Abstract
Owing to the sustainability, environmental friendliness, and structural diversity of biomass-derived materials, extensive efforts have been devoted to using them in high-energy rechargeable batteries. Alkali-metal–selenium batteries, one of the high-energy rechargeable batteries with a reasonable cost compared to up-to-date lithium-ion batteries, have also attracted significant attention. Therefore, a timely and comprehensive review of the biomass carbon structures/components to the mechanisms for enhancing alkali-metal–selenium batteries has been systematically introduced. In the end, advantages, challenges, and outlooks are pointed out for the future development of biomass-derived carbon materials in alkali-metal–selenium batteries. This review could help researchers think about using biomass carbon materials to improve battery performance and what other problems should be solved, thereby promoting the application of biomass materials in battery design.
1. Introduction
With the rapid growth of the market for electric vehicles and large-scale grid energy storage, a single lithium-ion battery system can no longer meet their demands. Therefore, it is imperative to seek new rechargeable metal battery systems [1,2]. As is known, the alkali metallic anodes have high theoretical capacity, for example, Li: 3860 mAh g−1, Na: 1166 mAh g−1, and K: 685 mAh g−1, and low redox potential (Li: −3.04 V, Na: −2.71 V, K: −2.93 V vs. standard hydrogen electrode) [1,3]. Simultaneously, Na and K elements are rich in the earth’s crust and seawater [1,4,5]. Sulfur is nontoxic, cost-effective, abundant in nature, and also exhibits high theoretical specific capacity and volumetric capacity density when it is used as a cathode materials [6,7]. Particularly when it is matched with alkali metallic anode, the alkali-metal–sulfur batteries exhibit various advantages and are a great prospect for commercial application [3].
Selenium, an element of the same main group as sulfur, shows a much higher electronic conductivity than sulfur (1 × 10−3 S m−1), and, when it is employed as the cathode material, it shows a high theoretical specific capacity of 675 mA h g−1 and a volumetric capacity of 3253 mA h cm−3 [6]. Due to the above advantages, rechargeable alkali-metal–selenium batteries have considerable potential in terms of high-volume energy density, earth-abundance, and cost-effectiveness.
During the discharge process, the alkali metal (Li, Na, and K) will be oxidized to metal ions, and metal ions migrate to the cathode. In the selenium cathode, there are various solid–liquid–solid phase transitions with reversible electrochemical reactions (redox between element Se, metal polyselenides, and metal selenides) [6,8]. However, these metal polyselenides are easily dissolved in the liquid organic electrolyte, particularly in an ether-based liquid organic electrolyte, to produce a severe “shuttle phenomenon” [6,9]. Moreover, there are also the inherent defects of selenium, i.e., low conductivity and high-volume changes during the charging–discharging process. All of the drawbacks will lead to short cycle life, low Coulombic efficiencies, and poor reproducibility of alkali-metal–selenium batteries.
To address these issues, numerous strategies, including designing various nano-structured carbon hosts [6,10], introducing polar groups or heteroatoms [9,11], developing new electrolytes [12,13,14], and modifying the separators [15,16], etc., have been proposed. Among them, biomass-derived carbon materials employed on selenium cathodes are of significant interest because of their sustainability, environmental friendliness, and reasonable cost [1,17].
Biomass carbon materials were mainly used as the anode materials for lithium-ion batteries, etc. [18,19], then they have shown continuous research hot in the field of Li–S batteries [20,21,22,23,24,25,26,27], and their utilization of Li–Se batteries was also making breakthroughs [28,29,30,31,32,33,34]. Differently, the research on biomass carbon materials in the field of Na–Se and K–Se batteries has gradually begun to develop in recent years [35,36]. Low electrochemical storage capacity is still a challenge for the practical application of biomass carbon materials in Na–Se and K–Se batteries [4,5,37]. As can be seen, many studies have summarized the application of biomass materials in designs of Li–S and lithium-ion batteries [38,39,40,41,42,43,44]. However, only a few systematic summaries have been reported on the biomass carbon materials’ utilization in emerging energy storage systems: alkali-metal–selenium batteries [1,17]. Therefore, it is urgent to summarize the latest development of biomass carbon materials in alkali-metal–selenium batteries. In this review, subjects from the different structures and components of biomass carbon, to how they inhibit the polyselenides shuttle effect, alleviate volume expansion, improve conductivity, and even inhibit dendrite growth in Li–Se, Na–Se and K–Se batteries have been systematically summarized as shown in Figure 1, which can provide a better research perspective in alkali-metal–selenium batteries for researchers.
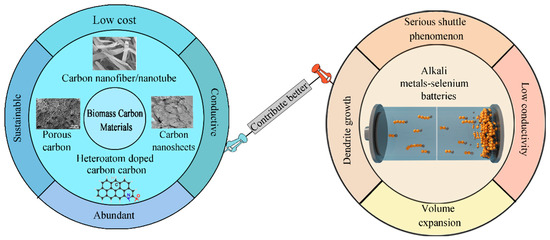
Figure 1.
Overview diagram of biochar promoting better alkali-metal–selenium batteries.
2. Biomass Carbon Utilization in Li–Se Batteries
Among the alkali-metal–selenium batteries, Li–Se batteries have received the most attention from researchers, which is mainly because they exhibit the highest theoretical specific energy (1155 W h kg−1) [3]. Similar to Li–S batteries, Li–Se batteries are also facing the weak conductivity of the electrode and the serious “shuttle phenomena” caused by the active materials. Therefore, biomass carbon materials have been widely developed to inhibit the lithium polyselenides shuttle phenomenon and enhance the conductivity of the Se cathode.
For example, Kang and his co-worker developed a new type of macro-/micro-porous biochar (MMPBc) derived from the inner sponge layer of pomelo pericarp, as shown in Figure 2a,b [29]. They used this kind of MMPBc as a porous matrix for selenium loading to form a Se/MMPBc composite. As can be seen in Figure 2c, the Se/MMPBc cathode showed one reduction peak at 1.5–1.8 V, indicating the reduction from element Se to high-order lithium polyselenides and even to lithium polyselenides; and one oxidation peak at around 2.08 V, indicating oxidation from lithium polyselenides to high-order lithium polyselenides. This one redox peak between 1.5–3.0 V corresponded to one discharge–charge plateau, as shown in Figure 2d. The best Se/MMPBc composite cathode could deliver a high reversible capacity of 597.4 or 466.8 mAh g−1 in the 2nd or 300th cycle at 0.2 C (1C = 675 mA g−1), as shown in Figure 2e, and even at 2 C high current, the best Se/MMPBc cathode could still deliver a capacity of around 400 mAh g−1 as shown in Figure 2f. Such outstanding electrochemical performances were attributed to the high BET surface area of 1539.4 m2 g−1 and a micropore volume of ca. 0.612 cm3 g−1, which effectively capture and homogeneously distribute elemental selenium within the porous nanostructures.
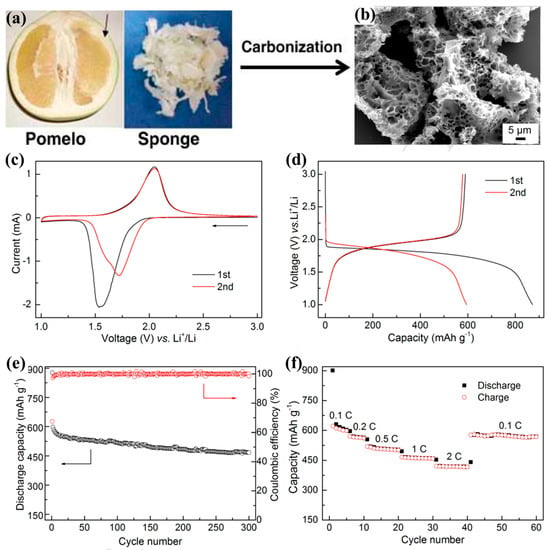
Figure 2.
Schematic illustration of the procedure adopted for the preparation of MMPBc: (a) digital photo of raw pomelo sponge and (b) SEM image of synthesized MMPBc; (c) initial two CV curves conducted at 0.1 mV s−1; (d) initial two voltage profiles recorded at 0.2 C; (e) cycling performance operated at 0.2 C; (f) rate performance performed at various C−rates. Reproduced with permissions from the ref. [29] Copyright © 2015 Elsevier Ltd.
In addition, a three-dimensional (3D) hierarchical porous carbon with a hollow tubular structure (HPTCs) has been synthesized by Xu et al. [45]. Such a unique structure (Figure 3a–c) can not only serve as a conductive path but also provide abundant porous space for loading Se and trapping polyselenides, as shown in Figure 3d. As a result, the HPTCs/Se illustrated excellent cycling stability, such that the capacity fading rate is only 0.02% per cycle over 900 cycles at 2 C. More importantly, the synthesized HPTCs process is simple and economic.
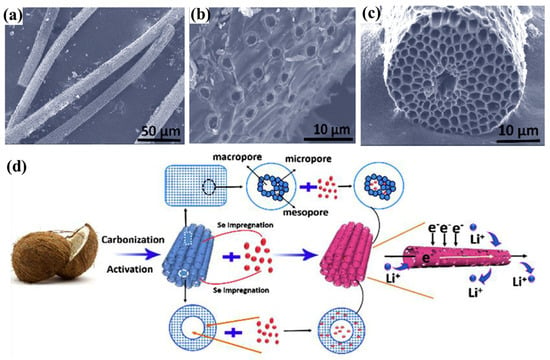
Figure 3.
(a–c) FESEM images of HPTCs, (d) schematic of loading Se active materials and the transport paths for Li+ and electrons in the HPTCs/Se tubular structure. Reproduced with permissions from the ref. [45] Copyright © 2017 Elsevier Ltd.
As we all know, biomass contains C and O elements, but some also contain N, S, and other elements. During the carbonization process, it is easy to form N-doped, S-doped, and other heteroatomic-doped biochar. Plenty of previous research has reported that the polar hetero-atom doped carbon is more favorable for the chemical adsorption of lithium polysulfide, thus limiting its shuttle effect [21,46,47,48,49,50,51,52,53]. Thus, hetero-atom doped carbon should have the same effect on lithium polyselenides. Based on this, in 2016, our research group successfully synthesized a nitrogen-doped porous carbon (N-LSC) with a high specific surface area by using a loofah sponge (Figure 4a) [54] and used it as the interlayer for the Se cathode. DFT calculation results (Figure 4b–d) revealed that pyrrolic N shows the highest adsorption energy on Li2Se8 (−1.60 eV) compared to pyridinic N (−0.99 eV) and graphitic N (−0.93 eV), which indicated that the pyrrolic N has the strongest chemical adsorption ability on lithium polyselenides. Due to the physical and chemical dual adsorption by the N-LSC-900 interlayer, the Se cathode could demonstrate an ultra-high reversible capacity of 350 mA h g−1 at 1356 mA g−1 after 1000 cycles as shown in Figure 4e. In 2018, Zhao et al. reported a N,O co-doped biochar from chitosan as the raw material [55]. When employing this N,O co-doped carbon as selenium host, the resulted composites delivered a high rate capability such that, at a high rate of 4.8 C, a stable discharge capacity of 342.8 mAh g−1 could be achieved.
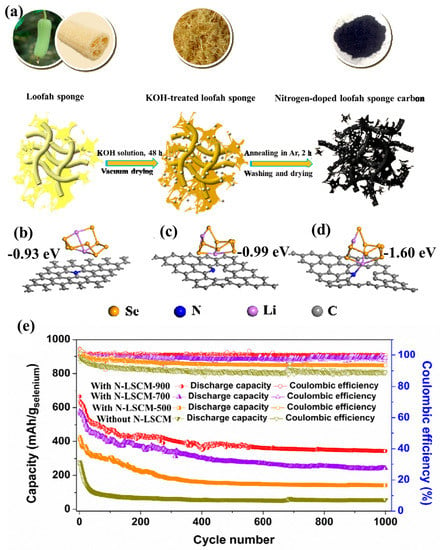
Figure 4.
(a) The synthesis process of the nitrogen−doped loofah sponge carbon, the most preferential adsorption structures of Li2Se8 on the different active sites: (b) graphitic N, (c) pyridinic N, (d) pyrrolic N, (e) cycling performances of the Li–Se cells with and without a N-LSCM interlayer at a current of 1356 mA g−1. Reproduced with permissions from the ref. [54] Copyright © 2019 American Chemical Society.
In addition to these listed biomass carbon materials, there are many other biomass carbons used to improve the electrochemical performance of Li–Se batteries, which are summarized in Table 1. As can be seen, the various biomass carbon materials are mainly obtained by carbonizing the biomass at high temperature under an inert atmosphere with a porogen, which acts in the roles of enhancing the electrode conductivity, accommodating the selenium and volume expansion, as well as physically or chemically inhibiting the polyselenide shuttle phenomenon. As a result, the Li–Se battery’s electrochemical performance could be enhanced.

Table 1.
Biomass-derived carbon material utilization in Li–Se batteries.
3. Biomass Carbon Utilization in Na–Se Batteries
Compared to Li–Se batteries, the large abundance of the element sodium contributed to Na–Se batteries arousing enormous attention. In 2012, a new class of selenium and selenium−sulfur (SexSy)-based cathode materials for rechargeable lithium and sodium batteries was reported by Khalil Amine [60]. Following that, in 2013, a pioneering work using mesoporous carbon as the selenium host on Na−Se batteries was reported by Wang et al. [35]. Since then, promising achievements have been obtained in Na−Se batteries, especially for carbon as the selenium host to enhance the electrochemical performances of Na–Se batteries.
For example, Wang et al. reported a N, O dual-doped biomass carbon (NOPCC) derived from poplar catkin as the selenium host as shown in Figure 5a [61]. The SEM images (Figure 5b,c) showed that the selenium has been incorporated into the porous NOPCC and distributed homogenously. The TEM image in Figure 5d further showed that no detectable Se particles or lattice fringes could be detected, which indicate poplar-catkin-derived carbon with low graphitization and amorphous selenium in it. In order to verify the changes of Se during the charge–discharge process, ex situ XPS characterization was conducted. As shown in Figure 5e,f, in the initial state, Se0 can be observed in the Se 3d XPS spectrum, but after full discharging, the binding energy shifts from of 53.6 to 54.5 eV, indicating the reduction of Se0 to Se2− species. Furthermore, in the pristine state, only the peak of −COO−Na in the Na 1s XPS spectra can be observed. While after discharge, two new peaks around 1073.3 and 1073.2 eV in the Na 1s spectra appear, which suggest the formation of the Na−Se bond and a solid-state electrolyte interphase (SEI) layer, respectively. More importantly, the XPS peaks of Se 3d and Na 1s almost completely returned to the initial state after being charged to 2.8 V, illustrating a highly reversible conversion reaction between Se0 and Se2− during cycling. In addition, density functional theory calculations were employed to theoretically verify the interaction between the NOPCC and sodium selenides, as shown in Figure 5g,h. Lower adsorption energies are observed for both NaSe2 (−0.87 eV) and Na2Se (−1.32 eV) on regular carbon substrates, while much higher adsorption energies of NaSe2 (−2.27 eV) and Na2Se (−3.87 eV) on NOPCC were observed, suggesting that the stronger chemical interaction between the NaSe2/Na2Se and N/O-doped carbon substrate. Due to such a strong chemical interaction, the Se@NOPCC electrode exhibited excellent cycling performance as shown in Figure 5i, which the reversible capacity could reach as high as 378.7 mA h g−1 at 1000 mA g−1 after 1600 cycles.
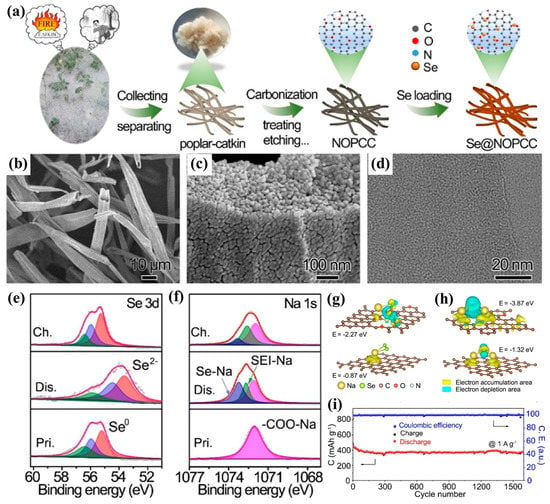
Figure 5.
(a) Schematic diagram of Se@NOPCC synthesis; (b,c) typical SEM images and (d) HRTEM image of Se@NOPCC. (e,f) Ex situ Se 3d and Na 1s XPS spectra of the Se@NOPCC electrode in the discharge/charge processes, respectively (Pri., pristine state; Dis., discharged state; Ch., charged state); (g) simulated charge distribution between NaSe2 and defective carbon (up) or regular carbon (down); (h) simulated charge distribution between Na2Se and defective carbon (up) or regular carbon (down). The yellow and cyan areas represent the increase and decrease in electron density, respectively. The isosurface is 0.002 e bohr−3. (i) Long cycling stability at 1.0 A g−1 of the Se@NOPCC electrode. Reproduced with permissions from the ref. [61] Copyright © 2021 American Chemical Society.
What’s more, Li et al. reported free-standing selenium impregnated carbonized leaf cathodes for high-performance Na-Se batteries [62]. The carbonized leaf possesses internal hierarchical porosity, thus guaranteeing the high mass loading of Se, and the unique natural three-dimensional structure and moderate graphitization degree of carbonized leaf facilitated Na+/e− transport to guarantee a high utilization of the selenium. As a result, the binder- and current-free cathode illustrated a high reversible specific 300 mA h g−1 at 2 A g−1 after 500 cycles without almost any capacity loss.
Finally, we also summarize the biomass-derived carbon utilization in Na–Se batteries in Table 2. As can be seen, the biomass carbon materials play the same roles in Na–Se batteries, but interestingly, compared to the electrochemical performances for Li–Se batteries in Table 1, the Na–Se batteries commonly demonstrate better cycling stability, which is because the shuttle effect in Na–Se batteries is not very pronounced. In addition, carbonized biomass under an NH3 atmosphere is an alternative strategy for the synthesis of the doped biochar.

Table 2.
Biomass-derived carbon materials utilization in Na–Se batteries.
4. Biomass Carbon Utilization in K–Se Batteries
Guo’s group first reported a new reversible and high-performance K–Se battery system in 2017 [36], using confined selenium/carbonized-polyacrylonitrile (PAN) composite (c-PAN–Se) as a cathode and metallic potassium as an anode. As another alternative for Li–Se batteries, K–Se batteries also have the advantage of the desirable natural abundance of the element potassium. Besides, the lower redox potential of the element potassium compared to the element Na is another important merit that captures intensive attention from researchers.
In the recent five years, research on K–Se batteries has still focused on the modification of the Se cathode. Therefore, employing the cost-effective, porous biochar materials to encapsulate the element Se as a composite cathode of K–Se batteries is still attracting the attention of some researchers. For instance, as shown in Figure 6a–c, a natural cotton-derived 3D interconnected foam-like N-doped porous carbon (FNDPC) being synthesized as a Se-container for K–Se batteries has been reported by Wang et al. [57]. As can be seen from Figure 6b,c, after encapsulating 50 wt% Se (FNDPC@Se-1), the morphology of FNDPC has barely changed, which is mainly because of the high pore volume of FNDPC that can completely incorporate the Se; as a result, the FNDPC@Se-1 cathode showed a reversible capacity of 108.5 mAh g−1 at 2 A g−1 (as shown in Figure 6d).
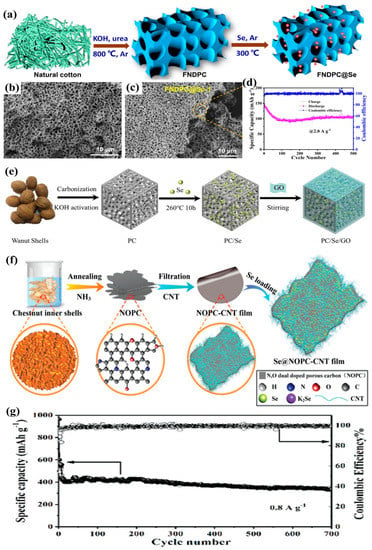
Figure 6.
(a) Schematic illustration of the FNDPC@Se synthesis process, (b) SEM image of FNDPC, (c) SEM images of FNDPC@Se-1 composite. (d) Cycling performances at 2.0 A g−1, reproduced with permissions from ref. [57] Copyright © 2020 Elsevier Ltd. (e) Schematic illustration of the preparation of PC/Se/GO, reproduced with permission from ref. [65] Copyright © 2020 WILEY-VCH; (f) synthesis procedure of the Se@NOPC-CNT film electrode, (g) long-term cycling performance of the Se@NOPC-CNT cathode at 0.8 A g−1 over 700 cycles. Reproduced with permissions from ref. [11] Copyright © 2018 WILEY-VCH.
In addition, combining the biochar with other functional carbon materials, such as graphene oxide (GO) and carbon nanotubes (CNT), as the co-host matrix for further enhancing the electrochemical performances of K–Se batteries were also reported. Liu et al. reported a novel walnut-shell-derived porous carbon/Se/graphene oxide (PC/Se/GO) cathode as shown in Figure 6e [65]. The PC/Se/GO delivered high discharge capacities of 316.8 mAh g−1 in the 150th cycle at 0.5 C. Yu’s group reported the encapsulation of Se into a CNT interwoven N,O dual-doped chestnut porous carbon nanosheet (Se@NOPC-CNT), as shown in Figure 6f [11]. The Se@NOPC-CNT delivered a remarkable reversible capacity of 335 mA h g−1 after 700 cycles at 0.8 A g−1, as shown in Figure 6g.
5. Conclusions
In this review, the current research status of biomass carbon materials utilization in alkali-metal–selenium batteries is discussed. Based on the above research, it can be concluded that: (i) biomass-derived porous carbon materials are most widely used as hosts for active selenium. They can not only improve the cathode conductivity and promote electron transport to accelerate the redox reaction but also accommodate a certain amount of active Se and polyselenides to inhibit their shuttle phenomenon, as well as alleviate the volume expansion during the charge/discharge process due to the large surface area and abundant porous structures. (ii) Non-polar biomass carbon materials have a limited ability to inhibit the shuttle effect of polyselenides. Therefore, the introduction of heteroatoms to play a role in the chemical adsorption of polar polyselenides or combining it with other carbon materials, i.e., GO, CNT, etc., to act as multiple qualifications for holding polar polyselenides, thus further improving the electrochemical performance of alkali-metal–selenium batteries.
However, there are still some potential problems in the application of biomass carbon materials in alkali-metal–selenium. For example, even almost all of the present research claimed that biomass carbon is cost-effective, which reduces the preparation cost of the battery, thereby creating great economic benefits, but rarely does research report a detailed cost calculation of converting biomass to biochar. In addition, converting the biomass to porous biochar occurs mainly through high-temperature calcination or hydrothermal treatment with a pore-forming agent, causing the low yield and uniform pores, which means that different batches of biochar materials may lack stability. Currently, there are few studies on the stability of different batches of biochar materials, which may greatly hinder the practical application of biochar materials in alkali-metal–selenium batteries.
What’s more, it should be noted that, at present, the Se content in porous biomass carbon is usually between 40 wt% and 60 wt%, and area loading is also very low (0.5 mg cm−2 to 1 mg cm−2), which is far from being able to meet the demands of practice application.
Apart from these drawbacks of biomass carbon materials, the cost problem of elemental Se is also a key factor limiting the development of alkali-metal–selenium batteries, as the natural reserves of Se in the earth’s crust are lower than those of S, and the fabrication cost is also higher. Thus, strategies to ensure both high performance and decreasing the cost of cathode materials are indispensable.
The above concerns focus on the cathode parts, it should be emphasized that other components, including types of electrolytes and separators, dendrite growth on metal anodes, and current collectors’ corrosions, also have great research value and necessity. Only by addressing all of these problems can high-performance alkali-metal–selenium batteries based on biomass carbon materials go one step further in practical applications.
Author Contributions
Y.D., S.M., J.L. and X.Z., data collection; Y.D., S.M.; X.G. and T.C., writing—original draft preparation; J.D., X.G. and T.C., writing—review and editing; X.G.—supervision; T.C., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC (Grant No. 51902036), Natural Science Foundation of Chongqing Science & Technology Commission (Grant No. 2022NSCQ-MSX3091 and cstc2019jcyj-msxm1407), National Key R&D Program of the Ministry of Science and Technology of China (Grant No. 2017YFC1700705), Sichuan Science and Technology Programs (Grant No. 2021JDRC0043), Scientific and Technological Research Special Project of Sichuan Provincial Administration of Traditional Chinese Medicine Science (Grant No. 2021MS228), the Venture & Innovation Support Program for Chongqing Overseas Returnees (Grant No. CX2021043), and Key Disciplines of Chemical Engineering and Technology in Chongqing Colleges and Universities during the 13th Five-Year Plan provided the financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, S.; Yang, J.; Liu, D.; Huang, Y.; Lee, Y. Bio-Derived Materials Achieving High Performance in Alkali Metal-Chalcogen Batteries. Adv. Funct. Mater. 2020, 31, 2008354. [Google Scholar] [CrossRef]
- Feng, C.; Huang, X.L.; Li, Y.; Wang, Y.; Li, C.; Qiu, W.; Zhang, S.; Liu, H.; Zhang, Y.; Liu, H.K.; et al. Towards rechargeable Na-SexSy batteries: From fundamental insights to improvement strategies. Chem. Eng. J. 2022, 442, 136189. [Google Scholar] [CrossRef]
- Gu, X.; Tang, T.; Liu, X.; Hou, Y. Rechargeable metal batteries based on selenium cathodes: Progress, challenges and perspectives. J. Mater. Chem. A 2019, 7, 11566–11583. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Wang, L.; Tong, X.; Dou, S.X.; Wang, Z.M. Advanced High-Performance Potassium-Chalcogen (S, Se, Te) Batteries. Small 2021, 17, e2004369. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhou, C.; He, W.; Sun, S.; Chueh, Y.L.; Wang, Z.M.; Liu, H.K.; Dou, S.X. An Emerging Energy Storage System: Advanced Na-Se Batteries. ACS Nano 2021, 15, 5876–5903. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C. One dimensional nanostructures contribute better Li–S and Li–Se batteries: Progress, challenges and perspectives. Energy Stor. Mater. 2019, 23, 190–224. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. Host Materials Anchoring Polysulfides in Li–S Batteries Reviewed. Adv. Energy Mater. 2020, 11, 2001304. [Google Scholar] [CrossRef]
- Yang, T.; Niu, Y.; Liu, Q.; Xu, M. Cathode host engineering for non-lithium (Na, K and Mg) sulfur/selenium batteries: A state-of-the-art review. Nano Mater. Sci. 2022, in press. [CrossRef]
- Gu, X.; Deng, L.; Ren, X. Metal Atom-Decorated Carbon Nanomaterials for Enhancing Li-S/Se Batteries Performances: A Mini Review. Front. Energy Res. 2021, 9, 626596. [Google Scholar] [CrossRef]
- Kim, J.K.; Kang, Y.C. Encapsulation of Se into Hierarchically Porous Carbon Microspheres with Optimized Pore Structure for Advanced Na-Se and K-Se Batteries. ACS Nano 2020, 14, 13203–13216. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, M.; Xu, R.; Zeng, S.; Yang, H.; Ye, S.; Liu, F.; Wu, X.; Yu, Y. CNT Interwoven Nitrogen and Oxygen Dual-Doped Porous Carbon Nanosheets as Free-Standing Electrodes for High-Performance Na-Se and K-Se Flexible Batteries. Adv. Mater. 2018, 30, e1805234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, J.; Kim, J.T.; Fu, J.; Duan, H.; Chen, N.; Li, R.; Zhao, S.; Wang, J.; Huang, H.; et al. Highly Stable Halide-Electrolyte-Based All-Solid-State Li-Se Batteries. Adv. Mater. 2022, 34, e2200856. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.C.; Nguyen, C.; Barlow, A.J.; Cherepanov, P.V.; Forsyth, M.; Howlett, P.C.; MacFarlane, D.R. A safe Li–Se battery in an ionic liquid-based electrolyte operating at 25–70 °C by using a N,S,O tri-doped mesoporous carbon host material. Sustain. Energy Fuels 2020, 4, 2322–2332. [Google Scholar] [CrossRef]
- Zuo, T.T.; Shi, Y.; Wu, X.W.; Wang, P.F.; Wang, S.H.; Yin, Y.X.; Wang, W.P.; Ma, Q.; Zeng, X.X.; Ye, H.; et al. Constructing a Stable Lithium Metal-Gel Electrolyte Interface for Quasi-Solid-State Lithium Batteries. ACS Appl. Mater. Interf. 2018, 10, 30065–30070. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Xiong, P.; Zhang, J.; Song, J.; Yan, K.; Gao, X.; Liu, H.; Wang, G. Interface Engineering of MXene Composite Separator for High-Performance Li–Se and Na–Se Batteries. Adv. Energy Mater. 2020, 10, 2000446. [Google Scholar] [CrossRef]
- Gu, X.; Xin, L.; Li, Y.; Dong, F.; Fu, M.; Hou, Y. Highly Reversible Li-Se Batteries with Ultra-Lightweight N,S-Codoped Graphene Blocking Layer. Nano Micro Lett. 2018, 10, 59. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.L.; Yang, Y.L.; Suo, G.; Zhang, L.; Lu, S.; Chen, Z.G. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Li, G.-D.; Chen, J.-S. Microporous carbon derived from pinecone hull as anode material for lithium secondary batteries. Mater. Lett. 2007, 61, 5209–5212. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, T.P.; Ramesh, R.; Thomas, S.; Jeong, S.K.; Nahm, K.S. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]
- Li, J.; Qin, F.; Zhang, L.; Zhang, K.; Li, Q.; Lai, Y.; Zhang, Z.; Fang, J. Mesoporous carbon from biomass: One-pot synthesis and application for Li–S batteries. J. Mater. Chem. A 2014, 2, 13916–13922. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Lai, Y.; Liu, Y.; Li, J. Highly ordered nitrogen-rich mesoporous carbon derived from biomass waste for high-performance lithium–sulfur batteries. Carbon 2015, 84, 399–408. [Google Scholar] [CrossRef]
- Schipper, F.; Vizintin, A.; Ren, J.; Dominko, R.; Fellinger, T.P. Biomass-Derived Heteroatom-Doped Carbon Aerogels from a Salt Melt Sol-Gel Synthesis and their Performance in Li-S Batteries. ChemSusChem 2015, 8, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Zhang, J.; Zafar, Z.A.; Ji, S.; Huang, T.; Anderson, J.A.; Zhang, Z.; Huang, Y. Biomass-derived nanostructured porous carbons for lithium-sulfur batteries. Sci. Chin. Mater. 2016, 59, 389–407. [Google Scholar] [CrossRef]
- Selvan, R.K.; Zhu, P.; Yan, C.; Zhu, J.; Dirican, M.; Shanmugavani, A.; Lee, Y.S.; Zhang, X. Biomass-derived porous carbon modified glass fiber separator as polysulfide reservoir for Li-S batteries. J. Colloid Interf. Sci. 2018, 513, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Guo, J.; Tao, J.; Li, X.; Zheng, W.; He, G.; Dai, Y. Low-Cost Biomass-Gel-Induced Conductive Polymer Networks for High-Efficiency Polysulfide Immobilization and Catalytic Conversion in Li–S Batteries. ACS Appl. Energy Mater. 2022, 5, 2308–2317. [Google Scholar] [CrossRef]
- Tian, X.; Yan, C.; Kang, J.; Yang, X.; Li, Q.; Yan, J.; Deng, N.; Cheng, B.; Kang, W. Working Mechanisms and Structure Engineering of Renewable Biomass-Derived Materials for Advanced Lithium-Sulfur Batteries: A Review. ChemElectroChem 2022, 9, e202100995. [Google Scholar] [CrossRef]
- Benítez, A.; Amaro-Gahete, J.; Chien, Y.-C.; Caballero, Á.; Morales, J.; Brandell, D. Recent advances in lithium-sulfur batteries using biomass-derived carbons as sulfur host. Renew. Sustain. Energy Rev. 2022, 154, 111783. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, H.; Zhang, S.; Yao, J.; Xu, J. Selenium/pomelo peel-derived carbon nanocomposite as advanced cathode for lithium-selenium batteries. Ionics 2015, 21, 2477–2484. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Kang, W.; Shen, Q. Encapsulating selenium into macro-/micro-porous biochar-based framework for high-performance lithium-selenium batteries. Carbon 2015, 95, 354–363. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, D.; Yang, Z.; Yu, F.; Su, Y.; Wang, D.; Shen, Q. Alkaline lignin derived porous carbon as an efficient scaffold for lithium-selenium battery cathode. Carbon 2017, 122, 547–555. [Google Scholar] [CrossRef]
- Zhao, P.; Shiraz, M.H.A.; Zhu, H.; Liu, Y.; Tao, L.; Liu, J. Hierarchically porous carbon from waste coffee grounds for high-performance Li–Se batteries. Electrochim. Acta 2019, 325, 134931. [Google Scholar] [CrossRef]
- Yang, Z.; Jia, D.; Wu, Y.; Song, D.; Sun, X.; Wang, C.; Yang, L.; Zhang, Y.; Gao, J.; Ohsaka, T.; et al. Novel lithium-chalcogenide batteries combining S, Se and C characteristics supported by chitosan-derived carbon intertwined with CNTs. Chem. Eng. J. 2022, 427, 131790. [Google Scholar] [CrossRef]
- Yangdan, L.; Yichuan, G.; Yang, T.; Haichao, T.; Zhizhen, Y.; Jianguo, L. Porous carbon derived from corncob as cathode host for Li–Se battery. Ionics 2022, 28, 2593–2601. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Jing, P.; Guo, Y.; Zhang, Y.; Wei, Y.; Wang, Q.; Zhang, Y.; Wu, H. Bioderived carbon fiber conductive networks with inlaid electrocatalysts as an ultralight freestanding interlayer for working Li–SeS2 pouch cells. Carbon 2022, 189, 10–20. [Google Scholar] [CrossRef]
- Luo, C.; Xu, Y.; Zhu, Y.; Liu, Y.; Zheng, S.; Liu, Y.; Langrock, A.; Wang, C. Selenium@Mesoporous Carbon Composite with Superior Lithium and Sodium Storage Capacity. ACS Nano 2013, 7, 8003–8010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tai, Z.; Zhang, Q.; Wang, H.; Pang, W.K.; Liu, H.K.; Konstantinov, K.; Guo, Z. A new energy storage system: Rechargeable potassium-selenium battery. Nano Energy 2017, 35, 36–43. [Google Scholar] [CrossRef]
- Huang, X.L.; Guo, Z.; Dou, S.X.; Wang, Z.M. Rechargeable Potassium–Selenium Batteries. Adv. Funct. Mater. 2021, 31, 2102326. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium-sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Tao, X.; Liang, Y.; Yang, S.J.; Huang, J.Q.; Yuan, T.Q.; Titirici, M.M.; Zhang, Q. Recent progress on biomass-derived ecomaterials toward advanced rechargeable lithium batteries. EcoMat 2020, 2, e12019. [Google Scholar] [CrossRef]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Liu, A.; Zhu, H.; Ma, T. Recent progress in biomass-derived carbon materials used for secondary batteries. Sustain. Energy Fuels 2021, 5, 3017–3038. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Z.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. The stable lithium metal cell with two-electrode biomass carbon. Electrochim. Acta 2020, 356, 136824. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
- Jia, M.; Lu, S.; Chen, Y.; Liu, T.; Han, J.; Shen, B.; Wu, X.; Bao, S.-J.; Jiang, J.; Xu, M. Three-dimensional hierarchical porous tubular carbon as a host matrix for long-term lithium-selenium batteries. J. Power Sources 2017, 367, 17–23. [Google Scholar] [CrossRef]
- Gu, X.; Tong, C.-J.; Lai, C.; Qiu, J.; Huang, X.; Yang, W.; Wen, B.; Liu, L.-M.; Hou, Y.; Zhang, S. Porous nitrogen and phosphorous dual doped graphene blocking layer for high performance Li–S batteries. J. Mater. Chem. A 2015, 3, 16670–16678. [Google Scholar] [CrossRef]
- Gu, X.; Li, H.; Wen, H.; Zhou, Y.; Kang, H.; Liao, H.; Gao, M.; Wang, Y.; Deng, L.; Yi, X.; et al. From agaric hydrogel to nitrogen-doped 3D porous carbon for high-performance Li–S batteries. J. Mater. Sci. 2019, 55, 1136–1147. [Google Scholar] [CrossRef]
- Ai, W.; Li, J.; Du, Z.; Zou, C.; Du, H.; Xu, X.; Chen, Y.; Zhang, H.; Zhao, J.; Li, C.; et al. Dual confinement of polysulfides in boron-doped porous carbon sphere/graphene hybrid for advanced Li-S batteries. Nano Res. 2018, 11, 4562–4573. [Google Scholar] [CrossRef]
- Park, S.-K.; Lee, J.-K.; Kang, Y.C. Yolk-Shell Structured Assembly of Bamboo-Like Nitrogen-Doped Carbon Nanotubes Embedded with Co Nanocrystals and Their Application as Cathode Material for Li-S Batteries. Adv. Funct. Mater. 2018, 28, 1705264. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, S.; Muheiyati, H.; Zhai, Y.; Li, C.; Ding, X.; Wang, L.; Wang, D.; Xu, L.; He, Y.; et al. Double-Shelled Ni–Fe–P/N-Doped Carbon Nanobox Derived from a Prussian Blue Analogue as an Electrode Material for K-Ion Batteries and Li–S Batteries. ACS Energy Lett. 2019, 4, 1496–1504. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Feng, Z.; Wu, F.; Wei, D.; Xi, B.; Xiong, S. P-doped BN nanosheets decorated graphene as the functional interlayer for Li–S batteries. J. Energy Chem. 2019, 39, 54–60. [Google Scholar] [CrossRef]
- Wei, L.; Li, W.; Zhao, T.; Zhang, N.; Li, L.; Wu, F.; Chen, R. Cobalt nanoparticles shielded in N-doped carbon nanotubes for high areal capacity Li-S batteries. Chem. Commun. 2020, 56, 3007–3010. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, X.; Li, B.; Shu, H.; Luo, L.; Xia, W.; Chen, M.; Zeng, P.; Yang, X.; Gao, P.; et al. NiMoO4 Nanosheets Anchored on N-S Doped Carbon Clothes with Hierarchical Structure as a Bidirectional Catalyst toward Accelerating Polysulfides Conversion for Li-S Battery. Adv. Funct. Mater. 2021, 31, 2101285. [Google Scholar] [CrossRef]
- Gu, X.; Tong, C.J.; Rehman, S.; Liu, L.M.; Hou, Y.; Zhang, S. Multifunctional Nitrogen-Doped Loofah Sponge Carbon Blocking Layer for High-Performance Rechargeable Lithium Batteries. ACS Appl. Mater. Interf. 2016, 8, 15991–16001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Luo, J.; Hu, Z. Hierarchical porous N,O Co-doped carbon/Se composite derived from hydrothermal treated chitosan as Li–Se battery cathode. Micro Nano Lett. 2018, 13, 1386–1389. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, H.; Zhang, H.; Tong, L.; Mitlin, D. Selenium Impregnated Monolithic Carbons as Free-Standing Cathodes for High Volumetric Energy Lithium and Sodium Metal Batteries. Adv. Energy Mater. 2017, 8, 1701918. [Google Scholar] [CrossRef]
- Qiu, R.; Fei, R.; Zhang, T.; Liu, X.; Jin, J.; Fan, H.; Wang, R.; He, B.; Gong, Y.; Wang, H. Biomass-derived, 3D interconnected N-doped carbon foam as a host matrix for Li/Na/K-selenium batteries. Electrochim. Acta 2020, 356, 136832. [Google Scholar] [CrossRef]
- Jia, D.; Yang, Z.; Zhang, H.; Liu, F.; Shen, Q. High performance of selenium cathode by encapsulating selenium into the micropores of chitosan-derived porous carbon framework. J. Alloys Compd. 2018, 746, 27–35. [Google Scholar] [CrossRef]
- Hoseini, A.H.A.; Shiraz, M.H.A.; Tao, L.; Lu, W.; Arjmand, M.; Liu, J. Synthesis of Soybean-derived Porous Carbon as Selenium Host for High-Performance Lithium-Selenium Batteries. Electrochim. Acta 2022, 44, 140954. [Google Scholar] [CrossRef]
- Abouimrane, A.; Dambournet, D.; Chapman, K.W.; Chupas, P.J.; Weng, W.; Amine, K. A new class of lithium and sodium rechargeable batteries based on selenium and selenium-sulfur as a positive electrode. J. Am. Chem. Soc. 2012, 134, 4505–4508. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, L.; Qiu, M.; Zhang, N. Amorphous Se Restrained by Biomass-Derived Defective Carbon for Stable Na–Se Batteries. ACS Appl. Energy Mater. 2021, 4, 7219–7225. [Google Scholar] [CrossRef]
- Guo, B.; Mi, H.; Zhang, P.; Ren, X.; Li, Y. Free-Standing Selenium Impregnated Carbonized Leaf Cathodes for High-Performance Sodium-Selenium Batteries. Nanoscale Res. Lett. 2019, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, Y.; Su, L.; Cen, W.; Wang, Q.; Xiao, D. Nitrogen/oxygen codoped hierarchical porous Carbons/Selenium cathode with excellent lithium and sodium storage behavior. J. Colloid Interf. Sci. 2022, 608, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, H.; Zhao, X.; Wang, X.; Miao, Y.; Cheng, L.; Wang, C.; Wang, L.; Yue, H.; Zhang, D. Porous Bamboo-Derived Carbon as Selenium Host for Advanced Lithium/Sodium–Selenium Batteries. Energy Technol. 2020, 8, 1901445. [Google Scholar] [CrossRef]
- Cai, R.; Chen, X.; Liu, P.; Chen, T.; Liu, W.; Fan, X.; Ouyang, B.; Liu, K. A Novel Cathode Based on Selenium Confined in Biomass Carbon and Graphene Oxide for Potassium–Selenium Battery. ChemElectroChem 2020, 7, 4477–4483. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).