Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators
Abstract
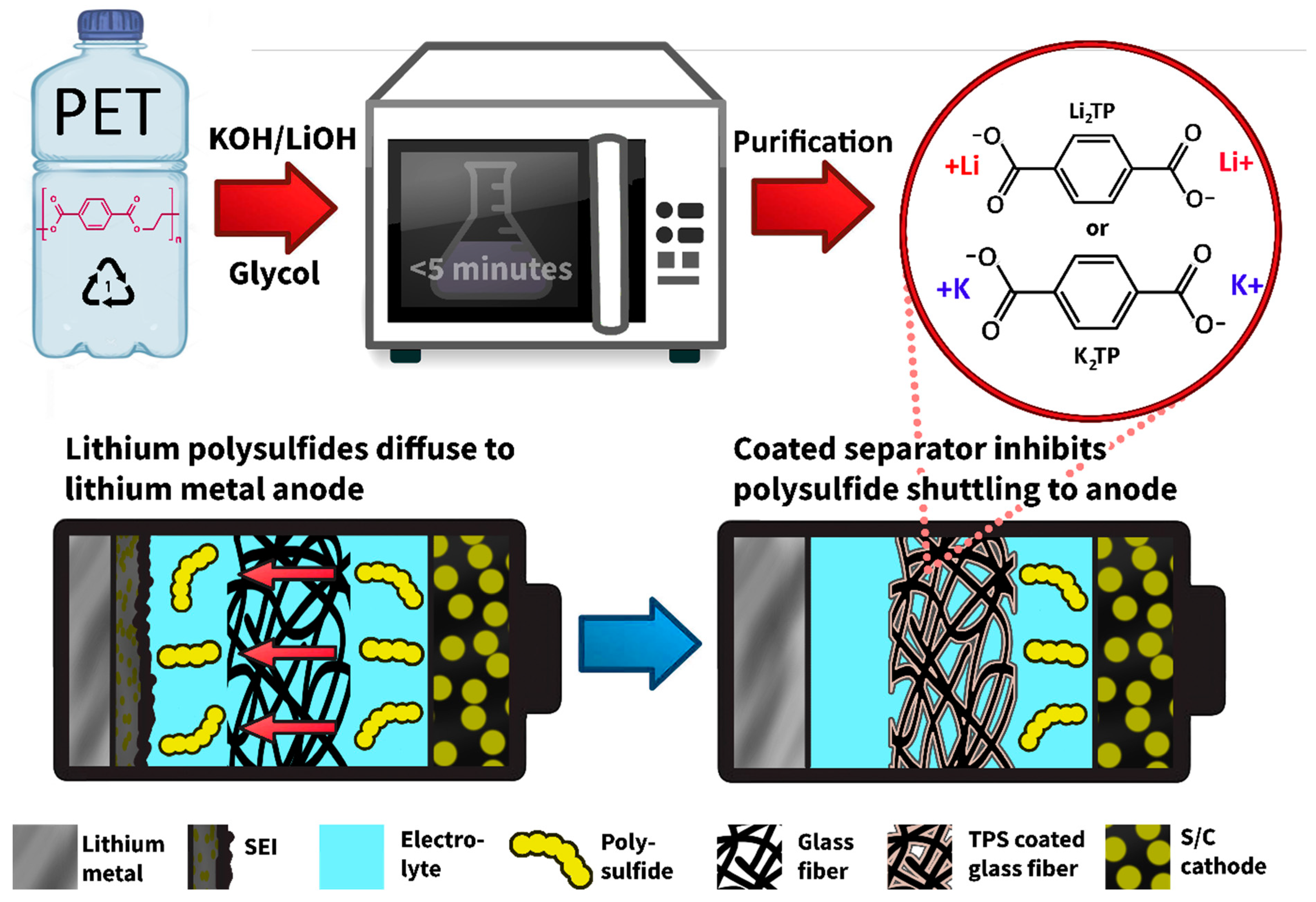
1. Introduction
2. Results and Discussion
2.1. Material Characterizations
2.2. Separator Characterizations
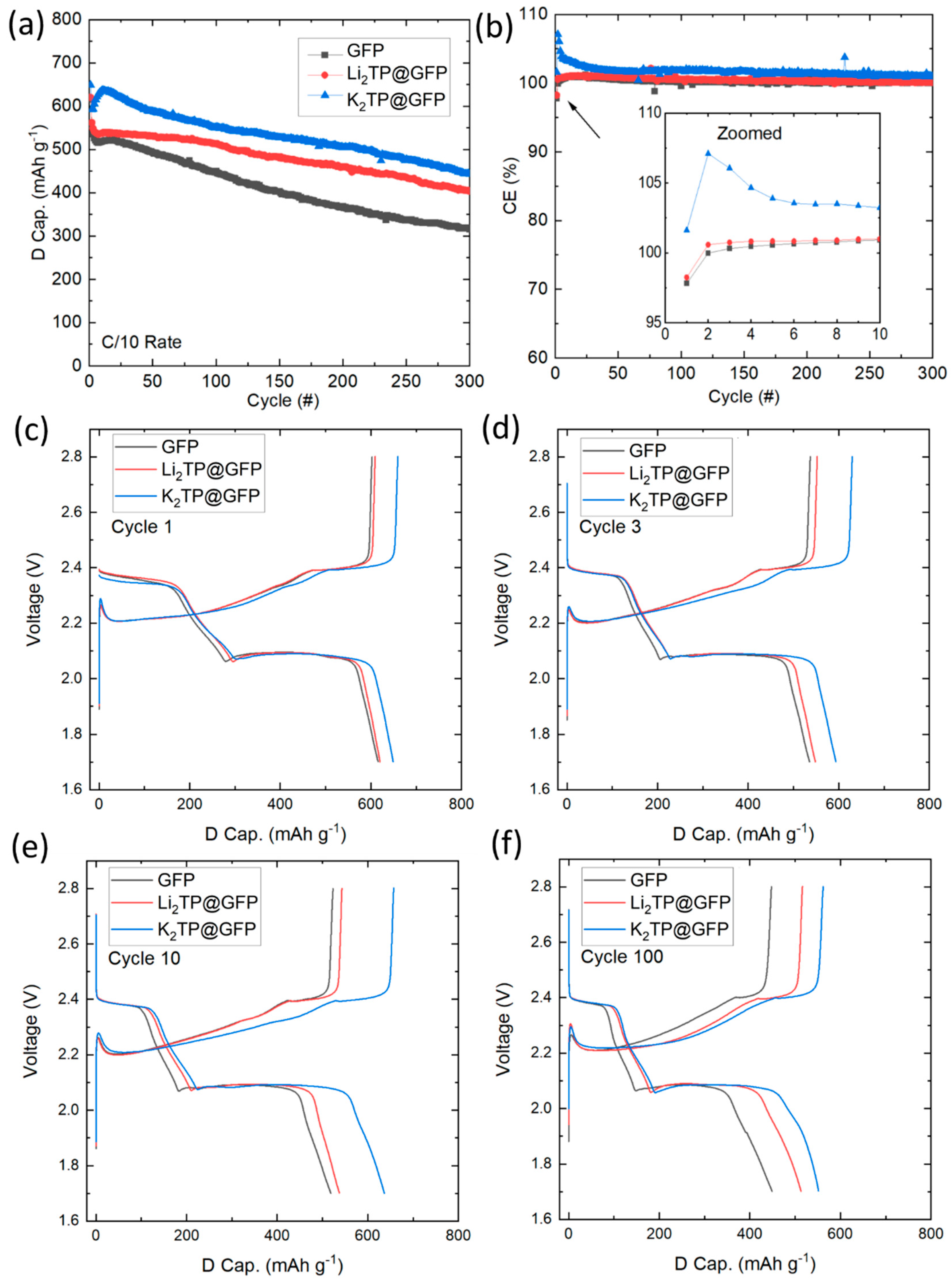
2.3. Electrochemical Cycling Analysis
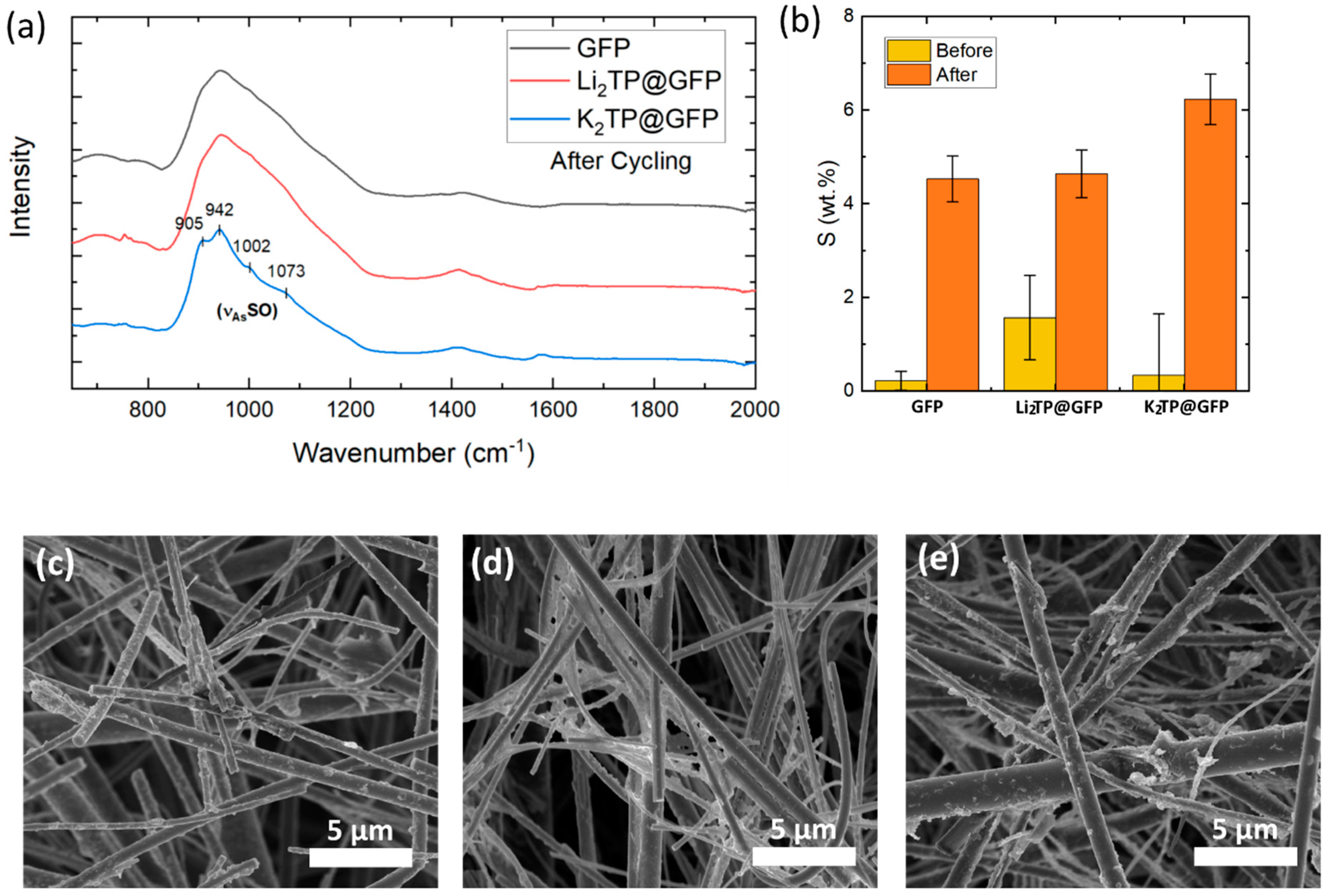
2.4. Post-Cycling Analysis
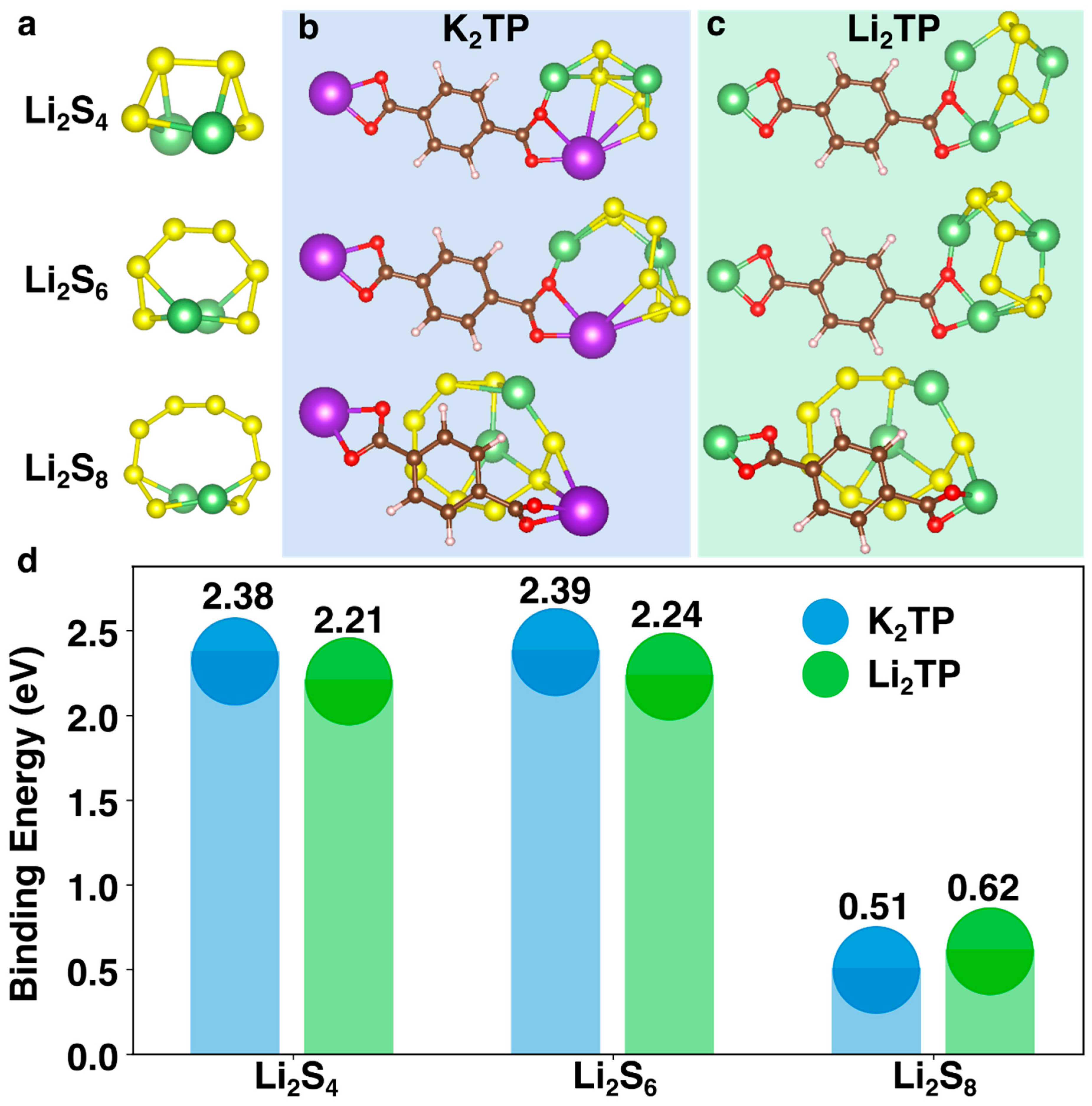
2.5. Binding Energy Analysis
3. Materials and Methods
3.1. Material Syntheses
3.2. Material Characterizations
3.3. Electrochemical Characterizations
3.4. Separator Characterizations
3.5. DFT Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Why Big Battery Phones Do Not Guarantee More Screen on Time? Hidden Facts, (n.d.). Available online: https://gadgetstouse.com/blog/2021/01/21/why-big-battery-phones-do-not-guarantee-more-screen-on-time-hidden-facts/ (accessed on 30 August 2022).
- Lithium Metal Batteries Can Power Drones for Longer, (n.d.). Available online: https://www.popularmechanics.com/flight/drones/a27155551/battery-boeing/ (accessed on 30 August 2022).
- Liu, Y.; Liu, S.; Li, G.; Gao, X. Strategy of Enhancing the Volumetric Energy Density for Lithium–Sulfur Batteries. Adv. Mater. 2021, 33, 2003955. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Q.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Chen, G.; Zhong, B.; Xiang, W.; Zhong, Y. A review of cathode materials in lithium-sulfur batteries. Ionics 2020, 26, 5299–5318. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, J.; Zheng, X.; Wen, J.; Dai, Y.; Wang, Z.; Luo, W.; Huang, Y. Lithium Metal-Based Composite: An Emerging Material for Next-Generation Batteries. Matter 2020, 3, 1009–1030. [Google Scholar] [CrossRef]
- Rana, M.; Ahad, S.A.; Li, M.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Review on areal capacities and long-term cycling performances of lithium sulfur battery at high sulfur loading. Energy Storage Mater. 2019, 18, 289–310. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.; He, P.; Zhou, H. A Review of Solid-State Lithium–Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy Fuels 2020, 34, 11942–11961. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Huang, J.; Hu, A.; Gong, C.; Yan, C.; Wang, X.; Xiong, J. Strategies toward High-Loading Lithium–Sulfur Battery. Adv. Energy Mater. 2020, 10, 2000082. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Kim, K.; Pol, V.G. Towards highly stable lithium sulfur batteries: Surface functionalization of carbon nanotube scaffolds. Carbon 2018, 131, 175–183. [Google Scholar] [CrossRef]
- Dysart, A.D.; Burgos, J.C.; Mistry, A.; Chen, C.-F.; Liu, Z.; Hong, C.N.; Balbuena, P.B.; Mukherjee, P.P.; Pol, V.G. Towards Next Generation Lithium-Sulfur Batteries: Non-Conventional Carbon Compartments/Sulfur Electrodes and Multi-Scale Analysis. J. Electrochem. Soc. 2016, 163, A730–A741. [Google Scholar] [CrossRef]
- Kim, P.J.; Fontecha, H.D.; Kim, K.; Pol, V.G. Toward High-Performance Lithium–Sulfur Batteries: Upcycling of LDPE Plastic into Sulfonated Carbon Scaffold via Microwave-Promoted Sulfonation. ACS Appl. Mater. Interfaces 2018, 10, 14827–14834. [Google Scholar] [CrossRef]
- Hu, C.; Chen, H.; Shen, Y.; Lu, D.; Zhao, Y.; Lu, A.-H.; Wu, X.; Lu, W.; Chen, L. In situ wrapping of the cathode material in lithium-sulfur batteries. Nat. Commun. 2017, 8, 479. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Surface Modification of Sulfur Cathodes with PEDOT:PSS Conducting Polymer in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A935–A939. [Google Scholar] [CrossRef]
- Kim, K.; Kim, P.J.H.; Youngblood, J.P.; Pol, V.G. Surface Functionalization of Carbon Architecture with Nano-MnO2 for Effective Polysulfide Confinement in Lithium–Sulfur Batteries. ChemSusChem 2018, 11, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Sun, M.-H.; Yu, Y.; Peng, Z.; Bulbula, S.T.; Li, Y.; Chen, L.-H.; Su, B.-L. Physical and chemical dual-confinement of polysulfides within hierarchically meso-microporous nitrogen-doped carbon nanocages for advanced Li–S batteries. RSC Adv. 2017, 7, 42627–42633. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, J.-Q.; Peng, H.-J.; Titirici, M.-M.; Xiang, R.; Chen, R.-J.; Liu, Q.-B.; Zhang, Q. A Review of Functional Binders in Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802107. [Google Scholar] [CrossRef]
- Liu, Y.; Elias, Y.; Meng, J.; Aurbach, D.; Zou, R.; Xia, D.; Pang, Q. Electrolyte solutions design for lithium-sulfur batteries. Joule 2021, 5, 2323–2364. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Shen, G.; Zhang, S. Recent progress in fluorinated electrolytes for improving the performance of Li–S batteries. J. Energy Chem. 2020, 41, 149–170. [Google Scholar] [CrossRef]
- Li, G.R.; Li, Z.; Zhang, B.; Lin, Z. Developments of electrolyte systems for lithium–sulfur batteries: A review. Front. Energy Res. 2015, 3, 5. [Google Scholar] [CrossRef]
- Weng, W.; Pol, V.G.; Amine, K. Ultrasound Assisted Design of Sulfur/Carbon Cathodes with Partially Fluorinated Ether Electrolytes for Highly Efficient Li/S Batteries. Adv. Mater. 2013, 25, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiong, X.; Zhu, Y.; Chen, Y.; Fu, L.; Zhang, Y.; Wu, Y. Modifications of Separators for Li–S Batteries with Improved Electrochemical Performance. Russ. J. Electrochem. 2020, 56, 365–377. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, Z.; Tang, J.; Xu, X.; Liu, J.; Jin, Y.; Zhang, Q.; Wang, H. Cation-Selective Separators for Addressing the Lithium–Sulfur Battery Challenges. ChemSusChem 2021, 14, 792–807. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ren, Y.; Sokolowski, J.; Zhu, X.; Wu, G. Mechanistic understanding of the role separators playing in advanced lithium-sulfur batteries. InfoMat 2020, 2, 483–508. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Wen, J.; Hu, X.; Li, Z.; Li, M.; Pan, F.; Xu, C. Separator engineering toward practical Li-S batteries: Targeted electrocatalytic sulfur conversion, lithium plating regulation, and thermal tolerance. Nano Energy 2022, 95, 106982. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, H.-J.; Lim, H.; Hwang, H.J.; Park, J.-W.; Lee, T.-G.; Cho, S.Y.; Jang, S.G.; Jun, Y.-S. Boron Nitride Nanotube-Based Separator for High-Performance Lithium-Sulfur Batteries. Nanomaterials 2021, 12, 11. [Google Scholar] [CrossRef]
- Song, X.; Wang, S.; Chen, G.; Gao, T.; Bao, Y.; Ding, L.-X.; Wang, H. Fe-N-doped carbon nanofiber and graphene modified separator for lithium-sulfur batteries. Chem. Eng. J. 2018, 333, 564–571. [Google Scholar] [CrossRef]
- Hu, S.; Yi, M.; Huang, X.; Wu, D.; Lu, B.; Wang, T.; Li, N.; Zhu, Z.; Liu, X.; Zhang, J. Cobalt-doped porphyrin-based porous organic polymer-modified separator for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 2792–2805. [Google Scholar] [CrossRef]
- CPaniagua-Vásquez, I.; Zuluaga-Gómez, C.C.; Chacón-Vargas, S.; Calvo, A.L.; Sáenz-Arce, G.; Katiyar, R.S.; Saavedra-Arias, J.J. High Specific Capacity of Lithium–Sulfur Batteries with Carbon Black/Chitosan-and Carbon Black/Polyvinylidene Fluoride-Coated Separators. Energies 2022, 15, 2183. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Goodship, V. Plastic Recycling. Sci. Prog. 2007, 90, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Makeev, M.A.; Qi, Z.; Wang, H.; Rajput, N.N.; Martha, S.K.; Pol, V.G. Rapid Upcycling of Waste Polyethylene Terephthalate to Energy Storing Disodium Terephthalate Flowers with DFT Calculations. ACS Sustain. Chem. Eng. 2020, 8, 6252–6262. [Google Scholar] [CrossRef]
- Ghosh, S.; Makeev, M.A.; Macaggi, M.L.; Qi, Z.; Wang, H.; Rajput, N.N.; Martha, S.K.; Pol, V.G. Dipotassium terephthalate as promising potassium storing anode with DFT calculations. Mater. Today Energy 2020, 17, 100454. [Google Scholar] [CrossRef]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef] [PubMed]
- Varghese, H.T.; Panicker, C.Y.; Philip, D.; Sreevalsan, K.; Anithakumary, V. IR, Raman and SERS spectra of disodium terephthalate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Babar, S.; Lekakou, C. Molecular modeling of electrolyte and polysulfide ions for lithium-sulfur batteries. Ionics 2021, 27, 635–642. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, C.; Wang, W.; Li, Y.; You, J.; Zhang, B.; Zou, J.; Abuelgasim, S.; Zhu, T.; Wu, J.; et al. Modification of lithium sulfur batteries by sieving effect: Long term investigation of carbon molecular sieve. J. Energy Storage 2022, 54, 105228. [Google Scholar] [CrossRef]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- FTIR Measurements of SiO2 Glass Prepared by Sol-Gel Technique. Chem. Sci. Trans. 2014, 3, 1064–1066. [CrossRef][Green Version]
- Jiang, K.; Gao, S.; Wang, R.; Jiang, M.; Han, J.; Gu, T.; Liu, M.; Cheng, S.; Wang, K. Lithium Sulfonate/Carboxylate-Anchored Polyvinyl Alcohol Separators for Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18310–18315. [Google Scholar] [CrossRef]
- Conder, J.; Urbonaite, S.; Streich, D.; Novák, P.; Gubler, L. Taming the polysulphide shuttle in Li–S batteries by plasma-induced asymmetric functionalisation of the separator. RSC Adv. 2015, 5, 79654–79660. [Google Scholar] [CrossRef]
- Kumaresan, L.; Kasiviswanathan, K.; Kirubakaran, K.P.; Priyadarshini, M.; Mathiyalagan, K.; Senthil, C.; Lee, C.W.; Vediappan, K. Band-Gap Tuned Dilithium Terephthalate from Environmentally Hazardous Material for Sustainable Lithium Storage Systems with DFT Modelling. ChemistrySelect 2022, 7, e202200527. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, N.; Park, J.; Park, J. Electrochemical Performance of Lithium/Sulfur Batteries with Protected Li Anodes. J. Power Sources 2003, 119–121, 964–972. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Wang, J.; Yang, H.; Wang, L.; Liu, T. Assessment on the Self-Discharge Behavior of Lithium–Sulfur Batteries with LiNO3-Possessing Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 35175–35183. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Zhang, S.; Jia, W.; Wang, X.; Guo, Y.; Jia, D.; Wang, L. Decoration of Silica Nanoparticles on Polypropylene Separator for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 7499–7504. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y. Dissolution and Reprecipitation of Sulfur on Carbon Surface. J. Electron. Mater. 2022, 51, 2926–2932. [Google Scholar] [CrossRef]
- Kam, W.; Liew, C.-W.; Lim, J.Y.; Ramesh, S. Electrical, structural, and thermal studies of antimony trioxide-doped poly(acrylic acid)-based composite polymer electrolytes. Ionics 2013, 20, 665–674. [Google Scholar] [CrossRef]
- Patil, V.S.; Vithya, K.; Premalatha, M.; Sundaresan, B. FTIR Studies on PMMA-LiNO3 Polymer Electrolyte. Macromol. Symp. 2019, 387, 1800177. [Google Scholar] [CrossRef]
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Insights into Li-S Battery Cathode Capacity Fading Mechanisms: Irreversible Oxidation of Active Mass during Cycling. J. Electrochem. Soc. 2012, 159, 1816–1821. [Google Scholar] [CrossRef]
- Lin, H.; Yang, D.-D.; Lou, N.; Wang, A.-L.; Zhu, S.-G.; Li, H.-Z. Defect engineering of black phosphorene towards an enhanced polysulfide host and catalyst for lithium-sulfur batteries: A first principles study. J. Appl. Phys. 2019, 125, 094303. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribble, D.A.; Lin, Z.-Y.; Ghosh, S.; Savoie, B.M.; Pol, V.G. Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators. Batteries 2022, 8, 253. https://doi.org/10.3390/batteries8120253
Gribble DA, Lin Z-Y, Ghosh S, Savoie BM, Pol VG. Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators. Batteries. 2022; 8(12):253. https://doi.org/10.3390/batteries8120253
Chicago/Turabian StyleGribble, Daniel A., Zih-Yu Lin, Sourav Ghosh, Brett M. Savoie, and Vilas G. Pol. 2022. "Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators" Batteries 8, no. 12: 253. https://doi.org/10.3390/batteries8120253
APA StyleGribble, D. A., Lin, Z.-Y., Ghosh, S., Savoie, B. M., & Pol, V. G. (2022). Mitigating Polysulfide Shuttles with Upcycled Alkali Metal Terephthalate Decorated Separators. Batteries, 8(12), 253. https://doi.org/10.3390/batteries8120253






