Zinc as a Promising Anodic Material for All-Solid-State Lithium-Ion Batteries
Abstract
:1. Introduction
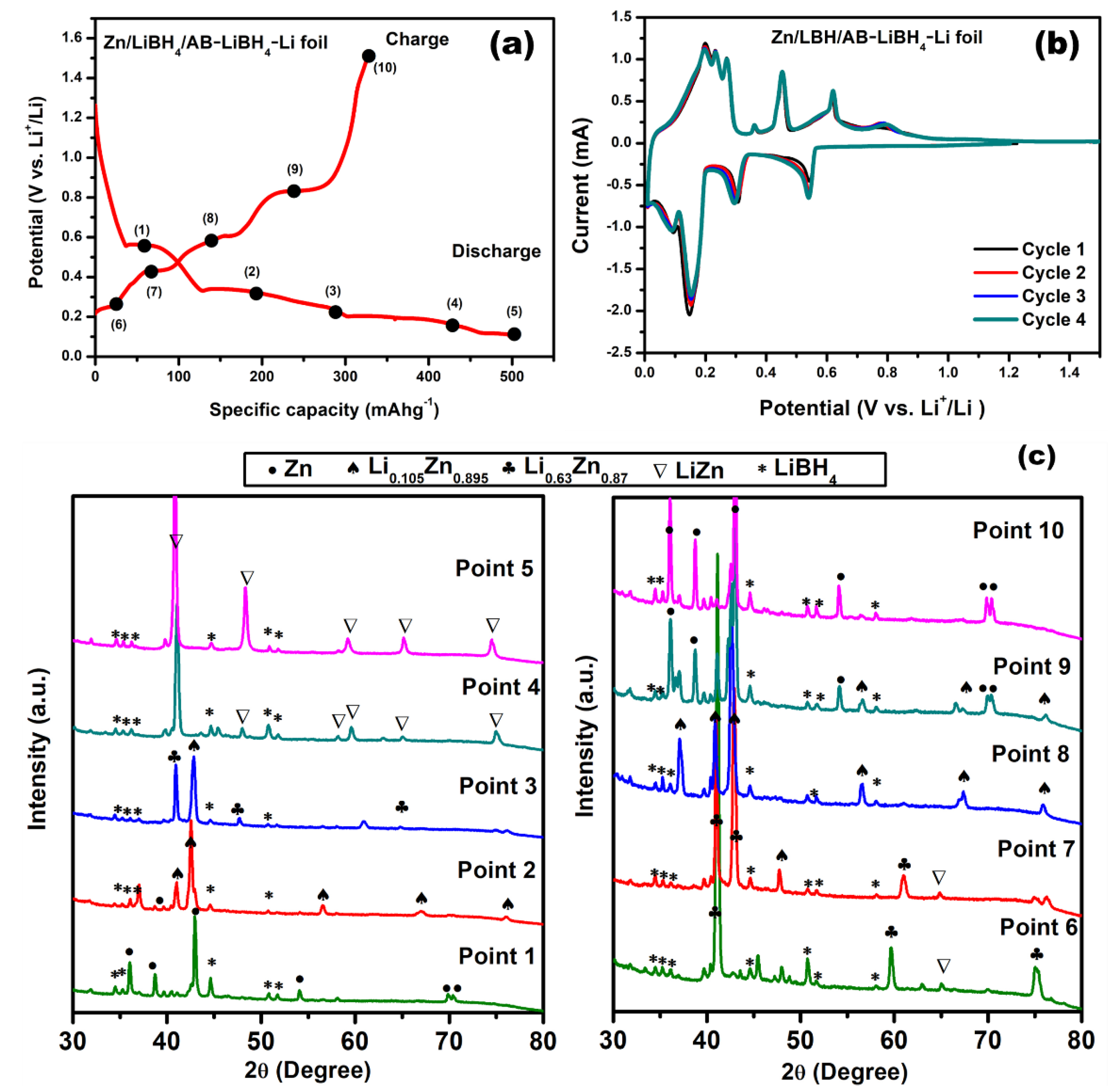
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Gross, R.; Leach, M.; Bauen, A. Progress in renewable energy. Environ. Int. 2003, 29, 105–122. [Google Scholar] [CrossRef]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Bull, S.R. Renewable energy today and tomorrow. Proc. IEEE 2001, 89, 1216–1226. [Google Scholar] [CrossRef]
- Harjanne, A.; Korhonen, J.M. Abandoning the concept of renewable energy. Energy Policy 2019, 127, 330–340. [Google Scholar] [CrossRef]
- Doughty, D.H.; Roth, E.P. A general discussion of Li ion battery safety. Electrochem. Soc. Interface 2012, 21, 37. [Google Scholar]
- Edstroem, K.; Gustafsson, T.; Thomas, J.O. The cathode–electrolyte interface in the Li-ion battery. Electrochim. Acta 2004, 50, 397–403. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Gao, H. A perspective on the Li-ion battery. Sci. China Chem. 2019, 62, 1555–1556. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Luntz, A.C.; Voss, J.; Reuter, K. Interfacial challenges in solid-state Li ion batteries. J. Phys. Chem. Lett. 2015, 6, 4599–4604. [Google Scholar] [CrossRef] [Green Version]
- Danilov, D.; Niessen, R.A.H.; Notten, P.H.L. Modeling all-solid-state Li-ion batteries. J. Electrochem. Soc. 2010, 158, A215. [Google Scholar] [CrossRef]
- Kim, D.H.; Oh, D.Y.; Park, K.H.; Choi, Y.E.; Nam, Y.J.; Lee, H.A.; Lee, S.-M.; Jung, Y.S. Infiltration of solution-processable solid electrolytes into conventional Li-ion-battery electrodes for all-solid-state Li-ion batteries. Nano Lett. 2017, 17, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liao, K.; Lu, Q.; Shao, Z. Recent advances in the interface engineering of solid-state Li-ion batteries with artificial buffer layers: Challenges, materials, construction, and characterization. Energy Environ. Sci. 2019, 2, 1780–1804. [Google Scholar] [CrossRef]
- Van Den Broek, J.; Afyon, S.; Rupp, J.L.M. Interface-engineered all-solid-state Li-ion batteries based on garnet-type fast Li+ conductors. Adv. Energy Mater. 2016, 6, 1600736. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Raj, R.; Wolfenstine, J. Current limit diagrams for dendrite formation in solid-state electrolytes for Li-ion batteries. J. Power Sources 2017, 343, 119–126. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, S.-B.; Liao, Y.-K.; Hu, S.-F.; Liu, R.-S. Interface between solid-state electrolytes and Li-metal anodes: Issues, materials, and processing routes. ACS Appl. Mater. Interfaces 2020, 12, 47181–47196. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan., A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Feng, Z.; Guerfi, A.; Vijh, A.; Zaghib, K. Recent progress in sulfide-based solid electrolytes for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Takada, K.; Inada, T.; Kajiyama, A.; Kouguchi, M.; Sasaki, H.; Kondo, S.; Michiue, Y.; Nakano, S.; Tabuchi, M.; Watanabe, M. Solid state batteries with sulfide-based solid electrolytes. Solid State Ion. 2004, 172, 25–30. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Sørby, M.H.; Nygård, M.M.; Hauback, B.C. Borohydride-based Solid-state Electrolytes for Lithium Batteries. In Proceedings of the IEEE 2019 7th International Renewable and Sustainable Energy Conference (IRSEC), Agadir, Morocco, 27–30 November 2019; pp. 1–4. [Google Scholar]
- Zhang, T.; He, W.; Zhang, W.; Wang, T.; Li, P.; Sun, Z.; Yu, X. Designing composite solid-state electrolytes for high performance lithium ion or lithium metal batteries. Chem. Sci. 2020, 11, 8686–8707. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, J.; Li, X.; Holmes, N.; Wang, C.; Wang, J.; Zhao, F.; Li, S.; Sun, Q.; Yang, X.; et al. Halide-based solid-state electrolyte as an interfacial modifier for high performance solid-state Li–O2 batteries. Nano Energy 2020, 75, 105036. [Google Scholar] [CrossRef]
- Park, K.-H.; Kaup, K.; Assoud, A.; Zhang, Q.; Wu, X.; Nazar, L.F. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 2020, 5, 533–539. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Yang, X.; Adair, K.R.; Wang, C.; Zhao, F.; Sun, X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 1429–1461. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Oguchi, H.; Toyama, N.; Orimo, S. Interfacial stability between LiBH4-based complex hydride solid electrolytes and Li metal anode for all-solid-state Li batteries. J. Power Sources 2019, 436, 226821. [Google Scholar] [CrossRef]
- Unemoto, A.; Yasaku, S.; Nogami, G.; Tazawa, M.; Taniguchi, M.; Matsuo, M.; Ikeshoji, T.; Orimo, S.-I. Development of bulk-type all-solid-state lithium-sulfur battery using LiBH4 electrolyte. Appl. Phys. Lett. 2014, 105, 83901. [Google Scholar] [CrossRef]
- Takahashi, K.; Hattori, K.; Yamazaki, T.; Takada, K.; Matsuo, M.; Orimo, S.; Maekawa, H.; Takamura, H. All-solid-state lithium battery with LiBH4 solid electrolyte. J. Power Sources 2013, 226, 61–64. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, R.; Tripathi, B.; Ichikawa, T.; Kumar, M.; Jain, A. All-Solid-State Li-Ion Batteries Using a Combination of Sb2S3/Li2S-P2S5/Acetylene Black as the Electrode Composite and LiBH4 as the Electrolyte. ACS Appl. Energy Mater. 2021, 4, 6269–6276. [Google Scholar] [CrossRef]
- Kumari, P.; Awasthi, K.; Agarwal, S.; Ichikawa, T.; Kumar, M.; Jain, A. Flower-like Bi2S3 nanostructures as highly efficient anodes for all-solid-state lithium-ion batteries. RSC Adv. 2019, 9, 29549–29555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kharbachi, A.; Uesato, H.; Kawai, H.; Wenner, S.; Miyaoka, H.; Sørby, M.H.; Fjellvåg, H.; Ichikawa, T.; Hauback, B.C. Hauback MgH2–CoO: A conversion-type composite electrode for LiBH4-based all-solid-state lithium ion batteries. RSC Adv. 2018, 8, 23468–23474. [Google Scholar] [CrossRef] [PubMed]
- Cano-Banda, F.; Gallardo-Gutierrez, A.; Luviano-Ortiz, L.; Hernandez-Guerrero, A.; Jain, A.; Ichikawa, T. High capacity MgH2 composite electrodes for all-solid-state Li-ion battery operating at ambient temperature. Int. J. Hydrogen Energy 2021, 46, 1030–1037. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S.-I. Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Hong, D.; Lee, H.-W.; Park, S. Practical considerations of Si-based anodes for lithium-ion battery applications. Nano Res. 2017, 10, 3970–4002. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, K.; Pal, P.; Kumar, M.; Ichikawa, T.; Jain, A. Highly efficient & stable Bi & Sb anodes using lithium borohydride as solid electrolyte in Li-ion batteries. RSC Adv. 2019, 9, 13077–13081. [Google Scholar] [PubMed]
- Tian, H.; Xin, F.; Wang, X.; He, W.; Han, W. High capacity group-IV elements (Si, Ge, Sn) based anodes for lithium-ion batteries. J. Mater. 2015, 1, 153–169. [Google Scholar] [CrossRef]
- Hwa, Y.; Sung, J.H.; Wang, B.; Park, C.-M.; Sohn, H.-J. Nanostructured Zn-based composite anodes for rechargeable Li-ion batteries. J. Mater. Chem. 2012, 22, 12767–12773. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, R.; Awasthi, K.; Ichikawa, T.; Jain, A.; Kumar, M. Electrochemical reaction mechanism for Bi2Te3-based anode material in highly durable all solid-state lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 16429–16436. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, R.; Awasthi, K.; Ichikawa, T.; Kumar, M.; Jain, A. Highly stable nanostructured Bi2Se3 anode material for all solid-state lithium-ion batteries. J. Alloys Compd. 2020, 838, 155403. [Google Scholar] [CrossRef]
- Kumari, P.; Pal, P.; Shinzato, K.; Awasthi, K.; Ichikawa, T.; Jain, A.; Kumar, M. Nanostructured Bi2Te3 as anode material as well as a destabilizing agent for LiBH4. Int. J. Hydrogen Energy 2019, 45, 16992–16999. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Yao, Y.; Ichikawa, T.; Jain, A.; Singh, R. Zinc as a Promising Anodic Material for All-Solid-State Lithium-Ion Batteries. Batteries 2022, 8, 113. https://doi.org/10.3390/batteries8090113
Singh K, Yao Y, Ichikawa T, Jain A, Singh R. Zinc as a Promising Anodic Material for All-Solid-State Lithium-Ion Batteries. Batteries. 2022; 8(9):113. https://doi.org/10.3390/batteries8090113
Chicago/Turabian StyleSingh, Kishore, Yuchen Yao, Takayuki Ichikawa, Ankur Jain, and Rini Singh. 2022. "Zinc as a Promising Anodic Material for All-Solid-State Lithium-Ion Batteries" Batteries 8, no. 9: 113. https://doi.org/10.3390/batteries8090113
APA StyleSingh, K., Yao, Y., Ichikawa, T., Jain, A., & Singh, R. (2022). Zinc as a Promising Anodic Material for All-Solid-State Lithium-Ion Batteries. Batteries, 8(9), 113. https://doi.org/10.3390/batteries8090113










