Identifying Anode and Cathode Contributions in Li-Ion Full-Cell Impedance Spectra
Abstract
1. Introduction
2. Materials and Methods
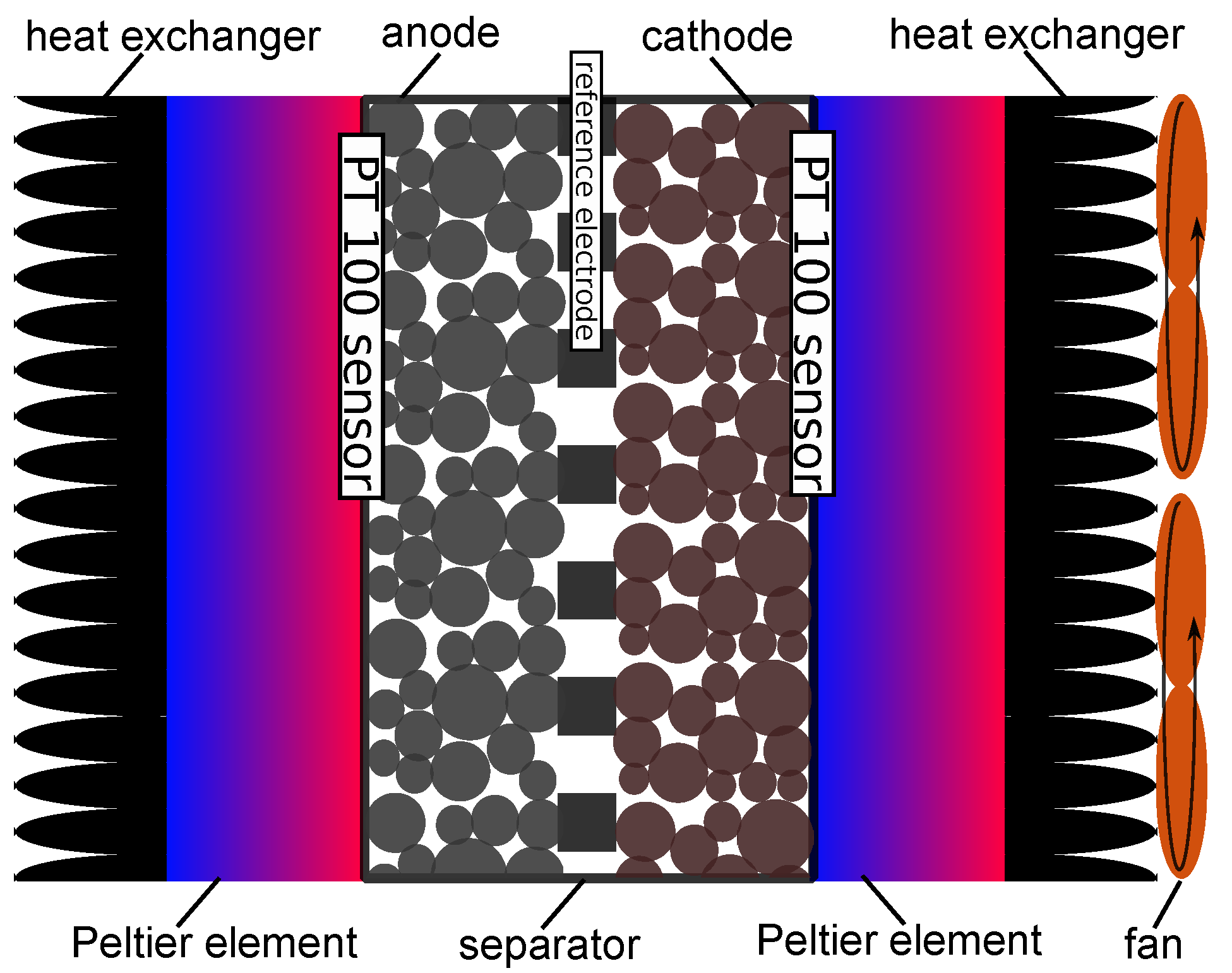
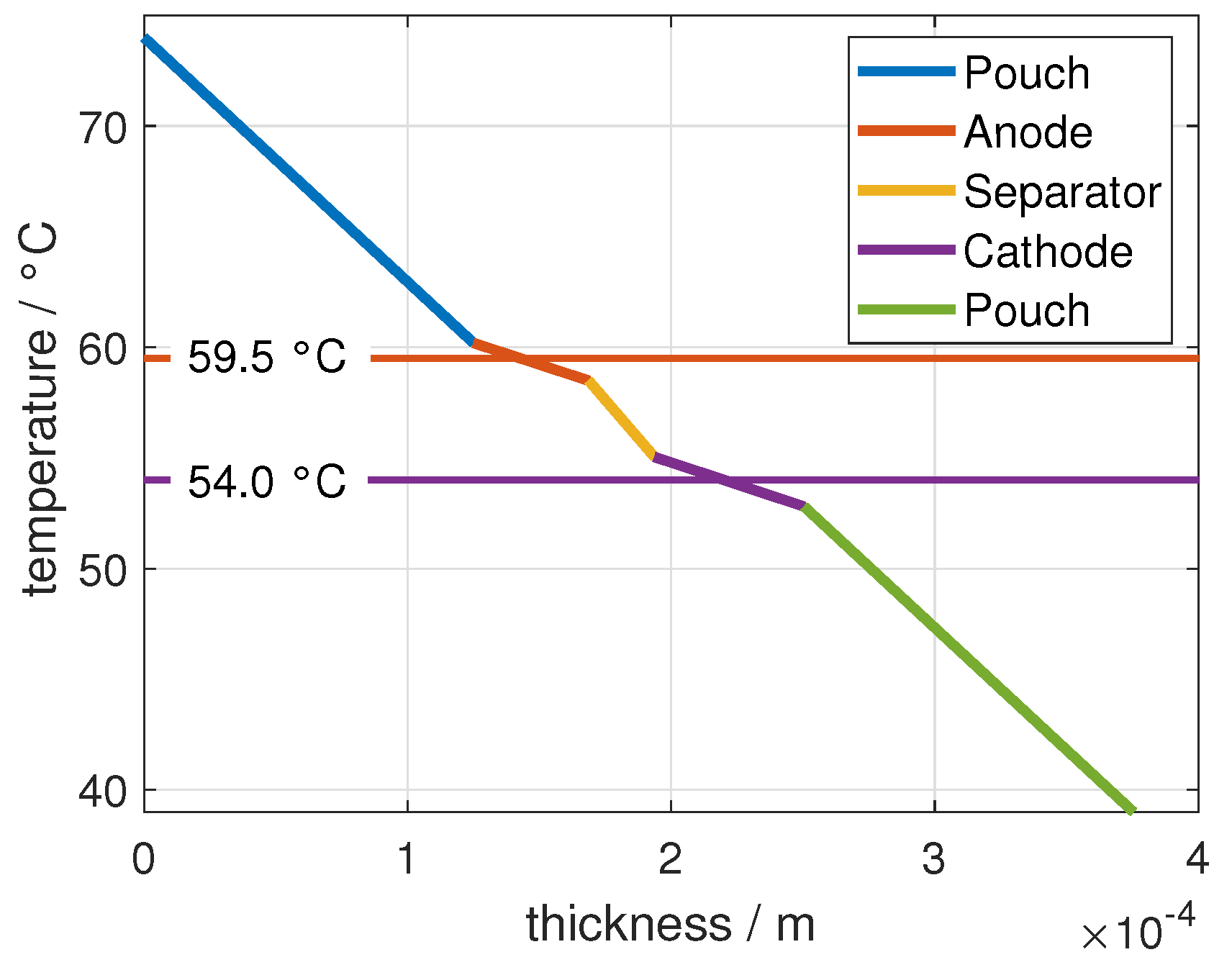
2.1. Temperature Distribution
2.2. Data Evaluation Method
2.2.1. Simplifications
2.2.2. Assumptions for Temperature Influence
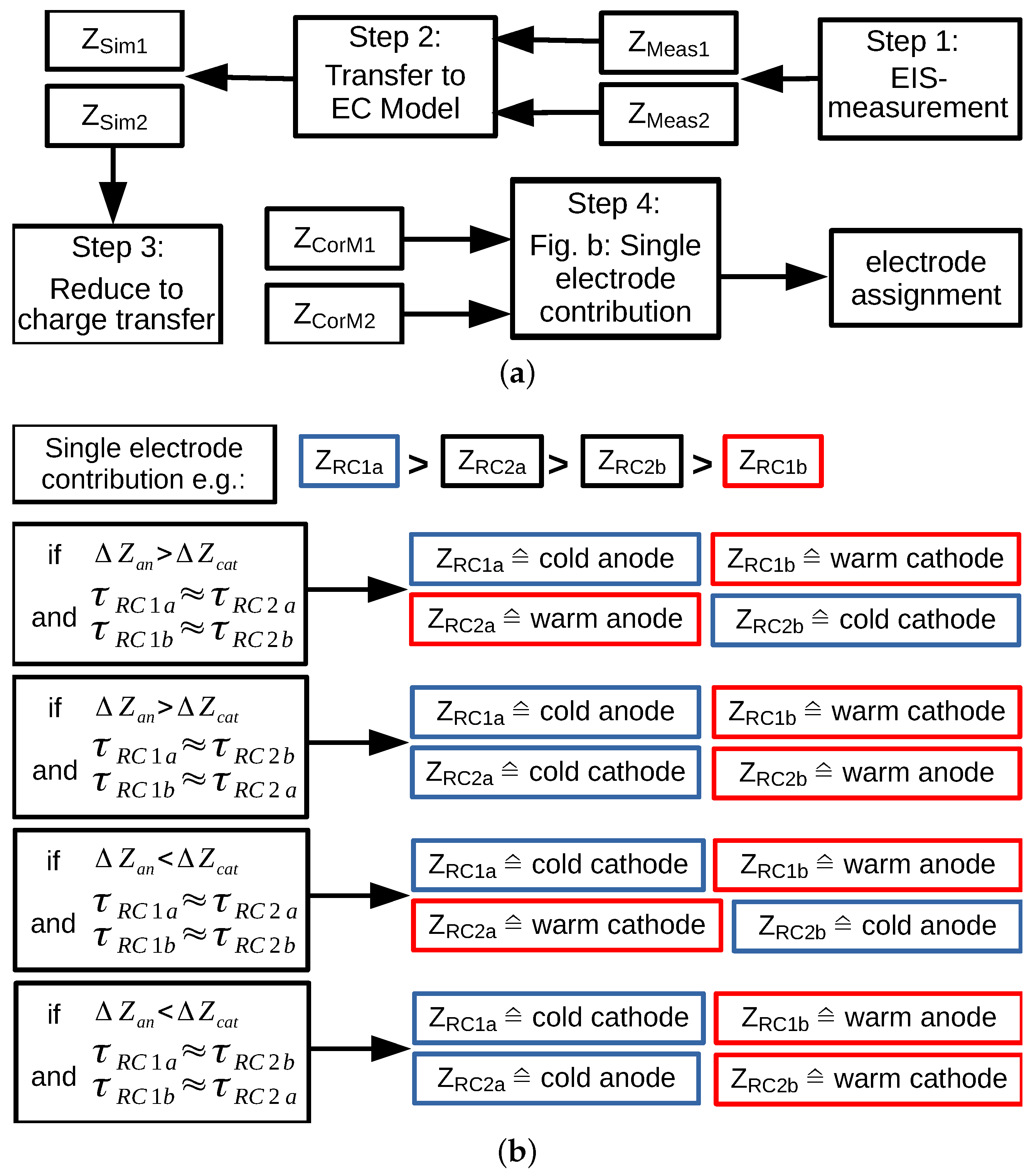
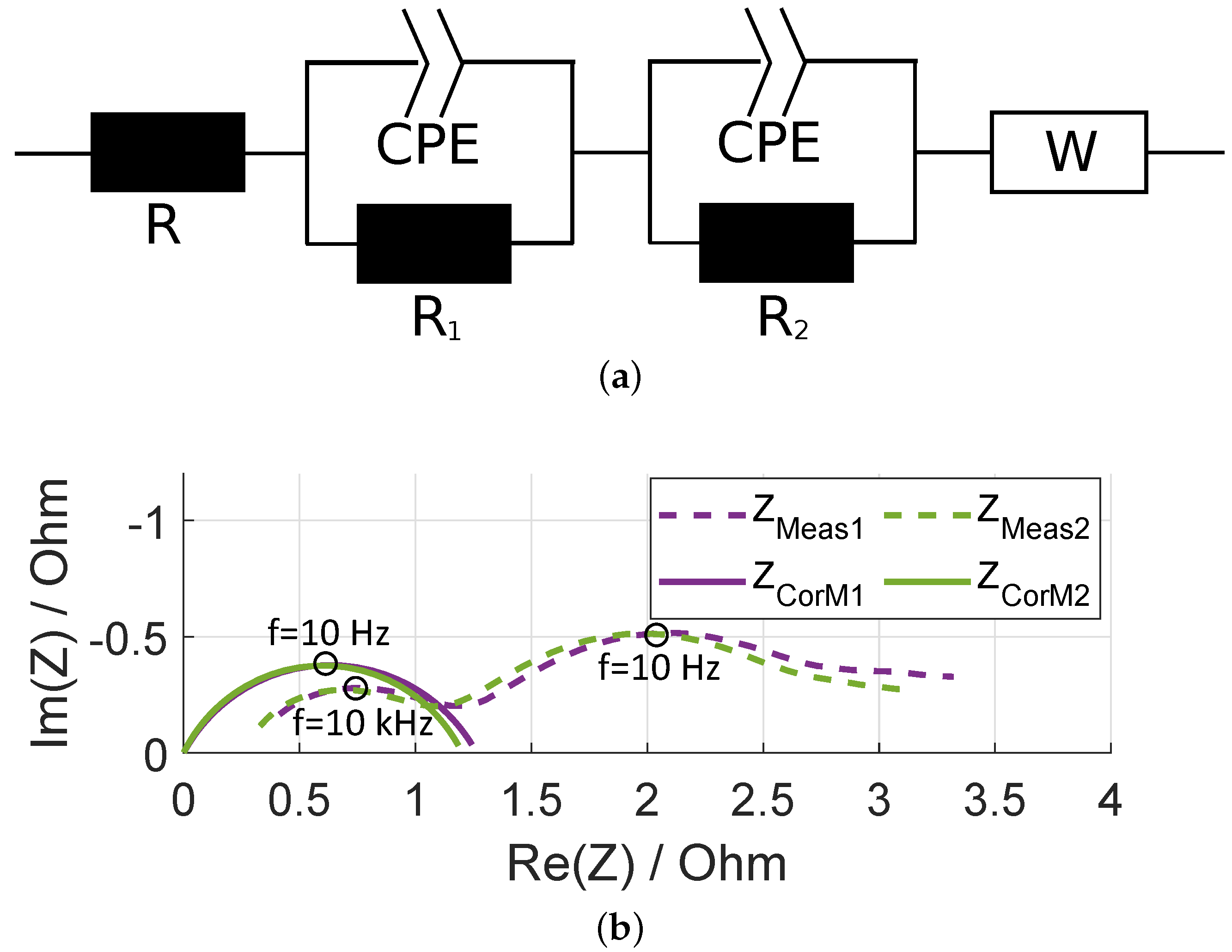
2.2.3. Identification of the Electrodes in Full-Cell Spectra
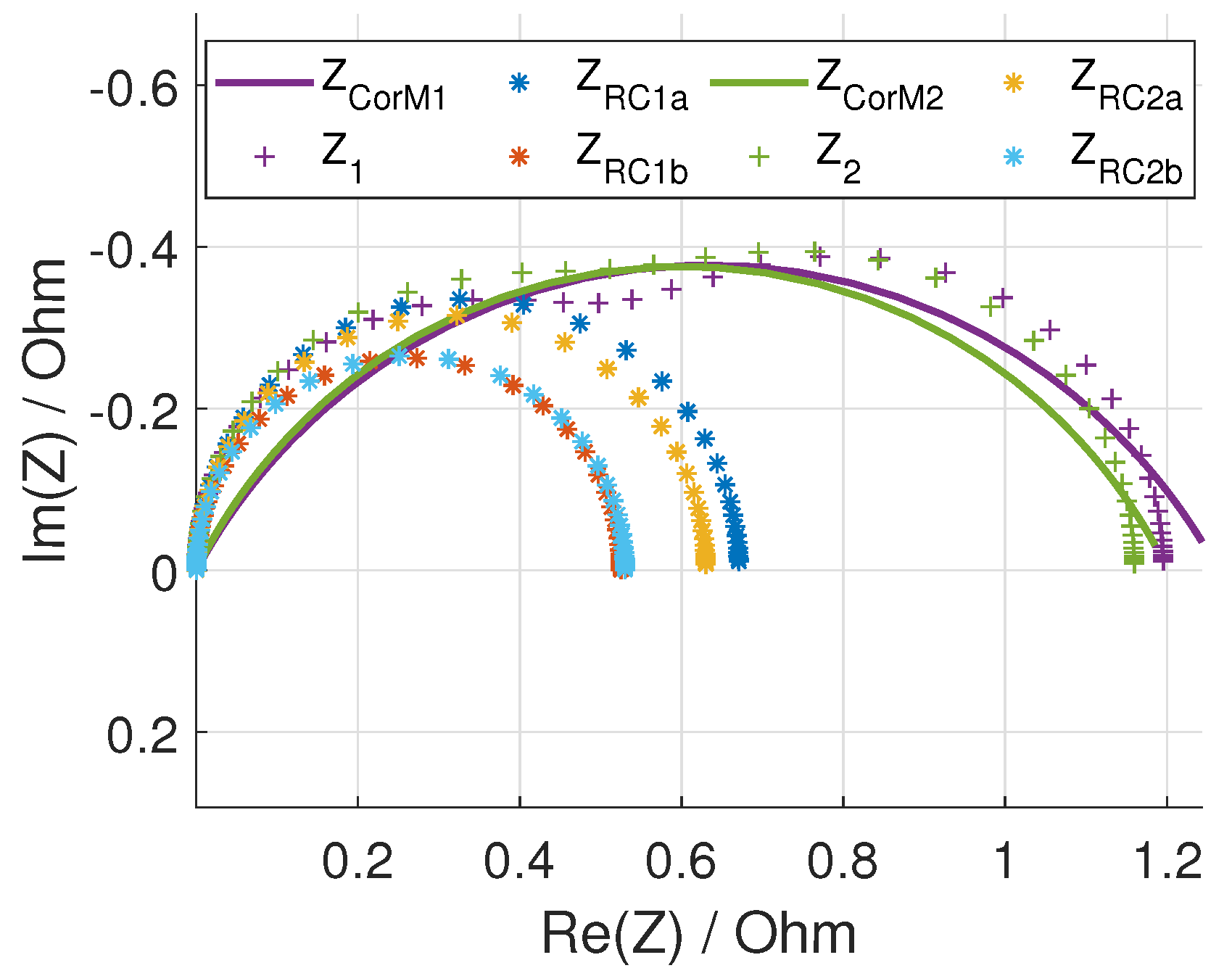
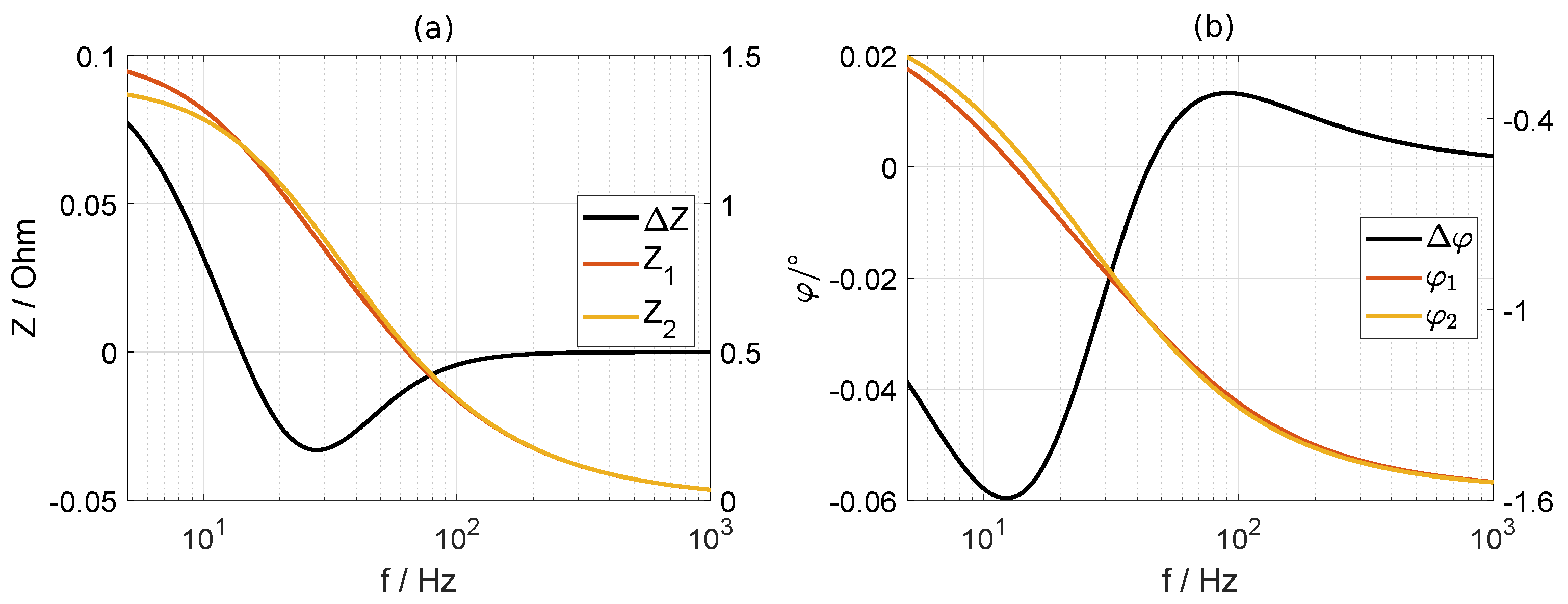
2.2.4. Consistency Checks
- The resistance values are in the same order of magnitude;
- The largest resistance is assigned to a cold anode;
- The change of the resistance at the anode and the cathode is smaller than the difference of the anode and cathode resistance;
- The double layer capacitance of the anode and the cathode are in the same order of magnitude;
- The double layer capacitance at an electrode does not change significantly with temperature.
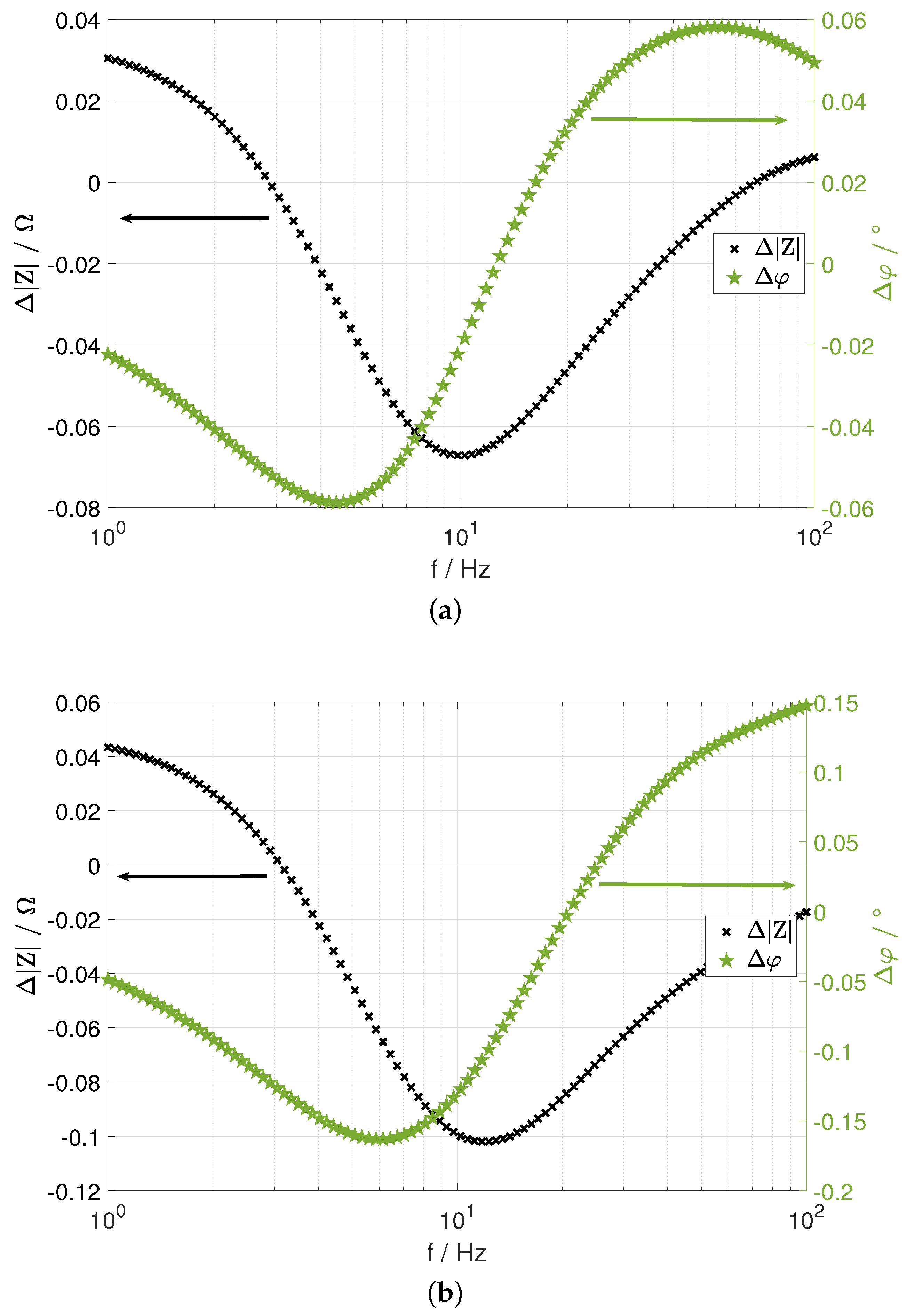
- first decreases with increasing frequency. It is positive at the low-frequency end because RC1a has been assigned to the cold anode, i.e., the highest resistance. With increasing frequency, the moduli of and cross each other. As a consequence, their difference becomes negative, and then, it shows a minimum. At high frequencies, gradually levels off to zero. If the highest resistance is assigned to the cold cathode, will be negative at the low frequencies and it will show a maximum.
- approaches zero at the low- and high-frequency end. It has a minimum at lower frequencies and a maximum at larger frequencies.
3. Results
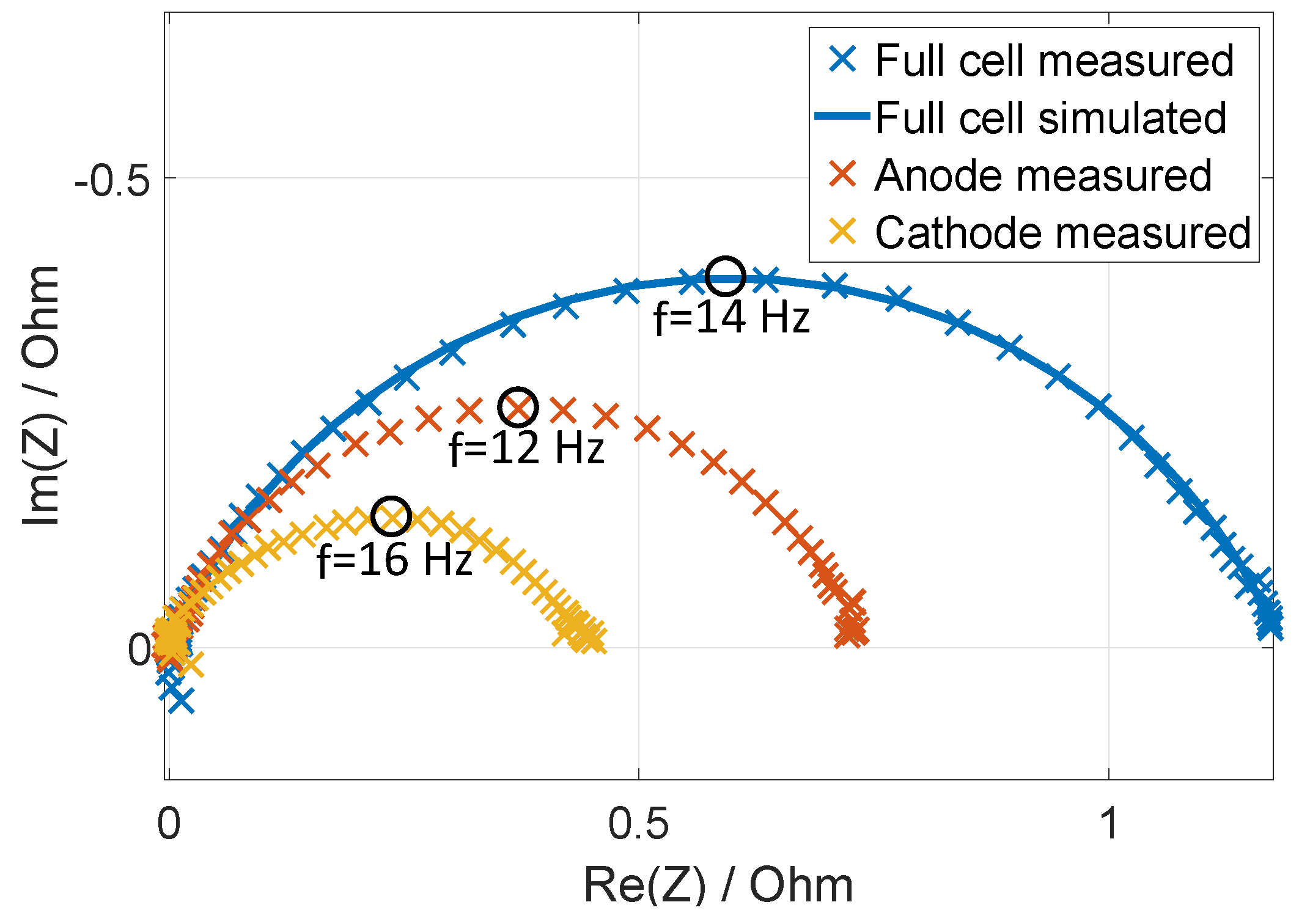
3.1. Method Application to Experimental Data
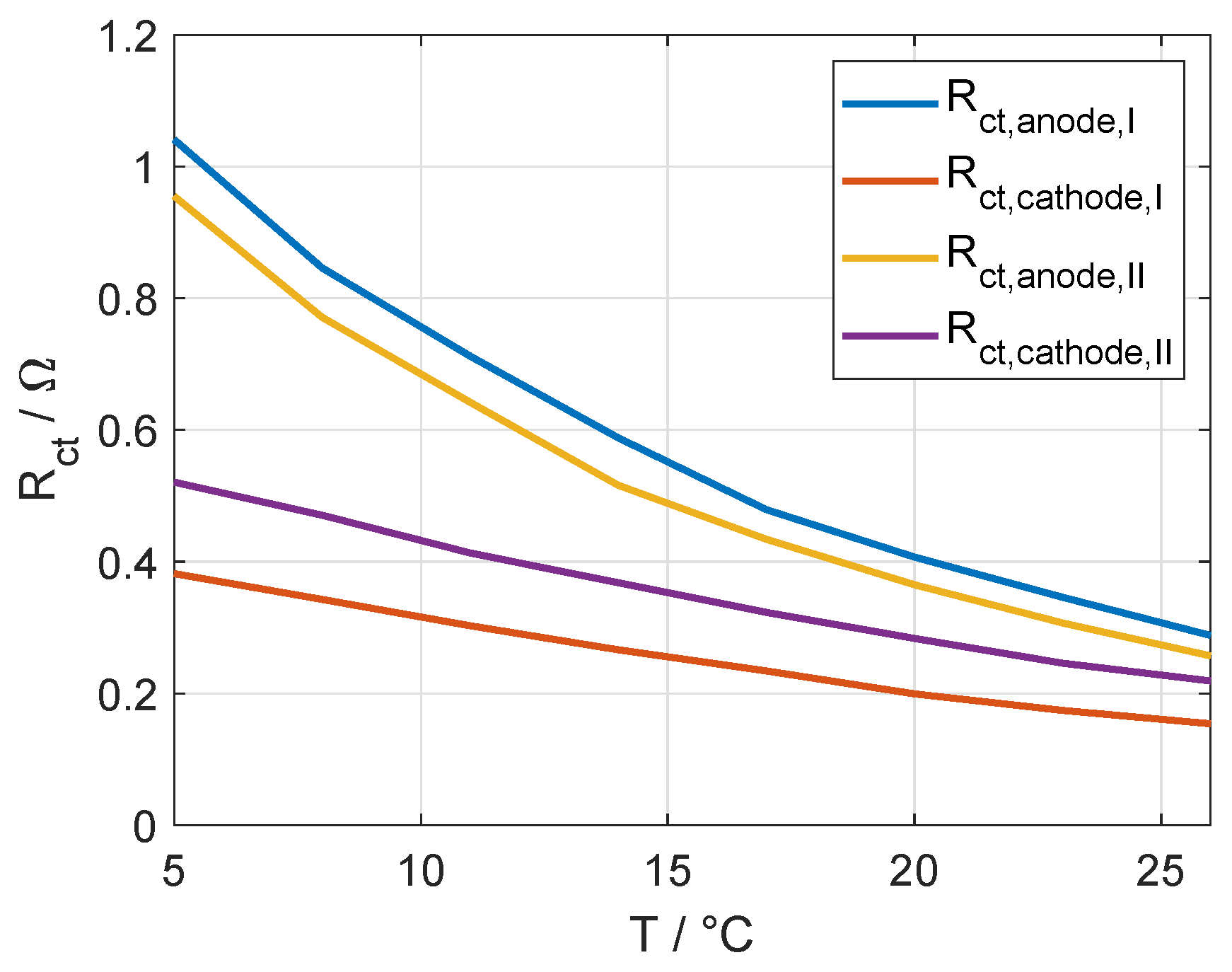
3.2. Validation of the Assignments with Half-Cell Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doyle, M.; Meyers, J.P.; Newman, J. Computer Simulations of the Impedance Response of Lithium Rechargeable Batteries. J. Electrochem. Soc. 2000, 147, 99. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Yuan, C. Mathematical Modeling of the Electrochemical Impedance Spectroscopy in Lithium Ion Battery Cycling. Electrochim. Acta 2014, 127, 266–275. [Google Scholar] [CrossRef]
- Levi, M.D.; Aurbach, D. Simultaneous Measurements and Modeling of the Electrochemical Impedance and the Cyclic Voltammetric Characteristics of Graphite Electrodes Doped with Lithium. J. Phys. Chem. B 1997, 101, 4630–4640. [Google Scholar] [CrossRef]
- Huang, R.W.J.M.; Chung, F.; Kelder, E.M. Impedance Simulation of a Li-Ion Battery with Porous Electrodes and Spherical Li+ Intercalation Particles. J. Electrochem. Soc. 2006, 153, A1459. [Google Scholar] [CrossRef][Green Version]
- Sikha, G.; White, R.E. Analytical Expression for the Impedance Response for a Lithium-Ion Cell. J. Electrochem. Soc. 2008, 155, A893–A902. [Google Scholar] [CrossRef]
- Abraham, D.P.; Knuth, J.L.; Dees, D.W.; Bloom, I.; Christophersen, J.P. Performance degradation of high-power lithium-ion cells-Electrochemistry of harvested electrodes. J. Power Source 2007, 170, 465–475. [Google Scholar] [CrossRef]
- Shafiei Sabet, P.; Sauer, D.U. Separation of predominant processes in electrochemical impedance spectra of lithium-ion batteries with nickel–manganese–cobalt cathodes. J. Power Sources 2019, 425, 121–129. [Google Scholar] [CrossRef]
- Shafiei Sabet, P.; Stahl, G.; Sauer, D.U. Non-invasive investigation of predominant processes in the impedance spectra of high energy lithium-ion batteries with Nickel-Cobalt-Aluminum cathodes. J. Power Source 2018, 406, 185–193. [Google Scholar] [CrossRef]
- Schindler, S.; Danzer, M.A. Influence of cell design on impedance characteristics of cylindrical lithium-ion cells: A model-based assessment from electrode to cell level. J. Energy Storage 2017, 12, 157–166. [Google Scholar] [CrossRef]
- Mussa, A.S.; Liivat, A.; Marzano, F.; Klett, M.; Philippe, B.; Tengstedt, C.; Lindbergh, G.; Edström, K.; Lindström, R.W.; Svens, P. Fast-charging effects on ageing for energy-optimized automotive LiNi 1/3 Mn 1/3 Co 1/3 O 2 /graphite prismatic lithium-ion cells. J. Power Source 2019, 422, 175–184. [Google Scholar] [CrossRef]
- Guo, M.; Meng, W.; Zhang, X.; Liu, X.; Bai, Z.; Chen, S.; Wang, Z.; Yang, F. Electrochemical behavior and self-organization of porous Sn nanocrystals@acetylene black microspheres in lithium-ion half cells. Appl. Surf. Sci. 2019, 470, 36–43. [Google Scholar] [CrossRef]
- Illig, J.; Chrobak, T.; Ender, M.; Schmidt, J.; Klotz, D.; Ivers-Tiffée, E. Studies on LiFePO 4 as cathode material in Li-ion batteries. ECS Trans. 2010, 28, 3–17. [Google Scholar] [CrossRef]
- Tong, B.; Wang, J.; Liu, Z.; Ma, L.; Zhou, Z.; Peng, Z. Identifying compatibility of lithium salts with LiFePO4 cathode using a symmetric cell. J. Power Source 2018, 384, 80–85. [Google Scholar] [CrossRef]
- Kisu, K.; Aoyagi, S.; Nagatomo, H.; Iwama, E.; Reid, M.T.H.; Naoi, W.; Naoi, K. Internal resistance mapping preparation to optimize electrode thickness and density using symmetric cell for high-performance lithium-ion batteries and capacitors. J. Power Source 2018, 396, 207–212. [Google Scholar] [CrossRef]
- Conder, J.; Villevieille, C.; Trabesinger, S.; Novák, P.; Gubler, L.; Bouchet, R. Electrochemical impedance spectroscopy of a Li–S battery: Part 1. Influence of the electrode and electrolyte compositions on the impedance of symmetric cells. Electrochim. Acta 2017, 244, 61–68. [Google Scholar] [CrossRef]
- Petibon, R.; Sinha, N.N.; Burns, J.C.; Aiken, C.P.; Ye, H.; Vanelzen, C.M.; Jain, G.; Trussler, S.; Dahn, J.R. Comparative study of electrolyte additives using electrochemical impedance spectroscopy on symmetric cells. J. Power Source 2014, 251, 187–194. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, J.; Amine, K. Symmetric cell approach and impedance spectroscopy of high power lithium-ion batteries. J. Power Source 2001, 96, 321–328. [Google Scholar] [CrossRef]
- Abraham, D.P.; Poppen, S.D.; Jansen, A.N.; Liu, J.; Dees, D.W. Application of a lithium-tin reference electrode to determine electrode contributions to impedance rise in high-power lithium-ion cells. Electrochim. Acta 2004, 49, 4763–4775. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Yokoshima, T.; Momma, T.; Osaka, T. Impedance Analysis with Transmission Line Model for Reaction Distribution in a Pouch Type Lithium-Ion Battery by Using Micro Reference Electrode. J. Electrochem. Soc. 2016, 163, A434–A441. [Google Scholar] [CrossRef]
- Gómez-Cámer, J.L.; Novák, P. Electrochemical impedance spectroscopy: Understanding the role of the reference electrode. Electrochem. Commun. 2013, 34, 208–210. [Google Scholar] [CrossRef]
- Dondelinger, M.; Swanson, J.; Nasymov, G.; Jahnke, C.; Qiao, Q.; Wu, J.; Widener, C.; Numan-Al-Mobin, A.M.; Smirnova, A. Electrochemical stability of lithium halide electrolyte with antiperovskite crystal structure. Electrochim. Acta 2019, 306, 498–505. [Google Scholar] [CrossRef]
- Raijmakers, L.H.; Lammers, M.J.; Notten, P.H. A new method to compensate impedance artefacts for Li-ion batteries with integrated micro-reference electrodes. Electrochim. Acta 2018, 259, 517–533. [Google Scholar] [CrossRef]
- Heinrich, M. Electrochemical Impedance Spectroscopy on ageing Lithium-Ion Batteries. Ph.D. Thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2020. [Google Scholar]
- Troxler, Y.; Wu, B.; Marinescu, M.; Yufit, V.; Patel, Y.; Marquis, A.J.; Brandon, N.P.; Offer, G.J. The effect of thermal gradients on the performance of lithium-ion batteries. J. Power Source 2014, 247, 1018–1025. [Google Scholar] [CrossRef]
- Burheim, O.S.; Onsrud, M.A.; Pharoah, J.G.; Vullum-Bruer, F.; Vie, P.J.S. Thermal Conductivity, Heat Sources and Temperature Profiles of Li-Ion Batteries. ECS Trans. 2014, 58, 145–171. [Google Scholar] [CrossRef]
- Korthauer, R. Handbuch Lithium-Ionen-Batterien; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Richter, F.; Vie, P.J.; Kjelstrup, S.; Burheim, O.S. Measurements of ageing and thermal conductivity in a secondary NMC-hard carbon Li-ion battery and the impact on internal temperature profiles. Electrochim. Acta 2017, 250, 228–237. [Google Scholar] [CrossRef]
- Heinrich, M.; Wolff, N.; Harting, N.; Laue, V.; Röder, F.; Seitz, S.; Krewer, U. Physico-Chemical Modeling of a Lithium-Ion Battery: An Ageing Study with Electrochemical Impedance Spectroscopy. Batter. Supercaps 2019, 2, 530–540. [Google Scholar] [CrossRef]
- Kaplan, T.; Gray, L.J.; Liu, S.H. Self-affine fractal model for a metal-electrolyte interface. Phys. Rev. B 1987, 35, 5–7. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Katzenmeier, L.; Lamprecht, X.; Bandarenka, A.S. Review on physical impedance models in modern battery research. Phys. Chem. Chem. Phys. 2021, 23, 12926–12944. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Bade Shrestha, S.O. Temperature Dependence of Double Layer Capacitance in Lithium-Ion Battery. In Proceedings of the 116th IIER International Conference, Phuket, Thailand, 9–10 August 2017; pp. 7–10. [Google Scholar]
- Xing, Z.; Tan, G.; Yuan, Y.; Wang, B.; Ma, L.; Xie, J.; Li, Z.; Wu, T.; Ren, Y.; Shahbazian-Yassar, R.; et al. Consolidating Lithiothermic-Ready Transition Metals for Li2S-Based Cathodes. Adv. Mater. 2020, 32, 2002403. [Google Scholar] [CrossRef]
- Tan, G.; Xu, R.; Xing, Z.; Yuan, Y.; Lu, J.; Wen, J.; Liu, C.; Ma, L.; Zhan, C.; Liu, Q.; et al. Burning lithium in CS2 for high-performing compact Li2S–graphene nanocapsules for Li–S batteries. Nat. Energy 2017, 2, 1–10. [Google Scholar] [CrossRef]

| Material | Conductivity | Thickness |
|---|---|---|
| Wm K | m | |
| Pouch bag foil | 0.25 [25] | 125 [26] |
| Graphite | 0.71 [27] | 43.66 [28] |
| NMC | 0.7 [27] | 56.75 [28] |
| Separator | 0.25 [25] | 20 [28] |
| Resistance | Capacitance | Time Constant | Assignment | |
|---|---|---|---|---|
| Ω | F | s | ||
| 0.671 | 0.0311 | cold anode | ||
| 0.525 | 0.00466 | warm cathode | ||
| 0.630 | 0.0319 | warm anode | ||
| 0.530 | 0.00464 | cold cathode |
| Resistance | Capacitance | Time Constant | Assignment | |
|---|---|---|---|---|
| Ω | F | s | ||
| 0.654 | 0.0282 | cold anode | ||
| 0.163 | 0.00534 | warm cathode | ||
| 0.595 | 0.0285 | warm anode | ||
| 0.172 | 0.00532 | cold cathode |
| Time constants of the simulation in seconds | ||||
| cold cathode | warm anode | cold anode | warm cathode | |
| Cell I | 0.002 | 0.020 | 0.021 | 0.002 |
| Cell II | 0.001 | 0.017 | 0.018 | 0.001 |
| Time constants of the experiment in seconds | ||||
| cold cathode | warm anode | cold anode | warm cathode | |
| Cell I | 0.011 | 0.013 | 0.013 | 0.008 |
| Cell II | 0.004 | 0.011 | 0.008 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinrich, M.; Wolff, N.; Seitz, S.; Krewer, U. Identifying Anode and Cathode Contributions in Li-Ion Full-Cell Impedance Spectra. Batteries 2022, 8, 40. https://doi.org/10.3390/batteries8050040
Heinrich M, Wolff N, Seitz S, Krewer U. Identifying Anode and Cathode Contributions in Li-Ion Full-Cell Impedance Spectra. Batteries. 2022; 8(5):40. https://doi.org/10.3390/batteries8050040
Chicago/Turabian StyleHeinrich, Marco, Nicolas Wolff, Steffen Seitz, and Ulrike Krewer. 2022. "Identifying Anode and Cathode Contributions in Li-Ion Full-Cell Impedance Spectra" Batteries 8, no. 5: 40. https://doi.org/10.3390/batteries8050040
APA StyleHeinrich, M., Wolff, N., Seitz, S., & Krewer, U. (2022). Identifying Anode and Cathode Contributions in Li-Ion Full-Cell Impedance Spectra. Batteries, 8(5), 40. https://doi.org/10.3390/batteries8050040






