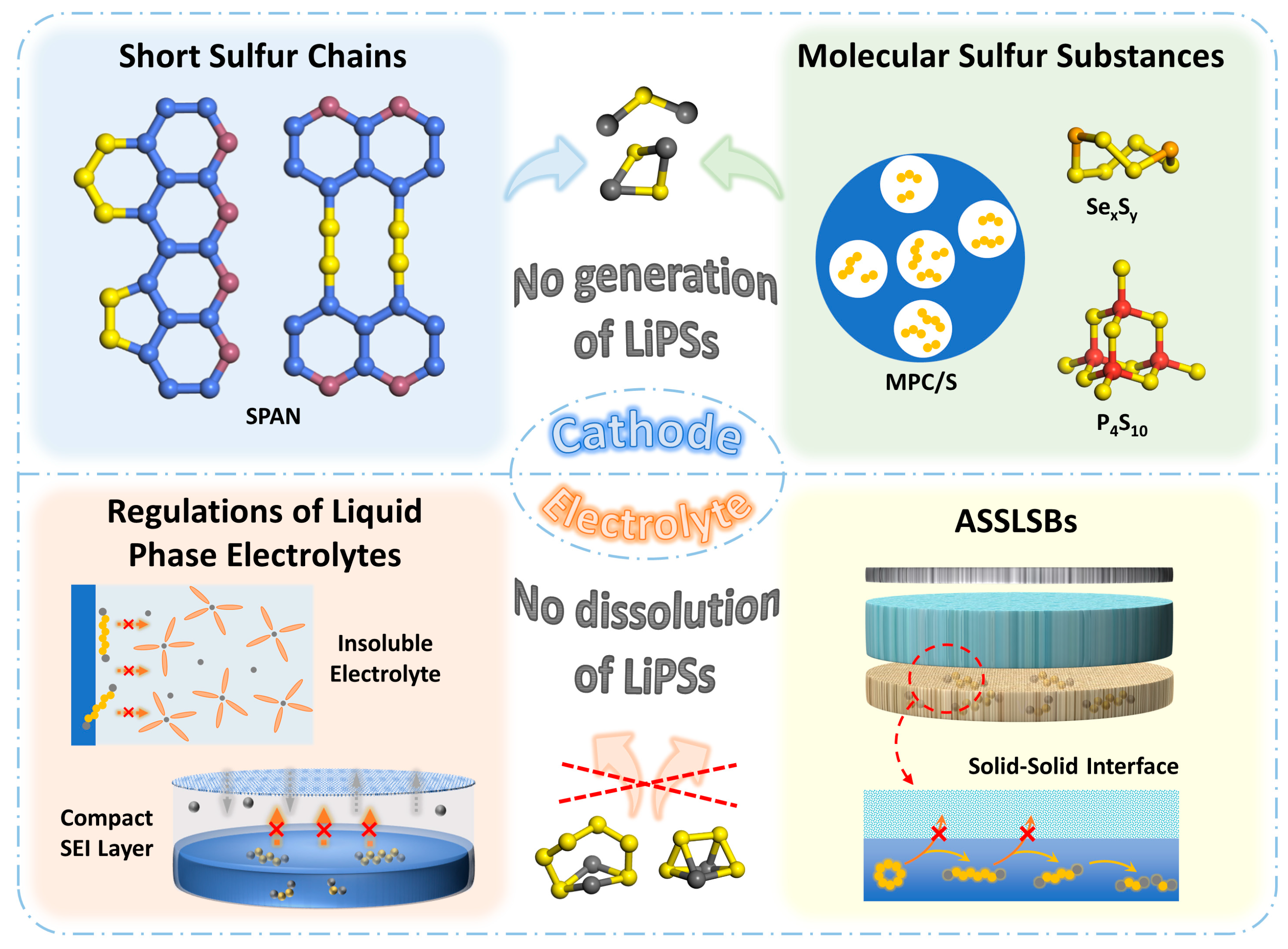
2. Modifications on Sulfur Cathodes
2.1. Organic Sulfur Polymers
2.1.1. Cathodic Design Based on SPAN Compounds
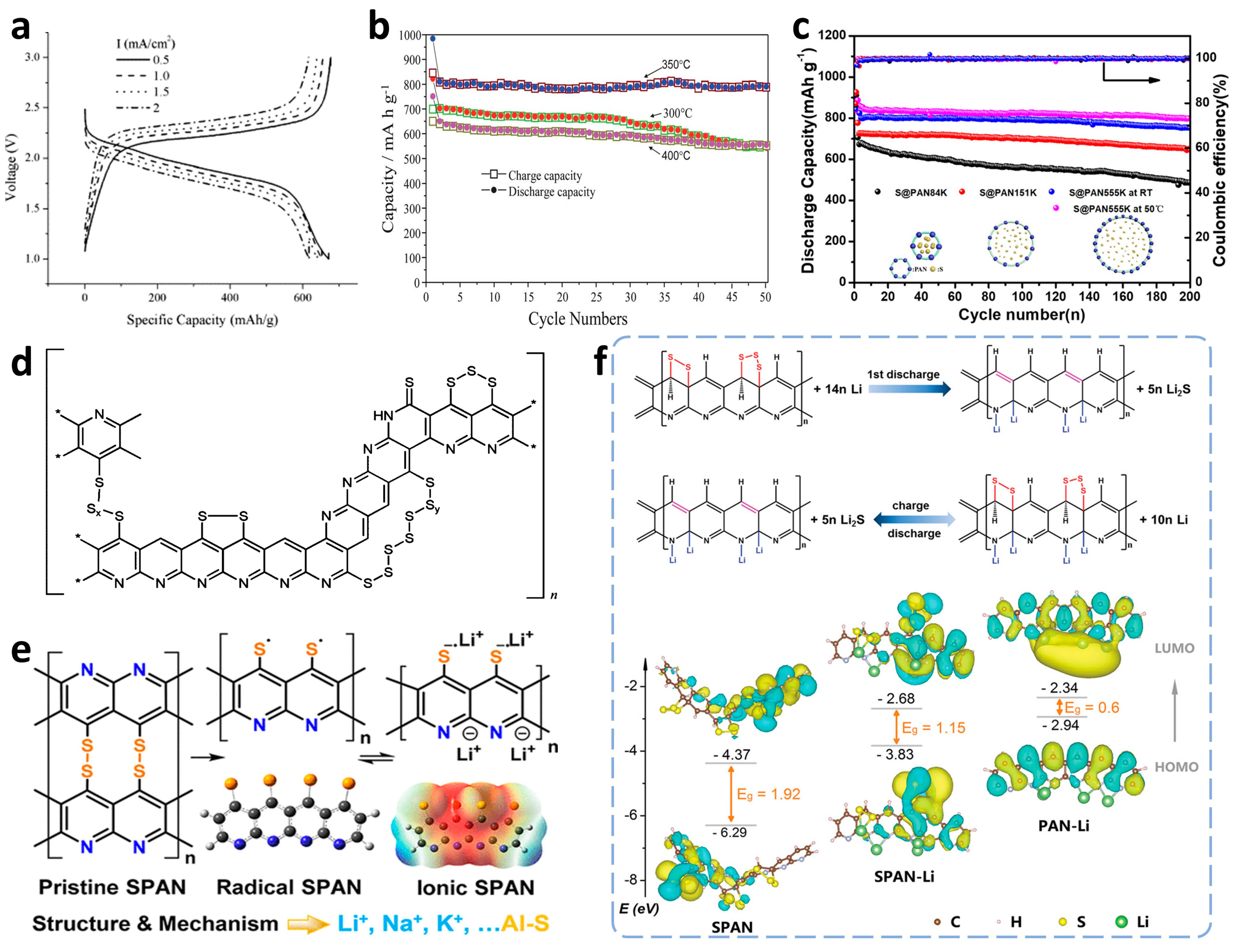
Origin and Preparation Optimizations of SPAN
Structure and Lithium Storage Mechanism of SPAN
2.1.2. Performance Optimizations of Li–SPAN Batteries
Optimizations of Cathodic Active Material Structure
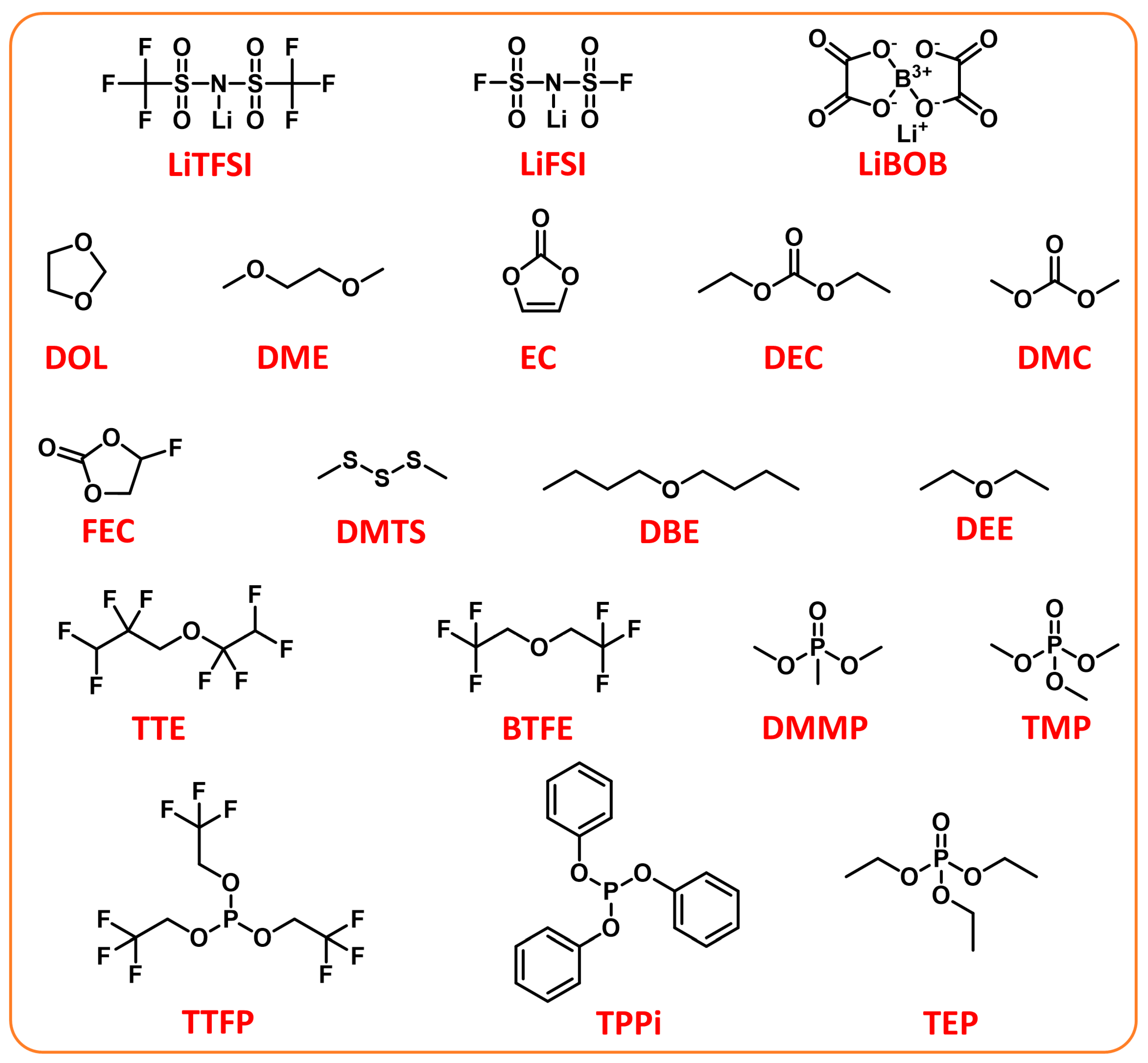
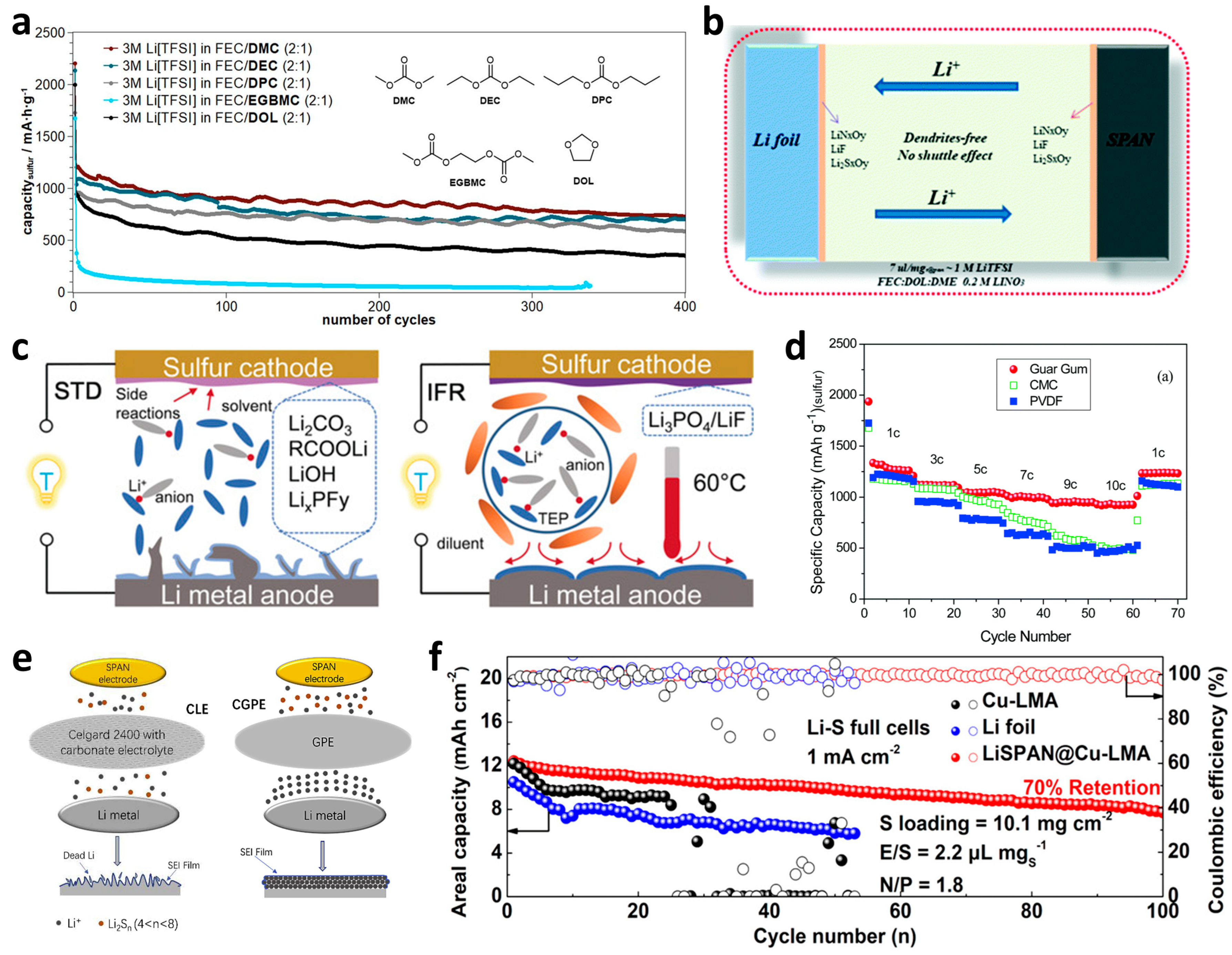
Electrolyte Regulations
Regulations of Other Components of Batteries
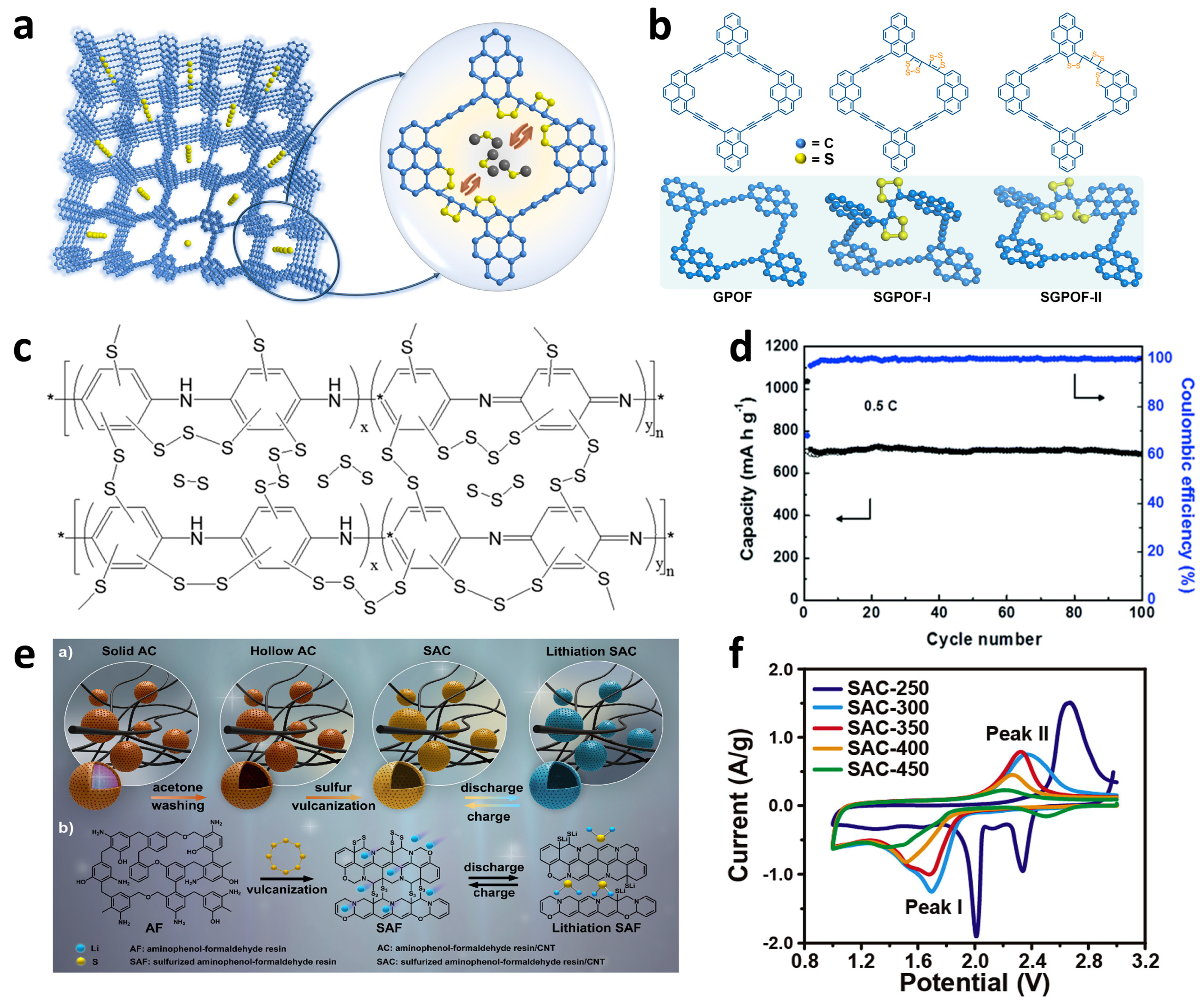
2.1.3. Other Sulfur–Polymer-Based Cathodes
2.2. Individual Molecular Forms of Sulfur Substances
2.2.1. Microporous Carbon–Sulfur Composites
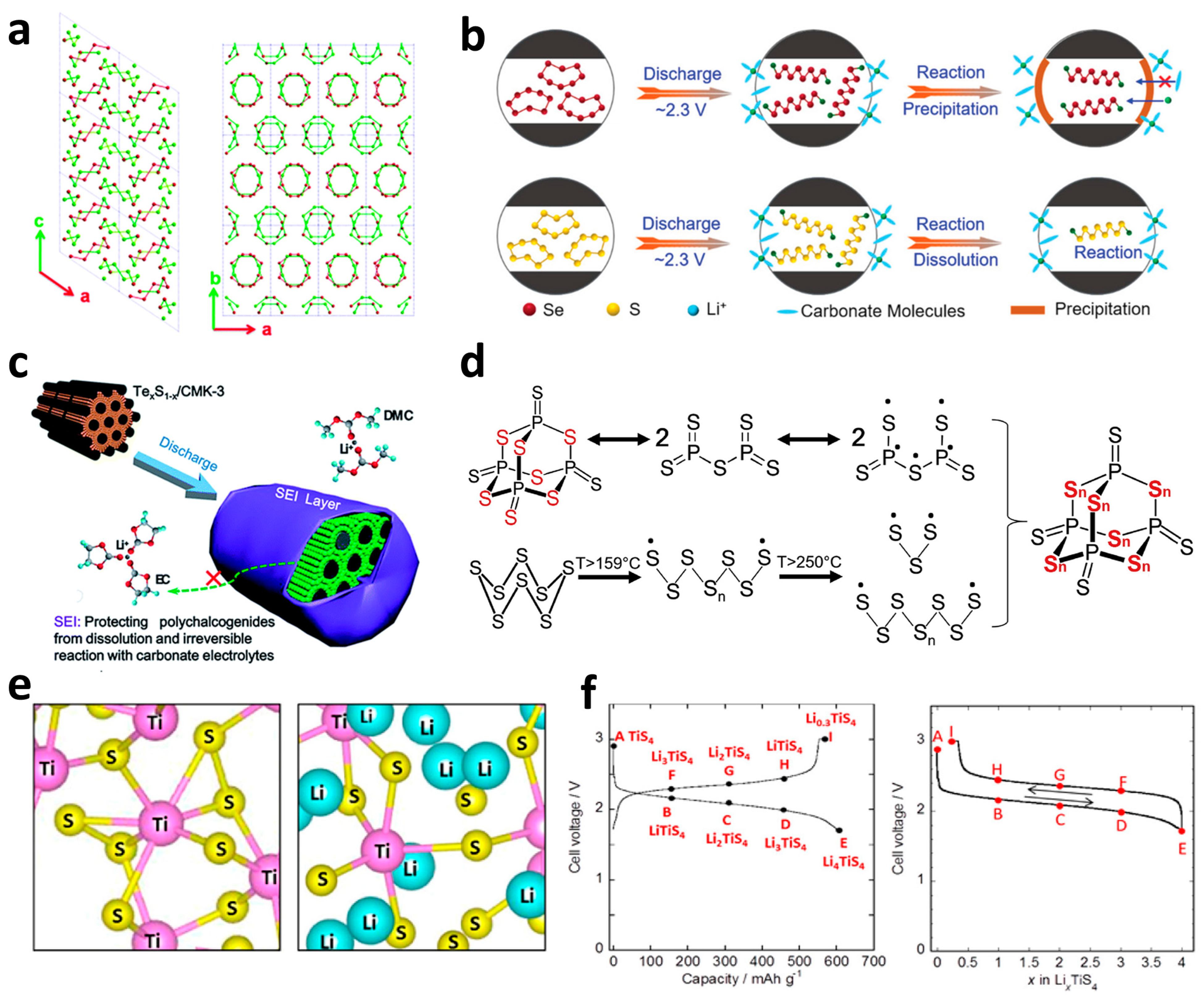
2.2.2. Non-Metallic Sulfides
2.2.3. Transition Metal–Sulfide Compound
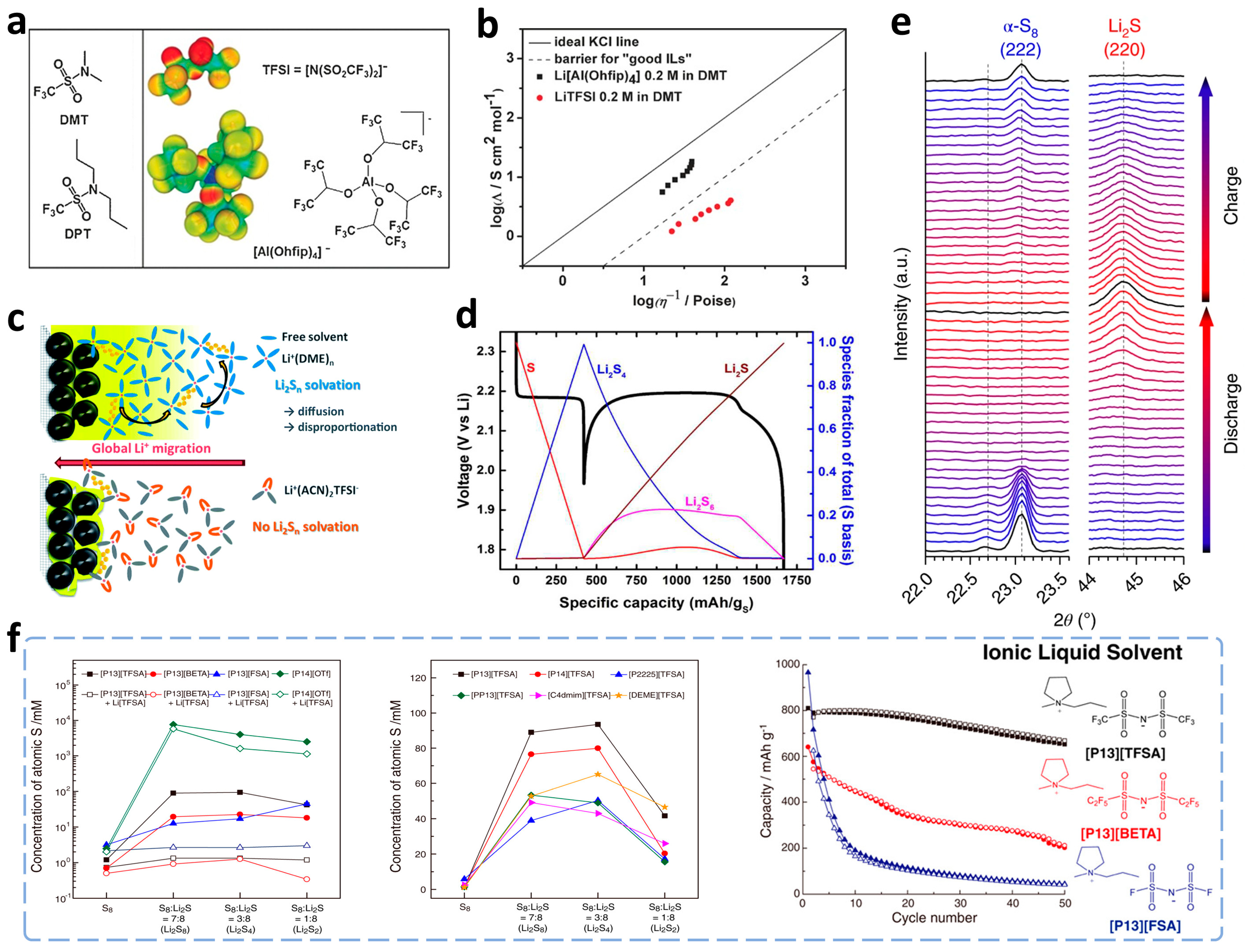
3. Modifications on Electrolytes
3.1. Regulations of Liquid Phase Electrolytes
3.1.1. Inhibition of Polysulfide Dissolution by Insoluble/Sparingly-Solvating Structures
Substitution of Insoluble Electrolytes
Modulations of Solventized Structure to Prevent Polysulfide Dissolution
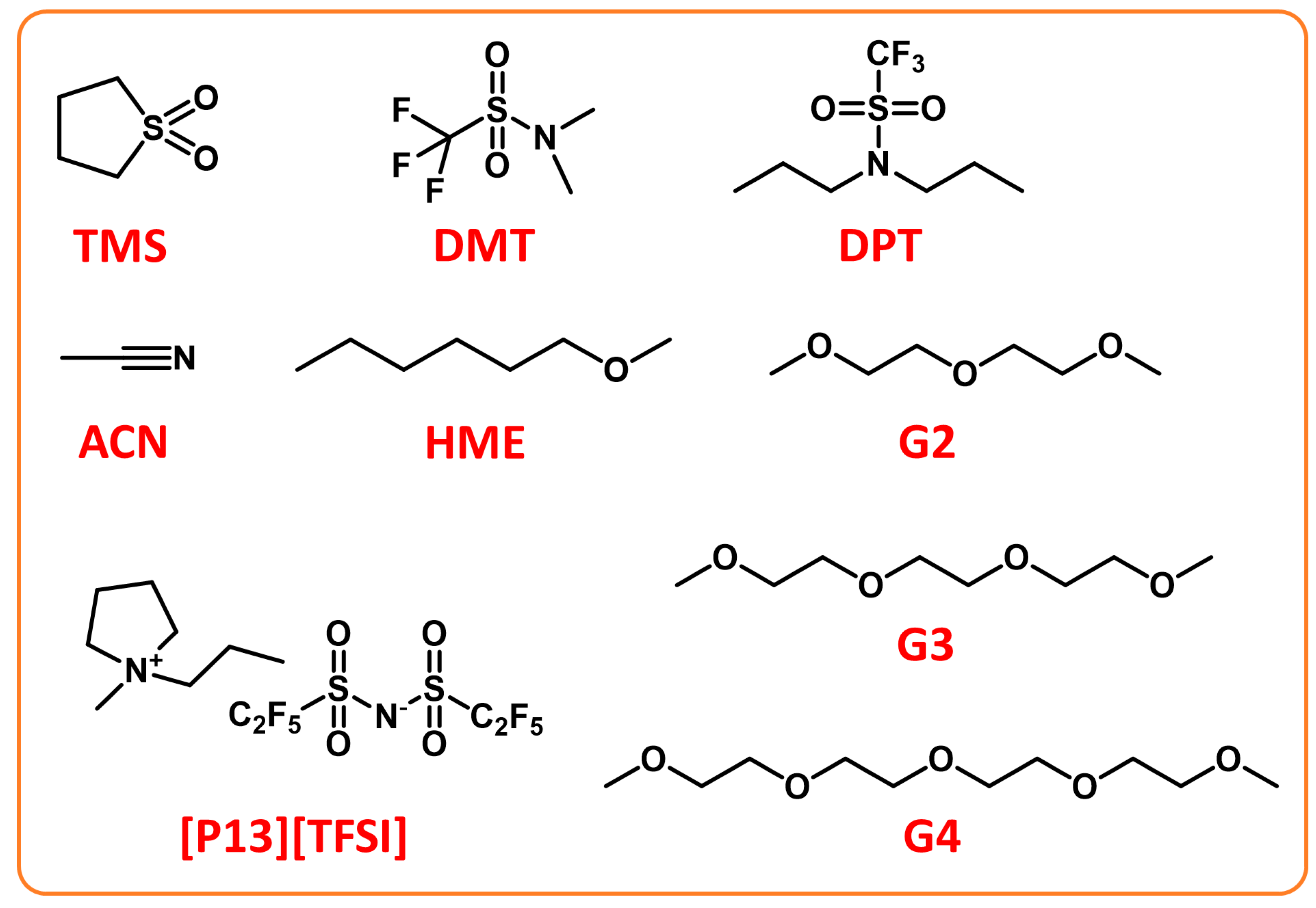
Ionic Liquids-Based Electrolytes
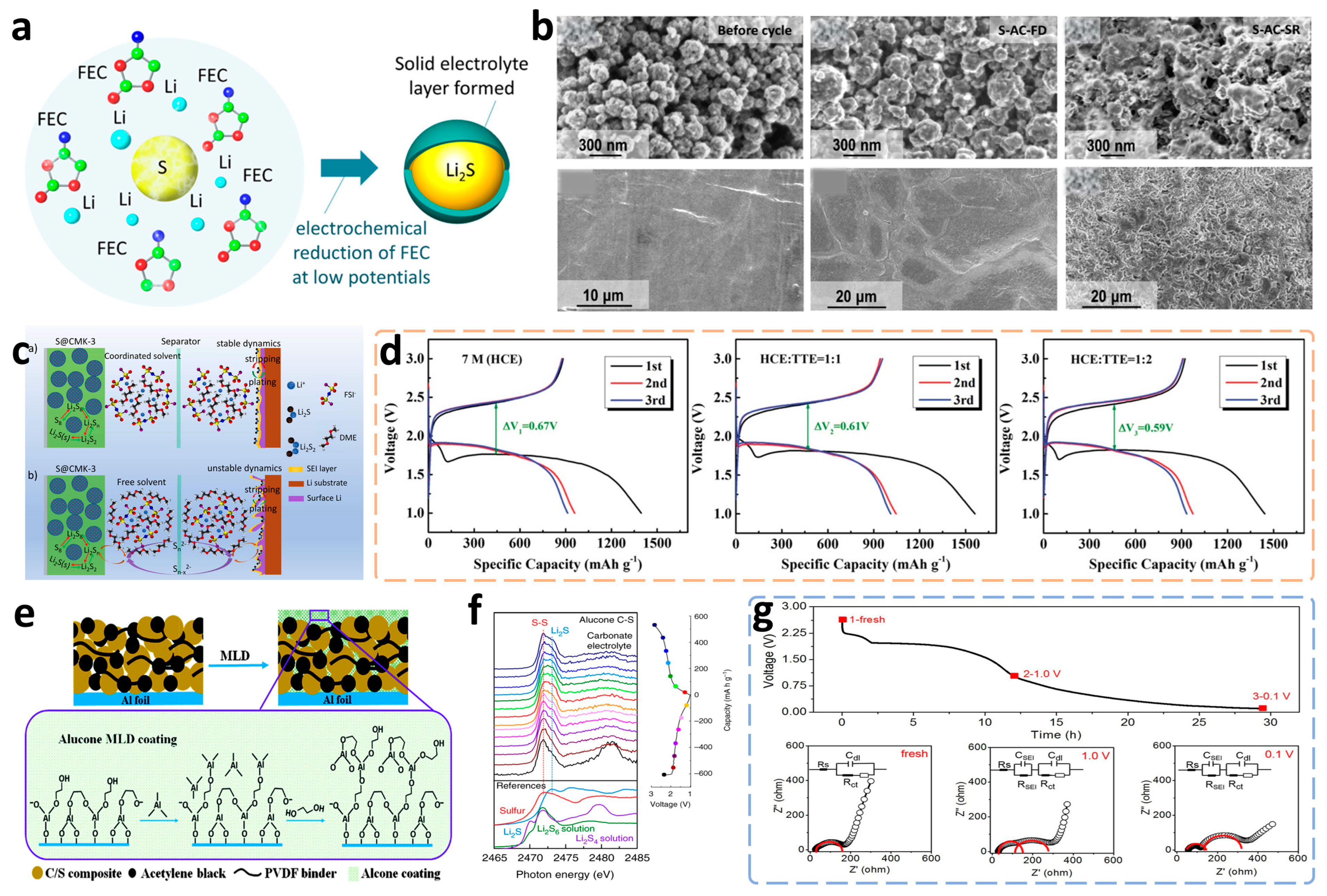
3.1.2. Construction of Dense SEI Films
Addition of Film-Forming Additives
Highly Concentrated Electrolytes
Ex-Situ Construction of SEI Films
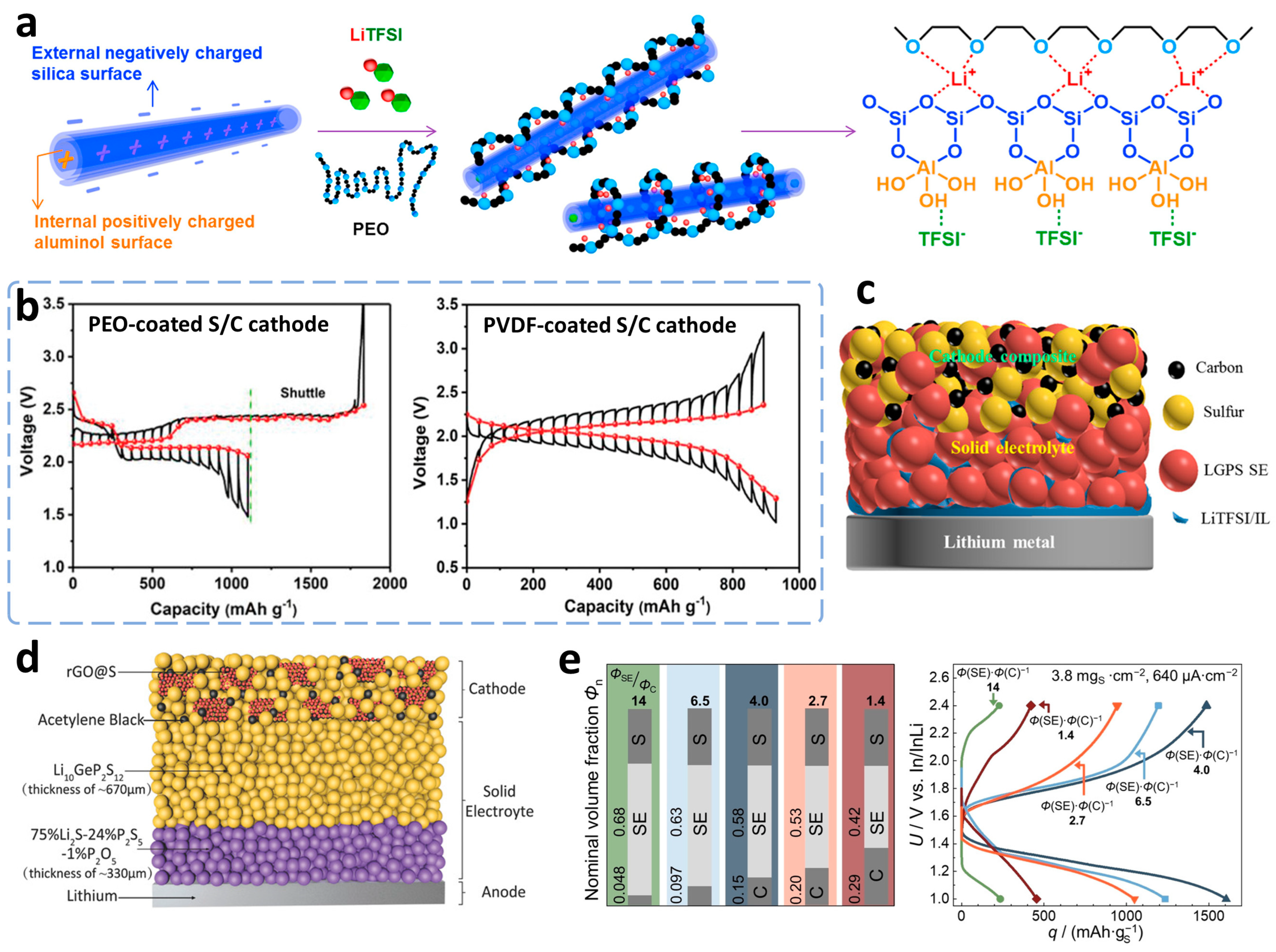
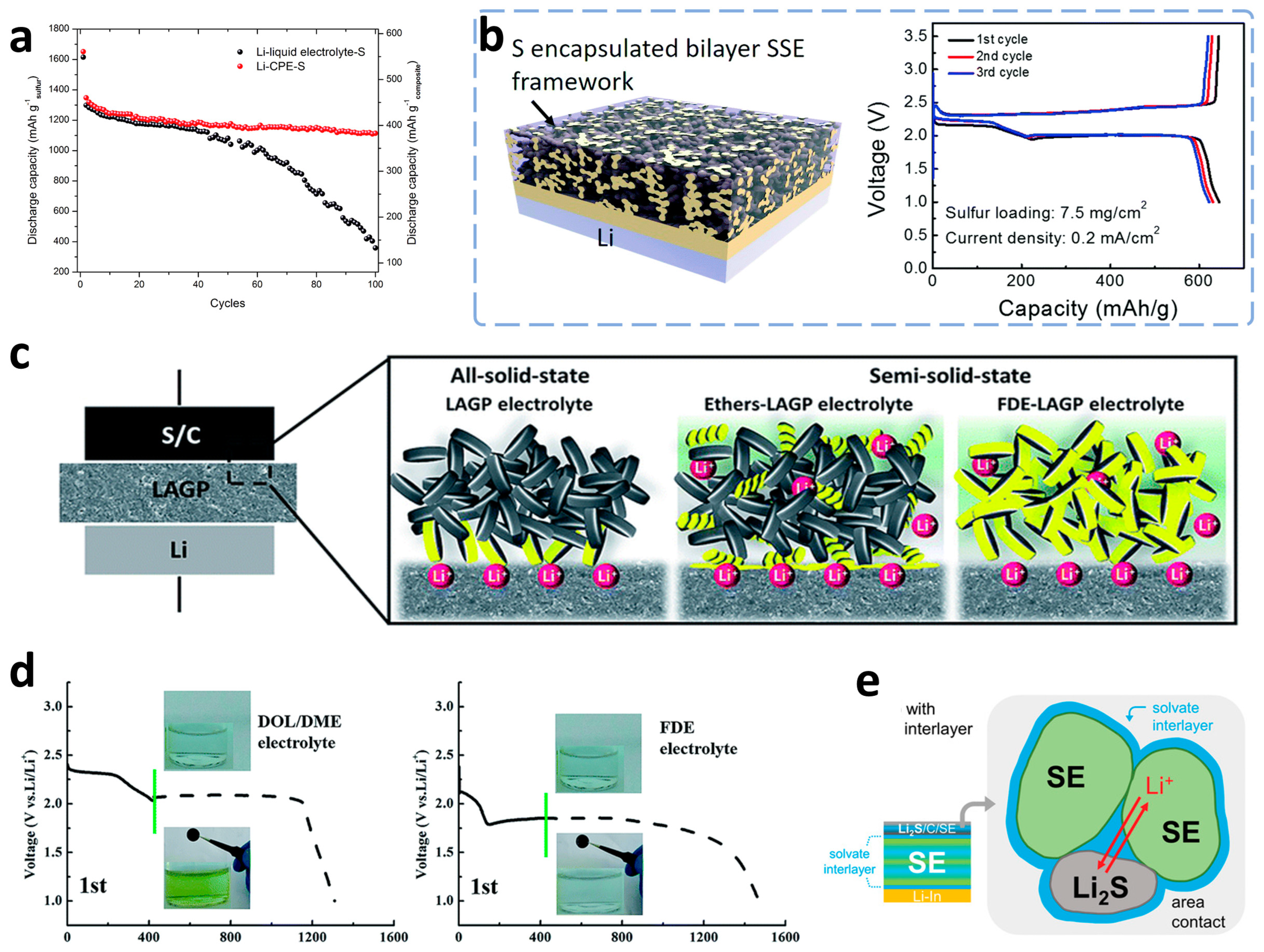
3.2. ASSLSBs Based on Solid-Phase Conversion
3.2.1. Polymer-Based Electrolytes
3.2.2. Inorganic-Based Solid Electrolytes
3.2.3. Hybrid Electrolytes
4. Summary and Perspectives
4.1. High Sulfur Loading
4.2. Low E/S Ratio
4.3. Modified Lithium Anode
4.4. Fast Reaction Kinetics
4.5. High Safety
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Lam, W.-Y.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.; Xu, H.; He, X. Cobalt-free cathode materials: Families and their prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]
- Ceder, G.; Chiang, Y.M.; Sadoway, D.R.; Aydinol, M.K.; Jang, Y.I.; Huang, B. Identification of cathode materials for lithium batteries guided by first-principles calculations. Nature 1998, 392, 694–696. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Cheng, X.-B.; Zhang, Q. Review on high-loading and high-energy lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar]
- Kang, W.; Deng, N.; Ju, J.; Li, Q.; Wu, D.; Ma, X.; Li, L.; Naebe, M.; Cheng, B. A review of recent developments in rechargeable lithium-sulfur batteries. Nanoscale 2016, 8, 16541–16588. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- He, Y.; Chang, Z.; Wu, S.; Zhou, H. Effective strategies for long-cycle life lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 6155–6182. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Lim, W.-G.; Kim, S.; Jo, C.; Lee, J. A comprehensive review of materials with catalytic effects in li–s batteries: Enhanced redox kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746–18757. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Li, J.; Zhang, Y.; Wook, A.; Yu, A.; Chen, Z. Structural and chemical synergistic encapsulation of polysulfides enables ultralong-life lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 2533–2538. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Review—Li metal anode in working lithium-sulfur batteries. J. Electrochem. Soc. 2018, 165, A6058. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Z.; Yu, Y.; Abruña, H.D.; Archer, L.A. Lithium–sulfur battery cathode enabled by lithium–nitrile interaction. J. Am. Chem. Soc. 2012, 135, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xiao, X.; Cai, M.; Yang, L. Polydopamine-coated, nitrogen-doped, hollow carbon-sulfur double-layered core-shell structure for improving lithium-sulfur batteries. Nano. Lett. 2014, 14, 5250–5256. [Google Scholar] [CrossRef]
- Chang, Z.; He, Y.; Deng, H.; Li, X.; Wu, S.; Qiao, Y.; Wang, P.; Zhou, H. A multifunctional silly-putty nanocomposite spontaneously repairs cathode composite for advanced li-s batteries. Adv. Funct. Mater. 2018, 28, 1804777. [Google Scholar] [CrossRef]
- Wang, J.; Chang, Z.; Ding, B.; Li, T.; Yang, G.; Pang, Z.; Nakato, T.; Eguchi, M.; Kang, Y.-M.; Na, J.; et al. Universal access to two-dimensional mesoporous heterostructures by micelle-directed interfacial assembly. Angew. Chem. Int. Ed. 2020, 59, 19570–19575. [Google Scholar] [CrossRef]
- Lin, Y.; He, S.; Ouyang, Z.; Li, J.; Zhao, J.; Xiao, Y.; Lei, S.; Cheng, B. Synergistic engineering of cobalt selenide and biomass-derived S, N, P co-doped hierarchical porous carbon for modulation of stable Li–S batteries. J. Mater. Sci. Technol. 2023, 134, 11–21. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Li, Y.; Zhao, Z.; Qiu, J. Selective catalytic oxidation of pollutant H2S over Co-decorated hollow N-doped carbon nanofibers for high-performance Li–S batteries. Appl. Catal. B-Environ. 2022, 317, 121763. [Google Scholar] [CrossRef]
- Chang, Z.; Ding, B.; Dou, H.; Wang, J.; Xu, G.; Zhang, X. Hierarchically porous multilayered carbon barriers for high-performance li–s batteries. Chem.—Eur. J. 2018, 24, 3768–3775. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Guo, S.; Wang, J.; Ouyang, Y.; Li, D.; Zeng, Q.; Gong, W.; Gan, L.; Zhang, Q.; et al. An ultralight electroconductive metal-organic framework membrane for multistep catalytic conversion and molecular sieving in lithium-sulfur batteries. Energy Storage Mater. 2022, 51, 882–889. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Liu, B.; Zhao, S.; Zhang, Y.; Qian, L.; Chen, Z.; Wang, J.; Wang, X.; Chen, Z. Creating edge sites within the 2D metal-organic framework boosts redox kinetics in lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2201960. [Google Scholar] [CrossRef]
- Yi, Y.; An, H.; Zhang, P.; Tian, X.; Yang, P.; Liu, P.; Wang, T.; Qu, L.; Li, M.; Yang, G.; et al. Oxygen deficiency driven conversion of polysulfide by electrocatalysis: MoO3-x nanobelts for an improved lithium-sulfur battery cathode. ChemNanoMat 2019, 5, 926–931. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, Z.; Yang, P.; Wang, T.; Zhao, X.; Huang, H.; Cheng, Y.; Zhang, J.; Li, M. MoS2 nanorods with inner caves through synchronous encapsulation of sulfur for high performance li–s cathodes. J. Energy Chem. 2020, 45, 18–24. [Google Scholar] [CrossRef]
- Fang, B.; Tian, X.; Wang, T.; Wang, T.; Qu, L.; Li, M. Restraining polysulfide with high-entropy metal nitride towards long cycle life and high capacity Li–S batteries. ChemElectroChem 2020, 7, 4737–4744. [Google Scholar] [CrossRef]
- Qu, L.; Liu, P.; Tian, X.; Shu, C.; Yi, Y.; Yang, P.; Wang, T.; Fang, B.; Li, M.; Yang, B. VN/S Nanoclusters encapsulated with graphene via zeta potential control: A pomegranate-like cathode for lithium-sulfur batteries with enhanced rate performance. ChemElectroChem 2020, 7, 1679–1688. [Google Scholar] [CrossRef]
- Wang, C.; Yi, Y.; Li, H.; Wu, P.; Li, M.; Jiang, W.; Chen, Z.; Li, H.; Zhu, W.; Dai, S. Rapid gas-assisted exfoliation promises V2O5 nanosheets for high performance lithium-sulfur batteries. Nano Energy 2020, 67, 104253. [Google Scholar] [CrossRef]
- Chang, Z.; Dou, H.; Ding, B.; Wang, J.; Wang, Y.; Hao, X.; MacFarlane, D.R. Co3O4 nanoneedle arrays as a multifunctional “super-reservoir” electrode for long cycle life Li–S batteries. J. Mater. Chem. A 2017, 5, 250–257. [Google Scholar] [CrossRef]
- Qu, L.; Liu, P.; Zhang, P.; Wang, T.; Yi, Y.; Yang, P.; Tian, X.; Li, M.; Yang, B. Carbon-nanotube/sulfur cathode with in-situ assembled Si3N4/graphene interlayer for high-rate and long cycling-life lithium-sulfur batteries. Electrochim. Acta 2019, 296, 155–164. [Google Scholar] [CrossRef]
- Qu, L.; Liu, P.; Yi, Y.; Wang, T.; Yang, P.; Tian, X.; Li, M.; Yang, B.; Dai, S. Enhanced cycling performance for Lithium-Sulfur batteries by a laminated 2D g-C3 N4/graphene cathode interlayer. ChemSusChem 2019, 12, 213–223. [Google Scholar] [CrossRef]
- Yi, Y.; Li, H.; Chang, H.; Yang, P.; Tian, X.; Liu, P.; Qu, L.; Li, M.; Yang, B.; Li, H.; et al. Few-Layer boron nitride with engineered nitrogen vacancies for promoting conversion of polysulfide as a cathode matrix for Lithium–Sulfur batteries. Chem.–Eur. J. 2019, 25, 8112–8117. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, Y.; Fan, M.; Liu, H.; Li, X.; Zhang, R.; Li, M.; Qiao, Z.-A. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries. Energy Storage Mater. 2019, 23, 678–683. [Google Scholar] [CrossRef]
- Ren, Y.; Zhai, Q.; Wang, B.; Hu, L.; Ma, Y.; Dai, Y.; Tang, S.; Meng, X. Synergistic Adsorption-Electrocatalysis of 2D/2D heterostructure toward high performance Li–S batteries. Chem. Eng. J. 2022, 439, 135535. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Liu, D.; Xiang, J.; Li, Z.; Huang, Y. Solid/Quasi-Solid phase conversion of sulfur in Lithium-Sulfur battery. Small 2022, 18, e2106970. [Google Scholar] [CrossRef] [PubMed]
- Mukkabla, R.; Buchmeiser, M.R. Cathode materials for lithium–sulfur batteries based on sulfur covalently bound to a polymeric backbone. J. Mater. Chem. A 2020, 8, 5379–5394. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Lee, S.; Agostini, M.; Jeong, M.G.; Jung, H.G.; Ming, J.; Sun, Y.K.; Kim, J.; Hwang, J.Y. Multiscale understanding of covalently fixed Sulfur-Polyacrylonitrile composite as advanced cathode for Metal-Sulfur batteries. Adv. Sci 2021, 8, e2101123. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Chen, H.; Qian, S.; Wu, Z.; Nizami, A.; Li, X.; Zhang, S.; Lai, C. Regulating liquid and solid-state electrolytes for solid-phase conversion in Li–S batteries. Chem 2022, 8, 1201–1230. [Google Scholar] [CrossRef]
- Cheng, L.; Curtiss, L.A.; Zavadil, K.R.; Gewirth, A.A.; Shao, Y.; Gallagher, K.G. Sparingly Solvating Electrolytes for High Energy Density Lithium–Sulfur Batteries. ACS Energy Lett. 2016, 1, 503–509. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal-Sulfur battery cathodes based on PAN-Sulfur composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Xin, S.; Gu, L.; Zhao, N.H.; Yin, Y.X.; Zhou, L.J.; Guo, Y.G.; Wan, L.J. Smaller sulfur molecules promise better lithium-sulfur batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef]
- Wang, D.-W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.-M.; Gentle, I.R.; Lu, G.Q.M. Carbon–sulfur composites for Li–S batteries: Status and prospects. J. Mater. Chem. A 2013, 1, 2106970. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, J.; Wang, J. Prospect of Sulfurized Pyrolyzed Poly(acrylonitrile) (S@pPAN) Cathode Materials for Rechargeable Lithium Batteries. Agew. Chem. Int. Ed. 2020, 59, 7306–7318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Xie, J.; Xu, N. A Novel Conductive Polymer–Sulfur composite cathode material for rechargeable lithium batteries. Adv. Mater. 2002, 14, 963–965. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; He, X.; Ren, J.; Jiang, C.; Wan, C. Electrochemical characteristics of sulfur composite cathode materials in rechargeable lithium batteries. J. Power Sources 2004, 138, 271–273. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Gao, J.; Guo, J.; Jiang, C.; Wan, C. Analysis of the synthesis process of sulphur–poly(acrylonitrile)-based cathode materials for lithium batteries. J. Mater. Chem. 2012, 22, 22077–22081. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Rolff, M.; Spera, M.B.M.; Tenzer, M.; Buchmeiser, M.R. Correlation of the electrochemistry of poly(acrylonitrile)–sulfur composite cathodes with their molecular structure. J. Mater. Chem. 2012, 22, 23240–23245. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, A.; Jin, Z.; Zhao, H.; Yang, Y. Effect of vapor pressure on performance of sulfurized polyacrylonitrile cathodes for Li/S batteries. RSC Adv. 2016, 6, 106625–106630. [Google Scholar] [CrossRef]
- Konarov, A.; Gosselink, D.; Doan, T.N.L.; Zhang, Y.; Zhao, Y.; Chen, P. Simple, scalable, and economical preparation of sulfur–PAN composite cathodes for Li/S batteries. J. Power Sources 2014, 259, 183–187. [Google Scholar] [CrossRef]
- Lei, J.; Chen, J.; Zhang, H.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. High molecular weight polyacrylonitrile precursor for S@pPAN composite cathode materials with high specific capacity for rechargeable lithium batteries. ACS Appl. Mater. Interfaces 2020, 12, 33702–33709. [Google Scholar] [CrossRef]
- Frey, M.; Zenn, R.K.; Warneke, S.; Müller, K.; Hintennach, A.; Dinnebier, R.E.; Buchmeiser, M.R. Easily accessible, textile fiber-based sulfurized poly(acrylonitrile) as Li/S cathode material: Correlating electrochemical performance with morphology and structure. ACS Energy Lett. 2017, 2, 595–604. [Google Scholar] [CrossRef]
- Lebherz, T.; Frey, M.; Hintennach, A.; Buchmeiser, M.R. Influence of morphology of monolithic sulfur-poly(acrylonitrile) composites used as cathode materials in lithium-sulfur batteries on electrochemical performance. RSC Adv. 2019, 9, 7181–7188. [Google Scholar] [CrossRef]
- Xiang, J.; Guo, Z.; Yi, Z.; Zhang, Y.; Yuan, L.; Cheng, Z.; Shen, Y.; Huang, Y. Facile synthesis of sulfurized polyacrylonitrile composite as cathode for high-rate lithium-sulfur batteries. J. Energy Chem. 2020, 49, 161–165. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Zhang, N.; Feng, T.; Li, L.; Wu, F.; Chen, R. Powering lithium-sulfur batteries by ultrathin sulfurized polyacrylonitrile nanosheets. Nanoscale 2021, 13, 16690–16695. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Chen, J.; Naveed, A.; Zhang, H.; Yang, J.; Nuli, Y.; Wang, J. Sulfurized polyacrylonitrile cathode derived from intermolecular cross-linked polyacrylonitrile for a rechargeable lithium battery. ACS Appl. Energy Mater. 2021, 4, 5706–5712. [Google Scholar] [CrossRef]
- He, B.; Rao, Z.; Cheng, Z.; Liu, D.; He, D.; Chen, J.; Miao, Z.; Yuan, L.; Li, Z.; Huang, Y. Rationally design a sulfur cathode with solid-phase conversion mechanism for high cycle-stable Li–S batteries. Adv. Energy Mater. 2021, 11, 2003690. [Google Scholar] [CrossRef]
- Yu, X.-G.; Xie, J.-Y.; Yang, J.; Huang, H.-J.; Wang, K.; Wen, Z.-S. Lithium storage in conductive sulfur-containing polymers. J. Electroanal. Chem. 2004, 573, 121–128. [Google Scholar]
- Fanous, J.; Wegner, M.; Grimminger, J.; Andresen, Ä.; Buchmeiser, M.R. Structure-related electrochemistry of sulfur-poly(acrylonitrile) composite cathode materials for rechargeable lithium batteries. Chem. Mater. 2011, 23, 5024–5028. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Jiao, X.; Zhang, C.; Dai, X.; Song, J. Stable cycling of phosphorus anode for sodium-ion batteries through chemical bonding with sulfurized polyacrylonitrile. Adv. Funct. Mater. 2018, 28, 1801010. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Wang, L.; Yang, H.; Li, H.; Zhao, Y.; Liu, T. Sulfurized polyacrylonitrile cathodes with high compatibility in both ether and carbonate electrolytes for ultrastable lithium–sulfur batteries. Adv. Funct. Mater. 2019, 29, 1902929. [Google Scholar] [CrossRef]
- Weret, M.A.; Jeffrey Kuo, C.-F.; Zeleke, T.S.; Beyene, T.T.; Tsai, M.-C.; Huang, C.-J.; Berhe, G.B.; Su, W.-N.; Hwang, B.-J. Mechanistic understanding of the Sulfurized-Poly(acrylonitrile) cathode for lithium-sulfur batteries. Energy Storage Mater. 2020, 26, 483–493. [Google Scholar] [CrossRef]
- Huang, C.J.; Lin, K.Y.; Hsieh, Y.C.; Su, W.N.; Wang, C.H.; Brunklaus, G.; Winter, M.; Jiang, J.C.; Hwang, B.J. New insights into the N-S bond formation of a sulfurized-polyacrylonitrile cathode material for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 14230–14238. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; He, X.; Wan, C. Kinetic investigation of sulfurized polyacrylonitrile cathode material by electrochemical impedance spectroscopy. Electrochim. Acta 2011, 56, 5252–5256. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Z.; Elia, G.A.; Wu, Y.; Wahyudi, W.; Abou-Hamad, E.; Emwas, A.-H.; Cavallo, L.; Li, L.-J.; Ming, J. Recognizing the mechanism of sulfurized polyacrylonitrile cathode materials for Li–S batteries and beyond in Al–S batteries. ACS Energy Lett. 2018, 3, 2899–2907. [Google Scholar] [CrossRef]
- Jin, Z.-Q.; Liu, Y.-G.; Wang, W.-K.; Wang, A.-B.; Hu, B.-W.; Shen, M.; Gao, T.; Zhao, P.-C.; Yang, Y.-S. A new insight into the lithium storage mechanism of sulfurized polyacrylonitrile with no soluble intermediates. Energy Storage Mater. 2018, 14, 272–278. [Google Scholar] [CrossRef]
- Wang, J.; Yin, L.; Jia, H.; Yu, H.; He, Y.; Yang, J.; Monroe, C.W. Hierarchical sulfur-based cathode materials with long cycle life for rechargeable lithium batteries. ChemSusChem 2014, 7, 563–569. [Google Scholar] [CrossRef]
- Konarov, A.; Bakenov, Z.; Yashiro, H.; Sun, Y.-K.; Myung, S.-T. Effect of carbon-sulphur bond in a sulphur/dehydrogenated polyacrylonitrile/reduced graphene oxide composite cathode for lithium-sulphur batteries. J. Power Sources 2017, 355, 140–146. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wu, Z.Z.; Pan, G.L.; Liu, S.; Gao, X.P. Microporous carbon polyhedrons encapsulated polyacrylonitrile nanofibers as sulfur immobilizer for lithium-sulfur battery. ACS Appl. Mater. Interfaces 2017, 9, 12436–12444. [Google Scholar] [CrossRef]
- Kuo, C.F.J.; Weret, M.A.; Hung, H.Y.; Tsai, M.C.; Huang, C.J.; Su, W.N.; Hwang, B.J. Sulfurized-poly(acrylonitrile) wrapped carbon sulfur composite cathode material for high performance rechargeable lithium sulfur batteries. J. Power Sources 2019, 412, 670–676. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, K.; Hong, B.; Zhang, L.; Zhang, Z.; Li, J.; Wang, X.; Lai, Y. A new insight into capacity fading of sulfurized polyacrylonitrile composite in carbonate electrolyte. J. Electroanal. Chem. 2021, 882, 114964. [Google Scholar] [CrossRef]
- Liu, Y.; Haridas, A.K.; Lee, Y.; Cho, K.-K.; Ahn, J.-H. Freestanding porous sulfurized polyacrylonitrile fiber as a cathode material for advanced lithium sulfur batteries. Appl. Surf. Sci. 2019, 472, 135–142. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Deng, H.; Cao, J.; Wang, L.; Peng, L.; Zhu, J.; Lu, B. 3D holey graphene/polyacrylonitrile sulfur composite architecture for high loading lithium sulfur batteries. Adv. Energy Mater. 2021, 11, 2100448. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C. One dimensional nanostructures contribute better Li–S and Li–Se batteries: Progress, challenges and perspectives. Energy Storage Mater. 2019, 23, 190–224. [Google Scholar]
- Xu, J.; Ma, J.; Fan, Q.; Guo, S.; Dou, S. Recent progress in the design of advanced cathode materials and battery models for high-performance lithium-X (X = O2, S, Se, Te, I2, Br2) batteries. Adv. Mater. 2017, 29, 1606454. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhu, Y.; Wen, Y.; Wang, J.; Wang, C. Carbonized Polyacrylonitrile-Stabilized SeSx Cathodes for Long Cycle Life and High Power Density Lithium Ion Batteries. Adv. Funct. Mater. 2014, 24, 4082–4089. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lu, Y.; Lou, X.W. A pyrolyzed polyacrylonitrile/selenium disulfide composite cathode with remarkable lithium and sodium storage performances. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef]
- Zhu, T.; Pang, Y.; Wang, Y.; Wang, C.; Xia, Y. S0.87Se0.13/CPAN composites as high capacity and stable cycling performance cathode for lithium sulfur battery. Electrochim. Acta 2018, 281, 789–795. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Gao, S.; Wang, R.; Han, J.; Yan, J.; Cheng, S.; Jiang, K. Selenium as Extra Binding Site for Sulfur Species in Sulfurized Polyacrylonitrile Cathodes for High Capacity Lithium-Sulfur Batteries. ChemElectroChem 2019, 6, 1365–1370. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.; Wang, L.; Yang, J.; Hao, Z.; Xiang, J.; Yuan, K.; Huang, Y.; Shan, B.; Yuan, L.; et al. Ether-compatible sulfurized polyacrylonitrile cathode with excellent performance enabled by fast kinetics via selenium doping. Nat. Commun. 2019, 10, 1021. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Peng, L.; Yang, J.; Jia, H.; Zhang, Z.; Shan, B.; Xie, J. Se as eutectic accelerator in sulfurized polyacrylonitrile for high performance all-solid-state lithium-sulfur battery. Energy Storage Mater. 2019, 21, 287–296. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Hu, W.; Peng, L.; Yang, J.; Wang, L.; Zhang, Y.; Shan, B.; Xie, J. Manipulating kinetics of sulfurized polyacrylonitrile with tellurium as eutectic accelerator to prevent polysulfide dissolution in lithium-sulfur battery under dissolution-deposition mechanism. Nano Energy 2019, 60, 153–161. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Peng, L.; Li, S.; Wang, X.; Cheng, S.; Xie, J. Elevating reactivity and cyclability of all-solid-state lithium-sulfur batteries by the combination of tellurium-doping and surface coating. Nano Energy 2020, 76, 105083. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Yuan, X.; Chen, Y.; Hu, J.; Mu, Q.; Ma, Y.; Zhao, X.; Miao, L.; Ahn, J.-H.; Peng, Y.; et al. Anchoring MOF-derived CoS2 on sulfurized polyacrylonitrile nanofibers for high areal capacity lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 1298–1306. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Chen, G.; Zhao, X.; Yuan, X.; Hu, J.; Li, Z.; Chen, Y.; Xu, J.; Shah, R.; Zhong, J.; et al. Cobalt coordination with pyridines in sulfurized polyacrylonitrile cathodes to form conductive pathways and catalytic M-N4S sites for accelerated Li–S kinetics. J. Energy Chem. 2021, 61, 170–178. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Chen, M.; Gao, J.; Jiang, C. Charge/discharge characteristics of sulfurized polyacrylonitrile composite with different sulfur content in carbonate based electrolyte for lithium batteries. Electrochim. Acta 2012, 72, 114–119. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, J.Y.; Aurbach, D.; Sun, Y.K. Electrochemical properties of sulfurized-polyacrylonitrile cathode for lithium-sulfur batteries: Effect of polyacrylic acid binder and fluoroethylene carbonate additive. J. Phys. Chem. Lett. 2017, 8, 5331–5337. [Google Scholar] [CrossRef]
- Warneke, S.; Hintennach, A.; Buchmeiser, M.R. Communication—Influence of carbonate-based electrolyte composition on cell performance of span-based lithium-sulfur-batteries. J. Electrochem. Soc. 2018, 165, A2093–A2095. [Google Scholar] [CrossRef]
- Yang, H.; Naveed, A.; Li, Q.; Guo, C.; Chen, J.; Lei, J.; Yang, J.; Nuli, Y.; Wang, J. Lithium sulfur batteries with compatible electrolyte both for stable cathode and dendrite-free anode. Energy Storage Mater. 2018, 15, 299–307. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Q.; Mu, D.; Ren, Y.; Li, Y.; Wang, L.; Xu, H.; Wu, F. New desolvated gel electrolyte for rechargeable lithium metal sulfurized polyacrylonitrile (S-PAN) battery. J. Phy. Chem. C 2014, 118, 28369–28376. [Google Scholar] [CrossRef]
- Jin, F.; Hu, C.; Liu, C.; Zheng, Y.; Chen, H.; Shen, Y.; Chen, L. Enhancing the performance of sulfurized polyacrylonitrile cathode by in-situ wrapping. J. Electroanal. Chem. 2019, 835, 156–160. [Google Scholar] [CrossRef]
- Kim, J.-S.; Hwang, T.H.; Kim, B.G.; Min, J.; Choi, J.W. A lithium-sulfur battery with a high areal energy density. Adv. Funct. Mater. 2014, 24, 5359–5367. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, Z.; Chen, K.; Lou, J.; Wang, Y. Highly stable lithium plating by a multifunctional electrolyte additive in a lithium-sulfurized polyacrylonitrile battery. Chem Commun. 2019, 55, 2376–2379. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Y.; Wang, D.; Chen, K.; Zhang, Z.; Wang, Y.; Lou, J. Highly stable performance of lithium-sulfurized polyacrylonitrile batteries using a lean ether-based electrolyte. Chem Commun. 2019, 55, 11271–11274. [Google Scholar] [CrossRef] [PubMed]
- Warneke, S.; Zenn, R.K.; Lebherz, T.; Müller, K.; Hintennach, A.; Starke, U.; Dinnebier, R.E.; Buchmeiser, M.R. Hybrid Li/S battery based on dimethyl trisulfide and sulfurized poly(acrylonitrile). Adv. Sustain. Syst. 2018, 2, 1700144. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, Y.; Liang, C.; Cao, L.; Pan, H.; Yang, J.; Wang, J. A new ether-based electrolyte for lithium sulfur batteries using a S@pPAN cathode. Chem. Commun. 2018, 54, 5478–5481. [Google Scholar] [CrossRef]
- Yang, H.; Qiao, Y.; Chang, Z.; He, P.; Zhou, H. Designing cation-solvent fully coordinated electrolyte for high-energy-density lithium-sulfur full cell based on solid-solid conversion. Angew. Chem. Int. Ed. Engl. 2021, 60, 17726–17734. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Z.; Hu, W.; Han, Z.; Cheng, S.; Xie, J. Diluted high concentration electrolyte with dual effects for practical lithium-sulfur batteries. Energy Storage Mater. 2021, 36, 333–340. [Google Scholar] [CrossRef]
- Liu, H.; Holoubek, J.; Zhou, H.; Chen, A.; Chang, N.; Wu, Z.; Yu, S.; Yan, Q.; Xing, X.; Li, Y.; et al. Ultrahigh coulombic efficiency electrolyte enables Li||SPAN batteries with superior cycling performance. Mater. Today 2021, 42, 17–28. [Google Scholar] [CrossRef]
- Lin, F.; Wang, J.; Jia, H.; Monroe, C.W.; Yang, J.; NuLi, Y. Nonflammable electrolyte for rechargeable lithium battery with sulfur based composite cathode materials. J. Power Sources 2013, 223, 18–22. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Jia, H.; Yang, J.; Monroe, C.W.; NuLi, Y. Towards a safe lithium-sulfur battery with a flame-inhibiting electrolyte and a sulfur-based composite cathode. Angew. Chem. Int. Ed. Engl. 2014, 53, 10099–10104. [Google Scholar] [CrossRef]
- Jia, H.; Wang, J.; Lin, F.; Monroe, C.W.; Yang, J.; NuLi, Y. TPPi as a flame retardant for rechargeable lithium batteries with sulfur composite cathodes. Chem. Commun. 2014, 50, 7011–7013. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Q.; Guo, C.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. Safer lithium-sulfur battery based on nonflammable electrolyte with sulfur composite cathode. Chem. Commun. 2018, 54, 4132–4135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Murugesan, V.; Han, K.S.; Jiang, X.; Cao, Y.; Xiao, L.; Ai, X.; Yang, H.; Zhang, J.-G.; Sushko, M.L.; et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 2018, 3, 674–681. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Zhang, X.; Lei, J.; Zhang, H.; Yuan, H.; Yang, J.; Nuli, Y.; Wang, J. Highly reversible lithium-metal anode and lithium-sulfur batteries enabled by an intrinsic safe electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 33419–33427. [Google Scholar] [CrossRef]
- Yang, H.; Guo, C.; Chen, J.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. An intrinsic flame-retardant organic electrolyte for safe lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2019, 58, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Z.; Monroe, C.W.; Yang, J.; Nuli, Y. Carbonyl-β-cyclodextrin as a novel binder for sulfur composite cathodes in rechargeable lithium batteries. Adv. Funct. Mater. 2013, 23, 1194–1201. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Xie, L.; Yang, J.; Nuli, Y.; Wang, J. Guar gum as a novel binder for sulfur composite cathodes in rechargeable lithium batteries. Chem. Commun. 2016, 52, 13479–13482. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Yang, H.; Lei, J.; Naveed, A.; Yang, J.; Nuli, Y.; Wang, J. Towards practical Li–S battery with dense and flexible electrode containing lean electrolyte. Energy Storage Mater. 2020, 27, 307–315. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.; Gentle, I.R.; Wang, D.-W. Carboxymethyl cellulose binders enable high-rate capability of sulfurized polyacrylonitrile cathodes for Li–S batteries. J. Mater. Chem. A 2017, 5, 5460–5465. [Google Scholar] [CrossRef]
- Wu, B.; Chen, F.; Mu, D.; Liao, W.; Wu, F. Cycleability of sulfurized polyacrylonitrile cathode in carbonate electrolyte containing lithium metasilicate. J. Power Sources 2015, 278, 27–31. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Yan, W.; Huang, Q.; Zhu, Y.; Fu, L.; Wu, Y. Synergy of sulfur/polyacrylonitrile composite and gel polymer electrolyte promises heat-resistant lithium-sulfur batteries. iScience 2019, 19, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.-B.; Jeong, J.-S.; Chae, M.-R.; Noh, J.-P.; Cho, K.-K.; Kim, J.-K.; Ahn, H.-J.; Nam, T.-H.; Kim, K.-W. Electrochemical properties of sulfurized poly-acrylonitrile (SPAN) cathode containing carbon fiber current collectors. Surf. Coat. Tech. 2017, 326, 443–449. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.; Wang, W.; Wang, A.; Jin, Z.; Wu, F.; Yang, Y. High-safety lithium-ion sulfur battery with sulfurized polyacrylonitrile cathode, prelithiated SiOx/C anode and carbonate-based electrolyte. J. Alloy. Compd. 2017, 723, 974–982. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, H.J.; Zeng, Z.; Han, Z.; Hu, W.; Wen, R.; Xie, J. Reconfiguring Organosulfur Cathode by Over-Lithiation to Enable Ultrathick Lithium Metal Anode toward Practical Lithium-Sulfur Batteries. ACS Nano 2020, 14, 13784–13793. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Xu, J.; Wang, D.-W. Covalent fixing of sulfur in metal–sulfur batteries. Energy Environ. Sci. 2020, 13, 432–471. [Google Scholar] [CrossRef]
- Duan, B.; Wang, W.; Wang, A.; Yuan, K.; Yu, Z.; Zhao, H.; Qiu, J.; Yang, Y. Carbyne polysulfide as a novel cathode material for lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 13261–13267. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Z.; He, J.; Cui, Z.; Chai, J.; Ma, J.; Yang, Z.; Huang, C.; Cui, G. A delicately designed sulfide graphdiyne compatible cathode for high-performance lithium/magnesium-sulfur batteries. Small 2017, 13, 1702277. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, W.; Tian, X.; Fang, B.; Wu, Z.; Zheng, S.; Li, M.; Ma, H. Graphdiyne-like Porous Organic Framework as a Solid-Phase Sulfur Conversion Cathodic Host for Stable Li–S Batteries. ACS Appl. Mater. Interfaces 2021, 13, 59983–59992. [Google Scholar] [CrossRef]
- Ma, J.; Xu, G.; Li, Y.; Ge, C.; Li, X. An in situ chemically and physically confined sulfur-polymer composite for lithium-sulfur batteries with carbonate-based electrolytes. Chem. Commun. 2018, 54, 14093–14096. [Google Scholar] [CrossRef]
- Chang, W.; Qu, J.; Sui, Y.; Ji, Q.-Y.; Zhang, T.-T.; Zhai, X.-Z.; Jing, Y.-Q.; Yu, Z.-Z. Constructing mesoporous hollow polysulfane spheres bonded with short-chain sulfurs (Sx, x ≤ 3) as high-performance sulfur cathodes in both ether and ester electrolytes. Energy Storage Mater. 2020, 27, 426–434. [Google Scholar] [CrossRef]
- Chang, W.; Qu, J.; Li, W.; Liu, Y.H.; Zhai, X.Z.; Liu, H.J.; Kang, Y.; Yu, Z.Z. Mesoporous yolk-shell structured organosulfur nanotubes with abundant internal joints for high-performance lithium-sulfur batteries by kinetics acceleration. Small 2021, 17, e2101857. [Google Scholar] [CrossRef] [PubMed]
- Markevich, E.; Salitra, G.; Talyosef, Y.; Chesneau, F.; Aurbach, D. Review—On the mechanism of quasi-solid-state lithiation of sulfur encapsulated in microporous carbons: Is the existence of small sulfur molecules necessary? J. Electrochem. Soc. 2016, 164, A6244–A6253. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon materials for lithium sulfur batteries-ten critical questions. Chemistry 2016, 22, 7324–7351. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xie, J.; Xu, N.; Li, Y. Sulfur–carbon nano-composite as cathode for rechargeable lithium battery based on gel electrolyte. Electrochem. Commun. 2002, 4, 499–502. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, D.; Pan, J.; Cao, Y.; Yang, H.; Ai, X. A Li+-conductive microporous carbon–sulfur composite for Li–S batteries. Electrochim. Acta 2013, 87, 497–502. [Google Scholar] [CrossRef]
- Zheng, S.; Han, P.; Han, Z.; Zhang, H.; Tang, Z.; Yang, J. High performance C/S composite cathodes with conventional carbonate-based electrolytes in Li–S battery. Sci. Rep. 2014, 4, 4842. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Y.; Zhu, Y.; Gaskell, K.; Cychosz, K.A.; Eichhorn, B.; Xu, K.; Wang, C. confined sulfur in microporous carbon renders superior cycling stability in li/s batteries. Adv. Funct. Mater. 2015, 25, 4312–4320. [Google Scholar] [CrossRef]
- Peng, Z.; Fang, W.; Zhao, H.; Fang, J.; Cheng, H.; Doan, T.N.L.; Xu, J.; Chen, P. Graphene-based ultrathin microporous carbon with smaller sulfur molecules for excellent rate performance of lithium-sulfur cathode. J. Power Sources 2015, 282, 70–78. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhou, G.; Li, F.; Wu, K.H.; Lu, G.Q.; Cheng, H.M.; Gentle, I.R. A microporous-mesoporous carbon with graphitic structure for a high-rate stable sulfur cathode in carbonate solvent-based Li–S batteries. Phys Chem Chem Phys 2012, 14, 8703–8710. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the electrode mechanism in lithium-sulfur batteries with ordered microporous carbon confined sulfur as the cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Li, L.-Y.; Chen, Y.-X.; Zhong, B.-H. Synthesis and electrochemical performance of a simple and low-cost sulfur/porous carbon composite cathode for rechargeable lithium sulfur battery. Compos. Part A Appl. Sci. Manuf. 2014, 62, 26–31. [Google Scholar] [CrossRef]
- Fu, C.; Wong, B.M.; Bozhilov, K.N.; Guo, J. Solid state lithiation-delithiation of sulphur in sub-nano confinement: A new concept for designing lithium-sulphur batteries. Chem. Sci. 2016, 7, 1224–1232. [Google Scholar] [CrossRef]
- Nojabaee, M.; Sievert, B.; Schwan, M.; Schettler, J.; Warth, F.; Wagner, N.; Milow, B.; Friedrich, K.A. Ultramicroporous carbon aerogels encapsulating sulfur as the cathode for lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 6508–6519. [Google Scholar] [CrossRef]
- Helen, M.; Reddy, M.A.; Diemant, T.; Golla-Schindler, U.; Behm, R.J.; Kaiser, U.; Fichtner, M. Single step transformation of sulphur to Li2S2/Li2S in Li–S batteries. Sci. Rep. 2015, 5, 12146. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Li, S.; Long, P.; Cao, C.; Cao, Y.; Wang, W.; Feng, Y.; Feng, W. A low cost ultra-microporous carbon scaffold with confined chain-like sulfur molecules as a superior cathode for lithium–sulfur batteries. Sustain. Energy Fuels 2018, 2, 2187–2196. [Google Scholar] [CrossRef]
- Rosenman, A.; Markevich, E.; Salitra, G.; Talyosef, Y.; Chesneau, F.; Aurbach, D. Facile Synthesis and Very Stable Cycling of Polyvinylidene Dichloride Derived Carbon: Sulfur Composite Cathode. J. Electrochem. Soc. 2016, 163, A1829–A1835. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, Q.; An, Y.; Anasori, B.; Wang, H.; Xu, B. Ultra-microporous carbons encapsulate small sulfur molecules for high performance lithium-sulfur battery. Nano Energy 2017, 33, 402–409. [Google Scholar] [CrossRef]
- Hu, L.; Lu, Y.; Li, X.; Liang, J.; Huang, T.; Zhu, Y.; Qian, Y. Optimization of Microporous Carbon Structures for Lithium-Sulfur Battery Applications in Carbonate-Based Electrolyte. Small 2017, 13, 1603533. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Miao, J.; Guan, Z.; Liu, H.; Chen, R.; An, Y.; Wu, F.; Xu, B. Three-Dimensional Carbon Current Collector Promises Small Sulfur Molecule Cathode with High Areal Loading for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 10882–10889. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Miao, J.; Zhang, P.; Xu, B. 2D MXene nanosheets enable small-sulfur electrodes to be flexible for lithium-sulfur batteries. Nanoscale 2019, 11, 8442–8448. [Google Scholar] [CrossRef] [PubMed]
- Helen, M.; Diemant, T.; Schindler, S.; Behm, R.J.; Danzer, M.; Kaiser, U.; Fichtner, M.; Anji Reddy, M. Insight into Sulfur Confined in Ultramicroporous Carbon. ACS Omega 2018, 3, 11290–11299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, Y.; DeCarlo, S.; Wang, Y.; Leung, K.; Qi, Y.; Xu, K.; Wang, C.; Eichhorn, B.W. Compositions and Formation Mechanisms of Solid-Electrolyte Interphase on Microporous Carbon/Sulfur Cathodes. Chem. Mater. 2020, 32, 3765–3775. [Google Scholar] [CrossRef]
- Peng, L.; Wei, Z.; Wan, C.; Li, J.; Chen, Z.; Zhu, D.; Baumann, D.; Liu, H.; Allen, C.S.; Xu, X.; et al. A fundamental look at electrocatalytic sulfur reduction reaction. Nat. Catalysis 2020, 3, 762–770. [Google Scholar] [CrossRef]
- Hou, T.-Z.; Chen, X.; Peng, H.-J.; Huang, J.-Q.; Li, B.-Q.; Zhang, Q.; Li, B. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium–Sulfur Batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Wu, H.B.; Wei, S.; Zhang, L.; Xu, R.; Hng, H.H.; Lou, X.W. Embedding sulfur in MOF-derived microporous carbon polyhedrons for lithium-sulfur batteries. Chemistry 2013, 19, 10804–10808. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L. Nitrogen-doped MOF-derived micropores carbon as immobilizer for small sulfur molecules as a cathode for lithium sulfur batteries with excellent electrochemical performance. ACS Appl. Mater. Interfaces 2015, 7, 4029–4038. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, G.; Lv, W.; Shi, H.; Luo, C.; He, Y.; Li, B.; Yang, Q.-H.; Kang, F. Sulfur confined in nitrogen-doped microporous carbon used in a carbonate-based electrolyte for long-life, safe lithium-sulfur batteries. Carbon 2016, 109, 1–6. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Zhang, Y.; Wang, X.; Zhang, C.; Zhang, S. Nitrogen-doped microporous carbon with narrow pore size distribution as sulfur host to encapsulate small sulfur molecules for highly stable lithium-sulfur batteries. J. Solid State Electrochem. 2021, 25, 1293–1302. [Google Scholar] [CrossRef]
- Xu, Q.-T.; Xue, H.-G.; Guo, S.-P. Status and prospects of SexSy cathodes for lithium/sodium storage. Inorg. Chem. Front. 2019, 6, 1326–1340. [Google Scholar] [CrossRef]
- Du, H.; Feng, S.; Luo, W.; Zhou, L.; Mai, L. Advanced Li–Se S battery system: Electrodes and electrolytes. J. Mater. Sci. Technol. 2020, 55, 1–15. [Google Scholar] [CrossRef]
- Susarla, S.; Puthirath, A.B.; Tsafack, T.; Salpekar, D.; Babu, G.; Ajayan, P.M. Atomic-Level Alloying of Sulfur and Selenium for Advanced Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, J.; Hou, Z.; Zhang, W.; Wang, Y.; Zhu, Y.; Qian, Y. A New Salt-Baked Approach for Confining Selenium in Metal Complex-Derived Porous Carbon with Superior Lithium Storage Properties. Adv. Funct. Mater. 2015, 25, 5229–5238. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Fan, L.; Zhu, Y.; Liang, J.; Qian, Y. Graphene–encapsulated selenium/polyaniline core–shell nanowires with enhanced electrochemical performance for Li–Se batteries. Nano Energy 2015, 13, 592–600. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Zhang, K.; Hou, Z.; Zhang, W.; Zhu, Y.; Qian, Y. Amorphous S-rich S1-xSex/C (x ≤ 0.1) composites promise better lithium–sulfur batteries in a carbonate-based electrolyte. Energy Environ. Sci. 2015, 8, 3181–3186. [Google Scholar] [CrossRef]
- Zeng, L.; Yao, Y.; Shi, J.; Jiang, Y.; Li, W.; Gu, L.; Yu, Y. A flexible S1-xSex@porous carbon nanofibers (x ≤ 0.1) thin film with high performance for Li–S batteries and room-temperature Na-S batteries. Energy Storage Mater. 2016, 5, 50–57. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.; Lee, S.; Eom, K.; Pak, C.; Kim, H.-J. Lithium-selenium sulfide batteries with long cycle life and high energy density via solvent washing treatment. Appl. Surface Sci. 2020, 512, 145632. [Google Scholar] [CrossRef]
- He, B.; Liu, D.; Cheng, Z.; Miao, Z.; Rao, Z.; Zhang, H.; Yuan, L.; Li, Z.; Huang, Y. Enabling Selenium-Rich SexSy Cathodes to Work in Carbonate-Based Electrolytes. Adv. Energy Mater. 2021, 12, 2102832. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, B.; Tang, H.; Yue, Z.; Li, X.; Yin, C.; Zhou, L. Heteroatomic TexS1-x molecule/C nanocomposites as stable cathode materials in carbonate-based electrolytes for lithium–chalcogen batteries. J. Mater. Chem. A 2018, 6, 10104–10110. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Liang, J.; Cai, J.; Zhang, K.; Lu, Y.; Wu, X.; Zhu, M.; Liu, Y.; Zhu, Y.; et al. Manipulating the Redox Kinetics of Li–S Chemistry by Tellurium Doping for Improved Li–S Batteries. ACS Energy Lett. 2018, 3, 420–427. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.; Fu, W.; Dudney, N.J.; Liang, C. Lithium polysulfidophosphates: A family of lithium-conducting sulfur-rich compounds for lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2013, 52, 7460–7463. [Google Scholar] [CrossRef] [PubMed]
- Tanibata, N.; Tsukasaki, H.; Deguchi, M.; Mori, S.; Hayashi, A.; Tatsumisago, M. A novel discharge–charge mechanism of a S–P2S5 composite electrode without electrolytes in all-solid-state Li/S batteries. J. Mater. Chem. A 2017, 5, 11224–11228. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Banis, M.N.; Luo, J.; Wang, C.; Li, W.; Li, X.; Sun, Q.; Hu, Y.; Xiao, Q.; et al. Totally compatible P4S10+n cathodes with self-generated Li+ pathways for sulfide-based all-solid-state batteries. Energy Storage Mater. 2020, 28, 325–333. [Google Scholar] [CrossRef]
- Sakuda, A.; Taguchi, N.; Takeuchi, T.; Kobayashi, H.; Sakaebe, H.; Tatsumi, K.; Ogumi, Z. Amorphous Niobium Sulfides as Novel Positive-Electrode Materials. ECS Electrochem. Lett. 2014, 3, A79–A81. [Google Scholar] [CrossRef]
- Matsuyama, T.; Hayashi, A.; Hart, C.J.; Nazar, L.F.; Tatsumisago, M. Amorphous TiS3/S/C Composite Positive Electrodes with High Capacity for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2016, 163, A1730–A1735. [Google Scholar] [CrossRef]
- Ye, H.; Ma, L.; Zhou, Y.; Wang, L.; Han, N.; Zhao, F.; Deng, J.; Wu, T.; Li, Y.; Lu, J. Amorphous MoS3 as the sulfur-equivalent cathode material for room-temperature Li–S and Na-S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 13091–13096. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Li, W.; Luo, J.; Li, X.; Yang, X.; Hu, Y.; Xiao, Q.; Zhang, W.; Li, R.; et al. Stabilizing sulfur cathode in carbonate and ether electrolytes: Excluding long-chain lithium polysulfide formation and switching lithiation/delithiation route. Chem. Mater. 2019, 31, 2002–2009. [Google Scholar] [CrossRef]
- Sakuda, A.; Ohara, K.; Fukuda, K.; Nakanishi, K.; Kawaguchi, T.; Arai, H.; Uchimoto, Y.; Ohta, T.; Matsubara, E.; Ogumi, Z.; et al. Amorphous Metal Polysulfides: Electrode Materials with Unique Insertion/Extraction Reactions. J. Am. Chem. Soc. 2017, 139, 8796–8799. [Google Scholar] [CrossRef]
- Umemura, Y.; Takeuchi, T.; Sakaebe, H.; Kiuchi, H.; Yaji, T.; Katayama, M. Improvement of Cycle Capability of VS4 by Addition of Phosphorus Element. Electrochemistry 2021, 89, 273–278. [Google Scholar]
- Lu, K.; Liu, Y.; Chen, J.; Zhang, Z.; Cheng, Y. Redox catalytic and quasi-solid sulfur conversion for high-capacity lean lithium sulfur batteries. ACS Nano 2019, 13, 14540–14548. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Peng, H.J.; Yuan, H.; Wei, J.Y.; Huang, J.Q. Lithium-sulfur batteries under lean electrolyte conditions: Challenges and opportunities. Angew. Chem. Int. Ed. Engl. 2020, 59, 12636–12652. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.; Pampel, J.; Dörfler, S.; Althues, H.; Kaskel, S. Polysulfide Shuttle Suppression by Electrolytes with Low-Density for High-Energy Lithium–Sulfur Batteries. Energy Technol. 2019, 7, 1900625. [Google Scholar] [CrossRef]
- Weller, C.; Thieme, S.; Härtel, P.; Althues, H.; Kaskel, S. Intrinsic shuttle suppression in lithium-sulfur batteries for pouch cell application. J. Electrochem. Soc. 2017, 164, A3766–A3771. [Google Scholar] [CrossRef]
- Kensy, C.; Schwotzer, F.; Dörfler, S.; Althues, H.; Kaskel, S. Impact of carbon porosity on sulfur conversion in li-s battery cathodes in a sparingly polysulfide solvating electrolyte. Batter. Supercaps 2021, 4, 823–833. [Google Scholar] [CrossRef]
- Shyamsunder, A.; Beichel, W.; Klose, P.; Pang, Q.; Scherer, H.; Hoffmann, A.; Murphy, G.K.; Krossing, I.; Nazar, L.F. Inhibiting polysulfide shuttle in lithium-sulfur batteries through low-ion-pairing salts and a triflamide solvent. Angew. Chem. Int. Ed. Engl. 2017, 56, 6192–6197. [Google Scholar] [CrossRef] [PubMed]
- Cuisinier, M.; Cabelguen, P.E.; Adams, B.D.; Garsuch, A.; Balasubramanian, M.; Nazar, L.F. Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries. Energy Environ. Sci. 2014, 7, 2697–2705. [Google Scholar] [CrossRef]
- Shin, M.; Wu, H.L.; Narayanan, B.; See, K.A.; Assary, R.S.; Zhu, L.; Haasch, R.T.; Zhang, S.; Zhang, Z.; Curtiss, L.A.; et al. Effect of the Hydrofluoroether Cosolvent Structure in Acetonitrile-Based Solvate Electrolytes on the Li(+) Solvation Structure and Li–S Battery Performance. ACS Appl. Mater. Interfaces 2017, 9, 39357–39370. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Pang, Q.; Ha, S.; Cheng, L.; Han, S.-D.; Zavadil, K.R.; Gallagher, K.G.; Nazar, L.F.; Balasubramanian, M. Directing the lithium–sulfur reaction pathway via sparingly solvating electrolytes for high energy density batteries. ACS Cent. Sci. 2017, 3, 605–613. [Google Scholar] [CrossRef]
- Pang, Q.; Shyamsunder, A.; Narayanan, B.; Kwok, C.Y.; Curtiss, L.A.; Nazar, L.F. Tuning the electrolyte network structure to invoke quasi-solid state sulfur conversion and suppress lithium dendrite formation in Li–S batteries. Nat. Energy 2018, 3, 783–791. [Google Scholar] [CrossRef]
- Ishino, Y.; Takahashi, K.; Murata, W.; Umebayashi, Y.; Tsuzuki, S.; Watanabe, M.; Kamaya, M.; Seki, S. Effect of Electrolyte Composition on Performance and Stability of Lithium–Sulfur Batteries. Energy Technol. 2019, 7, 1900197. [Google Scholar] [CrossRef]
- Matsumae, Y.; Obata, K.; Ando, A.; Yanagi, M.; Kamei, Y.; Ueno, K.; Dokko, K.; Watanabe, M. Effects of sulfur loading, cathode porosity, and electrolyte amount on li-s battery performance with solvate ionic liquid electrolyte. Electrochemistry 2019, 87, 254–259. [Google Scholar] [CrossRef]
- Nakanishi, A.; Ueno, K.; Watanabe, D.; Ugata, Y.; Matsumae, Y.; Liu, J.; Thomas, M.L.; Dokko, K.; Watanabe, M. Sulfolane-based highly concentrated electrolytes of lithium bis(trifluoromethanesulfonyl)amide: Ionic transport, li-ion coordination, and li–s battery performance. J. Phy. Chem. C 2019, 123, 14229–14238. [Google Scholar] [CrossRef]
- Park, J.-W.; Ueno, K.; Tachikawa, N.; Dokko, K.; Watanabe, M. Ionic liquid electrolytes for lithium–sulfur batteries. J. Phy. Chem. C 2013, 117, 20531–20541. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Rosenman, A.; Talyosef, Y.; Chesneau, F.; Aurbach, D. The effect of a solid electrolyte interphase on the mechanism of operation of lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 19873–19883. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Rao, Z.; Yuan, L.; Shen, Y.; Xu, H.; Li, Z.; Huang, Y. Insight into the fading mechanism of the solid-conversion sulfur cathodes and designing long cycle lithium–sulfur batteries. Adv. Energy Mater. 2021, 12, 2102774. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Rosenman, A.; Talyosef, Y.; Chesneau, F.; Aurbach, D. Fluoroethylene carbonate as an important component in organic carbonate electrolyte solutions for lithium sulfur batteries. Electrochem. Commun. 2015, 60, 42–46. [Google Scholar] [CrossRef]
- Lee, J.T.; Eom, K.; Wu, F.; Kim, H.; Lee, D.C.; Zdyrko, B.; Yushin, G. Enhancing the Stability of Sulfur Cathodes in Li–S Cells via in Situ Formation of a Solid Electrolyte Layer. ACS Energy Lett. 2016, 1, 373–379. [Google Scholar] [CrossRef]
- Xu, G.L.; Sun, H.; Luo, C.; Estevez, L.; Zhuang, M.; Gao, H.; Amine, R.; Wang, H.; Zhang, X.; Sun, C.J.; et al. Solid-state lithium/selenium–sulfur chemistry enabled via a robust solid-electrolyte interphase. Adv. Energy Mater. 2018, 9, 1802235. [Google Scholar] [CrossRef]
- He, F.; Wu, X.; Qian, J.; Cao, Y.; Yang, H.; Ai, X.; Xia, D. Building a cycle-stable sulphur cathode by tailoring its redox reaction into a solid-phase conversion mechanism. J. Mater. Chem. A 2018, 6, 23396–23407. [Google Scholar] [CrossRef]
- Li, S.; Ma, J.; Zeng, Z.; Hu, W.; Zhang, W.; Cheng, S.; Xie, J. Enhancing the kinetics of lithium–sulfur batteries under solid-state conversion by using tellurium as a eutectic accelerator. J. Mater. Chem. A 2020, 8, 3405–3412. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Q.; Tang, G.; Cao, Y.; Yang, H.; Li, H.; Ai, X. A Solid-Phase Conversion Sulfur Cathode with Full Capacity Utilization and Superior Cycle Stability for Lithium-Sulfur Batteries. Small 2022, 18, e2106144. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Hu, Y.S.; Li, H.; Armand, M.; Chen, L. A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fan, X.; Ji, G.; Wang, H.; Hou, S.; DeMella, K.C.; Raghavan, S.R.; Wang, J.; Xu, K.; Wang, C. Manipulating electrolyte and solid electrolyte interphase to enable safe and efficient Li–S batteries. Nano Energy 2018, 50, 431–440. [Google Scholar] [CrossRef]
- Amine, R.; Liu, J.; Acznik, I.; Sheng, T.; Lota, K.; Sun, H.; Sun, C.J.; Fic, K.; Zuo, X.; Ren, Y.; et al. Regulating the hidden solvation-ion-exchange in concentrated electrolytes for stable and safe lithium metal batteries. Adv. Energy Mater. 2020, 10, 2000901. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, J.; Gao, Y.; Hou, Q.; Ren, Z.; Qi, Y.; Zhang, K.; Shen, C.; Zhang, J.; Xie, K. A multifunctional electrolyte with highly-coordinated solvation structure-in-nonsolvent for rechargeable lithium batteries. J. Energy Chem. 2020, 51, 362–371. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, G.; Fan, X.; Chen, J.; Li, Q.; Wang, H.; Yang, Y.; DeMella, K.C.; Raghavan, S.R.; Wang, C. High-Fluorinated Electrolytes for Li–S Batteries. Adv. Energy Mater. 2019, 9, 1803774. [Google Scholar] [CrossRef]
- Huang, F.; Gao, L.; Zou, Y.; Ma, G.; Zhang, J.; Xu, S.; Li, Z.; Liang, X. Akin solid–solid biphasic conversion of a Li–S battery achieved by coordinated carbonate electrolytes. J. Mater. Chem. A 2019, 7, 12498–12506. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Yang, X.; Wang, C.; Zheng, M.L.; Li, R.; Zuo, P.; Yin, G.; Sun, X. Realizing solid-phase reaction in li–s batteries via localized high-concentration carbonate electrolyte. Adv. Energy Mater. 2021, 11, 2101004. [Google Scholar] [CrossRef]
- Li, X.; Lushington, A.; Liu, J.; Li, R.; Sun, X. Superior stable sulfur cathodes of Li–S batteries enabled by molecular layer deposition. Chem. Commun. 2014, 50, 9757–9760. [Google Scholar] [CrossRef]
- Li, X.; Lushington, A.; Sun, Q.; Xiao, W.; Liu, J.; Wang, B.; Ye, Y.; Nie, K.; Hu, Y.; Xiao, Q.; et al. Safe and durable high-temperature lithium-sulfur batteries via molecular layer deposited coating. Nano Lett. 2016, 16, 3545–3549. [Google Scholar] [CrossRef]
- Li, X.; Banis, M.; Lushington, A.; Yang, X.; Sun, Q.; Zhao, Y.; Liu, C.; Li, Q.; Wang, B.; Xiao, W.; et al. A high-energy sulfur cathode in carbonate electrolyte by eliminating polysulfides via solid-phase lithium-sulfur transformation. Nat. Commun. 2018, 9, 4509. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, L.; Li, Z.; Chen, S.; Ji, H.; Qin, Y.; Wu, L.; Shen, Y.; Wang, L.; Hu, J.; et al. Realizing an applicable “solid --> solid” cathode process via a transplantable solid electrolyte interface for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2019, 11, 29830–29837. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Z.; Lin, J.; Zhang, L.; Wang, L.; Chen, S.; Zhang, C.; Liu, X. Recent progress in quasi/all-solid-state electrolytes for lithium–sulfur batteries. Front. Energy Res. 2022, 10, 945003. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Chen, N.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Development and challenges of functional electrolytes for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2018, 28, 1800919. [Google Scholar] [CrossRef]
- Jeddi, K.; Ghaznavi, M.; Chen, P. A novel polymer electrolyte to improve the cycle life of high performance lithium–sulfur batteries. J. Mater. Chem. A 2013, 1, 2769–2772. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y.; Liu, J. Sulfur double locked by a macro-structural cathode and a solid polymer electrolyte for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 10760–10766. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Liu, J.; Miller, J.D. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries. Nano Energy 2017, 31, 478–485. [Google Scholar] [CrossRef]
- Gao, J.; Sun, C.; Xu, L.; Chen, J.; Wang, C.; Guo, D.; Chen, H. Lithiated Nafion as polymer electrolyte for solid-state lithium sulfur batteries using carbon-sulfur composite cathode. J. Power Sources 2018, 382, 179–189. [Google Scholar] [CrossRef]
- Fang, R.; Xu, H.; Xu, B.; Li, X.; Li, Y.; Goodenough, J.B. Reaction mechanism optimization of solid-state Li–S batteries with a PEO-based electrolyte. Adv. Funct. Mater. 2020, 31, 2001812. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Luo, J.; Wang, C.; Li, X.; Sun, Q.; Li, R.; Zhang, L.; Yang, R.; Lu, S.; et al. High-performance li-sesx all-solid-state lithium batteries. Adv. Mater. 2019, 31, e1808100. [Google Scholar] [CrossRef]
- Lefevr, J.; Cervini, L.; Griffin, J.M.; Blanchard, D. Lithium conductivity and ions dynamics in LiBH4/SiO2 solid electrolytes studied by solid-state nmr and quasi-elastic neutron scattering and applied in lithium–sulfur batteries. J. Phys. Chem. C 2018, 122, 15264–15275. [Google Scholar] [CrossRef]
- Park, K.H.; Bai, Q.; Kim, D.H.; Oh, D.Y.; Zhu, Y.; Mo, Y.; Jung, Y.S. Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all-solid-state batteries. Adv. Energy Mater. 2018, 8, 1800035. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv. Mater. 2021, 33, e2000751. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Huang, N.; Han, F.; Zhang, Q.; Wan, H.; Mwizerwa, J.P.; Wang, C.; Xu, X. High-performance all-solid-state lithium–sulfur batteries enabled by amorphous sulfur-coated reduced graphene oxide cathodes. Adv. Energy Mater. 2017, 7, 1602923. [Google Scholar] [CrossRef]
- Hou, L.-P.; Yuan, H.; Zhao, C.-Z.; Xu, L.; Zhu, G.-L.; Nan, H.-X.; Cheng, X.-B.; Liu, Q.-B.; He, C.-X.; Huang, J.-Q.; et al. Improved interfacial electronic contacts powering high sulfur utilization in all-solid-state lithium–sulfur batteries. Energy Storage Mater. 2020, 25, 436–442. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Shen, Y.; Li, L.; Nan, C.W. High-conductivity argyrodite li6ps5cl solid electrolytes prepared via optimized sintering processes for all-solid-state lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef] [PubMed]
- Trevey, J.E.; Gilsdorf, J.R.; Stoldt, C.R.; Lee, S.-H.; Liu, P. electrochemical investigation of all-solid-state lithium batteries with a high capacity sulfur-based electrode. J. Electrochem. Soc. 2012, 159, A1019. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, S.; Liu, D.; Peng, G.; Yao, X.; Xu, X. Lithium superionic conducting oxysulfide solid electrolyte with excellent stability against lithium metal for all-solid-state cells. J. Electrochem. Soc. 2015, 163, A96–A101. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Zheng, B.; Zhu, J.; Wang, H.; Li, Y.; Yang, Y. Stable cycling lithium-sulfur solid batteries with enhanced li/li10gep2s12 solid electrolyte interface stability. ACS Appl. Mater. Interfaces 2019, 11, 18436–18447. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Dewald, G.F.; Ohno, S.; Hering, J.G.C.; Janek, J.; Zeier, W.G. Analysis of charge carrier transport toward optimized cathode composites for all-solid-state li-s batteries. Batter. Supercaps 2020, 4, 183–194. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Fan, X.; Gao, T.; Luo, C.; Ma, Z.; Suo, L.; Wang, C. High-performance all-solid-state lithium-sulfur battery enabled by a mixed-conductive Li2s nanocomposite. Nano Lett. 2016, 16, 4521–4527. [Google Scholar] [CrossRef] [PubMed]
- Hakari, T.; Hayashi, A.; Tatsumisago, M. Li2S-based solid solutions as positive electrodes with full utilization and superlong cycle life in all-solid-state li/s batteries. Adv. Sustain. Syst. 2017, 1, 1700017. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; Wang, D.; Li, X.; Gong, Z.; Yang, Y. In situ generated Li2s-C nanocomposite for high-capacity and long-life all-solid-state lithium sulfur batteries with ultrahigh areal mass loading. Nano Lett. 2019, 19, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, Y.; Zheng, X.; Tang, S.; Gong, Z.; Yang, Y. Li2S@NC composite enable high active material loading and high Li2S utilization for all-solid-state lithium sulfur batteries. J. Power Sources 2020, 479, 228792. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.; Dudney, N.J.; Liang, C. Lithium superionic sulfide cathode for all-solid lithium–sulfur batteries. ACS Nano 2013, 7, 2829–2833. [Google Scholar] [CrossRef]
- Yao, X.; Liu, D.; Wang, C.; Long, P.; Peng, G.; Hu, Y.S.; Li, H.; Chen, L.; Xu, X. High-energy all-solid-state lithium batteries with ultralong cycle life. Nano Lett. 2016, 16, 7148–7154. [Google Scholar] [CrossRef]
- Yersak, T.A.; Stoldt, C.; Lee, S.-H. electrochemical evolution of an iron sulfide and sulfur based cathode for all-solid-state li-ion batteries. J. Electrochem. Soc. 2013, 160, A1009–A1015. [Google Scholar] [CrossRef]
- Jeddi, K.; Sarikhani, K.; Qazvini, N.T.; Chen, P. Stabilizing lithium/sulfur batteries by a composite polymer electrolyte containing mesoporous silica particles. J. Power Sources 2014, 245, 656–662. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.; Hitz, G.T.; McOwen, D.W.; Li, Y.; Xu, S.; Wen, Y.; Zhang, L.; Wang, C.; Pastel, G.; et al. Three-dimensional bilayer garnet solid electrolyte based high energy density lithium metal–sulfur batteries. Energy Environ. Sci. 2017, 10, 1568–1575. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, J.; Wu, X.; Ma, G.; Yang, J.; Wen, Z. A shuttle effect free lithium sulfur battery based on a hybrid electrolyte. Phys. Chem. Chem. Phys. 2014, 16, 21225–21229. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Huang, X.; Wang, Q.; Jin, J.; Wang, Q.; Wen, Z.; Qian, R. A hybrid electrolyte for long-life semi-solid-state lithium sulfur batteries. J. Mater. Chem. A 2017, 5, 13971–13975. [Google Scholar] [CrossRef]
- Shin, M.; Gewirth, A.A. Incorporating solvate and solid electrolytes for all-solid-state Li2s batteries with high capacity and long cycle life. Adv. Energy Mater. 2019, 9, 1900938. [Google Scholar] [CrossRef]






| Sample | Sulfur Content/wt% | Electrolyte | Current Density/mA g−1 | Cycle Number—Capacity (Retention)/mAh g−1 | Rate Capability/mAh g−1 | Ref. |
|---|---|---|---|---|---|---|
| S@PAN/S7Se | 68 (S + Se) | 1M LiPF6 + EC/DMC (1:1 by volume) + 5%FEC | 1000 | 100–675 | 6 A g−1—453.5 | [55] |
| pPAN-S@GNS | 47 | 1M LiPF6 + EC/DMC (1:1 by volume) | 0.2 C | 300–604.9 (88.8%) | 10 C—~330 | [65] |
| S/DPAN/rGO | 44.76 | 1M LiPF6 + EC/DMC (3:7 by volume) | 0.2 C | 100–613.6 (92%) | 2 C—313.3 | [66] |
| S/MCPs-PAN | 52 | 1M LiPF6 + PC/EC/DEC (1:4:5 by volume) | 160 | 200–666 (84.4%) | 4 C—369.7 | [67] |
| S/rSP@SPAN | 54.5 | 1M LiPF6 + EC/DEC (1:1 by volume) | 0.1 C | 100–681.8 | 10 C—268 | [68] |
| PBD622–400 | 31.31 | 1M LiPF6 + EMC/EC/DEC (1:1:1 by volume) | 1050 | 150–250.5 | [69] | |
| PAN/PS/VGCF | 37.78 | 1M LiPF6 + EC/DEC (1:1 by volume) | 1 C | 150–341.2 | 2 C—254.6 | [70] |
| 3DHG/PS | 40.2 | 1M LiTFSI + DOL/DME (1:1 by volume) + 1% LiNO3 | 0.5 C | 800–383.3 | 5 C—198.9 | [71] |
| SeS0.7/CPAN | 33 (SeS0.7) | 1M LiPF6 + EC/DEC (1:1 by volume) | 600 | 1200–257.4 | 6 A g−1—148.5 | [74] |
| pPAN/SeS2 | 63 (SeS2) | 1M LiPF6 + EC/DMC (1:1 by volume) | 500 | 100–642.6 | 5 A g−1—446.7 | [75] |
| S0.87Se0.13/CPAN | 29.79 (S) + 10.82 (Se) | 1M LiPF6 + EC/DMC/DEC (1:1:1 by volume) | 300 | 200–401.6 | [76] | |
| SeSPAN | 60 | 1M LiPF6 + EC/DEC (1:1 by volume) | 100 | 250–402 (80%) | 2 A g−1—360 | [77] |
| Se0.06SPAN | 47.25 (Se0.06) | 1M LiTFSI + DOL/DME (1:1 by volume) + 2% LiNO3 | 200 | 500–533.9 (91.6%) | 10 A g−1—425.3 | [78] |
| Te0.04S0.96@pPAN | 47.62 | 1M LiTFSI + DOL/DME (1:1 by volume) + 0.2 M LiNO3 | 500 | 200–482.4 (87.3%) | 10 A g−1—410 | [80] |
| S/PAN/Mg0.6Ni0.4O | 38.5 | 1M LiPF6 + EC/DMC/DEC (1:1:1 by volume) | 0.1 C | 100–470.9 | 1 C—171.3 | [82] |
| CoS2-SPAN-CNT | 43.2 | 1M LiPF6 + EC/DMC/DEC (1:1:1 by volume) | 0.2 C | 100–439.8 | 2 C—288.1 | [83] |
| Co10-SPAN-CNT | 41.9 | 1M LiPF6 + EC/DMC/DEC (1:1:1 by volume) | 0.2 C | 100–586.2 | 5 C—352.8 | [84] |
| Sample | Carbon Source | Method | Pore Size/nm | Sulfur Content/% | Current Density/mA g−1 | Cycle Number—Capacity (Retention)/mAh g−1 | Ref. |
|---|---|---|---|---|---|---|---|
| S/(CNT@MPC) | D-glucose | Solvothermal-calcination | 0.5 | 40 | 0.1 C | 200–1142 | [40] |
| Sulfur-carbon sphere | Sucrose | Solvothermal-calcination | 0.7 | 42 | 400 | 500–650 | [125] |
| S/C | Sucrose | Solvothermal-calcination | 1 | 40 | 100 | 100–720 | [126] |
| C/S-50-T | Sucrose | Solvothermal-calcination | 1.7–6 | 26 | 100 | 500–860 | [127] |
| S@UMPC | D-glucose/sucrose | Solvothermal-calcination | 0.6 | 40 | 0.1 C | 150–900 | [129] |
| CS-Ex | Phenolic resin | Template method | 0.65 | 16 | 150 | 50–900 | [130] |
| FDU/S-40 | Phenolic resin | Template method | 0.46 | 40 | 500 | 170–608 | [131] |
| 100 | 400–900 | ||||||
| 400 | 500–>600 | ||||||
| SPC2 | Phenolic resin | Template method | 4 | 58.12 | 600 | 100–730 | [132] |
| CA(Ar) + Sinf | Phenolic resin | Template method | <1 | 23 | 500 | 200–990 | [134] |
| CSC-S | Coconut shells | KOH activation | 0.53 | 45.8 | 0.2 C | 100–703 (75%) | [135] |
| c-MNS/S40 | Macadamia nut shell | KOH activation | 0.6 | 41 | 0.1 C | 100–998 | [136] |
| S-PVDCDC | Polyvinylidene dichloride | Direct calcination | <1 | 40 | 260 | 200–~750 | [137] |
| S/UMC-2 | PVDF | Direct calcination | 0.55 | 37.7 | 0.1 C | 150–852 | [138] |
| KC/S | Potassium tartrate | Direct calcination | 0.49 | 42.5 | 0.1 C | 50–968 | [139] |
| S2–4/UMC-MFC | PVDF | Direct calcination | 0.55 | 37.2 | 0.1 C | 100–693 | [140] |
| MXene-bonded S2–4/UMC | PVDF | Direct calcination | 0.55 | 37.2 | 0.1 C | 200–946 | [141] |
| MPCP-S-I | ZIF-8 | Direct calcination | <2 | 43 | ~230 | 100–490 | [146] |
| C-S-3 | ZIF-8 | Direct calcination | 0.5 | 27 | 335 | 100–936 (82.6%) | [147] |
| NPCS-S50 | Ppy/ZnCl2 | Template method | <0.8 | 53 | 0.3 C | 200–1002 | [148] |
| NDMC/S | Triblock copolymer F127 + 4,4′-bipyridine | Template method | 0.57 | 28 | 0.2 C | 500–902 | [149] |
| Cathode | Active Material Content/wt% | Electrolyte | Current Density/mA g−1 | Cycle Number—Capacity (Retention)/mAh g−1 | Rate Capability/mAh g−1 | Ref. |
|---|---|---|---|---|---|---|
| S0.94Se0.06/C | 50 | 1 M LiPF6 + EC/DMC (1:1 by volume) | 1000 | 500–910 | 20 A g−1—617 | [155] |
| S0.94Se0.06@PCNFs | 49 | 1 M LiPF6 + EC/DMC (1:1 by volume) | 100 | 100–840 | 10 A g−1—350 | [156] |
| w-SeS2/ OMC | 49 | 1 M LiPF6 + EC/DEC (1:1 by volume) | 1 C | 2000–360 | 2 C—273 | [157] |
| CMK-3/Se5S3 | 67 | 1.2 M LiPF6 + EC/DMC (1:1 by volume) + 5% FEC | 1000 | 300–609 | 6 A g−1—417 | [158] |
| Te0.1S0.9/CMK-3 | 70 | 1 M LiPF6 + EC/DMC (1:1 by volume) | 250 | 100–845 | 5 A g−1—590 | [159] |
| Li3PS4+5 | 47.1 | Li3PS4 solid electrolyte | 0.1 C | 300–329 300–565 (60 °C) | 2 C—346 (60 °C) | [161] |
| S-P2S5 | 50 | P2S5 solid electrolyte | 0.1 C | 471 | [162] | |
| P4S34/C | 40.2 | Li10GeP2S12 + Li3PS4 solid electrolyte | 200 | 180–458.5 | [163] |
| Cathode | Electrolyte | Current Density/mA g−1 | Cycle Number—Capacity (Retention)/mAh g−1 | Ref. |
|---|---|---|---|---|
| a-NbS3 | 1 M LiPF6 + EC/DMC (1:1 by volume) | 10–281 | [164] | |
| a-TiS3/S/KB | 80 Li2S · 20P2S5 solid electrolyte | 0.064 mA cm−2 | 50–650 | [165] |
| MoS3/MWCNTs | 1 M LiTFSI + DOL/DME (1:1 by volume) + 0.1 M LiNO3 | 0.5 mA cm−2 | 200–291.7 | [166] |
| a-FeS4/C | 1.2 M LiPF6 + EC/DMC (1:1 by volume) + 5% FEC | 100 | 500–644 | [167] |
| a-TiS4 | 1 M LiPF6 + EC/DMC (1:1 by volume) | 400 | 2–500 | [168] |
| P0.4VS5.0 | 1 M LiPF6 + EC/DMC (1:1 by volume) | 0.1 C | 50–445 | [169] |
| Cathode | Anode | Electrolyte Materials | Interlayer Material | Battery Operating Temperature/°C | Electrolyte Conductivity/S cm−1 | Rate-Cycle Number-Capacity/ mAh g−1 | Ref. |
|---|---|---|---|---|---|---|---|
| S–PAN | Li | PMMA-PVdF-HFP + 1M LiPF6 in EC/DMC | 25 | 0.2 C-100-441 (88%) | [205] | ||
| PANI@C/S-280 | Li | PEO–MIL-53(Al)–LiTFSI | 80 | 4 C-1000-325 | [206] | ||
| S | Li | PEO + LiTFSI + HNT | 100 | 2.14 × 10−3 | 4 C-400-386 | [207] | |
| C-S | Li | PC-Li-Nafion | Li-Nafion interlayer | 70 | 2.1 × 10−4 | 1C-100-796 (89%) | [208] |
| PVDF-coated S/C | Li | PEO-LiTFSI | 55 | 0.05 mA cm−2-60–630 | [209] | ||
| SeS2 | Li | Li10GeP2S12–Li3PS4 | 25 | [210] | |||
| C/S | Li | LiBH4/SiO2 | [211] | ||||
| rGO@S-Li10GeP2S12-AB | Li | Li10GeP2S12/75%Li2S-24%P2S5-1%P2O5 | 60 | 8.27 × 10−3 Li10GeP2S12 | 1 C-750–830 | [212] | |
| CNT@S-Li10GeP2S12 | Li–In | Li10GeP2S12 | In foil | 25 | 8.27 × 10−3 | 0.1 C-200–998.6 (87.7%) | [215] |
| nano-sulfur/MWCNT-Li6PS5Cl | Li–In | Li6PS5Cl | 25 | 3.15 × 10−3 | 0.1 C-50–1393 | [216] | |
| PAN-S | Li | Li2S-P2S5 | 60 | 2.5 × 10−3 | 0.1 C-50–487 (99%) | [217] | |
| S@KBC-Li10GeP2S12-AB | Li | Li10GeP2S12 | 1 M LiFSI/PYR13TFSI IL. | 25 | 2.04 × 10−3 | 83.5 mA g−1-25–868 (81%) | [219] |
| Li2S-Li6PS5Cl-C | Li-In | 80 Li2S·20P2S5 | 25 | 1.3 × 10−3 | 50 mA g−1-60–830 | [222] | |
| 80Li2S·20LiI-VGCF | Li-In | 75 Li2S·25P2S5 | In foil | 25 | 2 C-2000-980 | [223] | |
| Li2S@C-LPS-AB | Li-In | Li7P3S11 | 60 | 1.7 × 10−3 | 2 mA cm−2-700–643 (93%) | [224] | |
| Li2S@NC-Li7P3S11-AB | Li-In | Li7P3S11 | 60 | 0.5 mA cm−2-100–690 (80%) | [225] | ||
| Co9S8-Li7P3S11 | Li | Li10GeP2S12/70%Li2S-29% P2S5-1%-P2O5 | 25 | 1.5 × 10−3 Li7P3S11 | 1.27 mA cm−2-100–421 | [227] | |
| FeS + S | Li | 77.5Li2S:22.5P2S5 | 60 | 225-900 | [228] | ||
| S/PAN | Li | MPS/PVdF-HFP/f-PMMA + 1M LiPF6 in EC/DEC | 25 | 0.2 C-100–1143 | [229] | ||
| KB–S | Li | Li1.5Al0.5Ge1.5(PO4)3 | 25 | 1.77 × 10−4 | 0.2 C-40–720 | [231] | |
| S/C | Li | FDE-LAGP | 25 | 3.2 × 10−4 | 1 C-1200-668 (73%) | [232] | |
| Li2S | Li-In | Li7P3S11 | (ACN)2-LiTFSI:HFE | 25 | 0.1 C-100–760 | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Hai, F.; Guo, J.; Tian, X.; Zheng, S.; Wu, Z.; Wang, T.; Li, M. Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion. Batteries 2023, 9, 27. https://doi.org/10.3390/batteries9010027
Yi Y, Hai F, Guo J, Tian X, Zheng S, Wu Z, Wang T, Li M. Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion. Batteries. 2023; 9(1):27. https://doi.org/10.3390/batteries9010027
Chicago/Turabian StyleYi, Yikun, Feng Hai, Jingyu Guo, Xiaolu Tian, Shentuo Zheng, Zhendi Wu, Tao Wang, and Mingtao Li. 2023. "Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion" Batteries 9, no. 1: 27. https://doi.org/10.3390/batteries9010027
APA StyleYi, Y., Hai, F., Guo, J., Tian, X., Zheng, S., Wu, Z., Wang, T., & Li, M. (2023). Progress and Prospect of Practical Lithium-Sulfur Batteries Based on Solid-Phase Conversion. Batteries, 9(1), 27. https://doi.org/10.3390/batteries9010027







