Evaluation of Glyoxal-Based Electrolytes for Lithium-Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrolyte Characterization
2.3. Cathode Manufacturing
2.4. Cell Performance
3. Results
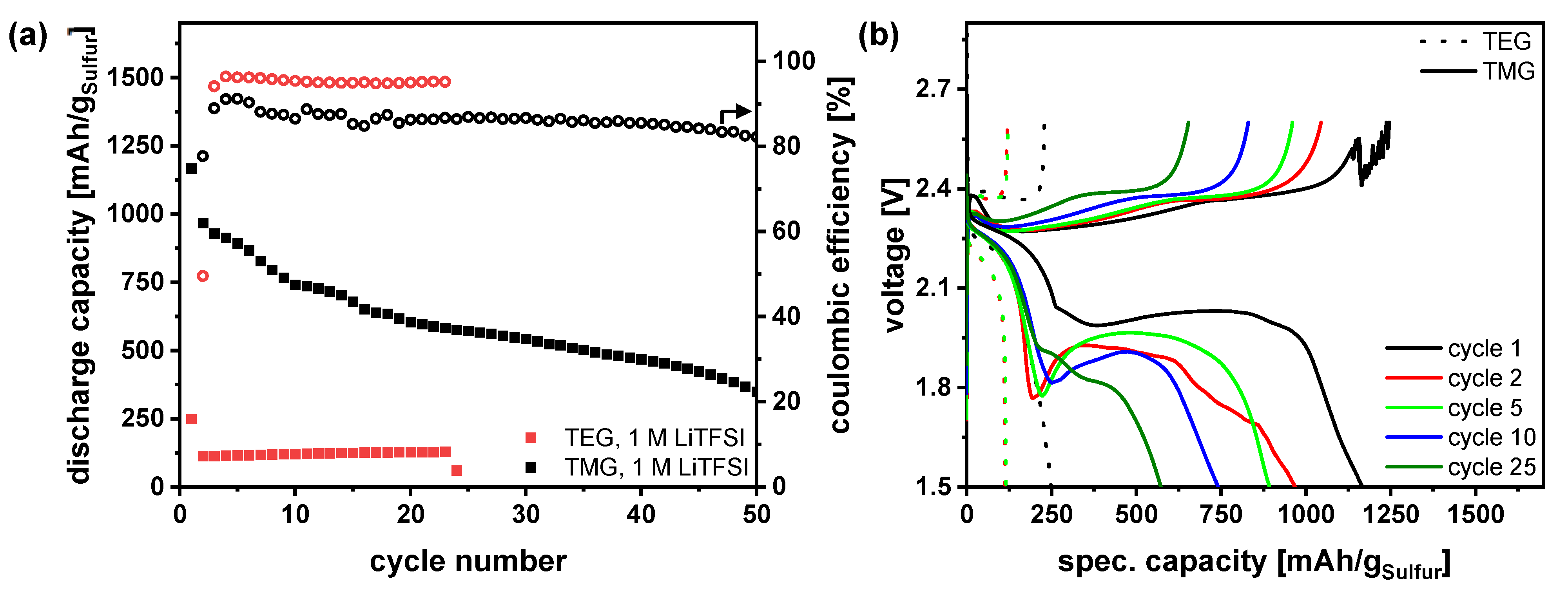
3.1. Pure Glyoxal-Solvent Electrolytes
3.2. Influence of Temperature on Electrolyte Performance
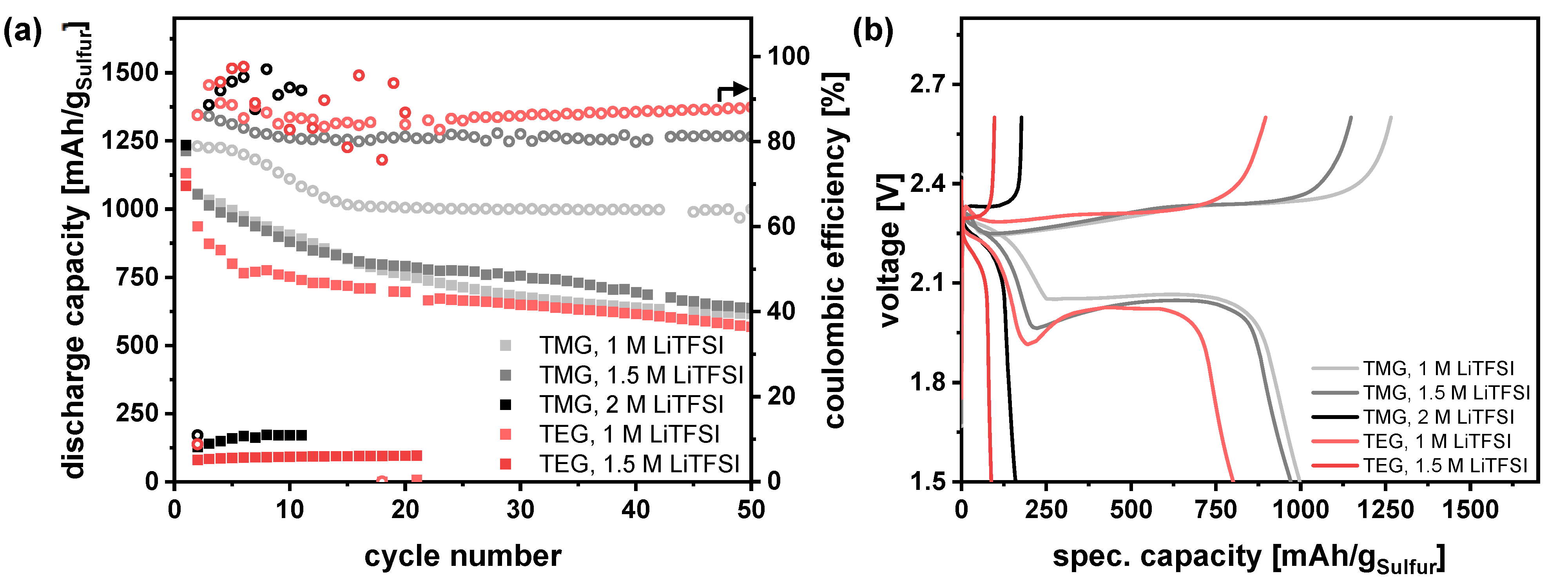
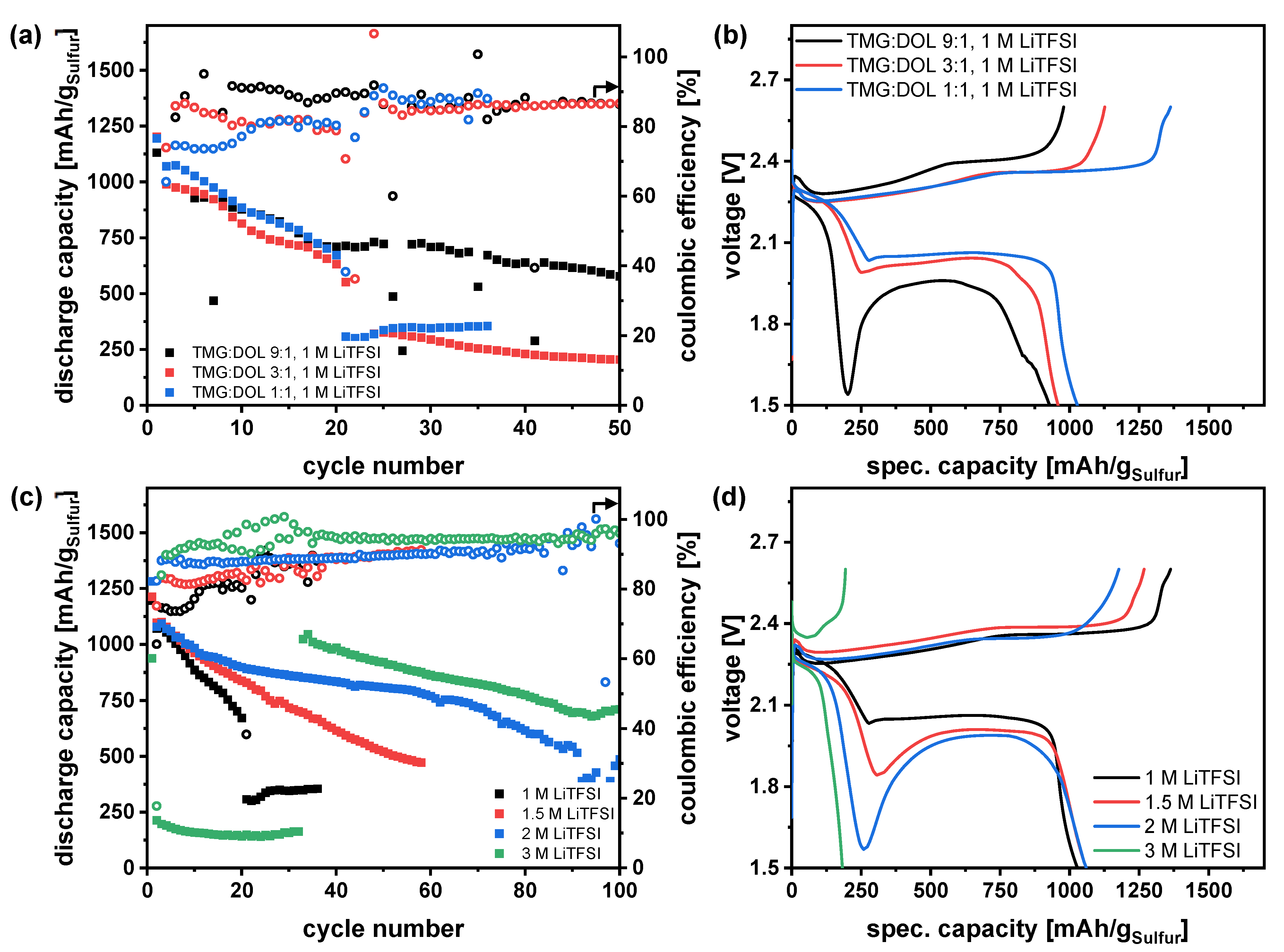
3.3. Addition of a Second Solvent—Adaption of the Electrolyte Composition
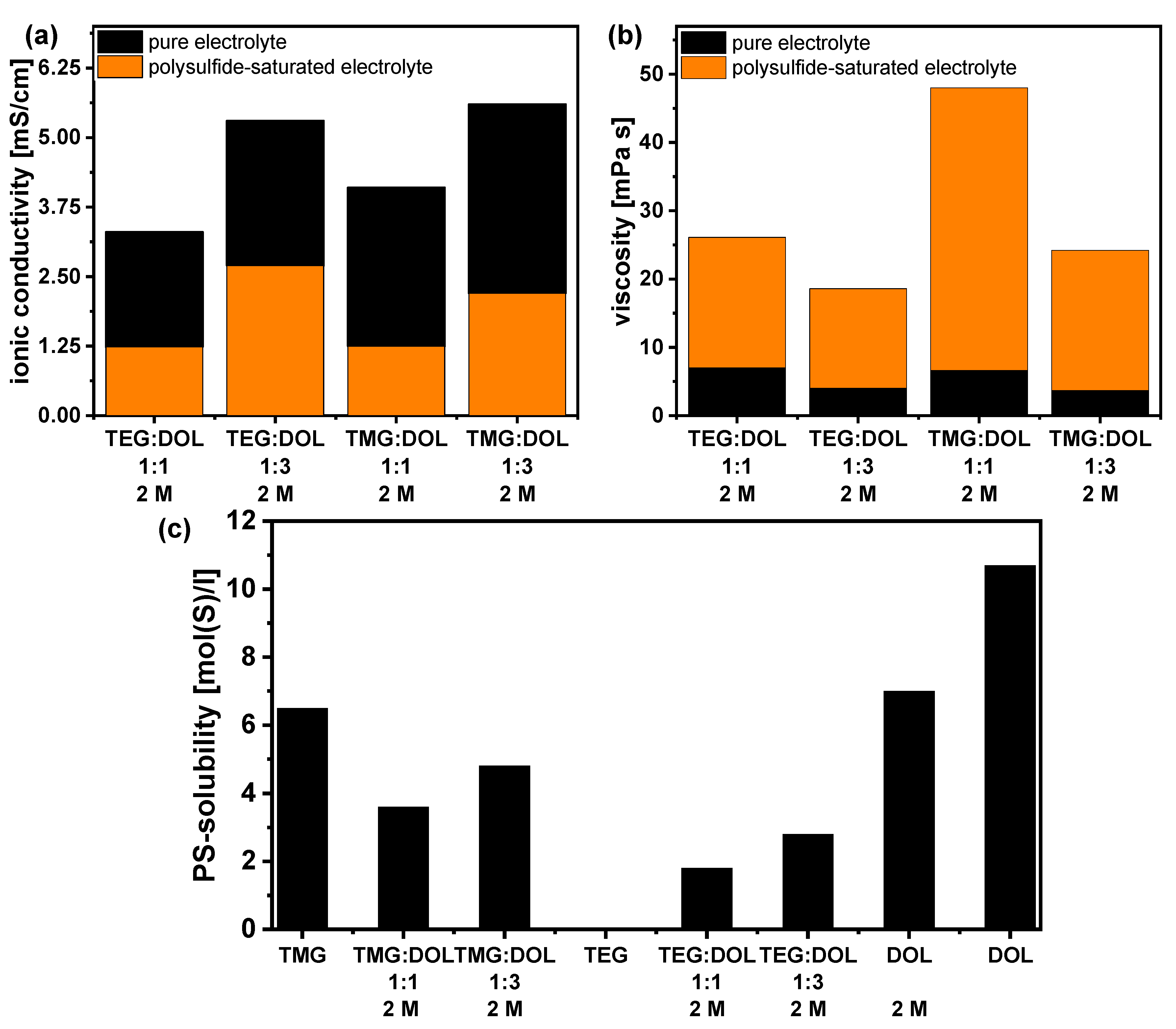
3.4. Physicochemical Characterization of the Adapted Glyoxal/Dioxolane-Based Electrolytes
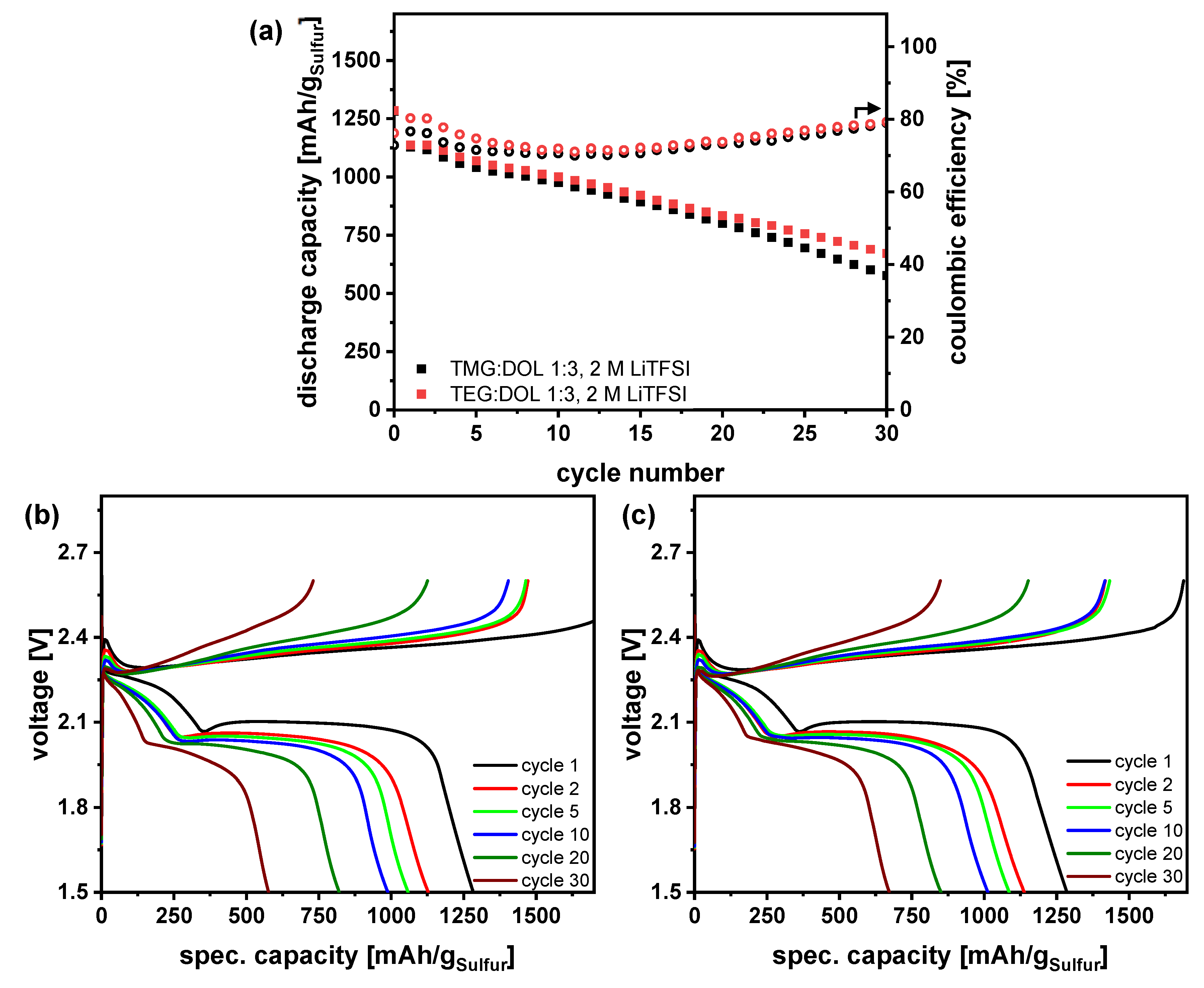
3.5. Influence of Polysulfides on Electrolyte Properties and Evaluation in Li-S Pouchcells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application. Electrochem. Energ. Rev. 2018, 1, 239–293. [Google Scholar] [CrossRef]
- Wang, X.; Deng, N.; Wei, L.; Yang, Q.; Xiang, H.; Wang, M.; Cheng, B.; Kang, W. Recent Progress in High-Performance Lithium Sulfur Batteries: The Emerging Strategies for Advanced Separators/Electrolytes Based on Nanomaterials and Corresponding Interfaces. Chem. Asian J. 2021, 16, 2852–2870. [Google Scholar] [CrossRef]
- OXIS Energy. OXIS Energy is Close to Achieving 500 Wh/kg and Is Targeting 600 Wh/kg with Solid State Lithium Sulfur Technology. Available online: https://www.prnewswire.com/news-releases/oxis-energy-is-close-to-achieving-500whkg-and-is-targeting-600whkg-with-solid-state-lithium-sulfur-technology-300990184.html (accessed on 1 November 2022).
- Dörfler, S.; Althues, H.; Härtel, P.; Abendroth, T.; Schumm, B.; Kaskel, S. Challenges and Key Parameters of Lithium-Sulfur Batteries on Pouch Cell Level. Joule 2020, 4, 539–554. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Dhanalakshmi, R.B.; Sathya, S.; Ahn, J.-H.; Stephan, A.M. Understanding the Electrolytes of Lithium−Sulfur Batteries. Batter. Supercaps 2021, 414, 359. [Google Scholar] [CrossRef]
- Gupta, A.; Bhargav, A.; Manthiram, A. Highly Solvating Electrolytes for Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1803096. [Google Scholar] [CrossRef]
- Gupta, A.; Bhargav, A.; Manthiram, A. Evoking High Donor Number-assisted and Organosulfur-mediated Conversion in Lithium-Sulfur Batteries. ACS Energy Lett. 2021, 6, 224–231. [Google Scholar] [CrossRef]
- Guo, J.; Pei, H.; Dou, Y.; Zhao, S.; Shao, G.; Liu, J. Rational Designs for Lithium-Sulfur Batteries with Low Electrolyte/Sulfur Ratio. Adv. Funct. Mater. 2021, 31, 2010499. [Google Scholar] [CrossRef]
- Su, C.-C.; He, M.; Amine, R.; Chen, Z.; Amine, K. The Relationship between the Relative Solvating Power of Electrolytes and Shuttling Effect of Lithium Polysulfides in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed Engl. 2018, 57, 12033–12036. [Google Scholar] [CrossRef]
- Xu, W.-T.; Peng, H.-J.; Huang, J.-Q.; Zhao, C.-Z.; Cheng, X.-B.; Zhang, Q. Towards Stable Lithium-Sulfur Batteries with a Low Self-Discharge Rate: Ion Diffusion Modulation and Anode Protection. ChemSusChem 2015, 8, 2892–2901. [Google Scholar] [CrossRef]
- Deng, N.; Kang, W.; Liu, Y.; Ju, J.; Wu, D.; Li, L.; Hassan, B.S.; Cheng, B. A review on separators for lithium sulfur battery: Progress and prospects. J. Power Sources 2016, 331, 132–155. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 652. [Google Scholar] [CrossRef]
- Chen, W.-J.; Li, B.-Q.; Zhao, C.-X.; Zhao, M.; Yuan, T.-Q.; Sun, R.-C.; Huang, J.-Q.; Zhang, Q. Electrolyte Regulation towards Stable Lithium-Metal Anodes in Lithium-Sulfur Batteries with Sulfurized Polyacrylonitrile Cathodes. Angew. Chem. Int. Ed Engl. 2020, 59, 10732–10745. [Google Scholar] [CrossRef]
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the Surface Chemical Aspects of Very High Energy Density, Rechargeable Li–Sulfur Batteries. J. Electrochem. Soc. 2009, 156, A694. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, W.; Si, Y.; Wang, D.; Fu, Y.; Manthiram, A. Artificial dual solid-electrolyte interfaces based on in situ organothiol transformation in lithium sulfur battery. Nat. Commun. 2021, 12, 3031. [Google Scholar] [CrossRef]
- Wei, J.-Y.; Zhang, X.-Q.; Hou, L.-P.; Shi, P.; Li, B.-Q.; Xiao, Y.; Yan, C.; Yuan, H.; Huang, J.-Q. Shielding Polysulfide Intermediates by an Organosulfur-Containing Solid Electrolyte Interphase on the Lithium Anode in Lithium-Sulfur Batteries. Adv. Mater. 2020, 32, e2003012. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Wu, M.; Jin, J.; Zhang, H.; Wu, X. Improved cycling performances of lithium sulfur batteries with LiNO3-modified electrolyte. J. Power Sources 2011, 196, 9839–9843. [Google Scholar] [CrossRef]
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Properties of surface film on lithium anode with LiNO3 as lithium salt in electrolyte solution for lithium–sulfur batteries. Electrochim. Acta 2012, 83, 78–86. [Google Scholar] [CrossRef]
- Weller, C.; Thieme, S.; Härtel, P.; Althues, H.; Kaskel, S. Intrinsic Shuttle Suppression in Lithium-Sulfur Batteries for Pouch Cell Application. J. Electrochem. Soc. 2017, 164, A3766. [Google Scholar] [CrossRef]
- Qu, C.; Chen, Y.; Yang, X.; Zhang, H.; Li, X.; Zhang, H. LiNO3-free electrolyte for Li-S battery: A solvent of choice with low Ksp of polysulfide and low dendrite of lithium. Nano Energy 2017, 39, 262–272. [Google Scholar] [CrossRef]
- Jozwiuk, A.; Berkes, B.B.; Weiß, T.; Sommer, H.; Janek, J.; Brezesinski, T. The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 2603–2608. [Google Scholar] [CrossRef]
- Liu, Y.; Elias, Y.; Meng, J.; Aurbach, D.; Zou, R.; Xia, D.; Pang, Q. Electrolyte solutions design for lithium-sulfur batteries. Joule 2021, 5, 2323–2364. [Google Scholar] [CrossRef]
- Cheng, L.; Curtiss, L.A.; Zavadil, K.R.; Gewirth, A.A.; Shao, Y.; Gallagher, K.G. Sparingly Solvating Electrolytes for High Energy Density Lithium–Sulfur Batteries. ACS Energy Lett. 2016, 1, 503–509. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Q.; Tong, X.; Gan, H. Electrolyte with Low Polysulfide Solubility for Li–S Batteries. ACS Appl. Energy Mater. 2018, 1, 2608–2618. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Jin, Q.; Nan, Y.-L.; Hou, L.-P.; Li, B.-Q.; Chen, X.; Jin, Z.-H.; Zhang, X.-T.; Huang, J.-Q.; Zhang, Q. Electrolyte Structure of Lithium Polysulfides with Anti-Reductive Solvent Shells for Practical Lithium-Sulfur Batteries. Angew. Chem. Int. Ed Engl. 2021, 60, 15503–15509. [Google Scholar] [CrossRef]
- Weller, C.; Pampel, J.; Dörfler, S.; Althues, H.; Kaskel, S. Polysulfide Shuttle Suppression by Electrolytes with Low-Density for High-Energy Lithium–Sulfur Batteries. Energy Technol. 2019, 20, 9821. [Google Scholar] [CrossRef]
- Glaser, R.; Borodin, O.; Johnson, B.; Jhulki, S.; Yushin, G. Minimizing Long-Chain Polysulfide Formation in Li-S Batteries by Using Localized Low Concentration Highly Fluorinated Electrolytes. J. Electrochem. Soc. 2021, 168, 90543. [Google Scholar] [CrossRef]
- Tang, B.; Wu, H.; Du, X.; Cheng, X.; Liu, X.; Yu, Z.; Yang, J.; Zhang, M.; Zhang, J.; Cui, G. Highly Safe Electrolyte Enabled via Controllable Polysulfide Release and Efficient Conversion for Advanced Lithium-Sulfur Batteries. Small 2020, 16, e1905737. [Google Scholar] [CrossRef]
- Lee, C.-W.; Pang, Q.; Ha, S.; Cheng, L.; Han, S.-D.; Zavadil, K.R.; Gallagher, K.G.; Nazar, L.F.; Balasubramanian, M. Directing the Lithium-Sulfur Reaction Pathway via Sparingly Solvating Electrolytes for High Energy Density Batteries. ACS Cent. Sci. 2017, 3, 605–613. [Google Scholar] [CrossRef]
- Suo, L.; Hu, Y.-S.; Li, H.; Armand, M.; Chen, L. A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, X.; Ji, G.; Wang, H.; Hou, S.; DeMella, K.C.; Raghavan, S.R.; Wang, J.; Xu, K.; Wang, C. Manipulating electrolyte and solid electrolyte interphase to enable safe and efficient Li-S batteries. Nano Energy 2018, 50, 431–440. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.-E.; Adams, B.D.; Garsuch, A.; Balasubramanian, M.; Nazar, L.F. Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries. Energy Environ. Sci. 2014, 7, 2697–2705. [Google Scholar] [CrossRef]
- Nakanishi, A.; Ueno, K.; Watanabe, D.; Ugata, Y.; Matsumae, Y.; Liu, J.; Thomas, M.L.; Dokko, K.; Watanabe, M. Sulfolane-Based Highly Concentrated Electrolytes of Lithium Bis(trifluoromethanesulfonyl)amide: Ionic Transport, Li-Ion Coordination, and Li–S Battery Performance. J. Phys. Chem. C 2019, 123, 14229–14238. [Google Scholar] [CrossRef]
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium–sulphur batteries. J. Power Sources 2014, 255, 204–218. [Google Scholar] [CrossRef]
- Cleaver, T.; Kovacik, P.; Marinescu, M.; Zhang, T.; Offer, G.J. Commercializing Lithium Sulfur Batteries: Are We Doing the Right Research? J. Electrochem. Soc. 2018, 165, A6029. [Google Scholar] [CrossRef]
- Heß, L.H.; Balducci, A. Glyoxal-Based Solvents for Electrochemical Energy-Storage Devices. ChemSusChem 2018, 11, 1919–1926. [Google Scholar] [CrossRef]
- Hess, L.H.; Wankmüller, S.; Köps, L.; Bothe, A.; Balducci, A. Stable Acetals of Glyoxal as Electrolyte Solvents for Lithium-Ion Batteries. Batteries Supercaps 2019, 2, 852–857. [Google Scholar] [CrossRef]
- Köps, L.; Leibing, C.; Hess, L.H.; Balducci, A. Mixtures of Glyoxylic Acetals and Organic Carbonates as Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 10513. [Google Scholar] [CrossRef]
- Schmidt, F.; Korzhenko, A.; Härtel, P.; Reuter, F.S.; Ehrling, S.; Dörfler, S.; Abendroth, T.; Althues, H.; Kaskel, S. Influence of external stack pressure on the performance of Li-S pouch cell. J. Phys. Energy 2022, 4, 14004. [Google Scholar] [CrossRef]
- Andersen, A.; Rajput, N.N.; Han, K.S.; Pan, H.; Govind, N.; Persson, K.A.; Mueller, K.T.; Murugesan, V. Structure and Dynamics of Polysulfide Clusters in a Nonaqueous Solvent Mixture of 1,3-Dioxolane and 1,2-Dimethoxyethane. Chem. Mater. 2019, 31, 2308–2319. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Chen, N.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Development and Challenges of Functional Electrolytes for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1800919. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Kovalev, I.; Schock, R.; Kumaresan, K.; Xu, J.; Affinito, J. High Energy Rechargeable Li-S Cells for EV Application: Status, Remaining Problems and Solutions. ECS Trans. 2010, 25, 23–34. [Google Scholar] [CrossRef]
- Barchasz, C.; Leprêtre, J.-C.; Patoux, S.; Alloin, F. Electrochemical properties of ether-based electrolytes for lithium/sulfur rechargeable batteries. Electrochim. Acta 2013, 89, 737–743. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, Y.; Liang, C.; Cao, L.; Pan, H.; Yang, J.; Wang, J. A new ether-based electrolyte for lithium sulfur batteries using a S@pPAN cathode. Chem. Commun. 2018, 54, 5478–5481. [Google Scholar] [CrossRef]
- Boenke, T.; Kirchhoff, S.; Reuter, F.S.; Schmidt, F.; Weller, C.; Dörfler, S.; Schwedtmann, K.; Härtel, P.; Abendroth, T.; Althues, H.; et al. The role of polysulfide-saturation in electrolytes for high power applications of real world Li-S pouch cells. Nano Res. 2022. [Google Scholar] [CrossRef]
- Liu, T.; Li, H.; Yue, J.; Feng, J.; Mao, M.; Zhu, X.; Hu, Y.-S.; Li, H.; Huang, X.; Chen, L.; et al. Ultralight Electrolyte for High-Energy Lithium-Sulfur Pouch Cells. Angew. Chem. Int. Ed Engl. 2021, 60, 17547–17555. [Google Scholar] [CrossRef]
- Pang, Q.; Shyamsunder, A.; Narayanan, B.; Kwok, C.Y.; Curtiss, L.A.; Nazar, L.F. Tuning the electrolyte network structure to invoke quasi-solid state sulfur conversion and suppress lithium dendrite formation in Li–S batteries. Nat. Energy 2018, 3, 783–791. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.-E.; Evers, S.; He, G.; Kolbeck, M.; Garsuch, A.; Bolin, T.; Balasubramanian, M.; Nazar, L.F. Sulfur Speciation in Li–S Batteries Determined by Operando X-ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3227–3232. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirchhoff, S.; Leibing, C.; Härtel, P.; Abendroth, T.; Dörfler, S.; Althues, H.; Kaskel, S.; Balducci, A. Evaluation of Glyoxal-Based Electrolytes for Lithium-Sulfur Batteries. Batteries 2023, 9, 210. https://doi.org/10.3390/batteries9040210
Kirchhoff S, Leibing C, Härtel P, Abendroth T, Dörfler S, Althues H, Kaskel S, Balducci A. Evaluation of Glyoxal-Based Electrolytes for Lithium-Sulfur Batteries. Batteries. 2023; 9(4):210. https://doi.org/10.3390/batteries9040210
Chicago/Turabian StyleKirchhoff, Sebastian, Christian Leibing, Paul Härtel, Thomas Abendroth, Susanne Dörfler, Holger Althues, Stefan Kaskel, and Andrea Balducci. 2023. "Evaluation of Glyoxal-Based Electrolytes for Lithium-Sulfur Batteries" Batteries 9, no. 4: 210. https://doi.org/10.3390/batteries9040210
APA StyleKirchhoff, S., Leibing, C., Härtel, P., Abendroth, T., Dörfler, S., Althues, H., Kaskel, S., & Balducci, A. (2023). Evaluation of Glyoxal-Based Electrolytes for Lithium-Sulfur Batteries. Batteries, 9(4), 210. https://doi.org/10.3390/batteries9040210







