Electrochemical Performance of Li2TiO3//LiCoO2 Li-Ion Aqueous Cell with Nanocrystalline Electrodes
Abstract
1. Introduction
2. Results and Discussion
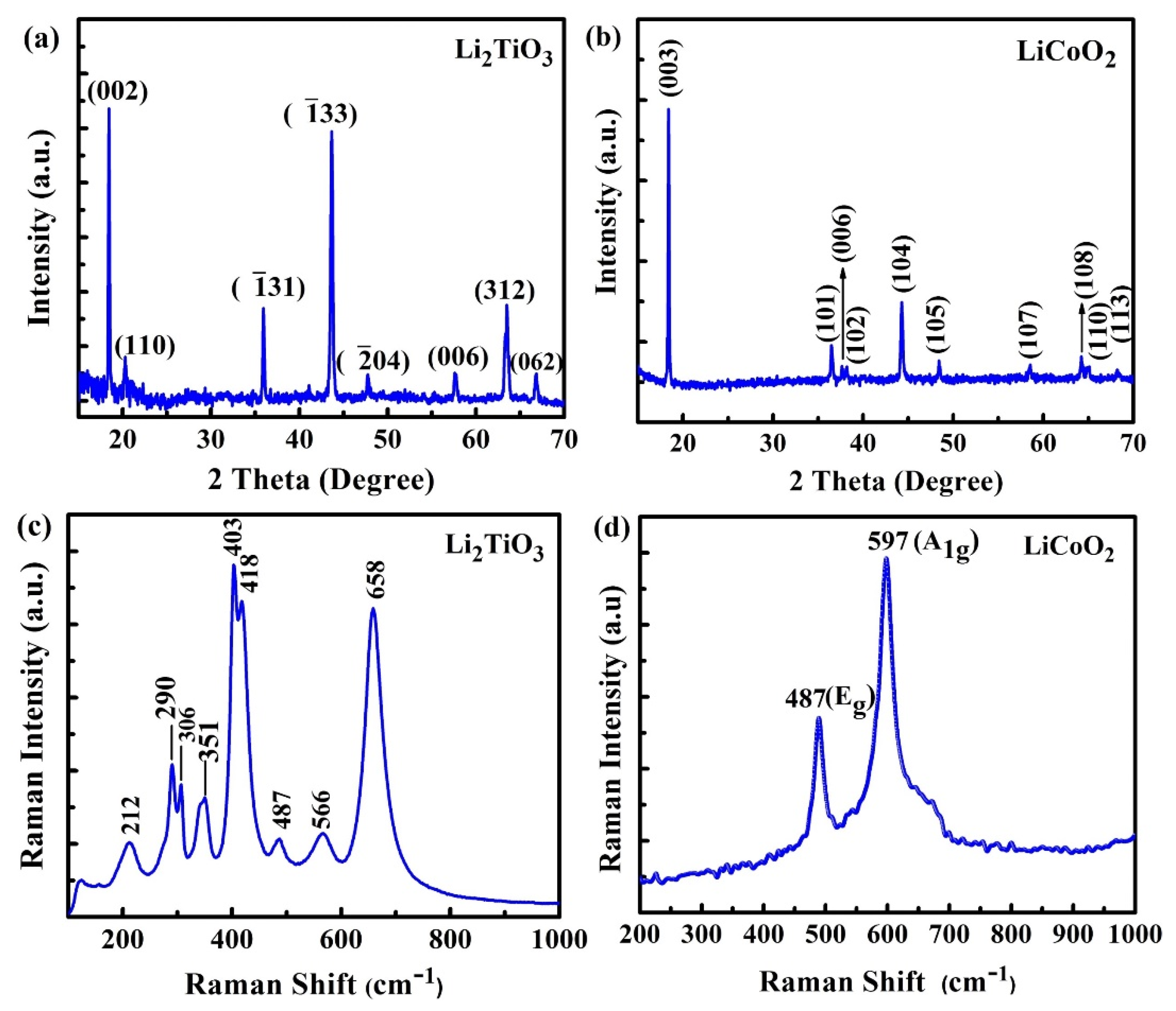
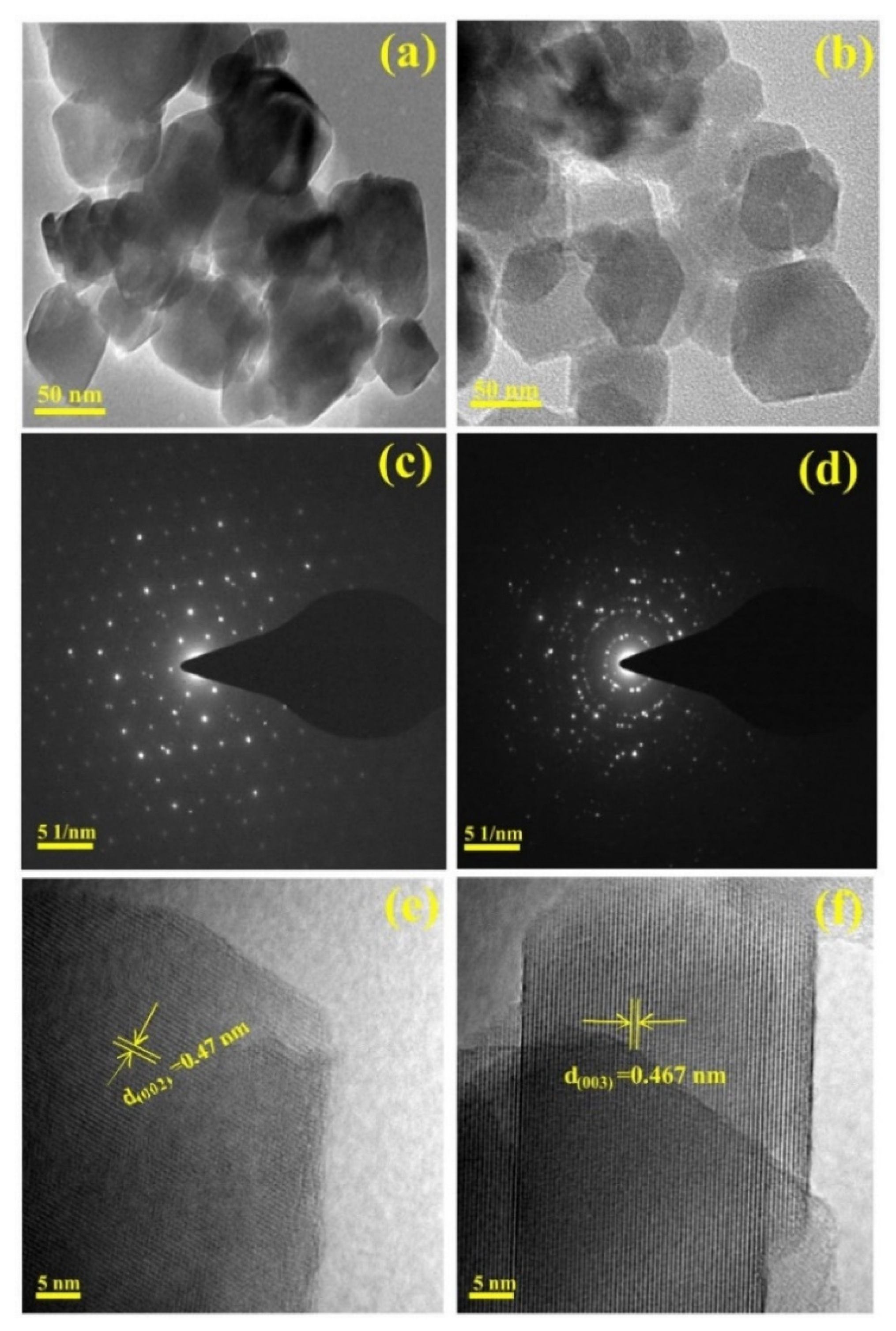
2.1. Structural and Elemental Analyses
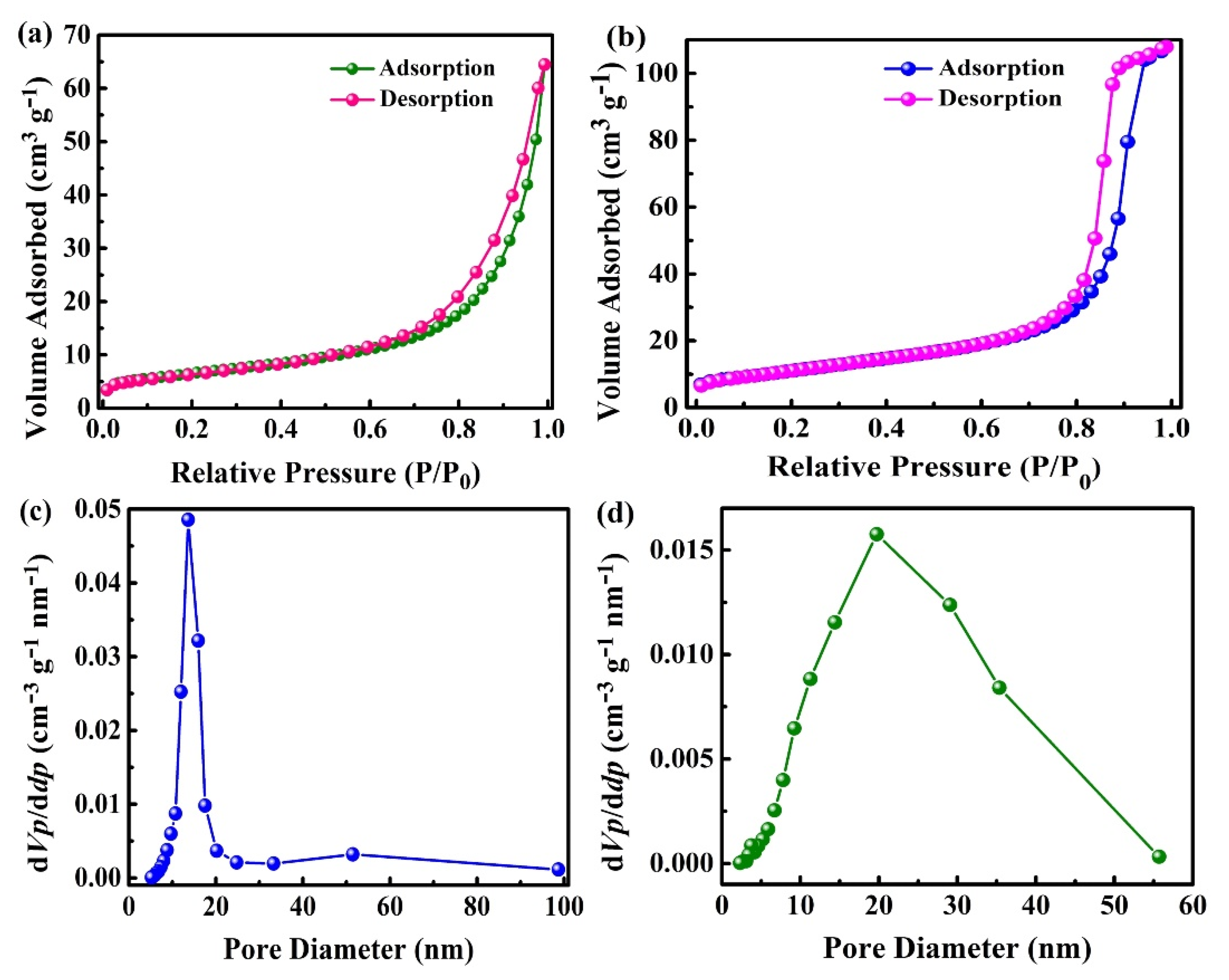
2.2. Brunauer–Emmett–Teller (BET) Analysis
2.3. Electrochemical Properties of Half-Cells
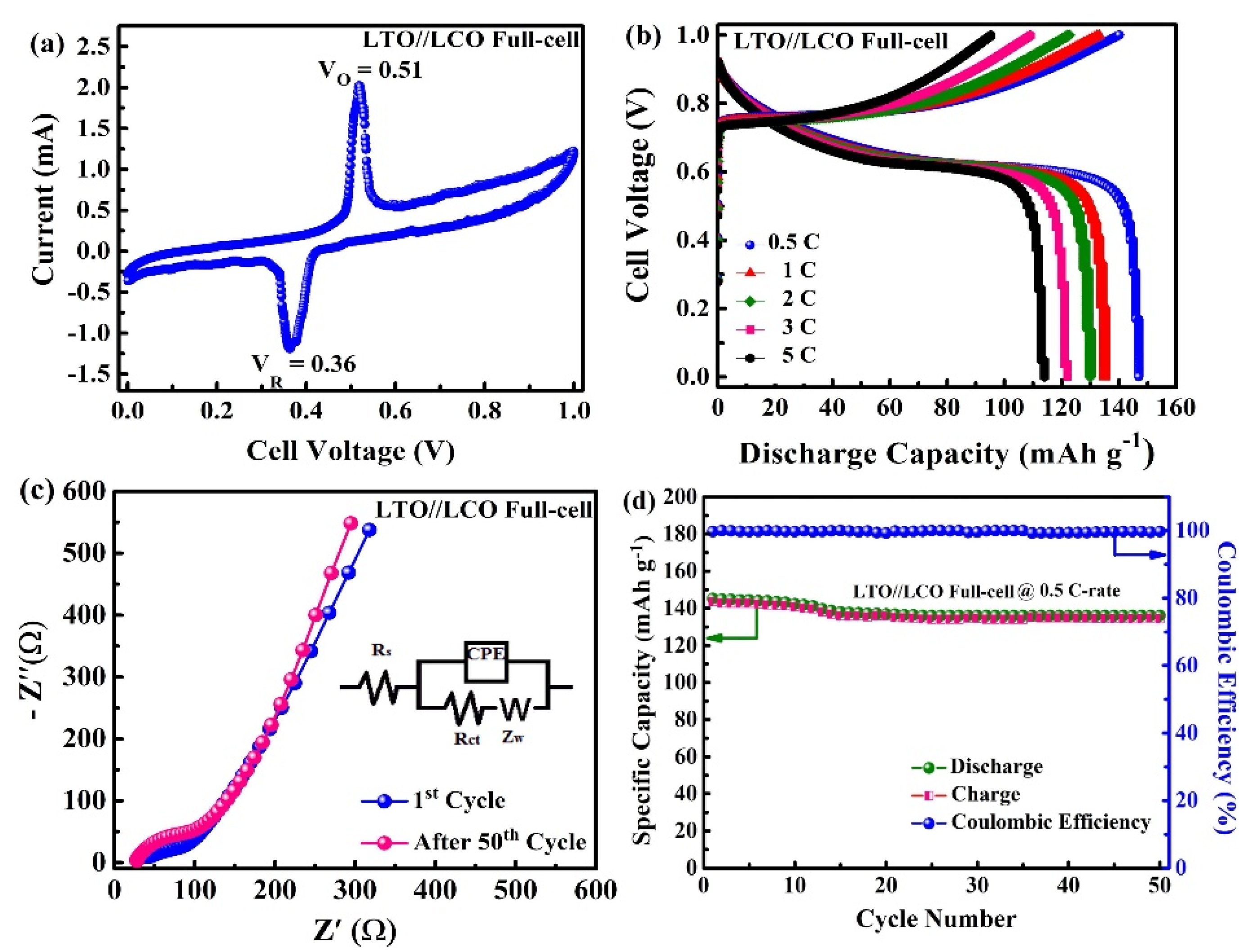
2.4. Electrochemical Properties of the LTO/LCO Full-Cell
2.5. Post-Mortem Analyses of the LTO//LCO Full-Cell
3. Materials and Methods
3.1. Synthesis of Li2TiO3
3.2. Synthesis of LiCoO2
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotech. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paollela, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R 2018, 134, 1–21. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Critical review on lithium-ion batteries: Are they safe? Sustainable? Ionics 2017, 23, 1933–1947. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Gu, X.; Liu, J.-L.; Yang, J.; Xiang, H.; Gong, X.; Xia, Y. First-principles study of H+ intercalation in layer-structured LiCoO2. J. Phys. Chem. C 2011, 115, 12672–12676. [Google Scholar] [CrossRef]
- Tang, W.; Hou, Y.; Wang, F.; Liu, L.; Wu, Y.; Zhu, K. LiMn2O4 nanotube as cathode material of second-level charge capability for aqueous rechargeable batteries. Nano Lett. 2013, 13, 2036–2040. [Google Scholar] [CrossRef]
- Zhu, X.; Long-Doan, T.N.; Yu, Y.; Tian, Y.; Sun, K.E.K.; Zhao, H.; Chen, P. Enhancing rate performance of LiMn2O4 cathode in rechargeable hybrid aqueous battery by hierarchical carbon nanotube/acetylene black conductive pathways. Ionics 2016, 22, 71–76. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Wang, X.J.; Zhu, Y.S.; Hu, C.L.; Chang, Z.; Wu, Y.P.; Holze, R. Macroporous LiFePO4 as a cathode for an aqueous rechargeable lithium battery of high energy density. J. Mater. Chem. A 2013, 1, 14713–14718. [Google Scholar] [CrossRef]
- Zheng, J.; Tan, G.; Shan, P.; Liu, T.; Hu, J.; Feng, Y.; Yang, L.; Zhang, M.; Chen, Z.; Lin, Y.; et al. Understanding thermodynamic and kinetic contributions in expanding the stability window of aqueous electrolytes. Chemistry 2018, 4, 2872–2882. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Ko, S.; Yamada, Y.; Miyazaki, K.; Shimada, T.; Watanabe, E.; Tateyama, Y.; Kamiya, T.; Honda, T.; Akikusa, J.; Yamada, A. Lithium-salt monohydrate melt: A stable electrolyte for aqueous lithium-ion batteries. Electrochem. Commun. 2019, 104, 106488. [Google Scholar] [CrossRef]
- Liu, S.; Ye, S.H.; Li, C.Z.; Pan, G.L.; Gao, X.P. Rechargeable aqueous lithium-ion battery of TiO2/LiMn2O4 with a high voltage. J. Electrochem. Soc. 2011, 158, A1490–A1497. [Google Scholar] [CrossRef]
- Wu, M.-S.; Wang, M.-J.; Jow, J.-J.; Yang, W.-D.; Hsieh, C.-Y.; Tsai, H.-M. Electrochemical fabrication of anatase TiO2 nanostructure as an anode material for aqueous lithium-ion batteries. J. Power Sources 2008, 185, 1420–1424. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, Y.; Zhu, X.; Li, X.; Yue, J.; Ma, X.; Gu, L.; Hu, Y.-S.; Li, H.; Huang, X.; et al. TiO2 (B) anode for high-voltage aqueous Li-ion batteries. Energy Storage Mater. 2021, 42, 438–444. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Q.; Zhu, X.; Gu, L.; Yue, J.; Xia, Q.; Xing, T.; Chen, T.; Yao, Y.; Xia, H. 3D LiCoO2 nanosheets assembled nanorod arrays via confined dissolution recrystallization for advanced aqueous lithium-ion batteries. Nano Energy 2019, 56, 463–472. [Google Scholar] [CrossRef]
- Bhatti, H.S.; Anjum, D.H.; Ullah, S.; Ahmed, B.; Habib, A.; Karim, A.; Hasanain, S.K. Electrochemical characteristics and Li+ ion intercalation kinetics of dual-phase Li4Ti5O12/Li2TiO3 composite in the voltage range 0–3 V. J. Phys. Chem. C 2016, 120, 9553–9561. [Google Scholar] [CrossRef]
- Suo, L.M.; Borodin, O.; Sun, W.; Fan, X.L.; Yang, C.Y.; Wang, F.; Gao, T.; Ma, Z.H.; Schroeder, M.; von Cresce, A.; et al. Advanced high-voltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte. Angew. Chem. Int. Ed. 2016, 55, 7136–7141. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Li, Q.; Vatamanu, J.; Ji, X.; Pollard, T.P.; Cui, C.; Hou, S.; Chen, J.; Yang, C.; et al. A 63 m superconcentrated aqueous electrolyte for high-energy Li-ion batteries. ACS Energy Lett. 2020, 5, 968–974. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, L.; Pang, Y.; Guo, Z.; Bin, D.; Wang, Y.G.; Wang, C.; Xia, Y. TiP2O7 and expanded graphite nanocomposite as anode material for aqueous lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 8075–8082. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, Y.X.; Suo, L.M.; Fan, X.L.; Gao, T.; Yang, C.Y.; Han, F.D.; Qi, Y.; Xu, K.; Wang, C.S. Stabilizing high voltage LiCoO2 cathode in aqueous electrolyte with interphase-forming additive. Energy Environ. Sci. 2016, 9, 3666–3673. [Google Scholar] [CrossRef]
- Ruffo, R.; Wessells, C.; Huggins, R.A.; Cui, Y. Electrochemical behavior of LiCoO2 as aqueous lithium-ion battery electrodes. Electrochem. Commun. 2009, 11, 247–249. [Google Scholar] [CrossRef]
- Ramanujapuram, A.; Gordon, D.; Magasinski, A.; Ward, B.; Nitta, N.; Huang, C.; Yushin, G. Degradation and stabilization of lithium cobalt oxide in aqueous electrolytes. Energy Environ. Sci. 2016, 9, 1841–1848. [Google Scholar] [CrossRef]
- Oh, H.; Yamagishi, H.; Ohta, T.; Byon, H.R. Understanding the interfacial reactions of LiCoO2 positive electrodes in aqueous lithium-ion batteries. Mater. Chem. Front. 2021, 5, 3657–3663. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Lin, B.; Xu, X. Sol–gel synthesis and sintering of nano-size Li2TiO3 powder. Mater. Lett. 2008, 62, 837–839. [Google Scholar] [CrossRef]
- Yu, L.; Yanagisawa, K.; Kamiya, S.; Kozawa, T.; Ueda, T. Monoclinic Li2TiO3 nanoparticles via hydrothermal reaction: Processing and structure. Ceram. Int. 2014, 40, 1901–1908. [Google Scholar] [CrossRef]
- Zhou, Q.; Oya, Y.; Chikada, T.; Zhang, W.; Xue, L.; Yan, Y. Preparation of Li2TiO3 by hydrothermal synthesis and its structure evolution under high energy Ar+ irradiation. J. Eur. Ceram. Soc. 2017, 37, 4955–4961. [Google Scholar] [CrossRef]
- Yu, C.L.; Wang, F.; Zhang, A.L.; Gao, D.P.; Cao, S.Y.; Guo, Y.Y.; Hui. H.B.; Hao, X.; Wang, D.Y.; Yanagisawa, K. Preparation of β-Li2TiO3 pebbles by a modified indirect wet chemistry method. Fusion. Eng. Des. 2015, 101, 73–79. [Google Scholar] [CrossRef]
- Kataoka, K.; Takahashi, Y.; Kijima, N.; Nagai, H.; Akimoto, J.; Idemoto, Y.; Ohshima, K.-I. Crystal growth and structure refinement of monoclinic Li2TiO3. Mater. Res. Bull. 2009, 44, 168–172. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueada, A. Solid-state redox reactions of LiCoO2 (R-3m) for 4-volt secondary lithium cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Calos, N.J.; Forrester, J.S.; Schaffer, G.B. A crystallographic contribution to the mechanism mechanically induced solid state reaction. J. Solid State Chem. 1996, 122, 272–280. [Google Scholar] [CrossRef]
- Denisova, T.A.; Maksimova, L.G.; Polyakov, E.V.; Zhuravlev, N.A.; Kovyazina, S.A.; Leonidova, O.N.; Khabibulin, D.F.; Yureva, E.I. Metatitanic acid: Synthesis and properties. Russian J. Inorg. Chem. 2006, 51, 691–699. [Google Scholar] [CrossRef]
- Julien, C. Local cationic environment in lithium nickel-cobalt oxides used as cathode materials for lithium batteries. Solid State Ion. 2000, 136, 887–896. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T.E. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227–231. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Shui, Y.; Zhao, L.-L.; Zhu, P.; Xu, W.-Y.; You, Y.H. Preparation of Ni-doped Li2TiO3 using an inorganic precipitation-peptization method. Coatings 2019, 9, 701. [Google Scholar] [CrossRef]
- Lu, J.; Peng, Q.; Wang, W.; Nan, C.; Li, L.; Li, Y. Nanoscale coating of LiMO2 (M = Ni, Co, Mn) nanobelts with Li+—conductive Li2TiO3: Toward better rate capabilities for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 1649–1652. [Google Scholar] [CrossRef]
- Ouatani, L.E.; Dedryvère, R.; Siret, C.; Biensan, P.; Gonbeau, D. Effect of vinylene carbonate additive in Li-ion batteries: Comparison of LiCoO2/C, LiFePO4/C, and LiCoO2/Li4Ti5O12 systems. J. Electrochem. Soc. 2009, 156, A468–A477. [Google Scholar] [CrossRef]
- Dedryvère, R.; Foix, D.; Franger, S.; Patoux, S.; Daniel, L.; Gonbeau, D. Electrode/electrolyte interface reactivity in high-voltage spinel LiMn1.6Ni0.4O4/Li4Ti5O12 lithium-ion battery. J. Phys. Chem. C 2010, 114, 10999–11008. [Google Scholar] [CrossRef]
- Daheron, L.; Martinez, H.; Dedryvere, R.; Baraille, I.; Menetrier, M.; Denage, C.; Delmas, C.; Gonbeau, D. Surface properties of LiCoO2 investigated by XPS analyses and theoretical calculations. J. Phys. Chem. C 2009, 113, 5843–5852. [Google Scholar] [CrossRef]
- Sivajee Ganesh, K.; Purusottam Reddy, B.; Jeevan Kumar, P.; Hussain, O.M. Influence of Zr dopant on microstructural and electrochemical properties of LiCoO2 thin film cathodes by RF sputtering. J. Electroanal. Chem. 2018, 828, 71–79. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Jiang, Y.; Wang, Y.; Chen, X. One-pot synthesis of highly greenish-yellow fluorescent nitrogen doped graphene quantum dots for pyrophosphate sensing via competitive coordination with Eu3+ ions. Nanoscale 2015, 7, 15427–15433. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Sun, X.; He, S.; Li, L. High capacity-favorable tap density cathode material based on three-dimensional carbonous framework supported Na3V2(PO4)2F3 nanoparticles. Chem. Eng. J. 2018, 331, 712–719. [Google Scholar] [CrossRef]
- Kavan, L.; Procházka, J.; Spitler, T.M.; Kalbáč, M.; Zukalová, M.; Drezen, T.; Grätzel, M. Li Insertion into Li4Ti5O12 (spinel): Charge capability vs. particle size in thin-film electrodes. J. Electrochem. Soc. 2003, 150, A1000–A1007. [Google Scholar] [CrossRef]
- Kojima, Y.; Suzuki, K.; Kawai, Y. Hydrogen generation from lithium borohydride solution over nano-sized platinum dispersed on LiCoO2. J. Power Sources 2006, 155, 325–328. [Google Scholar] [CrossRef]
- Lakshmi-Narayana, A.; Dhananjaya, M.; Guru-Prakash, N.; Hussain, O.M.; Julien, C.M. Nanocrystalline Li2TiO3 electrodes for supercapattery application. Ionics 2017, 23, 3419–3428. [Google Scholar] [CrossRef]
- Islam, M.M.; Bredow, T. Lithium diffusion pathways in β-Li2TiO3: A theoretical study. J. Phys. Chem. C 2016, 120, 7061–7066. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K. Li+-ion diffusion in LiCoO2 thin film prepared by the poly(vinylpyrrolidone) sol-gel method. J. Electrochem. Soc. 2004, 151, A1406–A1411. [Google Scholar] [CrossRef]
- Van der Ven, A.; Aydinol, M.K.; Ceder, G.; Kresse, G.; Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 1998, 58, 2975–2987. [Google Scholar] [CrossRef]
- Morales, J.; Santos-Pena, J.; Trocoli, R.; Franger, S. Electrochemical activity of rock-salt-structured LiFeO2-Li4/3Ti2/3O2 nanocomposites in lithium cells. J. Nanopart. Res. 2008, 10, 217–226. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Bae, J.-S. The ac impedance study of electrochemical lithium intercalation into porous vanadium oxide electrode. Electrochim. Acta 1996, 41, 919–925. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 <x <1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Lakshmi-Narayana, A.; Dhananjaya, M.; Guru-Prakash, N.; Hussain, O.M.; Mauger, A.; Julien, C.M. Li2TiO3/Graphene and Li2TiO3/CNT composites as anodes for high power Li–ion batteries. ChemSelect 2018, 3, 9150–9158. [Google Scholar]
- Schweikert, N.; Heinzmann, R.; Eichhöfer, A.; Hahn, H.; Indris, S. Electrochemical impedance spectroscopy of Li4Ti5O12 and LiCoO2 based half-cells and Li4Ti5O12/LiCoO2 cells: Internal interfaces and influence of state-of-charge and cycle number. Solid State Ion. 2012, 226, 15–23. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Wang, C.; Xia, Y. Hybrid aqueous energy storage cells using activated carbon and lithium-ion intercalated compounds: II. Comparison of LiMn2O4, LiCo1/3Ni1/3Mn1/3O2 and LiCoO2 positive electrodes. J. Electrochem. Soc. 2006, 153, A1425–A1431. [Google Scholar] [CrossRef]
- Sencanski, J.; Bajuk-Bogdanovi, D.; Majstorovic, D.; Tchernychova, E.; Papan, J.; Vujkovic, M. The synthesis of Li(Co-Mn-Ni)O2 cathode material from spent-Li ion batteries and the proof of its functionality in aqueous lithium and sodium electrolytic solutions. J. Power Sources 2017, 342, 690–703. [Google Scholar] [CrossRef]
- Wang, G.J.; Zhao, N.H.; Yang, L.C.; Wu, Y.P.; Wu, H.Q.; Holze, R. Characteristics of an aqueous rechargeable lithium battery (ARLB). Electrochim. Acta 2007, 52, 4911–4915. [Google Scholar] [CrossRef]
- Jeong, E.D.; Won, M.S.; Shim, Y.B. Cathodic properties of a lithium-ion secondary battery using LiCoO2 prepared by a complex formation reaction. J. Power Sources 1998, 70, 70–77. [Google Scholar] [CrossRef]
- Yoshio, M.; Tanaka, H.; Tominaga, K.; Noguchi, H. Synthesis of LiCoO2 from cobalt-organic acid complexes and its electrode behavior in a lithium secondary battery. J. Power Sources 1992, 40, 347–353. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Zheng, W. A semiconductor-electrochemistry model for design of high-rate Li ion battery, literature. J. Energy Chem. 2020, 41, 100–106. [Google Scholar] [CrossRef]




| Cell | Electrolyte | Averaged Cell Voltage (V) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| LiCoO2//AC | 1 M Li2SO4 solution | 0.6 | 126 @ 6 mA cm−2 | [56] |
| LiCoO2//Mo6S8 | TFSI in water-in-salt | 1.0 | 60 @ 0.5 C (20) | [23] |
| LiCoO2//Li4Ti5O12 | Li(TFSI)x(BETI)1−x salt | 2.3 | ~40 @ 10 C (200) | [13] |
| LiCoO2//Li0.5Mn2O4 | 1 M LiNO3 | 0.8 | 105 @ 1 C (25) | [24] |
| LiCo0.415Mn0.435Ni0.15O2//C | 6 M LiNO3 | 1.0 | 64 @ 40 C (1) | [57] |
| LiCoO2//LiTi2(PO4)3 | 1 M Li2SO4 (pH = 10) | 0.7 | 130 @ 1 C (1) | [18] |
| LiCoO2//Li4Ti5O12 | (PTFSI)0.6(TFSI)0.4·1.15H2O | 2.2 | ~75 @ 0.2 C (100) | [14] |
| LiCoO2//LiV3O8 | saturated LiNO3 | 1.0 | 55 @ 0.2 mA cm−2 (12) | [58] |
| LiCoO2//Li2TiO3 | 1 M Li2SO4 (pH ≈ 9) | 0.7 | 135 @ 0.5 C (50) | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshmi-Narayana, A.; Sivajee-Ganesh, K.; Dhananjaya, M.; Narayan-Banerjee, A.; Julien, C.M.; Joo, S.-W. Electrochemical Performance of Li2TiO3//LiCoO2 Li-Ion Aqueous Cell with Nanocrystalline Electrodes. Batteries 2022, 8, 149. https://doi.org/10.3390/batteries8100149
Lakshmi-Narayana A, Sivajee-Ganesh K, Dhananjaya M, Narayan-Banerjee A, Julien CM, Joo S-W. Electrochemical Performance of Li2TiO3//LiCoO2 Li-Ion Aqueous Cell with Nanocrystalline Electrodes. Batteries. 2022; 8(10):149. https://doi.org/10.3390/batteries8100149
Chicago/Turabian StyleLakshmi-Narayana, Ambadi, Kapu Sivajee-Ganesh, Merum Dhananjaya, Arghya Narayan-Banerjee, Christian M. Julien, and San-Woo Joo. 2022. "Electrochemical Performance of Li2TiO3//LiCoO2 Li-Ion Aqueous Cell with Nanocrystalline Electrodes" Batteries 8, no. 10: 149. https://doi.org/10.3390/batteries8100149
APA StyleLakshmi-Narayana, A., Sivajee-Ganesh, K., Dhananjaya, M., Narayan-Banerjee, A., Julien, C. M., & Joo, S.-W. (2022). Electrochemical Performance of Li2TiO3//LiCoO2 Li-Ion Aqueous Cell with Nanocrystalline Electrodes. Batteries, 8(10), 149. https://doi.org/10.3390/batteries8100149







