Research Trends on the Dispersibility of Carbon Nanotube Suspension with Surfactants in Their Application as Electrodes of Batteries: A Mini-Review
Abstract
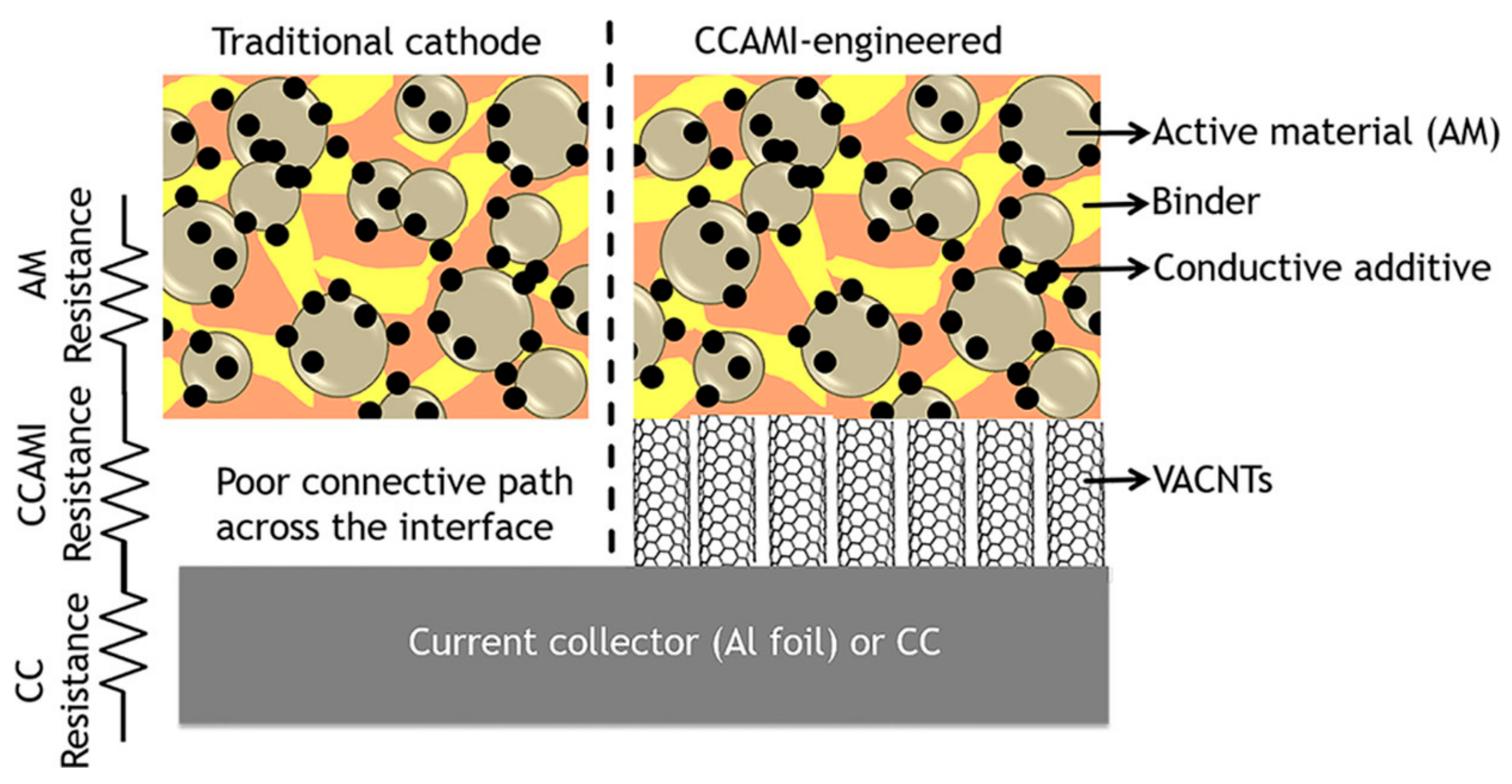
1. Introduction
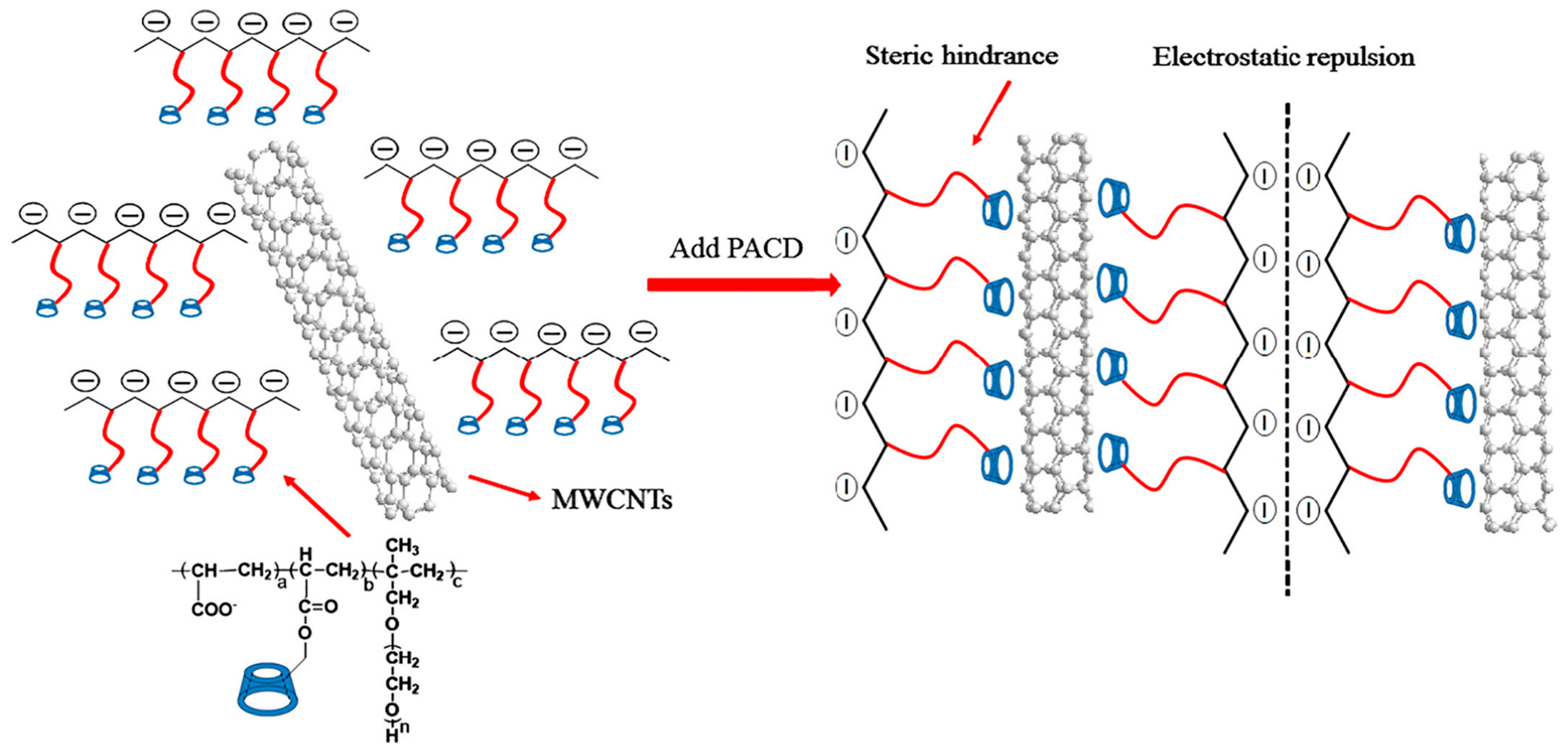
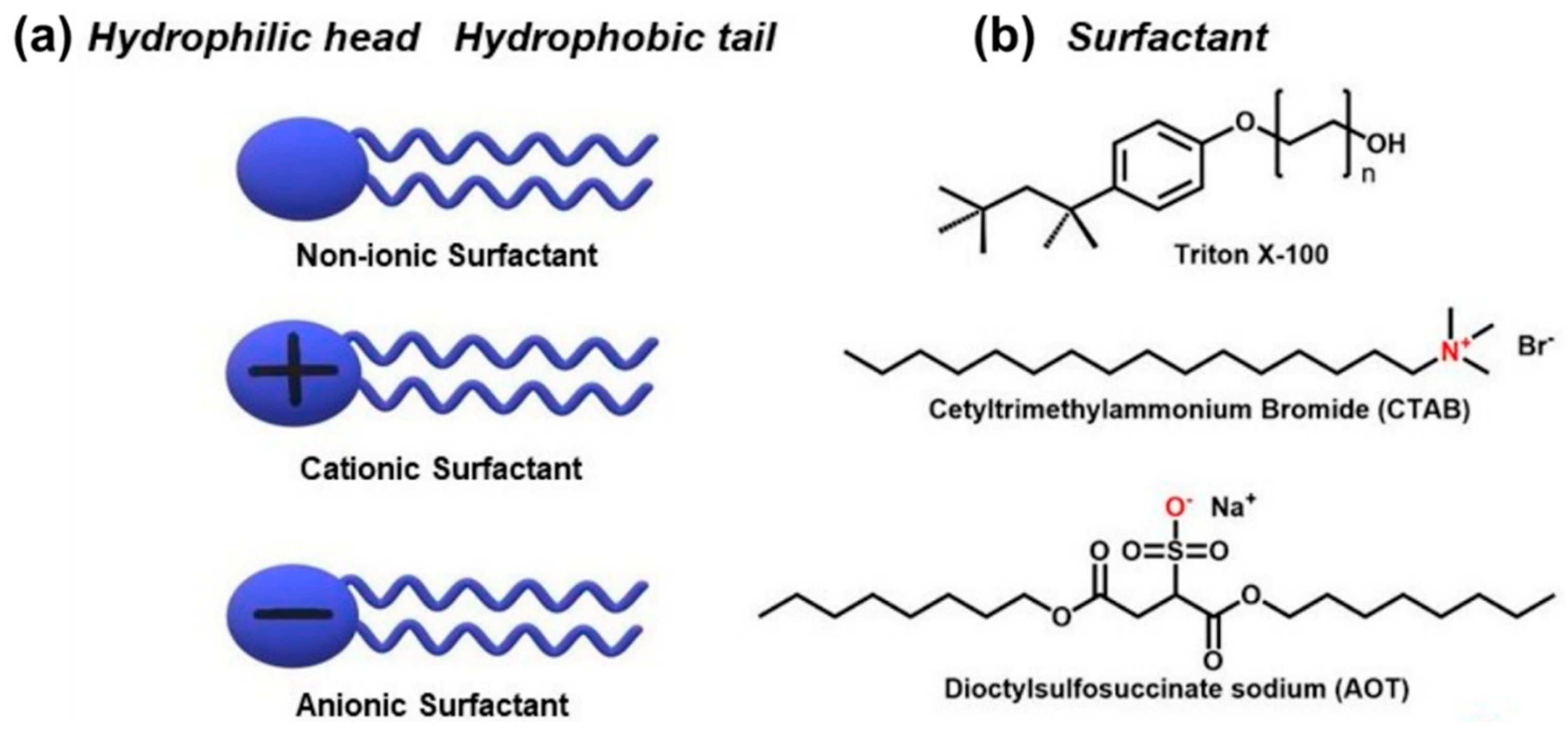
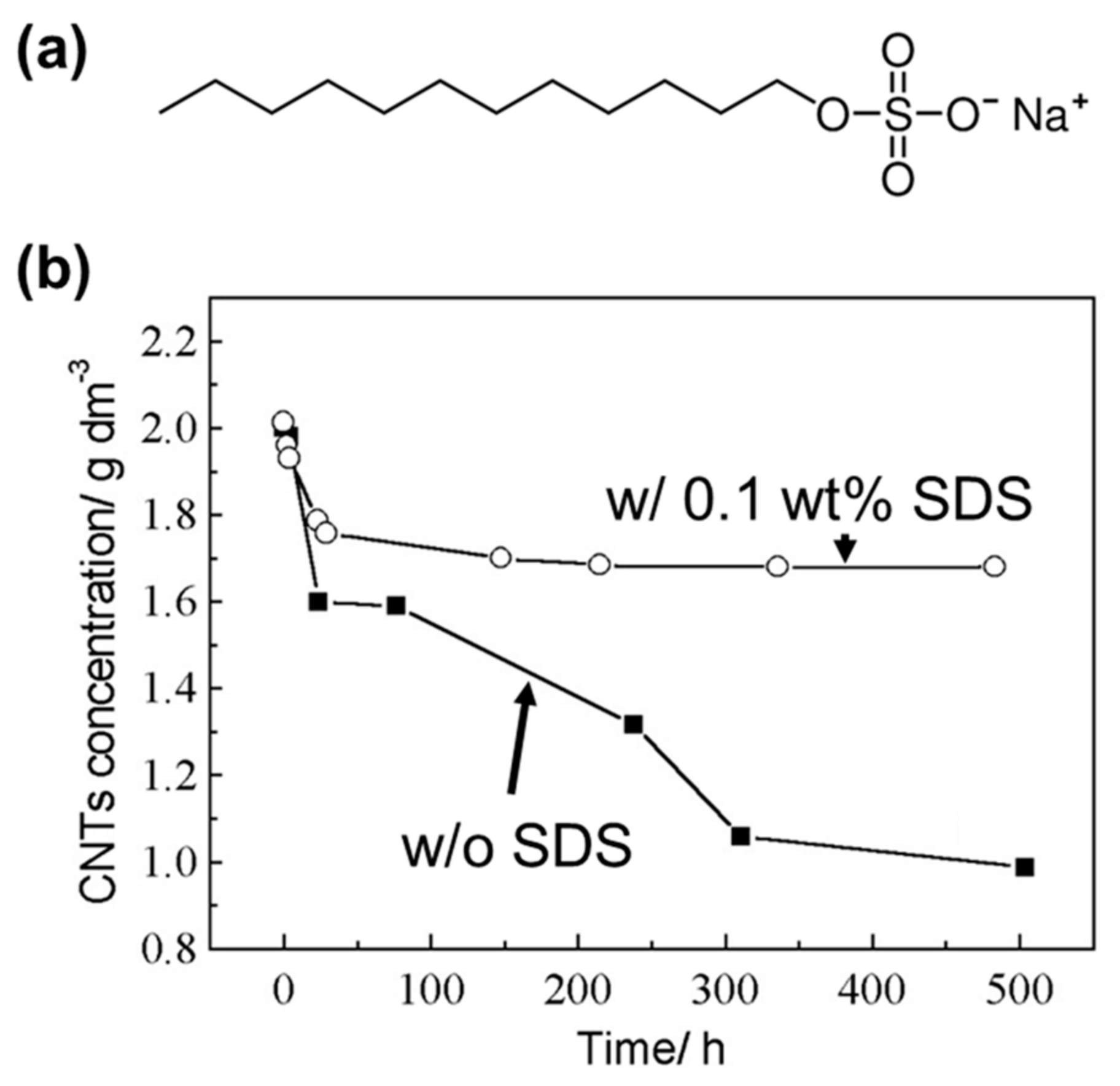
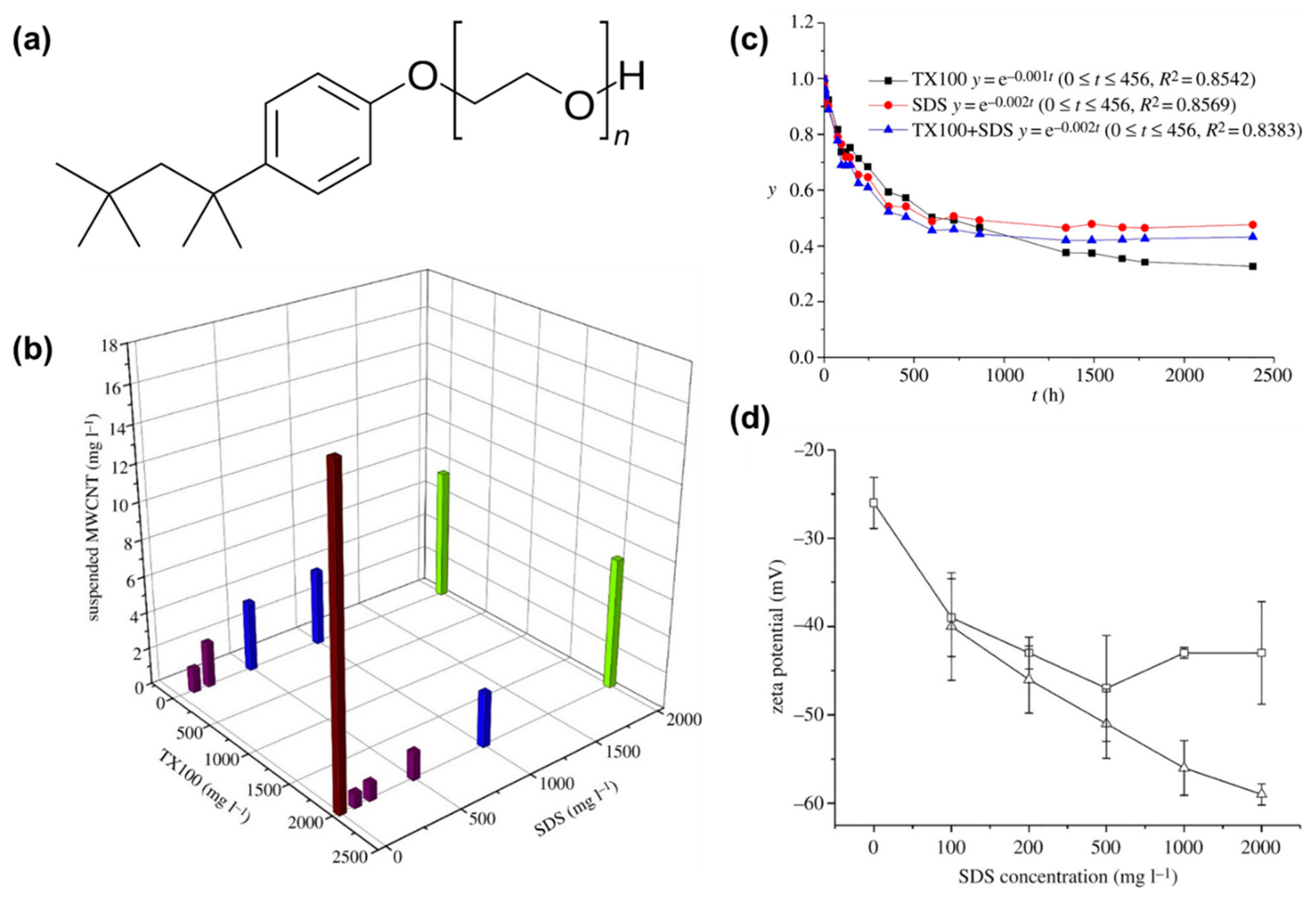
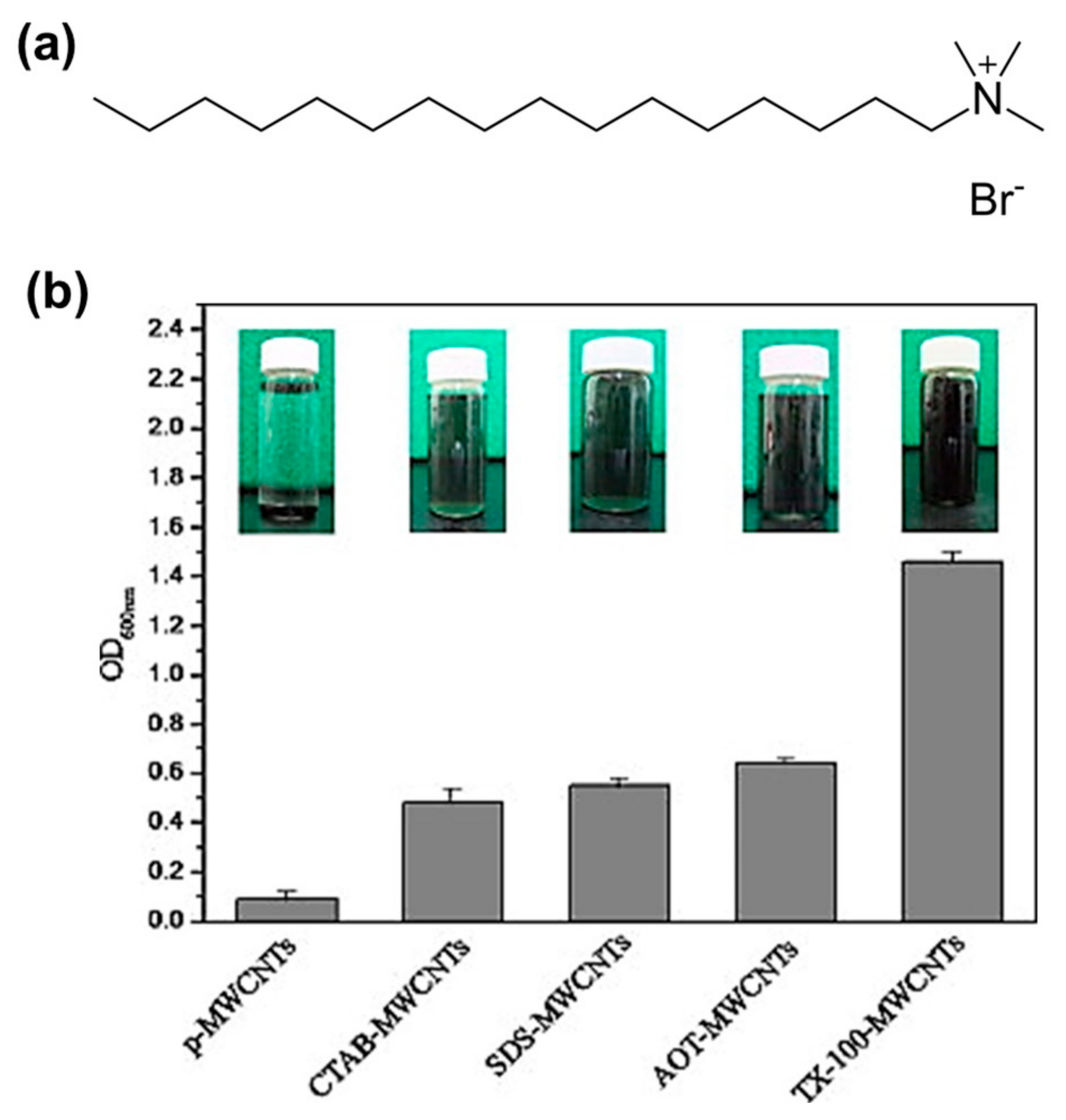
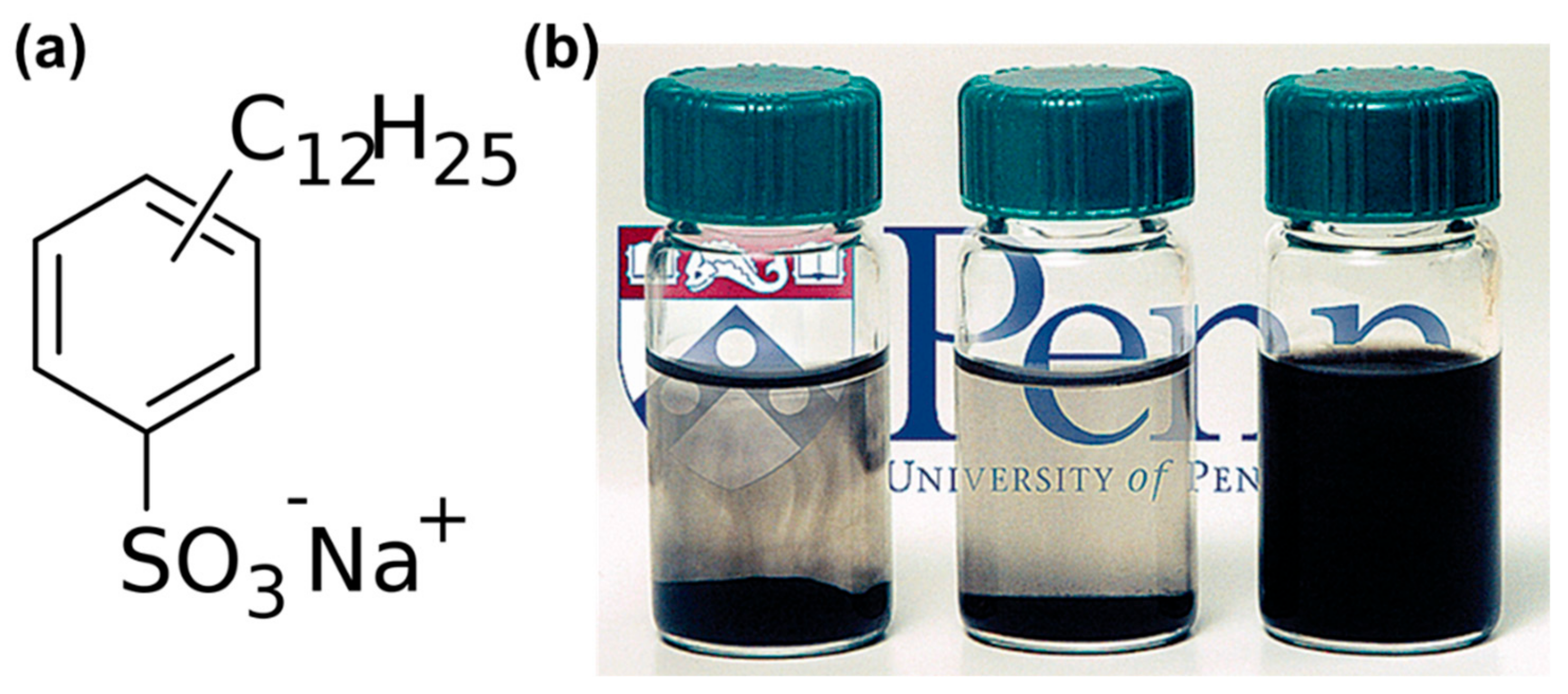
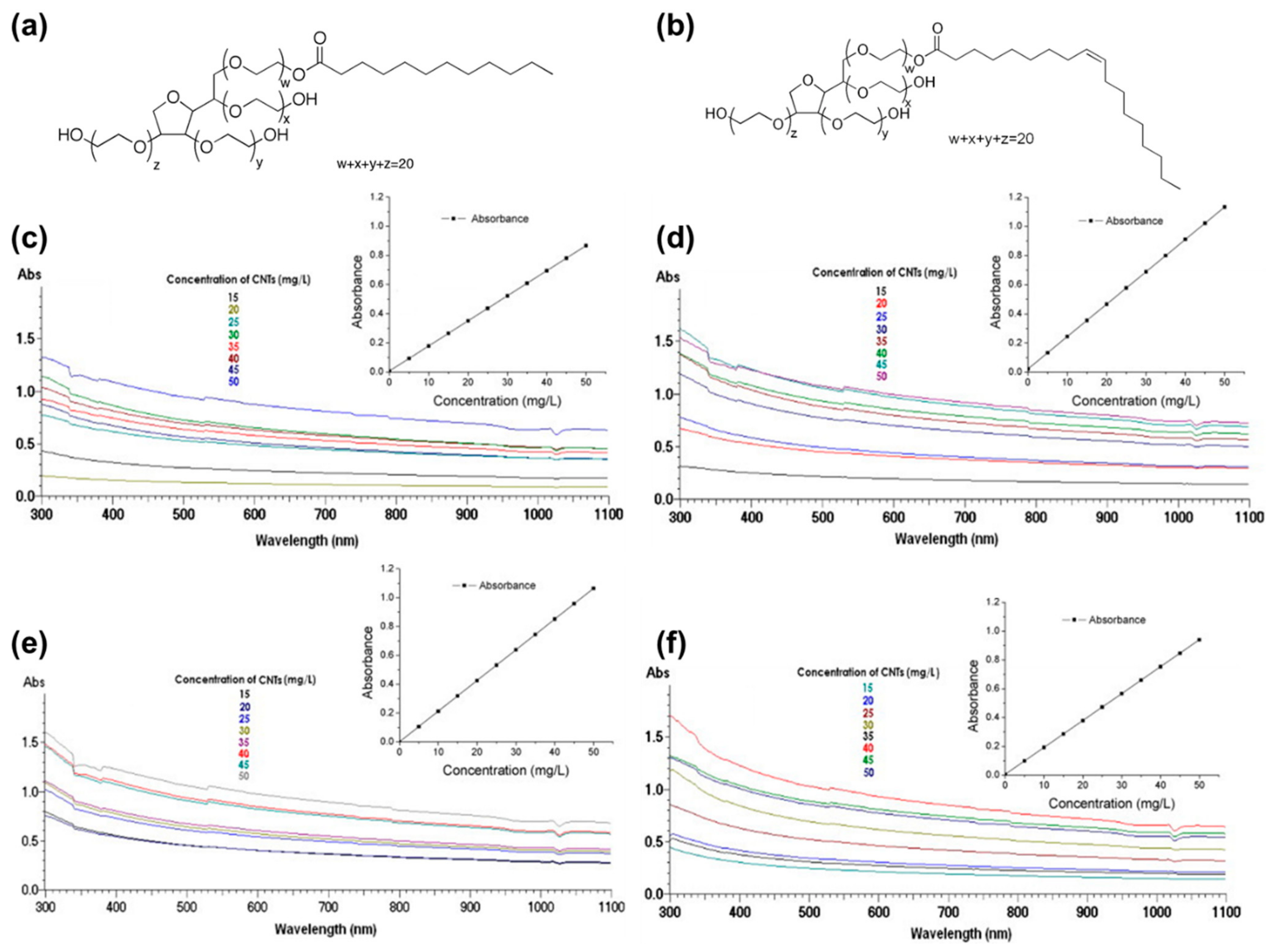
2. Enhancing the Dispersibility of CNTs Using Various Types of Surfactants
3. Summary
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheong, J.Y.; Lee, S.; Lee, J.; Lim, H.; Cho, S.-H.; Lee, D.C.; Kim, I.-D. CuFeO2–NiFe2O4 hybrid electrode for lithium-ion batteries with ultra-stable electrochemical performance. RSC Adv. 2019, 9, 27257–27263. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Han, Y.; Matteini, P. Bending fatigue behavior of Ag nanowire/Cu thin-film hybrid interconnects for wearable electronics. Facta Univ. Ser. Mech. Eng. 2022. [Google Scholar] [CrossRef]
- Ventrapragada, L.K.; Zhu, J.; Creager, S.E.; Rao, A.M.; Podila, R. A Versatile Carbon Nanotube-Based Scalable Approach for Improving Interfaces in Li-Ion Battery Electrodes. ACS Omega 2018, 3, 4502–4508. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, K.; Lv, C.; Zhong, S.; Wang, Q.; Liu, T.; Liu, X.; Yin, Y.; Hu, Y.; Wei, D. Ultrahigh-energy density lithium-ion cable battery based on the carbon-nanotube woven macrofilms. Small 2018, 14, 1800414. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, C.S.; Hwang, J.-Y.; Kim, S.-J.; Maglia, F.; Lamp, P.; Myung, S.-T.; Sun, Y.-K. High-energy-density lithium-ion battery using a carbon-nanotube–Si composite anode and a compositionally graded Li [Ni0.85 Co0.05 Mn0.10] O2 cathode. Energy Environ. Sci. 2016, 9, 2152–2158. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, W.; Cheng, X.-B.; Huang, J.-Q.; Peng, H.-J.; Yang, S.-H.; Zhang, Q. Cathode materials based on carbon nanotubes for high-energy-density lithium–sulfur batteries. Carbon 2014, 75, 161–168. [Google Scholar] [CrossRef]
- Hu, J.W.; Wu, Z.P.; Zhong, S.W.; Zhang, W.B.; Suresh, S.; Mehta, A.; Koratkar, N. Folding insensitive, high energy density lithium-ion battery featuring carbon nanotube current collectors. Carbon 2015, 87, 292–298. [Google Scholar] [CrossRef]
- Landi, B.J.; Cress, C.D.; Raffaelle, R.P. High energy density lithium-ion batteries with carbon nanotube anodes. J. Mater. Res. 2010, 25, 1636–1644. [Google Scholar] [CrossRef]
- Liu, X.-M.; dong Huang, Z.; woon Oh, S.; Zhang, B.; Ma, P.-C.; Yuen, M.M.; Kim, J.-K. Carbon nanotube (CNT)-based composites as electrode material for rechargeable Li-ion batteries: A review. Compos. Sci. Technol. 2012, 72, 121–144. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Y.; Cheng, L.; Wu, H.; Zheng, L.; Zhao, D. The applications of carbon nanotubes and graphene in advanced rechargeable lithium batteries. J. Mater. Chem. A 2016, 4, 8932–8951. [Google Scholar] [CrossRef]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett. 2009, 9, 1002–1006. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.G.; Dai, S. Soft-templated mesoporous carbon-carbon nanotube composites for high performance lithium-ion batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Nossol, E.; Souza, V.H.; Zarbin, A.J. Carbon nanotube/Prussian blue thin films as cathodes for flexible, transparent and ITO-free potassium secondary battery. J. Colloid Interface Sci. 2016, 478, 107–116. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Yang, Z. Improved performance of flower-like ZnAl LDH growing on carbon nanotubes used in zinc–nickel secondary battery. Electrochim. Acta 2018, 277, 67–76. [Google Scholar] [CrossRef]
- Kim, T.; Mo, Y.; Nahm, K.; Oh, S.M. Carbon nanotubes (CNTs) as a buffer layer in silicon/CNTs composite electrodes for lithium secondary batteries. J. Power Sources 2006, 162, 1275–1281. [Google Scholar] [CrossRef]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.-S.; Hammond, P.T.; Shao-Horn, Y. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Li, Q.; Fan, S.; Wang, J. Macroscopic carbon nanotube structures for lithium batteries. Small 2020, 16, 1902719. [Google Scholar] [CrossRef]
- Qaiser, N.; Al-Modaf, F.; Khan, S.M.; Shaikh, S.F.; El-Atab, N.; Hussain, M.M. A Robust Wearable Point-of-Care CNT-Based Strain Sensor for Wirelessly Monitoring Throat-Related Illnesses. Adv. Funct. Mater. 2021, 31, 2103375. [Google Scholar] [CrossRef]
- Mun, J.; Park, J.-H.; Choi, W.; Benayad, A.; Park, J.-H.; Lee, J.-M.; Doo, S.-G.; Oh, S.M. New dry carbon nanotube coating of over-lithiated layered oxide cathode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 19670–19677. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Y.; Luo, S.; He, X.; Wang, J.; Jiang, K.; Fan, S. Hybrid super-aligned carbon nanotube/carbon black conductive networks: A strategy to improve both electrical conductivity and capacity for lithium ion batteries. J. Power Sources 2013, 233, 209–215. [Google Scholar] [CrossRef]
- Hai, N.Q.; Kim, H.; Yoo, I.S.; Hur, J. Facile and scalable preparation of a MoS2/carbon nanotube nanocomposite anode for high-performance lithium-ion batteries: Effects of carbon nanotube content. J. Nanosci. Nanotechnol. 2019, 19, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Cho, S.; Lee, Y.-S. Effect of the addition of carbon black and carbon nanotube to FeS2 cathode on the electrochemical performance of thermal battery. J. Ind. Eng. Chem. 2014, 20, 3584–3589. [Google Scholar] [CrossRef]
- Qaiser, N.; Kim, Y.J.; Hong, C.S.; Han, S.M. Numerical Modeling of Fracture-Resistant Sn Micropillars as Anode for Lithium Ion Batteries. J. Phys. Chem. C 2016, 120, 6953–6962. [Google Scholar] [CrossRef]
- Palanisamy, R.; Karuppiah, D.; Venkatesan, S.; Mani, S.; Kuppusamy, M.; Marimuthu, S.; Karuppanan, A.; Govindaraju, R.; Marimuthu, S.; Rengapillai, S.; et al. High-performance asymmetric supercapacitor fabricated with a novel MoS2/Fe2O3/Graphene composite electrode. Colloid Interface Sci. Commun. 2022, 46, 100573. [Google Scholar] [CrossRef]
- Kapiamba, K.F. Mini-review of the microscale phenomena during emulsification of highly concentrated emulsions. Colloid Interface Sci. Commun. 2022, 47, 100597. [Google Scholar] [CrossRef]
- Islam, M.S.; Mitra, S. Development of nano structured graphene oxide incorporated dexamethasone with enhanced dissolution. Colloid Interface Sci. Commun. 2022, 47, 100599. [Google Scholar] [CrossRef]
- Singh, M.; Rana, S.; Singh, A.K. Advanced nanomaterials utilized as top transparent electrodes in semi-transparent photovoltaic. Colloid Interface Sci. Commun. 2022, 46, 100563. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Recent advances in utility of artificial intelligence towards multiscale colloidal based materials design. Colloid Interface Sci. Commun. 2022, 47, 100595. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Hyun, S.; Han, S.M. Fabrication of ultralight 3D porous composite for Ag nanowire/cellulose nanofiber with tunable mechanical and electrical properties via directional freeze casting. Extrem. Mech. Lett. 2019, 30, 100512. [Google Scholar] [CrossRef]
- Hu, C.Y.; Xu, Y.J.; Duo, S.W.; Zhang, R.F.; Li, M.S. Non-covalent functionalization of carbon nanotubes with surfactants and polymers. J. Chin. Chem. Soc. 2009, 56, 234–239. [Google Scholar] [CrossRef]
- Hofstra, A.A.; Morris, M.L.; Sample, J.L.; Powell, W.D. Non-covalent functionalized nanotubes in nylon 12. Phys. Chem. Interfaces Nanomater. VI SPIE 2007, 7–15. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Wong, E.H. Elucidation of antimicrobial activity of non-covalently dispersed carbon nanotubes. Materials 2020, 13, 1676. [Google Scholar] [CrossRef]
- Tuncel, D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale 2011, 3, 3545–3554. [Google Scholar] [CrossRef]
- Chen, G.-X.; Li, Y.; Shimizu, H. Ultrahigh-shear processing for the preparation of polymer/carbon nanotube composites. Carbon 2007, 45, 2334–2340. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Minot, M.; Rantell, T. Fabrication of carbon multiwall nanotube/polymer composites by shear mixing. Macromol. Mater. Eng. 2002, 287, 395–403. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.-W. Aligned multi-walled carbon nanotube-reinforced composites: Processing and mechanical characterization. J. Phys. D Appl. Phys. 2002, 35, L77. [Google Scholar] [CrossRef]
- Luo, Z.; Koo, J. Quantitative study of the dispersion degree in carbon nanofiber/polymer and carbon nanotube/polymer nanocomposites. Mater. Lett. 2008, 62, 3493–3496. [Google Scholar] [CrossRef]
- Gupta, M.L.; Sydlik, S.A.; Schnorr, J.M.; Woo, D.J.; Osswald, S.; Swager, T.M.; Raghavan, D. The effect of mixing methods on the dispersion of carbon nanotubes during the solvent-free processing of multiwalled carbon nanotube/epoxy composites. J. Polym. Sci. Part B: Polym. Phys. 2013, 51, 410–420. [Google Scholar] [CrossRef]
- Hwang, B.; An, Y.; Lee, H.; Lee, E.; Becker, S.; Kim, Y.-H.; Kim, H. Highly Flexible and Transparent Ag Nanowire Electrode Encapsulated with Ultra-Thin Al2O3: Thermal, Ambient, and Mechanical Stabilities. Sci. Rep. 2017, 7, 41336. [Google Scholar] [CrossRef]
- Inam, F.; Vo, T.; Jones, J.P.; Lee, X. Effect of carbon nanotube lengths on the mechanical properties of epoxy resin: An experimental study. J. Compos. Mater. 2013, 47, 2321–2330. [Google Scholar] [CrossRef]
- Cheng, Q.; Debnath, S.; Gregan, E.; Byrne, H.J. Ultrasound-assisted SWNTs dispersion: Effects of sonication parameters and solvent properties. J. Phys. Chem. C 2010, 114, 8821–8827. [Google Scholar] [CrossRef]
- Montazeri, A.; Chitsazzadeh, M. Effect of sonication parameters on the mechanical properties of multi-walled carbon nanotube/epoxy composites. Mater. Des. (1980–2015) 2014, 56, 500–508. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Z.; Jing, Q.; Yue, R.; Jiang, W.; Lin, D. Sonication-assisted dispersion of carbon nanotubes in aqueous solutions of the anionic surfactant SDBS: The role of sonication energy. Chin. Sci. Bull. 2013, 58, 2082–2090. [Google Scholar] [CrossRef]
- Montazeri, A.; Montazeri, N.; Pourshamsian, K.; Tcharkhtchi, A. The effect of sonication time and dispersing medium on the mechanical properties of multiwalled carbon nanotube (MWCNT)/epoxy composite. Int. J. Polym. Anal. Charact. 2011, 16, 465–476. [Google Scholar] [CrossRef]
- Gao, S.; Villacorta, B.S.; Ge, L.; Rufford, T.E.; Zhu, Z. Effect of sonication and hydrogen peroxide oxidation of carbon nanotube modifiers on the microstructure of pitch-derived activated carbon foam discs. Carbon 2017, 124, 142–151. [Google Scholar] [CrossRef]
- Meng, Y.; Liao, B.; Pang, H.; Zhang, J.; Song, L. Cyclodextrin-modified polycarboxylate superplasticizers as dispersant agents for multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2019, 136, 47311. [Google Scholar] [CrossRef]
- Soleimani Zohr Shiri, M.; Henderson, W.; Mucalo, M.R. A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh. Materials 2019, 12, 1896. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Holmberg, K. Surfactants. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 1–56. [Google Scholar]
- Li, H.; Qiu, Y. Dispersion, sedimentation and aggregation of multi-walled carbon nanotubes as affected by single and binary mixed surfactants. R. Soc. Open Sci. 2019, 6, 190241. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Gadhamshetty, V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: A review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef]
- Tan, Y.; Resasco, D.E. Dispersion of Single-Walled Carbon Nanotubes of Narrow Diameter Distribution. J. Phys. Chem. B 2005, 109, 14454–14460. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Bricha, M.; El Mabrouk, K. Effect of surfactants on the degree of dispersion of MWNTs in ethanol solvent. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 561, 57–69. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Ismail, A.F.; Aziz, M.; Sanip, S.M. Surfactant dispersed multi-walled carbon nanotube/polyetherimide nanocomposite membrane. Solid State Sci. 2010, 12, 2155–2162. [Google Scholar] [CrossRef]
- Samanta, S.K.; Fritsch, M.; Scherf, U.; Gomulya, W.; Bisri, S.Z.; Loi, M.A. Conjugated Polymer-Assisted Dispersion of Single-Wall Carbon Nanotubes: The Power of Polymer Wrapping. Acc. Chem. Res. 2014, 47, 2446–2456. [Google Scholar] [CrossRef]
- Gomulya, W.; Costanzo, G.D.; de Carvalho, E.J.F.; Bisri, S.Z.; Derenskyi, V.; Fritsch, M.; Fröhlich, N.; Allard, S.; Gordiichuk, P.; Herrmann, A.; et al. Semiconducting Single-Walled Carbon Nanotubes on Demand by Polymer Wrapping. Adv. Mater. 2013, 25, 2948–2956. [Google Scholar] [CrossRef]
- Lassi, U.; Hu, T.; Pohjalainen, E.; Kallio, T.; Kordas, K.; Jantunen, H. Effect of a Surfactant Assisted Synthesis on the Electrochemical Performance of a LiFePO4-CNT Composite Electrode. Int. J. Mater. Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Jing, L.; Zheng, X.; Xu, Z.; Yuan, Y.; Liu, X.; Fu, A.; Guo, Y.-G.; Li, H. Microspheres of Si@Carbon-CNTs composites with a stable 3D interpenetrating structure applied in high-performance lithium-ion battery. J. Colloid Interface Sci. 2023, 629, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jeong, Y.J.; Jang, J.; Lim, S.; Kim, S.H. The effect of surfactants on electrohydrodynamic jet printing and the performance of organic field-effect transistors. Phys. Chem. Chem. Phys. 2018, 20, 1210–1220. [Google Scholar] [CrossRef] [PubMed]

| Material | Type | Aromatic Unit | Solvent | Dispersibility | Ref. |
|---|---|---|---|---|---|
| SDS | Anionic | No | Water | Moderate | [49,51,52] |
| Ethanol | Excellent | [57] | |||
| NMP | Good | [58] | |||
| CTAB | Cationic | No | Water | Moderate | [52] |
| Ethanol | Bad | [57] | |||
| NMP | Bad | [58] | |||
| AOT | Anionic | No | Water | Moderate | [52] |
| Tween 20 | Non-ionic | No | Water | Good | [56] |
| Tween 80 | Non-ionic | No | Water | Good | [56] |
| TX 100 | Non-ionic | Yes | Water | Excellent | [51,52,63] |
| Ethanol | Good | [57] | |||
| NMP | Excellent | [58] | |||
| SDBS | Anionic | Yes | Water | Excellent | [63] |
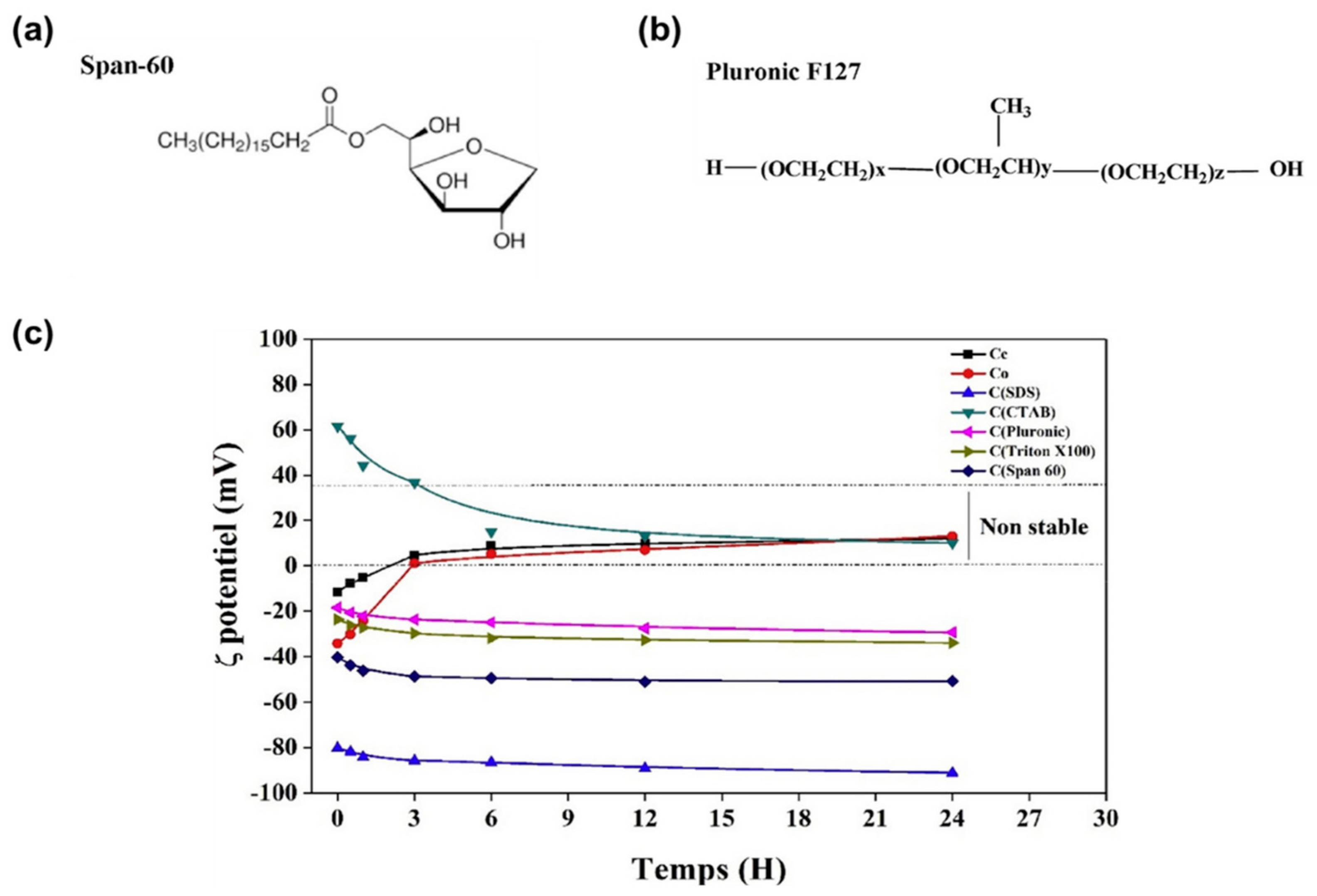
| Span 60 | Non-ionic | No | Ethanol | Excellent | [57] |
| Pluronic F127 | Non-ionic | No | Ethanol | Good | [57] |
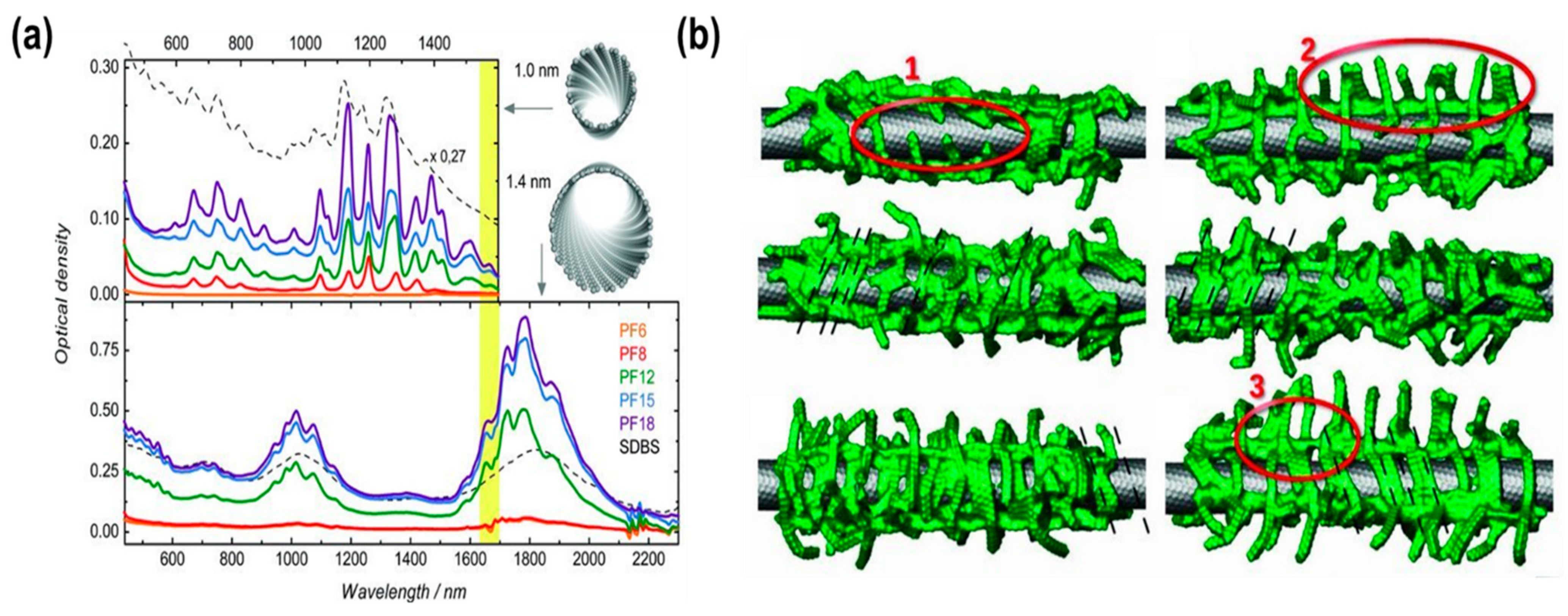
| PF6 | Polymer | Yes | Toluene | Bad | [60] |
| PF8 | Moderate | ||||
| PF12 | Good | ||||
| PF15 | Excellent | ||||
| PF18 | Excellent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.; Kim, H.; Matteini, P.; Hwang, B. Research Trends on the Dispersibility of Carbon Nanotube Suspension with Surfactants in Their Application as Electrodes of Batteries: A Mini-Review. Batteries 2022, 8, 254. https://doi.org/10.3390/batteries8120254
Yoon H, Kim H, Matteini P, Hwang B. Research Trends on the Dispersibility of Carbon Nanotube Suspension with Surfactants in Their Application as Electrodes of Batteries: A Mini-Review. Batteries. 2022; 8(12):254. https://doi.org/10.3390/batteries8120254
Chicago/Turabian StyleYoon, Hyungsub, Haeji Kim, Paolo Matteini, and Byungil Hwang. 2022. "Research Trends on the Dispersibility of Carbon Nanotube Suspension with Surfactants in Their Application as Electrodes of Batteries: A Mini-Review" Batteries 8, no. 12: 254. https://doi.org/10.3390/batteries8120254
APA StyleYoon, H., Kim, H., Matteini, P., & Hwang, B. (2022). Research Trends on the Dispersibility of Carbon Nanotube Suspension with Surfactants in Their Application as Electrodes of Batteries: A Mini-Review. Batteries, 8(12), 254. https://doi.org/10.3390/batteries8120254







