On the Specific Capacity and Cycle Stability of Si@void@C Anodes: Effects of Particle Size and Charge/Discharge Protocol
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Characteristics of Si@void@C Particles
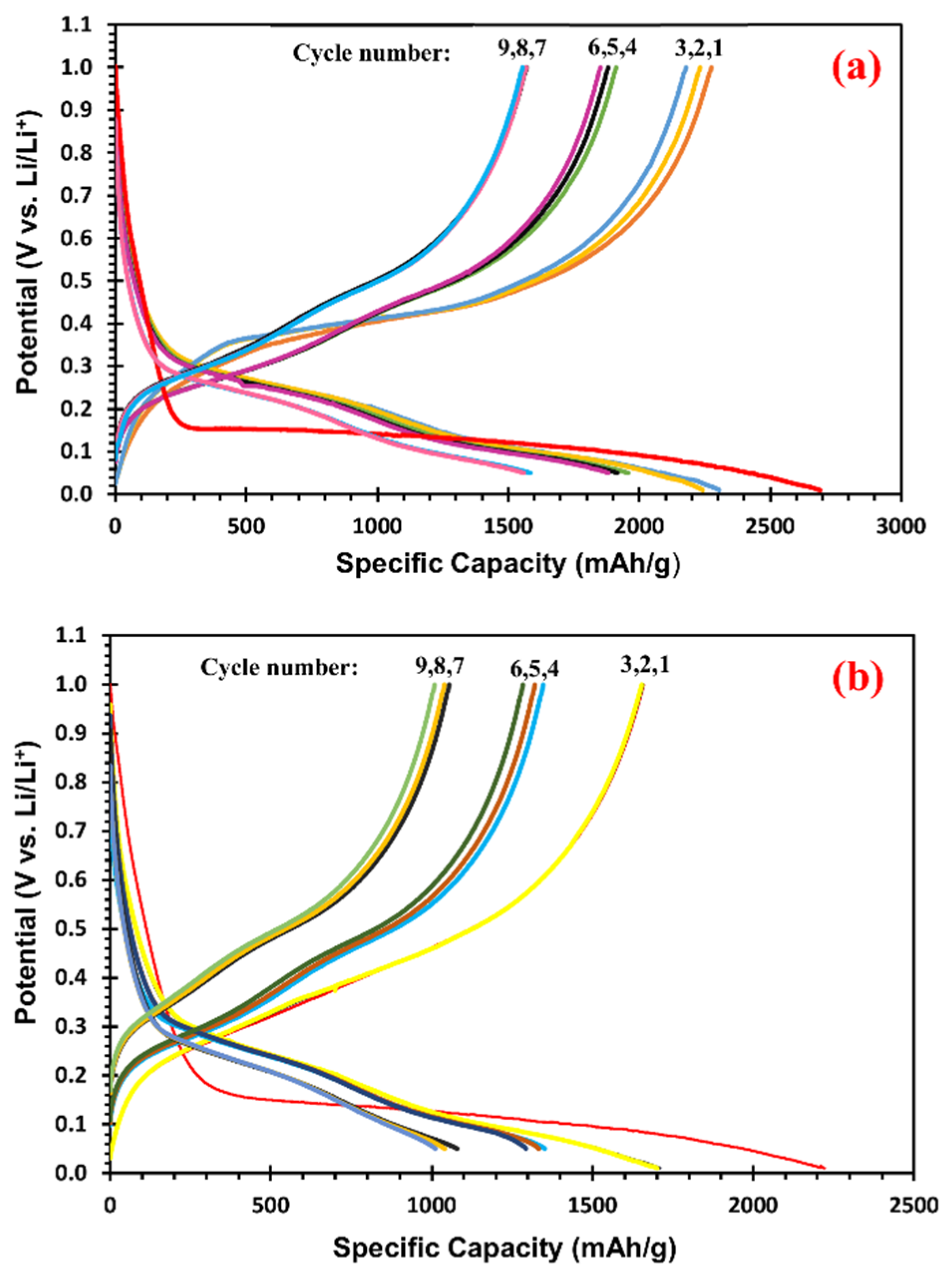
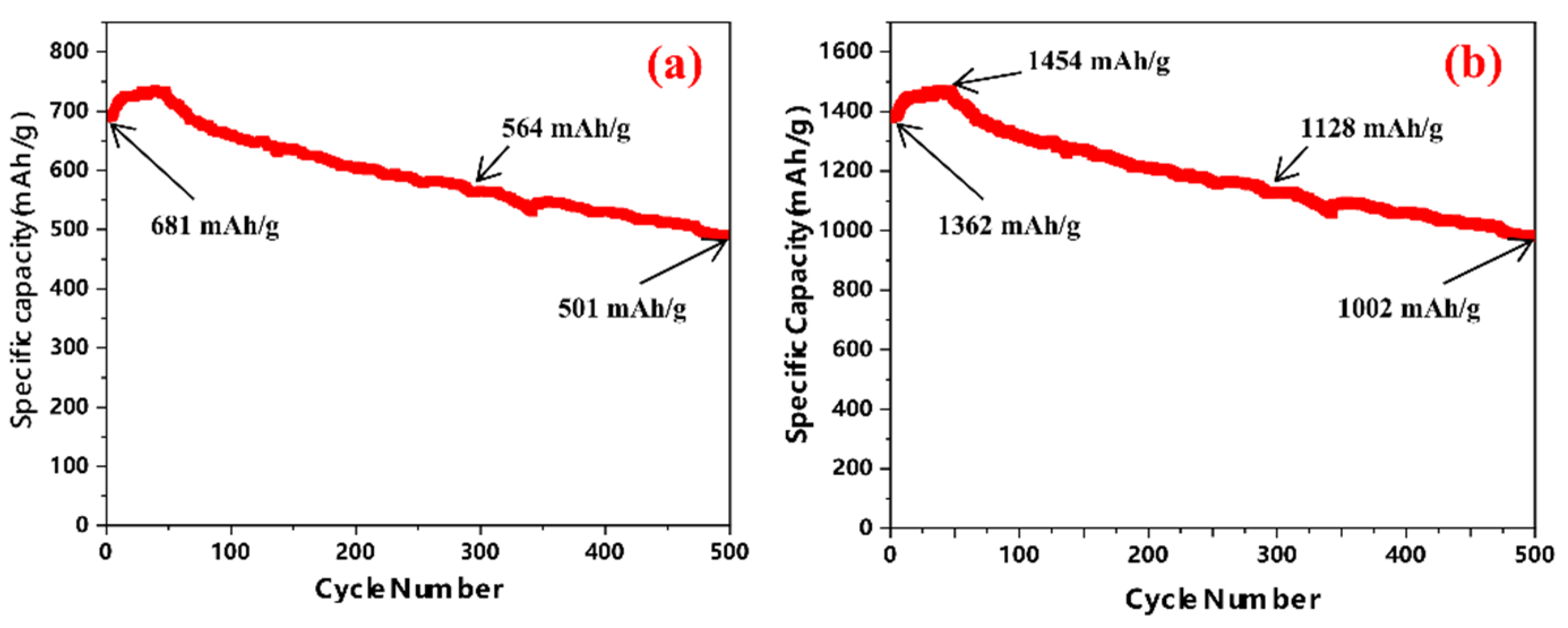
3.2. Specific Capacity of Si@void@C Anode as a Function of Particle Size
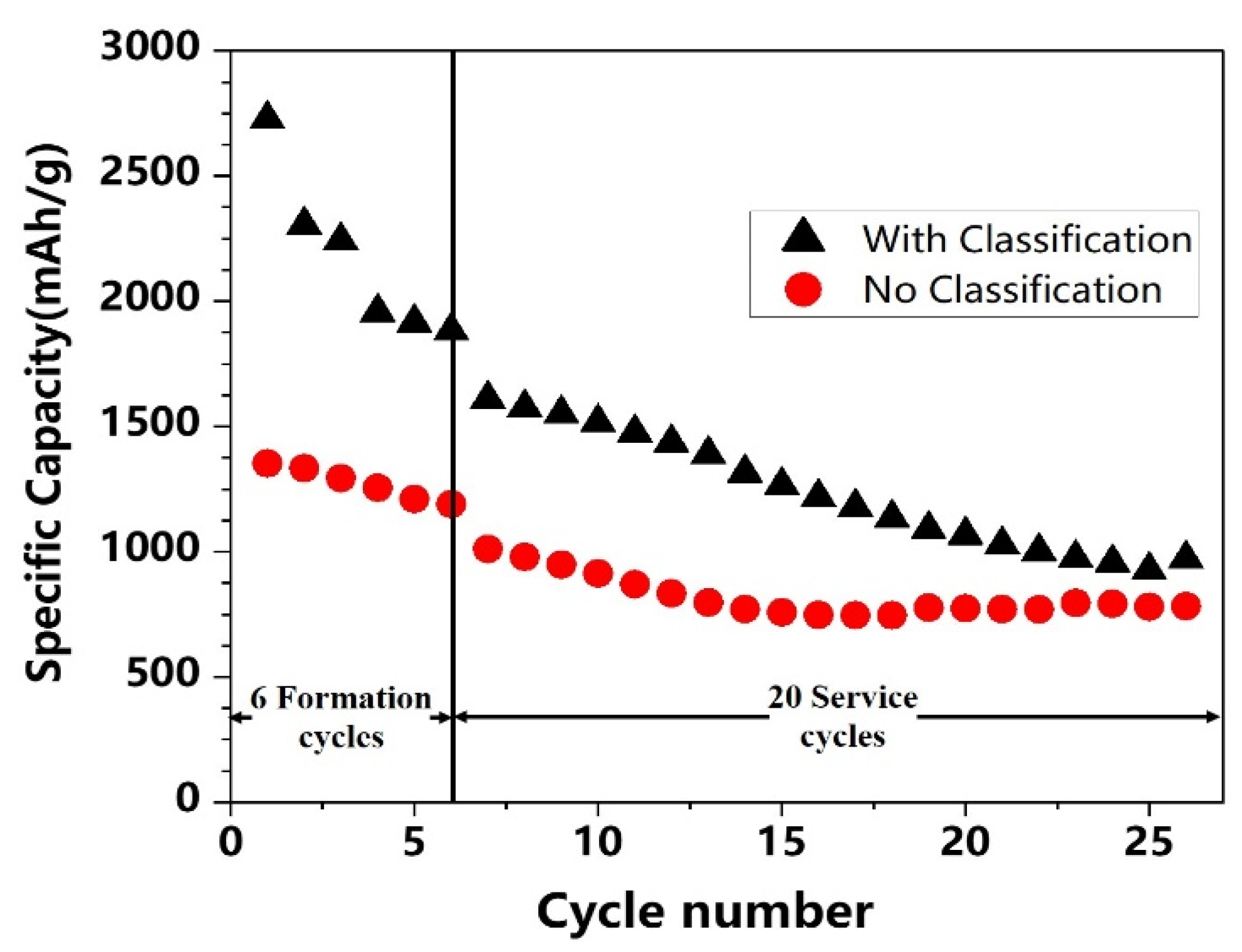
3.3. Effects of Si@void@C Particle Sizes on Cycle Stability
3.4. Enhancing the Cycle Stability of Si@void@C Anode via Charge/Discharge Protocol
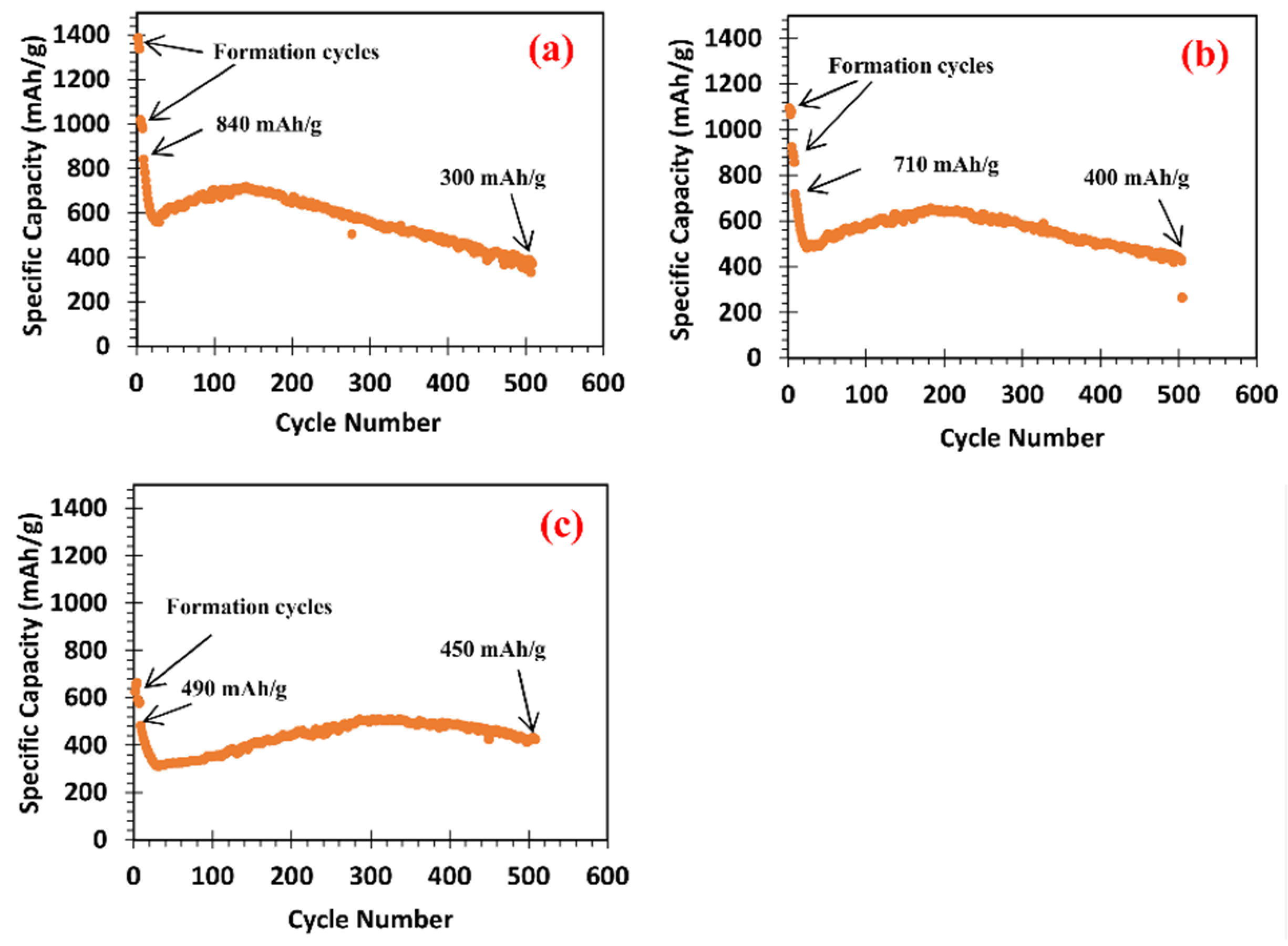
3.4.1. Effects of the Lower Cutoff Voltage in Formation Cycles on Specific Capacity and Cycle Stability
3.4.2. Effects of the Upper Cutoff Voltage on Specific Capacity and Cycle Stability
3.4.3. Effects of Constant Current (CC) and Constant Current-Constant Voltage (CCCV) Charge on Specific Capacity and Cycle Stability
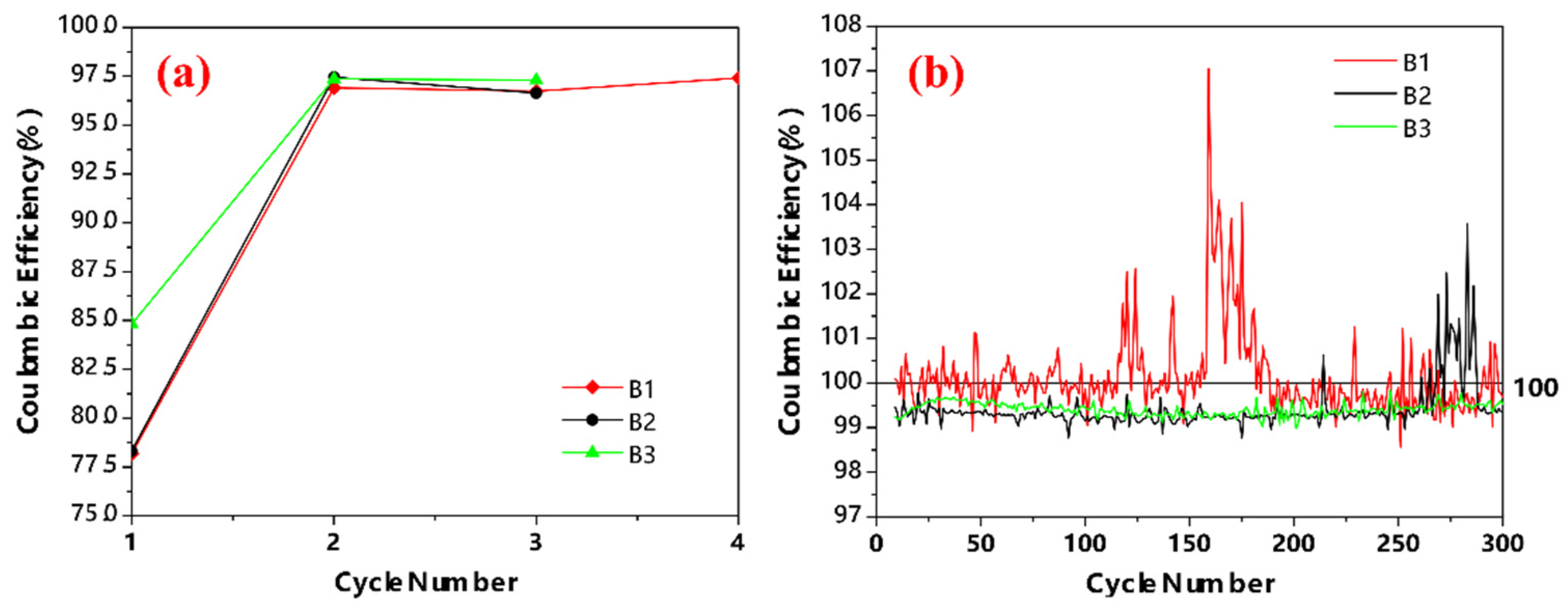
3.5. Coulombic Efficiency (CE) Analysis
3.6. Achieving High Specific Capacity and Good Cycle Stability Simultaneously
4. Conclusions
- Small Si@void@C particles have the advantage of higher specific capacity than large Si@void@C particles because the former has a larger electrode/electrolyte interfacial area for lithiation and delithiation. However, small Si@void@C particles do not have the advantage in the cycle stability over larger Si@void@C particles.
- Charge/discharge protocols in both formation and service cycles have a profound impact on the specific capacity and cycle stability of Si@void@C anodes. Although formation cycles are typically only a few cycles (e.g., 3 to 5 cycles), their influence is far-reaching and can be seen even after 500 service cycles.
- A high LCV (e.g., 0.1 V vs. Li+/Li) in formation cycles results in a small specific capacity at the 1st service cycle but has good cycle stability and high specific capacity after 500 service cycles because it has a small volume change in formation cycles. In contrast, a small LCV (e.g., 0.05 V vs. Li+/Li) in formation cycles results in a large specific capacity at the 1st service cycle but has poor cycle stability and low specific capacity after 500 service cycles because it has a large volume change in formation cycles.
- A high UCV (e.g., 1.5 V vs. Li+/Li) in both formation and service cycles results in high specific capacity initially but with poor cycle stability. In comparison, a low UCV (e.g., 1.0 V vs. Li+/Li) leads to low specific capacity initially but with good cycle stability. Such a phenomenon is attributed to the fact that lower UCV leads to less volume shrinkage of Si@void@C particles during delithiation and thus smaller compressive stresses on the SEI layer, thereby less fracture and reformation of the SEI layer.
- CC charge protocol always leads to rapid capacity decay in the first 20 to 40 cycles, followed by a gradual capacity increase in the next 50 to 150 cycles and then capacity decrease again as the cycle number increases.
- CCCV charge protocol can mitigate rapid capacity decay in the first 20 to 40 cycles because it can minimize polarization and lead to more uniform lithiation of most Si@void@C particles to avoid overcharge of some Si@void@C particles, thereby preventing loss of some Si@void@C particles in contact with CB conductive network and/or severe fracture and reformation of their SEI layers.
- The CE analysis reveals that the LCV at 0.01 V vs. Li+/Li in the formation cycles can provide a CE averaging around 100% in the service cycles, suggesting that the SEI layers formed in the formation cycles are durable due to their small LCV and there is little or no new SEI layer formation in the service cycles. In contrast, the CE in the service cycles is always below 100% for the LCV at high values (such as LCV = 0.1 V vs. Li+/Li) in the formation cycles, indicating that the SEI layers formed in the formation cycles is not durable enough and new SEI layers are formed throughout the entire service cycles.
- With proper charge/discharge protocols Si@void@C anodes can offer specific capacities of 544.8, 451.2 and 400.8 mAh/g-Si@void@C+CB+PAA at the electrode level for the 1st, 300th and 500th cycles. These specific capacities at the electrode level are 66.8%, 38.2% and 22.7% higher than those of graphite anodes at the 1st, 300th and 500th cycles, respectively. Thus, Si@void@C anodes investigated in this study have the potential to replace the state-of-the-art graphite anodes for applications with 500 cycle requirements in the future.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Beattie, S.; Morcrette, M.; Edström, K.; Jumas, J.C.; Tarascon, J.M. Recent findings and prospects in the field of pure metals as negative electrodes for Li-ion batteries. J. Mater. Chem. 2007, 17, 3759–3772. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Xu, Q.; Li, G.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Research progress regarding Si-based anode materials towards practical application in high energy density Li-ion batteries. Mater. Chem. Front. 2017, 1, 1691–1708. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Wetjen, M.; Solchenbach, S.; Pritzl, D.; Hou, J.; Tileli, V.; Gasteiger, H.A. Morphological changes of silicon nanoparticles and the influence of cutoff potentials in silicon-graphite electrodes. J. Electrochem. Soc. 2018, 7, A1503–A1514. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, J.; Zhang, H.P.; Yang, L.C.; Wu, Y.P.; Wu, H.Q. Preparation and electrochemical properties of core-shell Si/SiO nanocomposite as anode material for lithium ion batteries. Electrochem. Commun. 2007, 9, 886–890. [Google Scholar] [CrossRef]
- Hwa, Y.; Kim, W.-S.; Hong, S.-H.; Sohn, H.-J. High capacity and rate capability of core–shell structured nano-Si/C anode for Li-ion batteries. Electrochim. Acta 2012, 71, 201–205. [Google Scholar] [CrossRef]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef]
- Park, Y.; Choi, N.-S.; Park, S.; Woo, S.H.; Sim, S.; Jang, B.Y.; Oh, S.M.; Park, S.; Cho, J.; Lee, K.T. Si-encapsulating hollow carbon electrodes via electroless etching for lithium-ion batteries. Adv. Energy Mater. 2013, 3, 206–212. [Google Scholar] [CrossRef]
- Yang, L.Y.; Li, H.Z.; Liu, J.; Sun, Z.Q.; Tang, S.S.; Lei, M. Dual yolk-shell structure of carbon and silica-coated silicon for high-performance lithium-ion batteries. Sci. Rep. 2015, 5, 10908. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tong, L.; Su, L.; Xu, Y.; Wang, L.; Wang, Y. Core-shell yolk-shell Si@C@Void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance. J. Power Sources 2017, 342, 529–536. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gao, D.; Chen, S.; Tan, L.; Li, L. Facile synthesis of yolk–shell structured Si–C nanocomposites as anodes for lithium-ion batteries. Chem. Commun. 2014, 50, 5878–5880. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- An, Y.; Fei, H.; Zeng, G.; Ci, L.; Xiong, S.; Feng, J.; Qian, Y. Green, scalable, and controllable fabrication of nanoporous silicon from commercial alloy precursors for high-energy lithium-ion batteries. ACS Nano 2018, 12, 4993–5002. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Dai, F.; Gordin, M.L.; Chen, S.; Wang, D. Micro-sized Si-C composite with interconnected nanoscale building blocks as high-performance anodes for practical application in lithium-ion batteries. Adv. Energy Mater. 2013, 3, 295–300. [Google Scholar] [CrossRef]
- Tian, H.; Tan, X.; Xin, F.; Wang, C.; Han, W. Micro-sized nano-porous Si/C anodes for lithium ion batteries. Nano Energy 2015, 11, 490–499. [Google Scholar] [CrossRef]
- Cui, L.-F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core−shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xia, J.; Lee, J.-H.; Lee, D.H.; Kwon, M.-S.; Choi, J.-M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.; Ryou, M.-H.; Han, G.-B.; Lee, J.-N.; Song, J.; Choi, J.; Cho, K.Y.; Lee, Y.M.; Park, J.-K. Electrospun three-dimensional mesoporous silicon nanofibers as an anode material for high-performance lithium secondary batteries. ACS Appl. Mater. Interfaces 2013, 5, 12005–12010. [Google Scholar] [CrossRef]
- Xue, L.; Fu, K.; Li, Y.; Xu, G.; Lu, Y.; Zhang, S.; Toprakci, O.; Zhang, X. Si/C composite nanofibers with stable electric conductive network for use as durable lithium-ion battery anode. Nano Energy 2013, 2, 361–367. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, M.; Chen, G.; Dudko, N.; Li, Y.; Liu, H.; Shi, L.; Wu, G.; Zhang, D. High-performance microsized Si anodes for lithium-ion batteries: Insights into the polymer configuration conversion mechanism. Adv. Mater. 2022, 34, 2109658. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; Savio D’Souza, M.; Doux, J.-M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q.; et al. Role of polyacrylic acid (PAA) binder on the solid electrolyte interphase in silicon anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2019, 2, 3004–3010. [Google Scholar] [CrossRef]
- Song, J.; Zhou, M.; Yi, R.; Xu, T.; Tang, D.; Yu, Z.; Regula, M.; Wang, D. Interpenetrated gel polymer binder for high-performance silicon anodes in lithium-ion batteries. Adv. Funct. Mater. 2014, 24, 5904–5910. [Google Scholar] [CrossRef]
- Jiao, X.; Yin, J.; Xu, X.; Wang, J.; Liu, Y.; Xiong, S.; Zhang, Q.; Song, J. Highly energy-dissipative, fast self-healing binder for stable Si anode in lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2005699. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Yu, X.; Chen, S. Improving rate capacity and cycling stability of Si-anode lithium ion battery by using copper nanowire as conductive additive. J. Alloys Compd. 2020, 822, 153664. [Google Scholar] [CrossRef]
- Martinez De La Hoz, J.M.; Soto, F.A.; Balbuena, P.B. Effect of the electrolyte composition on SEI reactions at Si anodes of Li ion batteries. J. Phys. Chem. C 2015, 119, 7060–7068. [Google Scholar] [CrossRef]
- Nie, M.; Song, J.; Zhou, M.; Yi, R.; Xu, T.; Tang, D.; Yu, Z.; Regula, M.; Wang, D.; Abraham, D.P.; et al. Silicon solid electrolyte interphase (SEI) of lithium ion battery characterized by microscopy and spectroscopy. J. Phys. Chem. C 2013, 117, 13403–13412. [Google Scholar] [CrossRef]
- Jia, H.; Zou, L.; Gao, P.; Cao, X.; Zhao, W.; He, Y.; Engelhard, M.H.; Burton, S.D.; Wang, H.; Ren, X.; et al. High-performance silicon anodes enabled by nonflammable localized high-concentration electrolytes. Adv. Energy Mater. 2019, 9, 1900784. [Google Scholar] [CrossRef]
- Jin, Y.; Kneusels, N.-J.H.; Marbella, L.E.; Castillo-Martínez, E.; Magusin, P.C.M.M.; Weatherup, R.S.; Jónsson, E.; Liu, T.; Paul, S.; Grey, C.P. Understanding fluoroethylene carbonate and vinylene carbonate based electrolytes for Si anodes in lithium ion batteries with NMR spectroscopy. J. Am. Chem. Soc. 2018, 140, 9854–9867. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Han, X.; Xiang, Y.; Zhong, G.; Wang, J.; Zheng, B.; Zhou, J.; Yang, Y. Identification of the solid electrolyte interface on the Si/C composite anode with FEC as the additive. ACS Appl. Mater. Interfaces 2019, 11, 14066–14075. [Google Scholar] [CrossRef]
- He, Q.; Ashuri, M.; Liu, Y.; Liu, B.; Shaw, L. Silicon micro-reactor as a fast charge, long cycle life anode with high initial coulombic efficiency for Li-ion batteries. ACS Appl. Energy Mater. 2021, 4, 4744–4757. [Google Scholar] [CrossRef]
- Klett, M.; Gilbert, J.A.; Pupek, K.Z.; Trask, S.E.; Abraham, D.P. Layered oxide, graphite and silicon-graphite electrodes for lithium-ion cells: Effect of electrolyte composition and cycling windows. J. Electrochem. Soc. 2017, 164, A6095–A6102. [Google Scholar] [CrossRef]
- Prado, A.Y.R.; Rodrigues, M.-T.F.; Trask, S.E.; Shaw, L.; Abraham, D.P. Electrochemical dilatometry of Si-bearing electrodes: Dimensional changes and experimental design. J. Electrochem. Soc. 2020, 167, 160551. [Google Scholar] [CrossRef]
- Strobridge, F.C.; Orvananos, B.; Croft, M.; Yu, H.-C.; Robert, R.; Liu, H.; Zhong, Z.; Connolley, T.; Drakopoulos, M.; Thornton, K.; et al. Mapping the inhomogeneous electrochemical reaction through porous LiFePO4-electrodes in a standard coin cell battery. Chem. Mater. 2015, 27, 2374–2386. [Google Scholar] [CrossRef]
- Markevich, E.; Fridman, K.; Sharabi, R.; Elazari, R.; Salitra, G.; Gottlieb, H.E.; Gershinsky, G.; Garsuch, A.; Semrau, A.; Schmidt, M.A.; et al. Amorphous columnar silicon anodes for advanced high voltage lithium ion full cells: Dominant factors governing cycling performance. J. Electrochem. Soc. 2013, 160, A1824–A1833. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.; Mu, Y.; Zhao, Y.; Wang, Y.; Li, X.; Yao, P.; Deng, Y.; Cui, Y.; Zhu, J. Minimized lithium trapping by isovalent isomorphism for high initial Coulombic efficiency of silicon anodes. Sci. Adv. 2019, 5, eaax0651. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, Y.E.; Kim, H. Improved fast charging capability of graphite anodes via amorphous Al2O3 coating for high power lithium ion batteries. J. Power Sources 2019, 422, 18–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, X.; Li, L.; Su, P.; Huang, W.; Wang, J.; Xu, J.; Li, C.; Chen, S.; Yang, Y. Tailoring the interfaces of silicon/carbon nanotube for high rate lithium-ion battery anodes. J. Power Sources 2020, 450, 227593. [Google Scholar] [CrossRef]
- Yi, R.; Dai, F.; Gordin, M.L.; Sohn, H.; Wang, D. Influence of silicon nanoscale building blocks size and carbon coating on the performance of micro-sized Si-C composite Li-ion anodes. Adv. Energy Mater. 2013, 3, 1507–1515. [Google Scholar] [CrossRef]
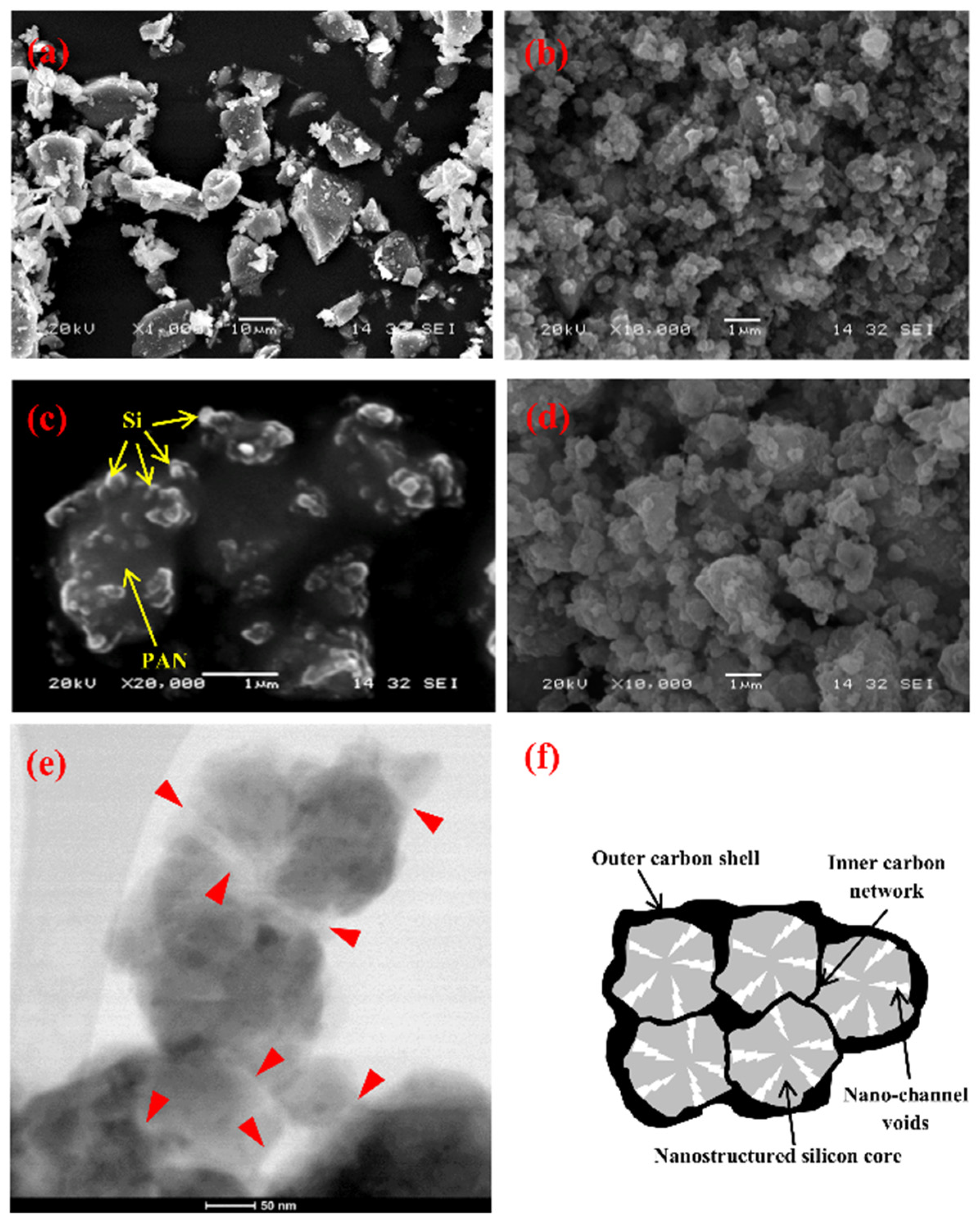


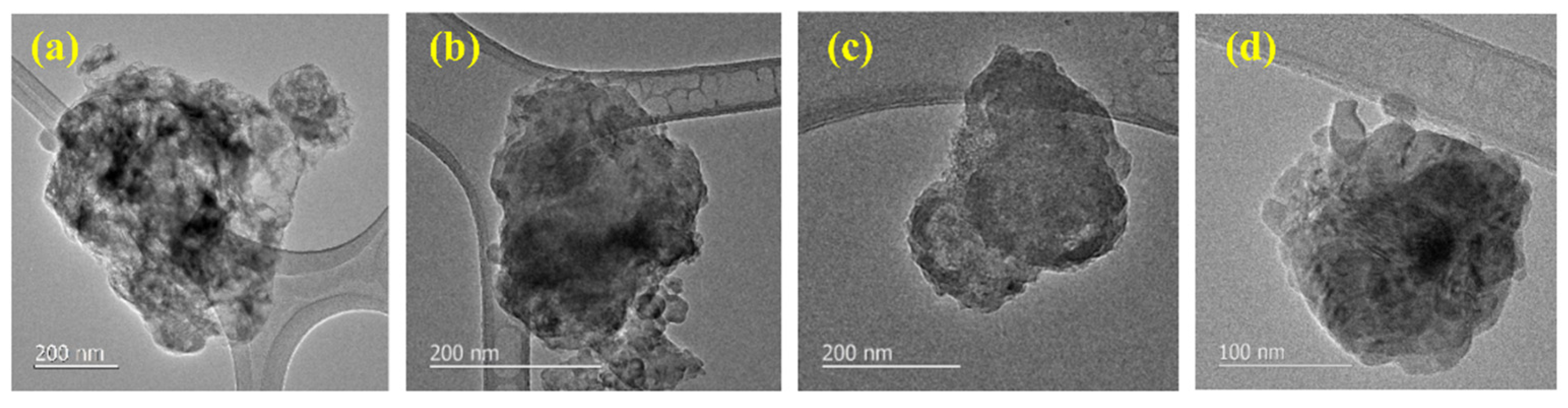
| (a) 100 to 650 nm | (b) 650 to 1200 nm | (c) >1200 nm | (d) 100 to >1200 nm |
|---|---|---|---|
| 45 wt% | 34 wt% | 21 wt% | No classification |
| Sample ID | Formation Cycles | Service Cycles | Specific Capacity at the 1st Service Cycle | Specific Capacity at the 500th Service Cycle |
|---|---|---|---|---|
| M1 | (a) 3 cycles at 0.05 A/g between 0.05 V and 1.5 V vs. Li+/Li. (b) 3 cycles at 0.1 A/g between 0.05 V and 1.5 V vs. Li+/Li. | 500 cycles at 0.5 A/g between 0.1 and 1.5 V vs. Li+/Li. | 840 mAh/g | 300 mAh/g |
| M2 | (a) 3 cycles at 0.05 A/g between 0.075 V and 1.5 V. (b) 3 cycles at 0.1 A/g between 0.075 V and 1.5 V. | 500 cycles at 0.5 A/g between 0.1 and 1.5 V. | 710 mAh/g | 400 mAh/g |
| M3 | (a) 3 cycles at 0.05 A/g between 0.1 V and 1.5 V. (b) 3 cycles at 0.1 A/g between 0.1 V and 1.5 V. | 500 cycles at 0.5 A/g between 0.1 and 1.5 V. | 490 mAh/g | 450 mAh/g |
| M4 | (a) 3 cycles at 0.1 A/g between 0.05 V and 1.0 V. | 800 cycles at 0.5 A/g between 0.1 and 1.0 V. | 305 mAh/g | 252 mAh/g |
| M5 | (a) 3 cycles at 0.1 A/g between 0.05 V and 1.5 V. | 800 cycles at 0.5 A/g between 0.1 and 1.5 V. | 430 mAh/g | 221 mAh/g |
| Protocol ID | Formation Cycles | Service Cycles |
|---|---|---|
| P1 (CCCV in formation cycles and CC in service cycles) | (a) Lithiate Si@void@C at 0.05 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.05 A/g to 1.5 V. (b) Lithiate Si@void@C at 0.1 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.1 A/g to 1.5 V. (c) Lithiate Si@void@C at 0.5 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.5 A/g to 1.5 V. | Lithiate and delithiate at 0.5 A/g between 0.1 V and 1.5 V vs. Li+/Li for 300 cycles. |
| P2 (CC in both formation and service cycles) | (a) Lithiate Si@void@C at 0.05 A/g to 0.01 V vs. Li+/Li and then delithiate the cell at 0.05 A/g to 1.0 V vs. Li+/Li. Repeat this for 4 times. | Lithiate and delithiate at 1.0 A/g between 0.1 V and 1.0 V vs. Li+/Li for 300 cycles. |
| P3 (CCCV in both formation and service cycles) | (a) Lithiate Si@void@C at 0.05 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.05 A/g to 1.0 V. (b) Lithiate Si@void@C at 0.1 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.1 A/g to 1.0 V. (c) Lithiate Si@void@C at 0.5 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.5 A/g to 1.0 V. | Lithiate at 1.0 A/g to 0.15 V vs. Li+/Li and hold at this potential until the current density becomes 0.05 A/g. Delithiate the cell at 0.5 A/g to 1.0 V. Cycle for 300 times. |
| P4 (CCCV in both formation and service cycles) | (a) Lithiate Si@void@C at 0.05 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.05 A/g to 1.0 V. (b) Lithiate Si@void@C at 0.1 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.1 A/g to 1.0 V. (c) Lithiate Si@void@C at 0.5 A/g to 0.1 V vs. Li+/Li and hold at this potential until the current density becomes 0.005 A/g. Delithiate the cell at 0.5 A/g to 1.0 V. | Lithiate at 1.0 A/g to 0.10 V vs. Li+/Li and hold at this potential until the current density becomes 0.05 A/g. Delithiate the cell at 0.5 A/g to 1.0 V. Cycle for 500 times. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Luo, M.; Wang, Z.; Passolano, C.; Shaw, L. On the Specific Capacity and Cycle Stability of Si@void@C Anodes: Effects of Particle Size and Charge/Discharge Protocol. Batteries 2022, 8, 154. https://doi.org/10.3390/batteries8100154
Liu B, Luo M, Wang Z, Passolano C, Shaw L. On the Specific Capacity and Cycle Stability of Si@void@C Anodes: Effects of Particle Size and Charge/Discharge Protocol. Batteries. 2022; 8(10):154. https://doi.org/10.3390/batteries8100154
Chicago/Turabian StyleLiu, Bingyu, Mei Luo, Ziyong Wang, Christopher Passolano, and Leon Shaw. 2022. "On the Specific Capacity and Cycle Stability of Si@void@C Anodes: Effects of Particle Size and Charge/Discharge Protocol" Batteries 8, no. 10: 154. https://doi.org/10.3390/batteries8100154
APA StyleLiu, B., Luo, M., Wang, Z., Passolano, C., & Shaw, L. (2022). On the Specific Capacity and Cycle Stability of Si@void@C Anodes: Effects of Particle Size and Charge/Discharge Protocol. Batteries, 8(10), 154. https://doi.org/10.3390/batteries8100154









