In Situ Solidified Gel Polymer Electrolytes for Stable Solid−State Lithium Batteries at High Temperatures
Abstract
1. Introduction
2. Experimental Section
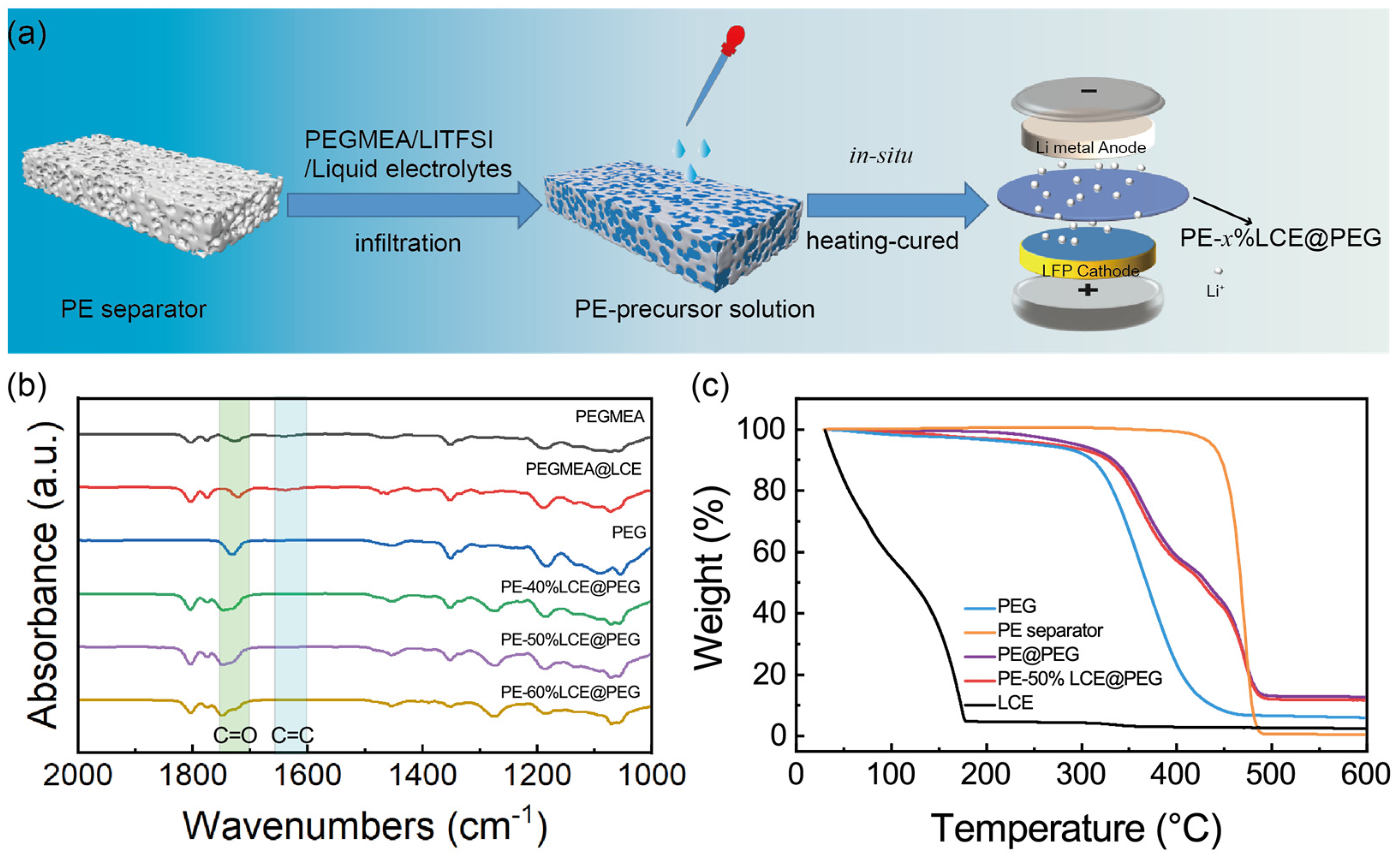
2.1. Synthesis of PE−Based Gel Polymer Electrolytes
2.2. Physical Characterization
2.3. Electrochemical Measurements
2.4. Battery Fabrication and Evaluation
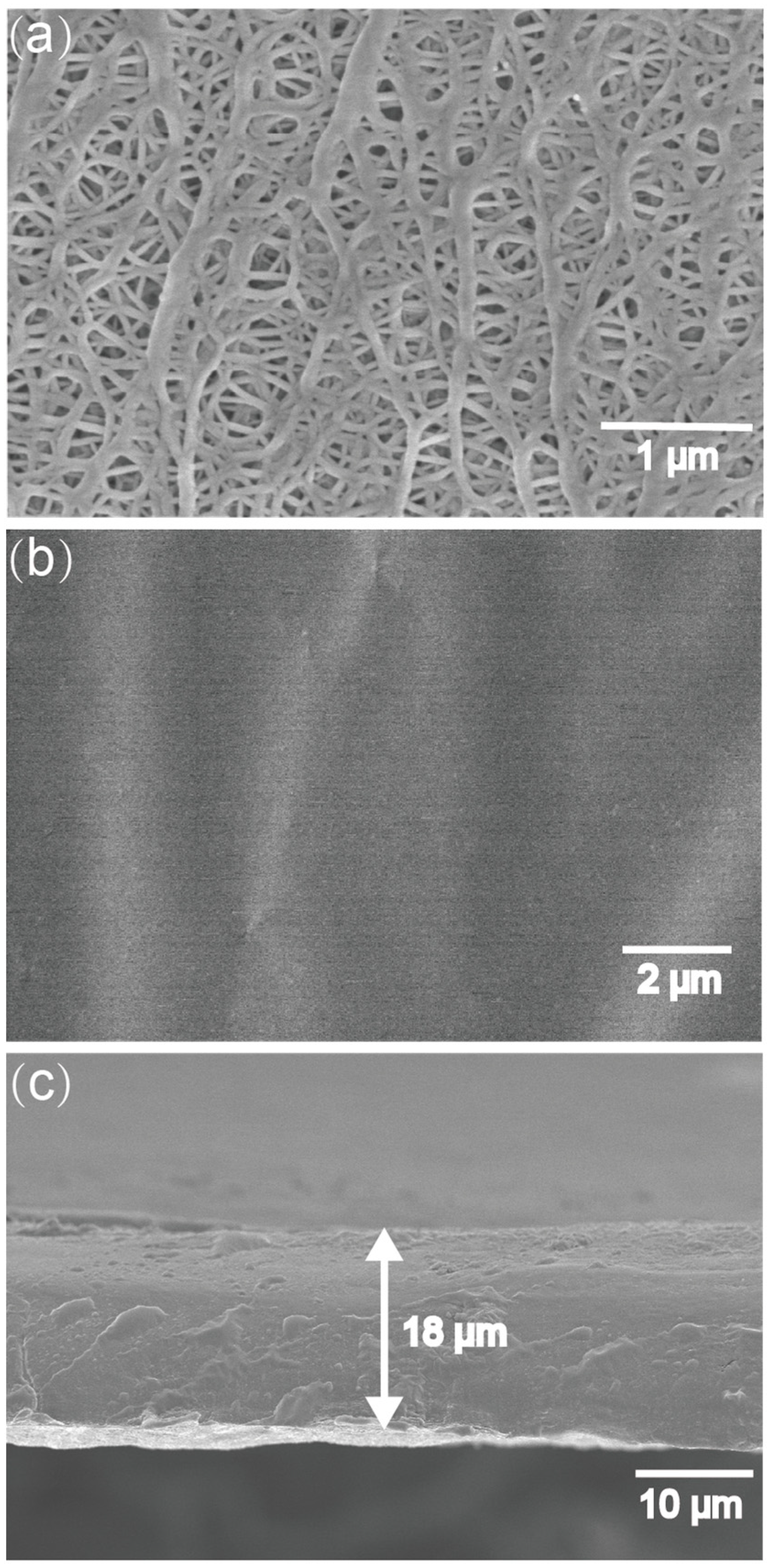
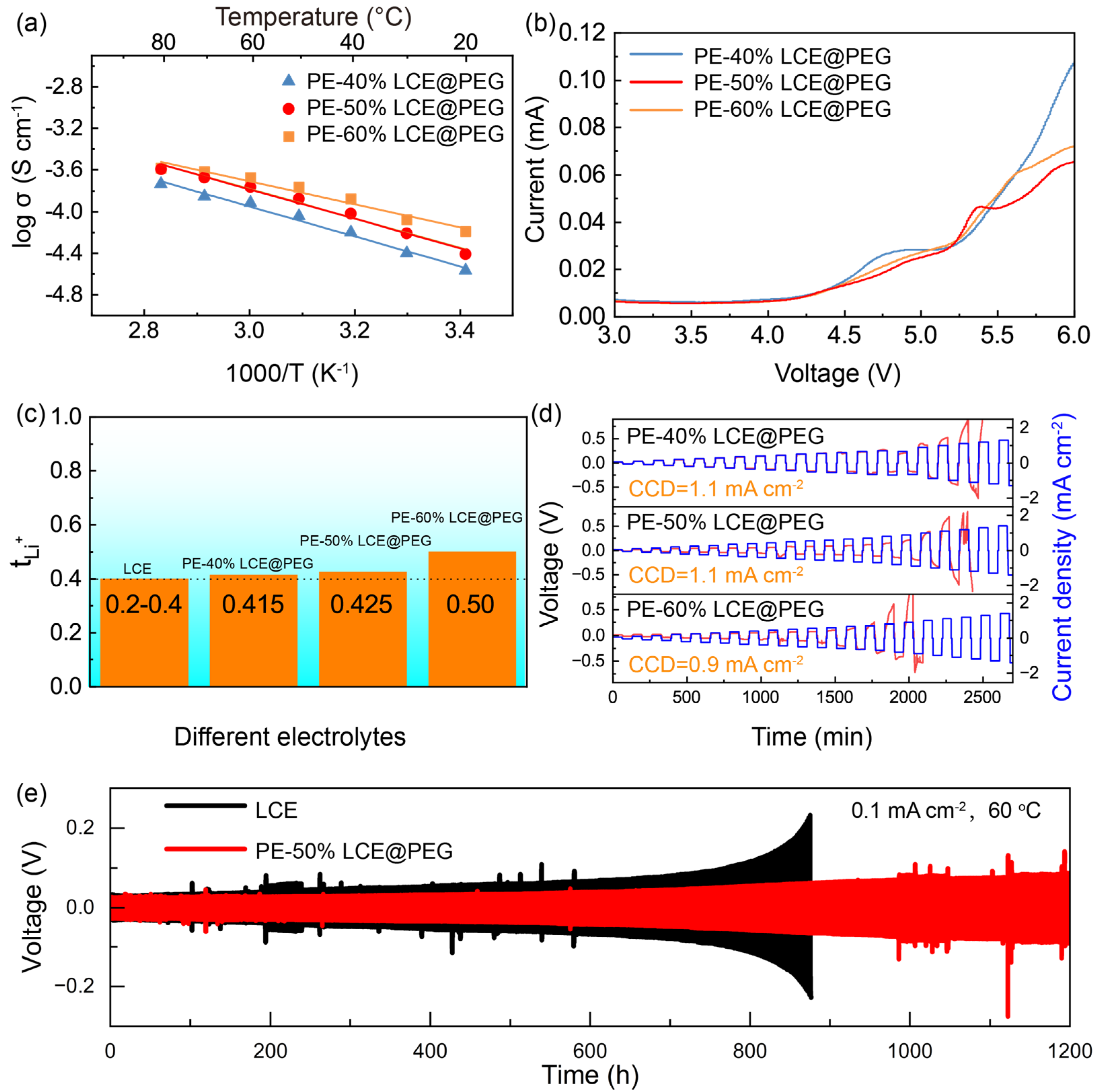
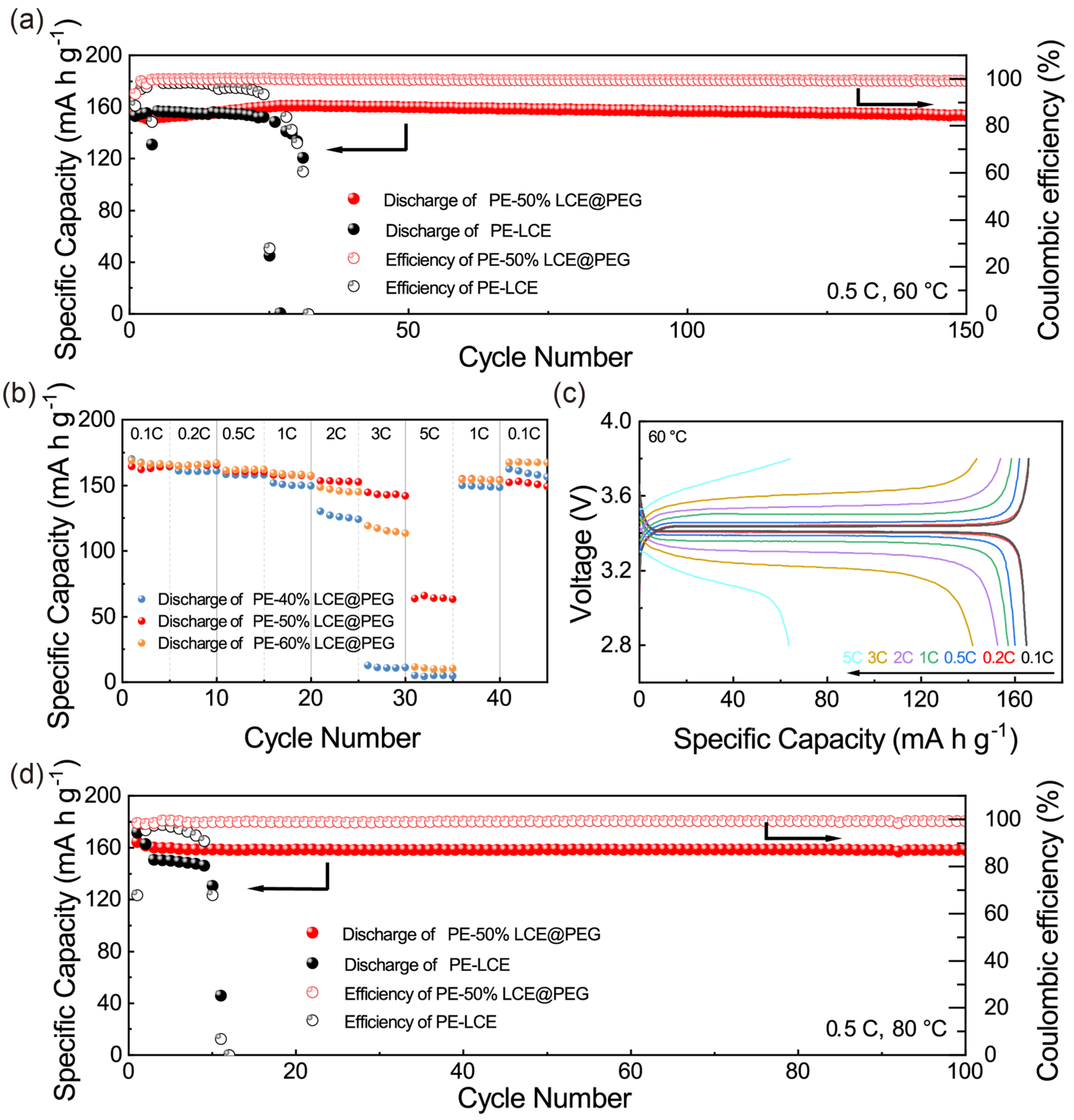
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. A BETTER BATTERY. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Safronova, E.Y.; Novikova, S.A.; Yaroslavtsev, A.B. Recent progress in lithium-ion and lithium metal batteries. Mendeleev Commun. 2022, 32, 287–297. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; He, Y.-B.; Kang, F.; Chen, L.; Li, H.; Yang, Q.-H. Solid-state lithium batteries: Safety and prospects. eScience 2022, 2, 138–163. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, Y.Y.; Zhang, Z.H.; Chen, X.W.; Lie, W.; He, Y.B.; Zhou, Z.; Xia, G.L.; Guo, Z.P. Building Artificial Solid-Electrolyte Interphase with Uniform Intermolecular Ionic Bonds toward Dendrite-Free Lithium Metal Anodes. Adv. Funct. Mater. 2020, 30, 2002414. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.L.; Xian, F.; Guo, Z.Y.; Lu, C.L.; Du, X.F.; Cheng, X.Y.; Zhang, S.; Dong, S.M.; Cui, G.L.; Chen, L.Q. Nonflammable Nitrile Deep Eutectic Electrolyte Enables High-Voltage Lithium Metal Batteries. Chem. Mater. 2020, 32, 3405–3413. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Borgel, V.; Ziv, B.; Leifer, N.; Dargel, V.; Aurbach, D.; Kim, J.H.; Liu, Z.Y.; Huang, X.S.; Krachkovskiy, S.A.; et al. Lithium Polyacrylate (LiPAA) as an Advanced Binder and a Passivating Agent for High-Voltage Li-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501008. [Google Scholar] [CrossRef]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Wood, S.M.; Pham, C.H.; Rodriguez, R.; Nathan, S.S.; Dolocan, A.D.; Celio, H.; de Souza, J.P.; Klavetter, K.C.; Heller, A.; Mullins, C.B. K+ Reduces Lithium Dendrite Growth by Forming a Thin, Less-Resistive Solid Electrolyte Interphase. ACS Energy Lett. 2016, 1, 414–419. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Chen, X.D. Artificial interphase engineering of electrode materials to improve the overall performance of lithium-ion batteries. Nano Res. 2017, 10, 4115–4138. [Google Scholar] [CrossRef]
- Chi, S.S.; Liu, Y.C.; Song, W.L.; Fan, L.Z.; Zhang, Q. Prestoring Lithium into Stable 3D Nickel Foam Host as Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2017, 27, 1700348. [Google Scholar] [CrossRef]
- Deng, K.R.; Zeng, Q.G.; Wang, D.; Liu, Z.; Wang, G.X.; Qiu, Z.P.; Zhang, Y.F.; Xiao, M.; Meng, Y.Z. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, X.; Ding, F.; Huang, J.; Xu, R.; Chen, X.; Yan, C.; Su, F.; Chen, C.; Liu, X.; et al. Advanced Electrode Materials in Lithium Batteries: Retrospect and Prospect. Energy Mater. Adv. 2021, 2021, 1205324. [Google Scholar] [CrossRef]
- Zhao, B.L.; Ma, L.X.; Wu, K.; Cao, M.X.; Xu, M.G.; Zhang, X.X.; Liu, W.; Chen, J.T. Asymmetric double-layer composite electrolyte with enhanced ionic conductivity and interface stability for all-solid-state lithium metal batteries. Chin. Chem. Lett. 2021, 32, 125–131. [Google Scholar] [CrossRef]
- Zheng, J.; Tang, M.X.; Hu, Y.Y. Lithium Ion Pathway within Li7La3Zr2O12-Polyethylene Oxide Composite Electrolytes. Angew. Chem.-Int. Ed. 2016, 55, 12538–12542. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Shen, L.; Deng, S.G.; Cui, P.; Yao, X.Y. 10 μm-Thick High-Strength Solid Polymer Electrolytes with Excellent Interface Compatibility for Flexible All-Solid-State Lithium-Metal Batteries. Adv. Mater. 2021, 33, 2100353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, H.; Li, D.; Li, Y.; Zeng, Z.; Ji, F.; Wei, Y.; Xu, X.; Sun, Q.; Wang, S.; et al. Agglomeration-Free and Air-Inert Garnet for Upgrading PEO/Garnet Composite Solid State Electrolyte. Batteries 2022, 8, 141. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhao, S.Y.; Fan, W.; Wang, J.N.; Li, C.J. Long cycling, thermal stable, dendrites free gel polymer electrolyte for flexible lithium metal batteries. Electrochim. Acta 2019, 301, 304–311. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Liu, H.D.; Li, Y.J.; Yue, X.J.; Wang, X.F.; Gonzalez, M.; Meng, Y.S.; Liu, P. In situ formed polymer gel electrolytes for lithium batteries with inherent thermal shutdown safety features. J. Mater. Chem. A 2019, 7, 16984–16991. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Xia, X.H.; Xie, D.; Xu, R.C.; Xu, Y.J.; Xia, Y.; Wu, J.B.; Yao, Z.J.; Wang, X.L.; Tu, J.P. Facile interfacial modification via in-situ ultraviolet solidified gel polymer electrolyte for high-performance solid-state lithium ion batteries. J. Power Sources 2019, 409, 31–37. [Google Scholar] [CrossRef]
- Xu, D.; Su, J.M.; Jin, J.; Sun, C.; Ruan, Y.D.; Chen, C.H.; Wen, Z.Y. In Situ Generated Fireproof Gel Polymer Electrolyte with Li6.4Ga0.2La3Zr2O12 As Initiator and Ion-Conductive Filler. Adv. Energy Mater. 2019, 9, 1900611. [Google Scholar] [CrossRef]
- Cho, Y.G.; Hwang, C.; Cheong, D.S.; Kim, Y.S.; Song, H.K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Liu, X.; Yu, B.Z.; Mateti, S.; O’Dell, L.A.; Rong, Q.Z.; Chen, Y. Amine-Functionalized Boron Nitride Nanosheets: A New Functional Additive for Robust, Flexible Ion Gel Electrolyte with High Lithium-Ion Transference Number. Adv. Funct. Mater. 2020, 30, 1910813. [Google Scholar] [CrossRef]
- Liu, F.Q.; Wang, W.P.; Yin, Y.X.; Zhang, S.F.; Shi, J.L.; Wang, L.; Zhang, X.D.; Zheng, Y.; Zhou, J.J.; Li, L.; et al. Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries. Sci. Adv. 2018, 4, eaat5383. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Li, M.; Li, G.Q.; Li, B.; Zhang, S.T.; Ming, H.; Qiu, J.Y.; Chen, J.H.; Zhao, P.C. A new composite gel polymer electrolyte based on matrix of PEGDA with high ionic conductivity for lithium-ion batteries. Electrochim. Acta 2020, 354, 136622. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.T.; Stalin, S.; Khan, K.; Archer, L.A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 2019, 4, 365–373. [Google Scholar] [CrossRef]
- Liu, X.C.; Ding, G.L.; Zhou, X.H.; Li, S.Z.; He, W.S.; Chai, J.C.; Pang, C.G.; Liu, Z.H.; Cui, G.L. An interpenetrating network poly(diethylene glycol carbonate)-based polymer electrolyte for solid state lithium batteries. J. Mater. Chem. A 2017, 5, 11124–11130. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Feng, Y.Y.; Wang, J.L.; Liang, B.; Li, Y.H.; Song, Z.X.; Itkis, D.M.; Song, J.X. A robust, highly stretchable ion-conducive skin for stable lithium metal batteries. Chem. Eng. J. 2020, 396, 125254. [Google Scholar] [CrossRef]
- Fan, W.; Li, N.W.; Zhang, X.L.; Zhao, S.Y.; Cao, R.; Yin, Y.Y.; Xing, Y.; Wang, J.N.; Guo, Y.G.; Li, C.J. A Dual-Salt Gel Polymer Electrolyte with 3D Cross-Linked Polymer Network for Dendrite-Free Lithium Metal Batteries. Adv. Sci. 2018, 5, 1800559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Xu, X.Q.; Hong, B.; Bai, M.H.; Li, J.; Zhang, Z.; Lai, Y.Q. Molecular engineering of a gel polymer electrolyte via in-situ polymerization for high performance lithium metal batteries. Chem. Eng. J. 2022, 428, 131331. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, L.; Shi, L.; Wang, Z.; Zhu, J.; Zhao, Y.; Yuan, S. Gel Polymer Electrolyte with High Li+ Transference Number Enhancing the Cycling Stability of Lithium Anodes. ACS Appl. Mater. Interfaces 2019, 11, 5168–5175. [Google Scholar] [CrossRef]
- Xu, H.; Xie, J.; Liu, Z.; Wang, J.; Deng, Y. Carbonyl-coordinating polymers for high-voltage solid-state lithium batteries: Solid polymer electrolytes. MRS Energy Sustain. 2020, 7, 2. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, X.; Wang, Z.; Wang, B.; He, S.; O’Dell, L.A.; Huang, J.; Li, H.; Yu, H.; Chen, L. A wide-temperature superior ionic conductive polymer electrolyte for lithium metal battery. Nano Energy 2020, 73, 104786. [Google Scholar] [CrossRef]
- Shen, L.; Deng, S.G.; Jiang, R.R.; Liu, G.Z.; Yang, J.; Yao, X.Y. Flexible composite solid electrolyte with 80 wt% Na3.4Zr1.9Zn0.1Si2.2P0.8O12 for solid-state sodium batteries. Energy Storage Mater. 2022, 46, 175–181. [Google Scholar] [CrossRef]
- Zhou, W.D.; Wang, S.F.; Li, Y.T.; Xin, S.; Manthiram, A.; Goodenough, J.B. Plating a Dendrite-Free Lithium Anode with a Polymer/Ceramic/Polymer Sandwich Electrolyte. J. Am. Chem. Soc. 2016, 138, 9385–9388. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, H.J.; Zhu, X.Y.; He, P.; Zhou, H.S. A stable quasi-solid electrolyte improves the safe operation of highly efficient lithium-metal pouch cells in harsh environments. Nat. Commun. 2022, 13, 1510. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, Z.; Wu, J.; Gu, Z.; Xin, X.; Yao, X. In Situ Solidified Gel Polymer Electrolytes for Stable Solid−State Lithium Batteries at High Temperatures. Batteries 2023, 9, 28. https://doi.org/10.3390/batteries9010028
Ma J, Wang Z, Wu J, Gu Z, Xin X, Yao X. In Situ Solidified Gel Polymer Electrolytes for Stable Solid−State Lithium Batteries at High Temperatures. Batteries. 2023; 9(1):28. https://doi.org/10.3390/batteries9010028
Chicago/Turabian StyleMa, Junfeng, Zhiyan Wang, Jinghua Wu, Zhi Gu, Xing Xin, and Xiayin Yao. 2023. "In Situ Solidified Gel Polymer Electrolytes for Stable Solid−State Lithium Batteries at High Temperatures" Batteries 9, no. 1: 28. https://doi.org/10.3390/batteries9010028
APA StyleMa, J., Wang, Z., Wu, J., Gu, Z., Xin, X., & Yao, X. (2023). In Situ Solidified Gel Polymer Electrolytes for Stable Solid−State Lithium Batteries at High Temperatures. Batteries, 9(1), 28. https://doi.org/10.3390/batteries9010028





