Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage
Abstract
:1. Introduction
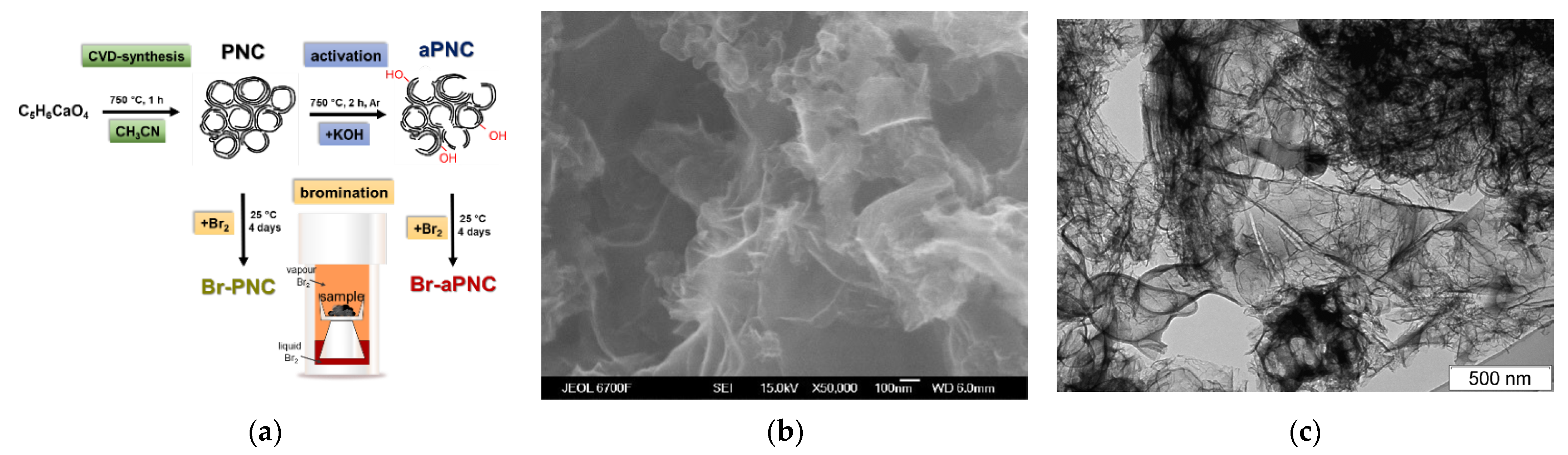
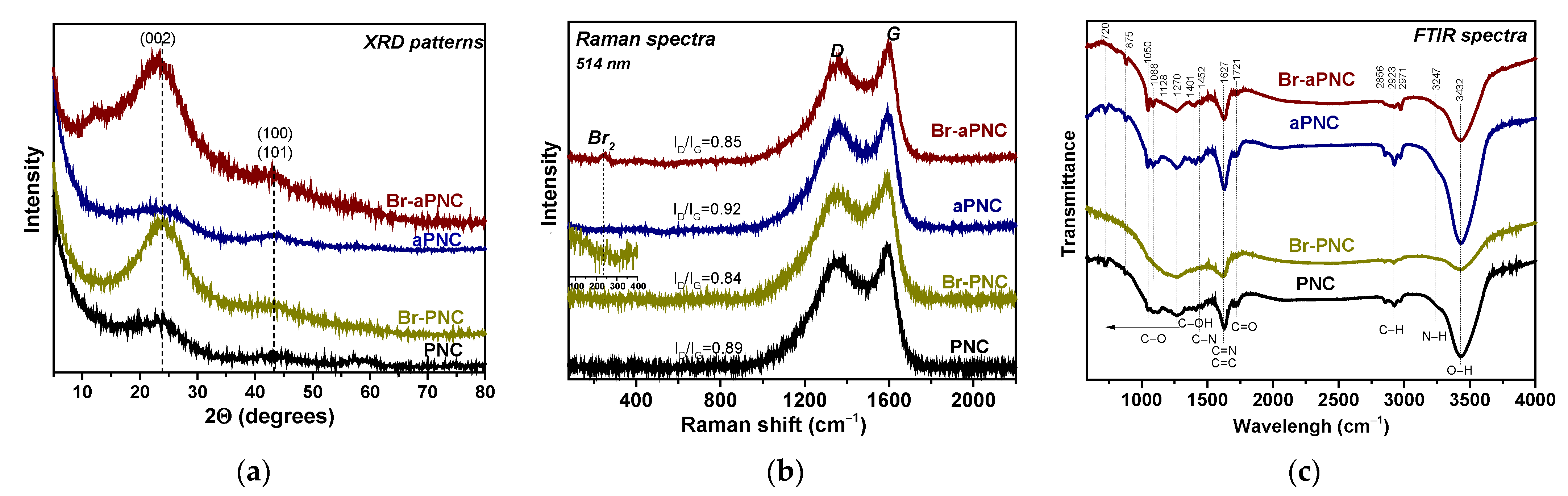
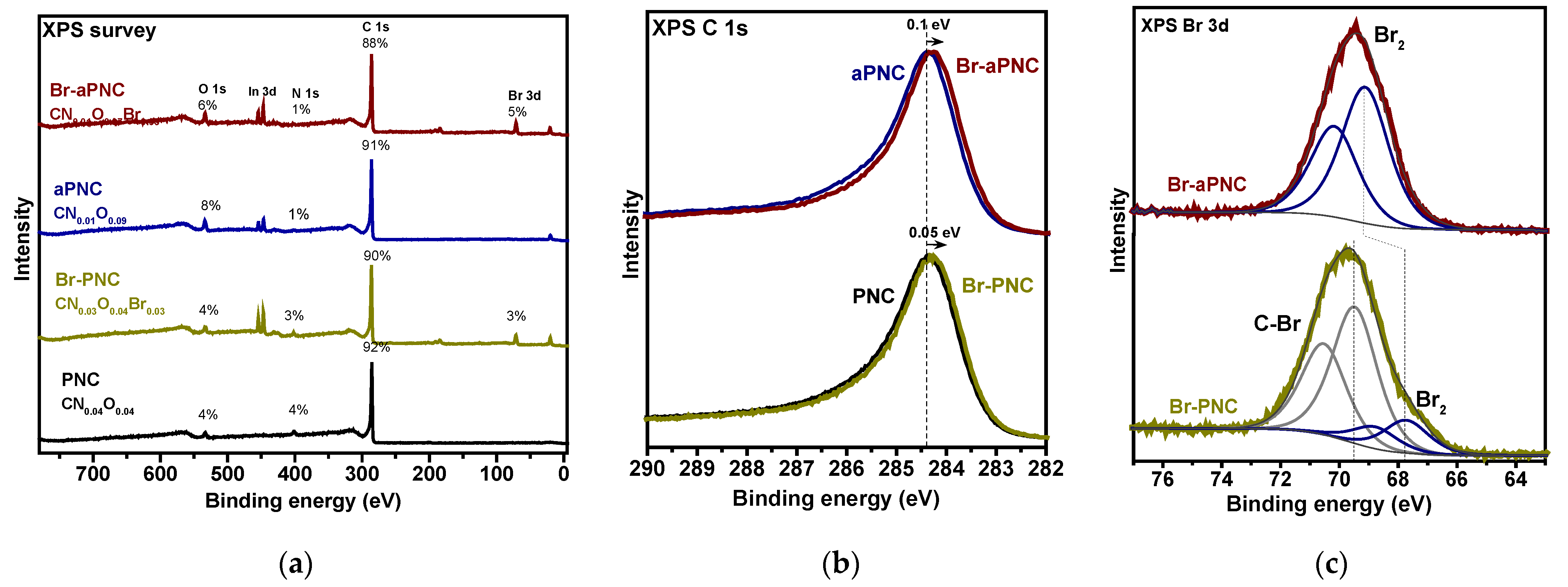
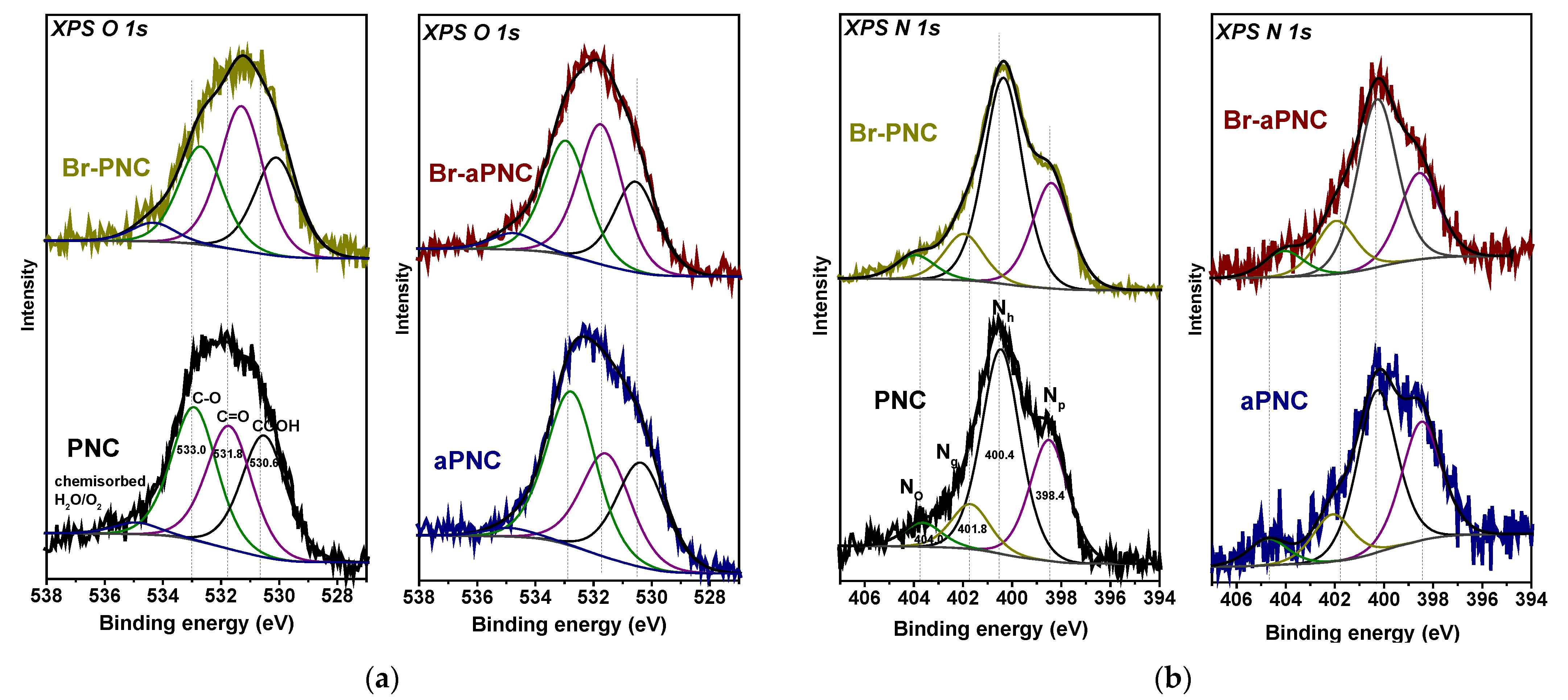
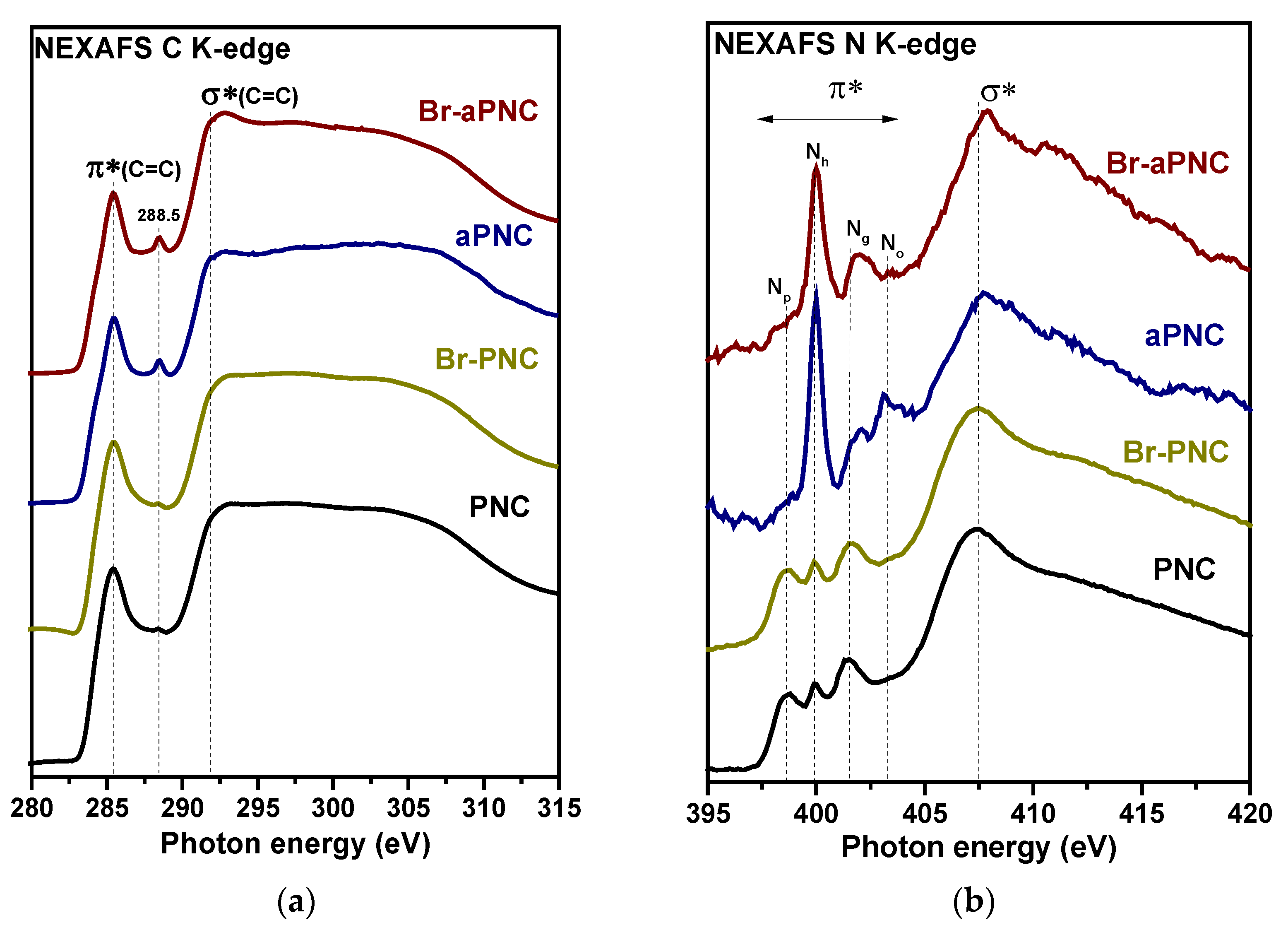
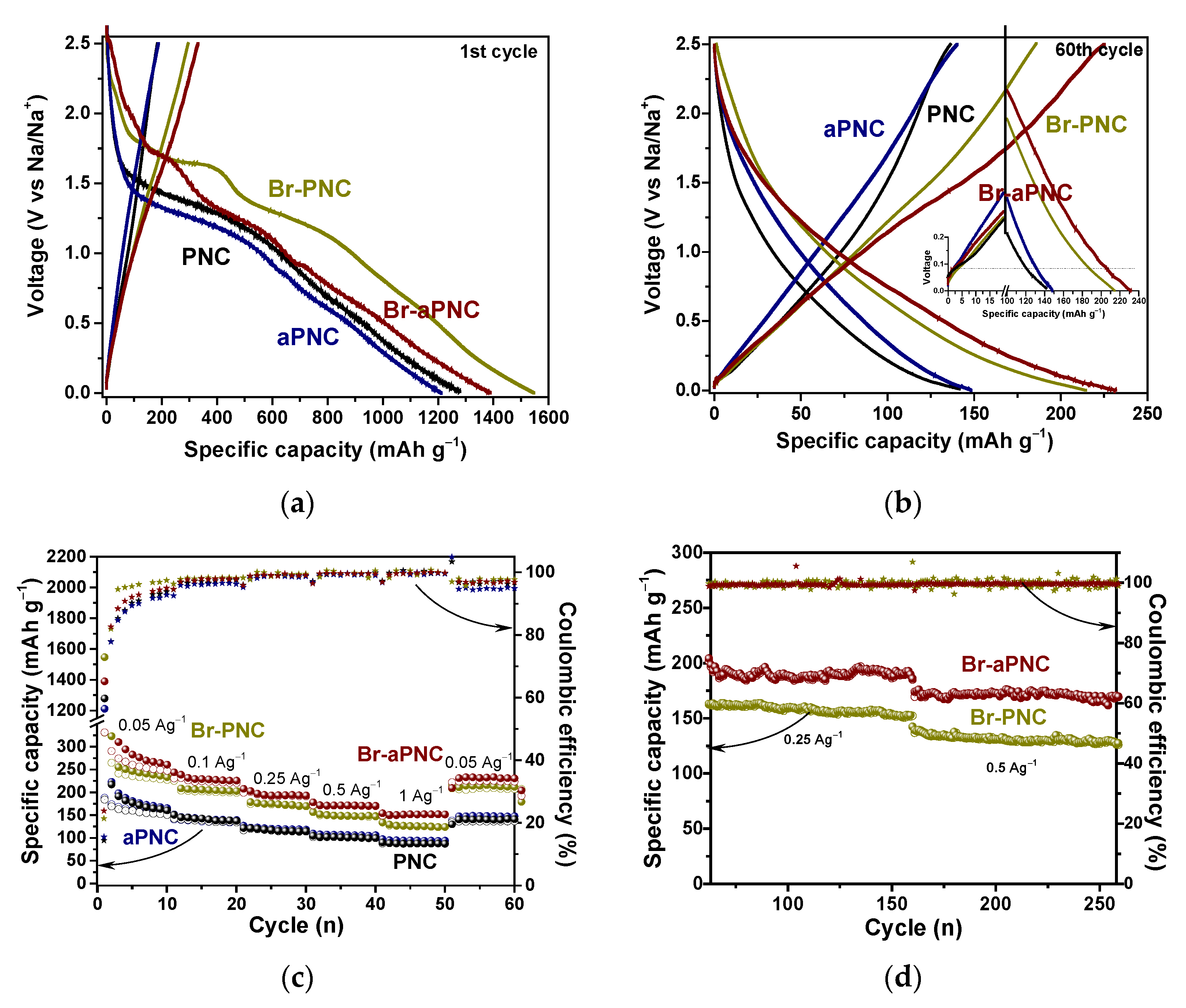
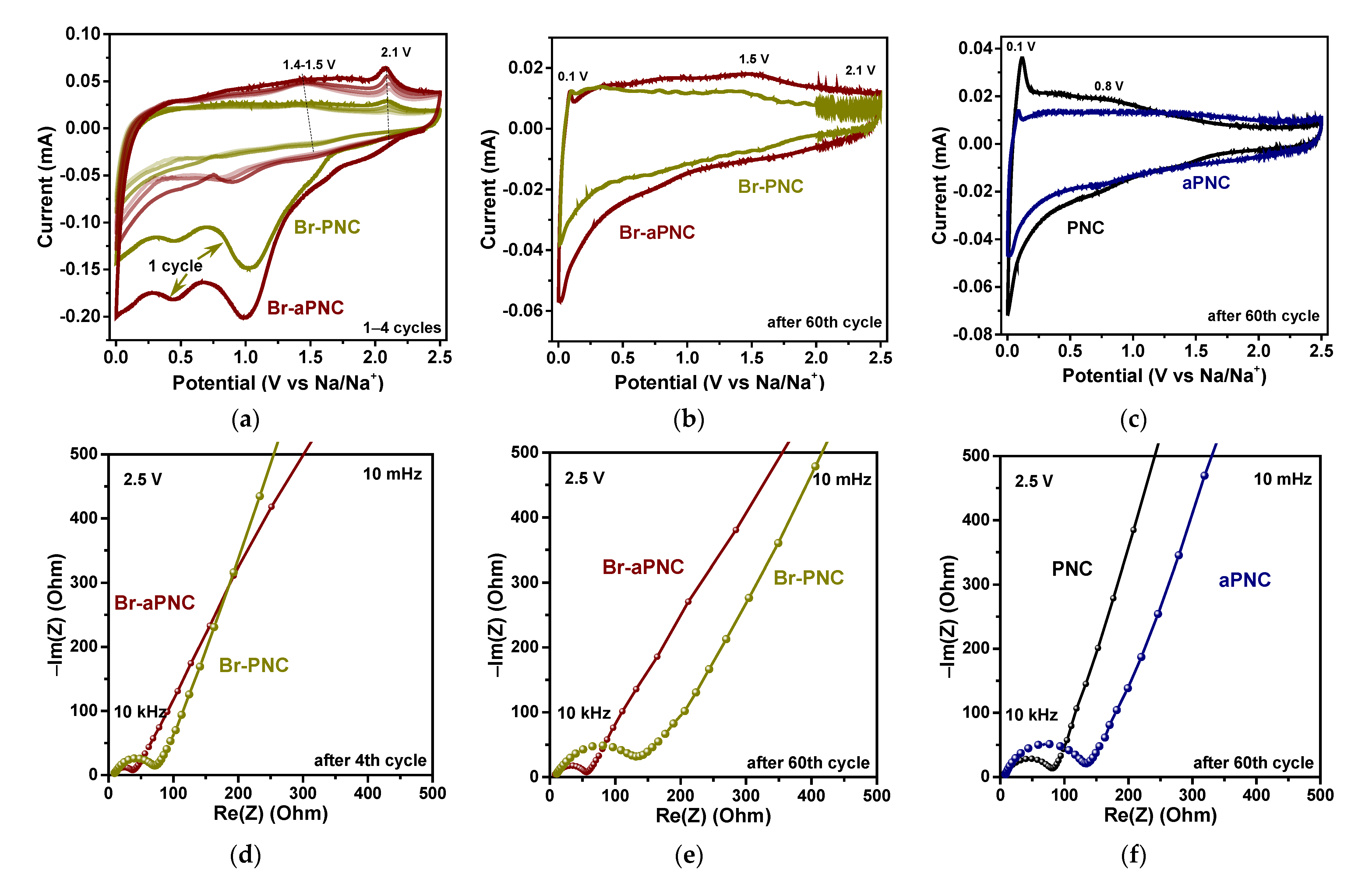
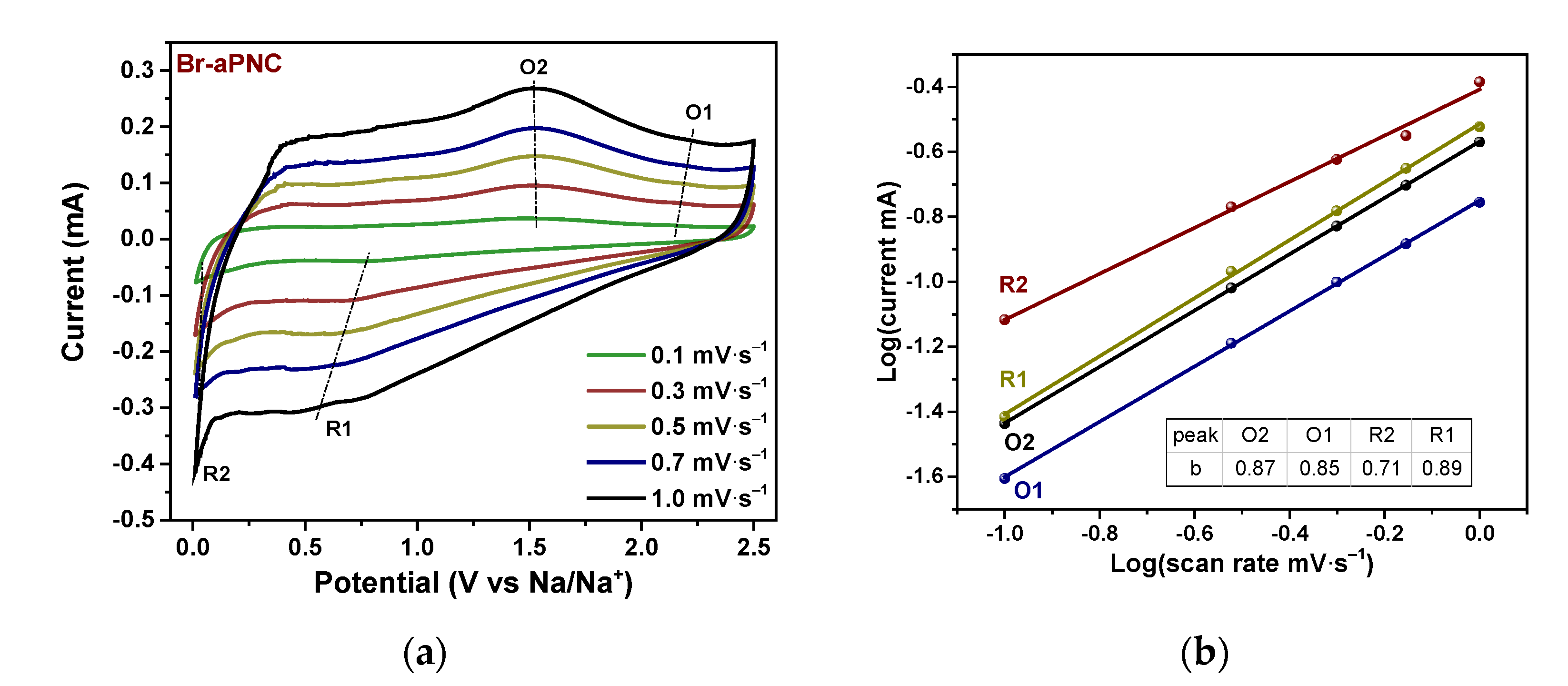
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Characterisation
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A brief review of post-lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Wang, F.; Noori, A.; Mousavi, M.F.; Xia, X.; Zhan, Y. Recent advances in carbon anodes for sodium-ion batteries. Chem. Rec. 2022, e202200083. [Google Scholar] [CrossRef] [PubMed]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. Nanostructured electrode materials for advanced sodium-ion batteries. Matter 2019, 1, 90–114. [Google Scholar] [CrossRef]
- Ge, P.; Fouletier, M. Electrochemical intercalation of sodium in graphite. Solid State Ionics 1988, 28–30, 1172–1175. [Google Scholar] [CrossRef]
- Li, Z.; Bommier, C.; Chong, Z.S.; Jian, Z.; Surta, T.W.; Wang, X.; Xing, Z.; Neuefeind, J.C.; Stickle, W.F.; Dolgos, M.; et al. Mechanism of Na-ion storage in hard carbon anodes revealed by heteroatom doping. Adv. Energy Mater. 2017, 7, 1602894. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An initial review of the status of electrode materials for potassium-ion. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High capacity anode materials for rechargeable sodium-ion batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Cao, B.; Liu, H.; Xu, B.; Lei, Y.; Chen, X.; Song, H. Mesoporous soft carbon as an anode material for sodium ion batteries with superior rate and cycling performance. J. Mater. Chem. A 2016, 4, 6472–6478. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef] [PubMed]
- Alvira, D.; Antorán, D.; Manyà, J.J. Plant-derived hard carbon as anode for sodium-ion batteries: A comprehensive review to guide interdisciplinary research. Chem. Eng. J. 2022, 447, 137468. [Google Scholar] [CrossRef]
- Tsai, P.; Chung, S.-C.; Lin, S.; Yamada, A. Ab initio study of sodium intercalation into disordered carbon. J. Mater. Chem. A 2015, 3, 9763–9768. [Google Scholar] [CrossRef]
- Yang, C.; Sun, X.; Zhang, X.; Li, J.; Ma, J.; Li, Y.; Xu, L.; Liu, S.; Yang, J.; Fang, S.; et al. Is graphite nanomesh a promising anode for the Na/K-ions batteries? Carbon 2021, 176, 242–252. [Google Scholar] [CrossRef]
- Yuan, M.; Cao, B.; Liu, H.; Meng, C.; Wu, J.; Zhang, S.; Li, A.; Chen, X.; Song, H. Sodium storage mechanism of nongraphitic carbons: A general model and the function of accessible closed pores. Chem. Mater. 2022, 34, 3489–3500. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Guan, Z.; Tong, Y.; Xu, B.; Wang, X.; Wang, Z.; Chen, L. Insights into lithium and sodium storage in porous carbon. Nano Lett. 2020, 20, 3836–3843. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Shi, G.-S.; Wang, Z.-G.; Zhao, J.-J.; Hu, J.; Fang, H.-P. Adsorption of sodium ions and hydrated sodium ions on a hydrophobic graphite surface via cation-π interactions. Chin. Phys. B 2011, 20, 068101. [Google Scholar] [CrossRef]
- Xiao, B.; Rojo, T.; Li, X. Hard carbon as sodium-ion battery anodes progress and challenges. ChemSusChem 2019, 12, 133–144. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Agrawal, A.; Janakiraman, S.; Biswas, K.; Venimadhav, A.; Srivastava, S.K.; Ghosh, S. Understanding the improved electrochemical performance of nitrogen-doped hard carbons as an anode for sodium ion battery. Electrochim. Acta 2019, 317, 164–172. [Google Scholar] [CrossRef]
- Vu, N.H.; Le, H.T.T.; Hoang, V.H.; Dao, V.D.; Huu, H.T.; Jun, Y.S.; Im, W. Bin highly N-doped, H-containing mesoporous carbon with modulated physicochemical properties as high-performance anode materials for Li-ion and Na-ion batteries. J. Alloys Compd. 2021, 851, 156881. [Google Scholar] [CrossRef]
- Shlyakhova, E.V.; Bulusheva, L.G.; Kanygin, M.A.; Plyusnin, P.E.; Kovalenko, K.A.; Senkovskiy, B.V.; Okotrub, A.V. Synthesis of nitrogen-containing porous carbon using calcium oxide nanoparticles. Phys. Status Solidi 2014, 251, 2607–2612. [Google Scholar] [CrossRef]
- Nishchakova, A.D.; Grebenkina, M.A.; Shlyakhova, E.V.; Shubin, Y.V.; Kovalenko, K.A.; Asanov, I.P.; Fedoseeva, Y.V.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Porosity and composition of nitrogen-doped carbon materials templated by the thermolysis products of calcium tartrate and their performance in electrochemical capacitors. J. Alloys Compd. 2021, 858, 158259. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Fluorine-doped hard carbon as the advanced performance anode material of sodium-ion batteries. Trans. Tianjin Univ. 2022, 28, 123–131. [Google Scholar] [CrossRef]
- Hong, S.M.; Etacheri, V.; Hong, C.N.; Choi, S.W.; Lee, K.B.; Pol, V.G. Enhanced lithium- and sodium-ion storage in an interconnected carbon network comprising electronegative fluorine. ACS Appl. Mater. Interfaces 2017, 9, 18790–18798. [Google Scholar] [CrossRef]
- Stolyarova, S.G.; Fedoseeva, Y.V.; Baskakova, K.I.; Vorfolomeeva, A.A.; Shubin, Y.V.; Makarova, A.A.; Bulusheva, L.G.; Okotrub, A.V. Bromination of carbon nanohorns to improve sodium-ion storage performance. Appl. Surf. Sci. 2022, 580, 152238. [Google Scholar] [CrossRef]
- Hamdan, R.; Kemper, A.F.; Cao, C.; Cheng, H.P. Structure and functionality of bromine doped graphite. J. Chem. Phys. 2013, 138, 164702. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.; Ma, J. Influence of the pore structure and surface chemistry on adsorption of ethylbenzene and xylene isomers by KOH-activated multi-walled carbon nanotubes. J. Hazard. Mater. 2012, 237–238, 102–109. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, F.; Dou, Y.; Zhai, Y.; Wang, J.; Liu, H.; Xia, Y.; Tu, B.; Zhao, D. A comprehensive study on KOH activation of ordered mesoporous carbons and their supercapacitor application. J. Mater. Chem. 2012, 22, 93–99. [Google Scholar] [CrossRef]
- Williams, N.E.; Oba, O.A.; Aydinlik, N.P. Modification, production and methods of KOH-activated carbon. ChemBioEng Rev. 2022, 9, 164–189. [Google Scholar] [CrossRef]
- Sevilla, M.; Gu, W.; Falco, C.; Titirici, M.M.; Fuertes, A.B.; Yushin, G. Hydrothermal synthesis of microalgae-derived microporous carbons for electrochemical capacitors. J. Power Sources 2014, 267, 26–32. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Wang, X.; Cao, M. Enhancement of lithium storage performance of carbon microflowers by achieving a high surface area. Chem. An Asian J. 2014, 9, 1957–1963. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Gevko, P.N.; Koroteev, V.O.; Fedoseeva, Y.V.; Yaya, A.; Ewels, C.P. Bromination of double-walled carbon nanotubes. Chem. Mater. 2012, 24, 2708–2715. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Lobiak, E.V.; Fedoseeva, Y.V.; Mevellec, J.Y.; Makarova, A.A.; Flahaut, E.; Okotrub, A.V. Effect of ultrasound pretreatment on bromination of double-walled carbon nanotubes. Synth. Met. 2020, 259, 116233. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Ewels, C.P.; Pinakov, D.V.; Chekhova, G.N.; Flahaut, E.; Okotrub, A.V.; Bulusheva, L.G. Bromine polycondensation in pristine and fluorinated graphitic carbons. Nanoscale 2019, 11, 15298–15306. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Lobiak, E.V.; Shlyakhova, E.V.; Kovalenko, K.A.; Kuznetsova, V.R.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Nishchakova, A.D.; Makarova, A.A.; Bulusheva, L.G.; et al. Hydrothermal activation of porous nitrogen-doped carbon materials for electrochemical capacitors and sodium-ion batteries. Nanomaterials 2020, 10, 2163. [Google Scholar] [CrossRef]
- Geng, D.; Yang, S.; Zhang, Y.; Yang, J.; Liu, J.; Li, R.; Sham, T.K.; Sun, X.; Ye, S.; Knights, S. Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 2011, 257, 9193–9198. [Google Scholar] [CrossRef]
- Pan, N.; Guan, D.; Yang, Y.; Huang, Z.; Wang, R.; Jin, Y.; Xia, C. A rapid low-temperature synthetic method leading to large-scale carboxyl graphene. Chem. Eng. J. 2014, 236, 471–479. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Brito, F.S.; Franceschi, W.; Simonetti, E.A.N.; Cividanes, L.S.; Chipara, M.; Lozano, K. Functionalized graphene oxide as reinforcement in epoxy based nanocomposites. Surfaces Interfaces 2018, 10, 100–109. [Google Scholar] [CrossRef]
- Yao, Y.; Velpari, V.; Economy, J. In search of brominated activated carbon fibers for elemental mercury removal from power plant effluents. J. Mater. Chem. A 2013, 1, 12103–12108. [Google Scholar] [CrossRef]
- Papirer, E.; Lacroix, R.; Donnet, J.-B.; Nanse, G.; Fioux, P. XPS study of the halogenation of carbon black-Part 1. Bromination. Carbon 1994, 32, 1341–1358. [Google Scholar] [CrossRef]
- Lippitz, A.; Friedrich, J.F.; Unger, W.E.S. Plasma bromination of HOPG surfaces: A NEXAFS and synchrotron XPS study. Surf. Sci. 2013, 611, L1–L7. [Google Scholar] [CrossRef]
- Colomer, J.-F.; Marega, R.; Traboulsi, H.; Meneghetti, M.; Van Tendeloo, G.; Bonifazi, D. Microwave-assisted bromination of double-walled carbon nanotubes. Chem. Mater. 2009, 21, 4747–4749. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour. Technol. Rep. 2019, 7, 100266. [Google Scholar] [CrossRef]
- Lapteva, L.L.; Fedoseeva, Y.V.; Shlyakhova, E.V.; Makarova, A.A.; Bulusheva, L.G.; Okotrub, A.V. NEXAFS spectroscopy study of lithium interaction with nitrogen incorporated in porous graphitic material. J. Mater. Sci. 2019, 54, 11168–11178. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Kanygin, M.A.; Arkhipov, V.E.; Popov, K.M.; Fedoseeva, Y.V.; Smirnov, D.A.; Okotrub, A.V. In situ X-ray photoelectron spectroscopy study of lithium interaction with graphene and nitrogen-doped graphene films produced by chemical vapor deposition. J. Phys. Chem. C 2017, 121, 5108–5114. [Google Scholar] [CrossRef]
- Aarva, A.; Deringer, V.L.; Sainio, S.; Laurila, T.; Caro, M.A. Understanding X-ray spectroscopy of carbonaceous materials by combining experiments, density functional theory, and machine learning. Part I: Fingerprint spectra. Chem. Mater. 2019, 31, 9243–9255. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Donnet, C.; Lefèbvre, F.; Fernéndez-Ramos, C.; Fernández, A. Bonding structure in amorphous carbon nitride: A spectroscopic and nuclear magnetic resonance study. J. Appl. Phys. 2001, 90, 675–681. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, X.; Bai, Y.; Yang, H.; Dong, R.; Wang, X.; Xu, H.; Wang, Q.; Li, H.; Gao, H.; et al. Probing the energy storage mechanism of quasi-metallic Na in hard carbon for sodium-ion batteries. Adv. Energy Mater. 2021, 11, 2003854. [Google Scholar] [CrossRef]
- Liang, H.J.; Gu, Z.Y.; Zheng, X.Y.; Li, W.H.; Zhu, L.Y.; Sun, Z.H.; Meng, Y.F.; Yu, H.Y.; Hou, X.K.; Wu, X.L. Tempura-like carbon/carbon composite as advanced anode materials for K-Ion batteries. J. Energy Chem. 2021, 59, 589–598. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Liu, Y.; Bai, Y.; Chen, G.; Li, Y.; Wang, X.; Xu, H.; Wu, C.; Lu, J. Analysis of the stable interphase responsible for the excellent electrochemical performance of graphite electrodes in sodium-ion batteries. Small 2020, 16, 2003268. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective—guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Come, J.; Taberna, P.-L.; Hamelet, S.; Masquelier, C.; Simon, P. Electrochemical kinetic study of LiFePO4 using cavity microelectrode. J. Electrochem. Soc. 2011, 158, A1090. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoseeva, Y.V.; Shlyakhova, E.V.; Stolyarova, S.G.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Makarova, A.A.; Shubin, Y.V.; Okotrub, A.V.; Bulusheva, L.G. Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage. Batteries 2022, 8, 114. https://doi.org/10.3390/batteries8090114
Fedoseeva YV, Shlyakhova EV, Stolyarova SG, Vorfolomeeva AA, Grebenkina MA, Makarova AA, Shubin YV, Okotrub AV, Bulusheva LG. Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage. Batteries. 2022; 8(9):114. https://doi.org/10.3390/batteries8090114
Chicago/Turabian StyleFedoseeva, Yuliya V., Elena V. Shlyakhova, Svetlana G. Stolyarova, Anna A. Vorfolomeeva, Mariya A. Grebenkina, Anna A. Makarova, Yuriy V. Shubin, Alexander V. Okotrub, and Lyubov G. Bulusheva. 2022. "Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage" Batteries 8, no. 9: 114. https://doi.org/10.3390/batteries8090114
APA StyleFedoseeva, Y. V., Shlyakhova, E. V., Stolyarova, S. G., Vorfolomeeva, A. A., Grebenkina, M. A., Makarova, A. A., Shubin, Y. V., Okotrub, A. V., & Bulusheva, L. G. (2022). Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage. Batteries, 8(9), 114. https://doi.org/10.3390/batteries8090114






