Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries
Abstract
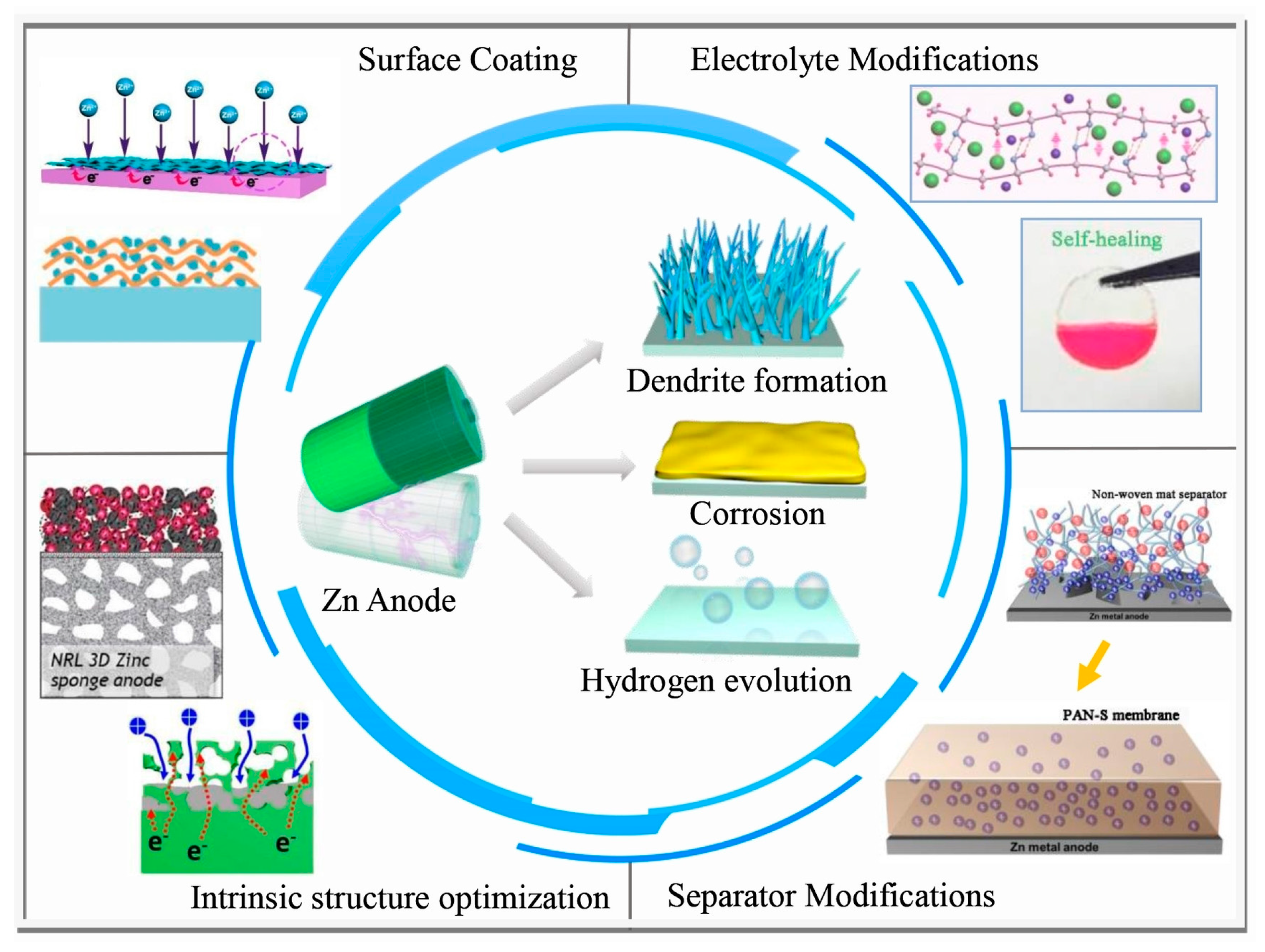
1. Introduction
2. Surface Reactions on Zn Anode
2.1. Zinc Dendrites
2.2. Hydrogen Evolution Parasitic Reaction
2.3. Corrosion Passivation
3. Optimization of the Electrolytes
3.1. Electrolyte Additives for Zinc Dendrites
3.2. Ionic Additives
3.3. Organic Additives
3.4. Inorganic Additives
4. Conclusions and Future Perspectives
- (1)
- More in-depth research on inorganic types of electrolyte additives. As mentioned above, the development of diverse low cost and non-toxic inorganic additives may lead to breakthroughs and fundamental improvements in zinc dendritic problems. In the future, inorganic additives with different characteristics could be designed to give full play to their own advantages and explore highly efficient inorganic additives that can minimize dendrite generation and side reactions and optimize battery performance.
- (2)
- Building mixed electrolyte system and the action mechanism. Currently, most research is limited to adding single component electrolyte additives or complementary additives of the same type in a system, but the exploration of multi-component electrolyte additives added together has not been well carried out, and establishing a link between ionic additives, organic additives, and inorganic additives to achieve synergistic effects in the electrolyte system may become a breakthrough. The influence of multiple additives can reduce the formation of dendrites more efficiently, and it is worthwhile to further study whether different additives can be linked and the mechanism of action.
- (3)
- Exploring the effect of electrolyte additives on the cathode. While electrolyte additives play the largest role in inhibiting anodic zinc dendrites and side reactions, and the principle and process of action are studied in detail, the effect of EAs on cathode properties has not been well described in the cathode. Therefore, the role of EAs on the cathode of the battery should be noted in the subsequent research to achieve a high level of overall battery performance.
- (4)
- Development of multifunctional EAs. With the development of flexible devices and low-temperature resistant batteries, a lot of attention has been focused on the wide temperature operating range and ductile energy storage devices, which put forward higher requirements for the electrolytes, and the use of EAs can change the nature of the electrolyte, thereby moving toward multifunctional and high performance. Some EAs can already meet these requirements, but they can be accompanied by problems, such as decreasing conductivity and insufficient mechanical properties, to meet the electrochemical performance and lifetime requirements. Therefore, the material selection and preparation methods of EAs need to be further explored.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| electrolyte additives | EAs |
| zinc-ion batteries | ZIBs |
| li-ion batteries | LIBs |
| hydrogen evolution reaction | HER |
| zinc | Zn |
| water-in-salt electrolyte | WISE |
| lithium bistrifluoromethanesulfonimide | LiTFSI |
| zinc trifluoromethanesulfonate | Zn(TfO)2 |
| X-ray absorption near edge structure | XANES |
| fibronectin | FI |
| polyaspartic acid | PASP |
| dimethyl sulfoxide | DMSO |
| polyethylene glycol | PEG |
| propylene glycol | PG |
| tin sulfide nanosheets | TS-Ns |
| graphene quantum dots | GQDs |
References
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S.-Z. Recent Advances in Atomic Metal Doping of Carbon-based Nanomaterials for Energy Conversion. Small 2017, 13, 1700191. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Perumal, P.; Andersen, S.M.; Nikoloski, A.; Basu, S.; Mohapatra, M. Leading strategies and research advances for the restoration of graphite from expired Li+ energy storage devices. J. Environ. Chem. Eng. 2021, 9, 106455. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Feng, W. Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li–S Batteries. Small Methods 2018, 2, 1800156. [Google Scholar] [CrossRef]
- Zhang, S.S. Problem, Status, and Possible Solutions for Lithium Metal Anode of Rechargeable Batteries. ACS Appl. Energy Mater. 2018, 1, 910–920. [Google Scholar] [CrossRef]
- Gandoman, F.H.; Jaguemont, J.; Goutam, S.; Gopalakrishnan, R.; Firouz, Y.; Kalogiannis, T.; Omar, N.; Van Mierlo, J. Concept of reliability and safety assessment of lithium-ion batteries in electric vehicles: Basics, progress, and challenges. Appl. Energy 2019, 251, 113343. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, X.; Liang, X.; Zhang, F.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Few-layer methyl-terminated germanene–graphene nanocomposite with high capacity for stable lithium storage. Carbon 2020, 161, 287–298. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.-P.; Fu, N.; Zhou, W.-B.; Liu, B.; Deng, Q.; Wu, X.-W. Comprehensive review on zinc-ion battery anode: Challenges and strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, R.; Or, T.; Deng, Y.-P.; Yu, A.; Chen, Z. Recent Progress on High-Performance Cathode Materials for Zinc-Ion Batteries. Small Struct. 2021, 2, 2000064. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Zhang, H.; Li, X. Anode for Zinc-Based Batteries: Challenges, Strategies, and Prospects. ACS Energy Lett. 2021, 6, 2765–2785. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Cai, Y.; Pam, M.E.; Yang, Y.; Zhang, D.; Wang, Y.; Huang, S. Designing Advanced Aqueous Zinc-Ion Batteries: Principles, Strategies, and Perspectives. Energy Environ. Mater. 2021, 5, 823–851. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Yang, F.; Liu, S.; Zhang, S.; Mao, J.; Guo, Z. Electrolyte Engineering Enables High Performance Zinc-Ion Batteries. Small 2022, 2107033. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Toward Long-Life Aqueous Zinc Ion Batteries by Constructing Stable Zinc Anodes. Chem. Rec. 2022, e202200088. [Google Scholar] [CrossRef]
- Jiao, S.; Fu, J.; Wu, M.; Hua, T.; Hu, H. Ion Sieve: Tailoring Zn2+ Desolvation Kinetics and Flux toward Dendrite-Free Metallic Zinc Anodes. ACS Nano 2022, 16, 1013–1024. [Google Scholar] [CrossRef]
- Jiao, Q.; Zhou, T.; Zhang, N.; Liu, S.; Huang, Q.; Bi, W.; Chu, W.; Wu, X.; Zhu, Y.; Feng, Y.; et al. High-surface-area titanium nitride nanosheets as zinc anode coating for dendrite-free rechargeable aqueous batteries. Sci. China Mater. 2022, 65, 1771–1778. [Google Scholar] [CrossRef]
- Han, X.; Leng, H.; Qi, Y.; Yang, P.; Qiu, J.; Zheng, B.; Wu, J.; Li, S.; Huo, F. Hydrophilic silica spheres layer as ions shunt for enhanced Zn metal anode. Chem. Eng. J. 2022, 431, 133931. [Google Scholar] [CrossRef]
- Dai, L.; Wang, T.; Jin, B.; Liu, N.; Niu, Y.; Meng, W.; Gao, Z.; Wu, X.; Wang, L.; He, Z. γ-Al2O3 coating layer confining zinc dendrite growth for high stability aqueous rechargeable zinc-ion batteries. Surf. Coat. Technol. 2021, 427, 127813. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.; Yin, J.; Tian, Z.; Ma, Y.; Liu, Z.; Zhu, Y.; Alshareef, H.N. Controlled Deposition of Zinc-Metal Anodes via Selectively Polarized Ferroelectric Polymers. Adv. Mater. 2022, 34, 2106937. [Google Scholar] [CrossRef]
- Yuan, C.; Yin, L.; Du, P.; Yu, Y.; Zhang, K.; Ren, X.; Zhan, X.; Gao, S. Microgroove-patterned Zn metal anode enables ultra-stable and low-overpotential Zn deposition for long-cycling aqueous batteries. Chem. Eng. J. 2022, 442, 136231. [Google Scholar] [CrossRef]
- Yun, K.; Jang, H.; An, G.-H. Stable anode enabled by an embossed and punched structure for a high-rate performance Zn-ion hybrid capacitor. Int. J. Energy Res. 2022, 46, 7175–7185. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, F.; Fan, G.; Liu, J.; Yu, M.; Yan, Z.; Zhang, N.; Cheng, F. Stabilizing Zinc Electrodes with a Vanillin Additive in Mild Aqueous Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 47650–47658. [Google Scholar] [CrossRef]
- Hoang, T.K.A.; Acton, M.; Chen, H.T.H.; Huang, Y.; Doan, T.N.L.; Chen, P. Sustainable gel electrolyte containing Pb2+ as corrosion inhibitor and dendrite suppressor for the zinc anode in the rechargeable hybrid aqueous battery. Mater. Today Energy 2017, 4, 34–40. [Google Scholar] [CrossRef]
- Guo, S.; Qin, L.; Zhang, T.; Zhou, M.; Zhou, J.; Fang, G.; Liang, S. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries. Energy Storage Mater. 2021, 34, 545–562. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Peng, M.; Zhou, X.; Cao, Y.; Liu, J.; Shen, X.; Yan, C.; Qian, T. Diminishing Interfacial Turbulence by Colloid-Polymer Electrolyte to Stabilize Zinc Ion Flux for Deep-Cycling Zn Metal Batteries. Adv. Mater. 2022, 34, 2200131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Li, C.; Wu, Q.; Ma, J.; Pan, H.; Liu, Y.; Lu, Y. Regulating zinc metal anodes via novel electrolytes in rechargeable zinc-based batteries. J. Mater. Chem. A 2022, 10, 14692–14708. [Google Scholar] [CrossRef]
- Cao, L.; Skyllas-Kazacos, M.; Menictas, C.; Noack, J. A review of electrolyte additives and impurities in vanadium redox flow batteries. J. Energy Chem. 2018, 27, 1269–1291. [Google Scholar] [CrossRef]
- Geng, Y.; Pan, L.; Peng, Z.; Sun, Z.; Lin, H.; Mao, C.; Wang, L.; Dai, L.; Liu, H.; Pan, K.; et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022, 51, 733–755. [Google Scholar] [CrossRef]
- Han, M.; Zheng, D.; Song, P.; Ding, Y. Theoretical study on fluoroethylene carbonate as an additive for the electrolyte of lithium ion batteries. Chem. Phys. Lett. 2021, 771, 138538. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Hao, J.; Luo, D.; Zhang, P.-F.; Zhang, B.; Davey, K.; Lin, Z.; Qiao, S.-Z. Dual-Function Electrolyte Additive for Highly Reversible Zn Anode. Adv. Energy Mater. 2021, 11, 2102010. [Google Scholar] [CrossRef]
- Kim, J.; Adiraju, V.A.K.; Chae, O.B.; Lucht, B.L. Lithium Bis(trimethylsilyl) Phosphate as an Electrolyte Additive to Improve the Low-Temperature Performance for LiNi0.8Co0.1Mn0.1O2/Graphite Cells. J. Electrochem. Soc. 2021, 168, 080538. [Google Scholar] [CrossRef]
- Li, T.C.; Fang, D.; Zhang, J.; Pam, M.E.; Leong, Z.Y.; Yu, J.; Li, X.L.; Yan, D.; Yang, H.Y. Recent progress in aqueous zinc-ion batteries: A deep insight into zinc metal anodes. J. Mater. Chem. A 2021, 9, 6013–6028. [Google Scholar] [CrossRef]
- He, P.; Chen, Q.; Yan, M.; Xu, X.; Zhou, L.; Mai, L.; Nan, C.-W. Building better zinc-ion batteries: A materials perspective. Energy Chem. 2019, 1, 100022. [Google Scholar] [CrossRef]
- Sze, Y.-K.; Irish, D.E. Vibrational spectral studies of ion-ion and ion-solvent interactions. I. Zinc nitrate in water. J. Solut. Chem. 1978, 7, 395–415. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for zinc at 25–300 °C. Corros. Sci. 1997, 39, 107–114. [Google Scholar] [CrossRef]
- Cao, Z.; Zhuang, P.; Zhang, X.; Ye, M.; Shen, J.; Ajayan, P.M. Strategies for Dendrite-Free Anode in Aqueous Rechargeable Zinc Ion Batteries. Adv. Energy Mater. 2020, 10, 2001599. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- McLarnon, F.R.; Cairns, E.J. The Secondary Alkaline Zinc Electrode. J. Electrochem. Soc. 1991, 138, 645–656. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef] [PubMed]
- Mitha, A.; Yazdi, A.Z.; Ahmed, M.; Chen, P. Surface Adsorption of Polyethylene Glycol to Suppress Dendrite Formation on Zinc Anodes in Rechargeable Aqueous Batteries. ChemElectroChem 2018, 5, 2409–2418. [Google Scholar] [CrossRef]
- Luan, J.; Zhang, Q.; Yuan, H.; Sun, D.; Peng, Z.; Tang, Y.; Ji, X.; Wang, H. Plasma-Strengthened Lithiophilicity of Copper Oxide Nanosheet–Decorated Cu Foil for Stable Lithium Metal Anode. Adv. Sci. 2019, 6, 1901433. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode Materials for Aqueous Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yamamoto, O.; Imanishi, N. Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes. Electrochemistry 2016, 84, 210–218. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Zheng, W.; Cui, X.; Rojo, T.; Zhang, Q. Towards High-Safe Lithium Metal Anodes: Suppressing Lithium Dendrites via Tuning Surface Energy. Adv. Sci. 2017, 4, 1600168. [Google Scholar] [CrossRef]
- Xu, M.; Chen, J.; Zhang, Y.; Raza, B.; Lai, C.; Wang, J. Electrolyte design strategies towards long-term Zn metal anode for rechargeable batteries. J. Energy Chem. 2022, 73, 575–587. [Google Scholar] [CrossRef]
- Yang, J.; Yin, B.; Sun, Y.; Pan, H.; Sun, W.; Jia, B.; Zhang, S.; Ma, T. Zinc Anode for Mild Aqueous Zinc-Ion Batteries: Challenges, Strategies, and Perspectives. Nano-Micro Lett. 2022, 14, 32–78. [Google Scholar] [CrossRef]
- Cai, Z.; Ou, Y.; Wang, J.; Xiao, R.; Fu, L.; Yuan, Z.; Zhan, R.; Sun, Y. Chemically resistant Cu–Zn/Zn composite anode for long cycling aqueous batteries. Energy Storage Mater. 2020, 27, 205–211. [Google Scholar] [CrossRef]
- Lei, L.; Sun, Y.; Wang, X.; Jiang, Y.; Li, J. Strategies to Enhance Corrosion Resistance of Zn Electrodes for Next Generation Batteries. Front. Mater. 2020, 7, 96. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Guo, S.; Cao, X.; Pan, A.; Fang, G.; Zhou, J.; Liang, S. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Geng, Y.; Miao, L.; Yan, Z.; Xin, W.; Zhang, L.; Peng, H.; Li, J.; Zhu, Z. Super-zincophilic additive induced interphase modulation enables long-life Zn anodes at high current density and areal capacity. J. Mater. Chem. A 2022, 10, 10132–10138. [Google Scholar] [CrossRef]
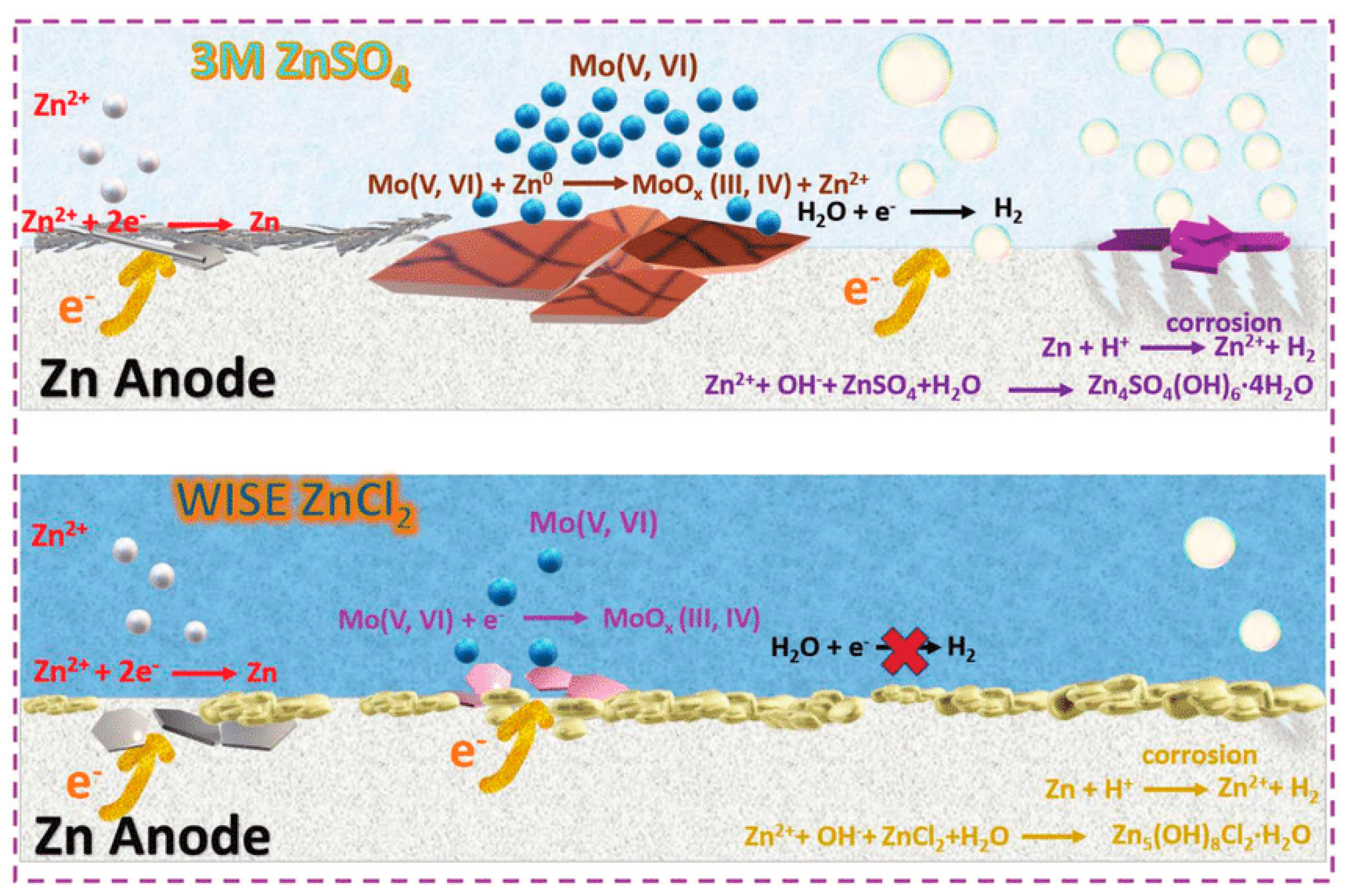
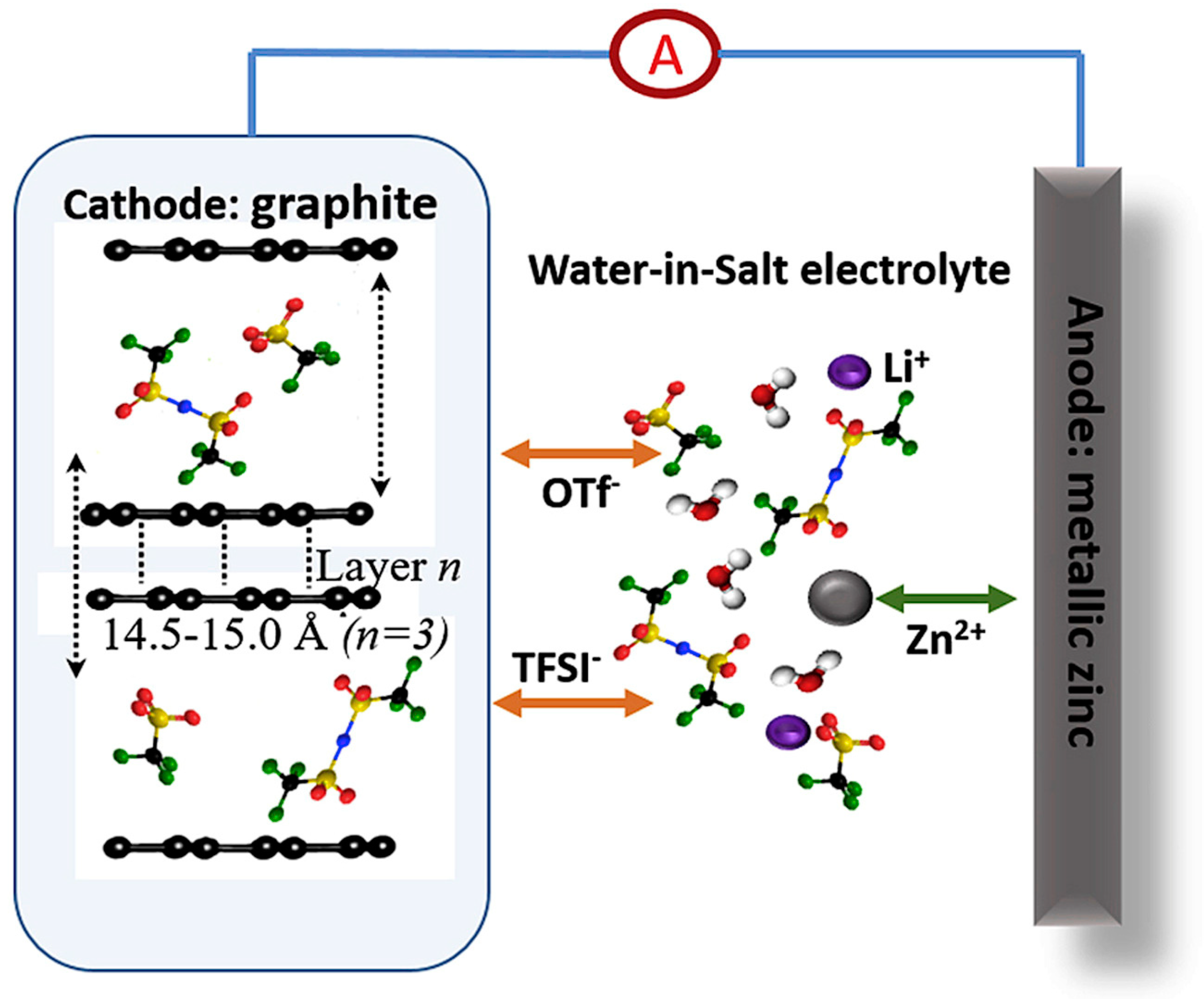
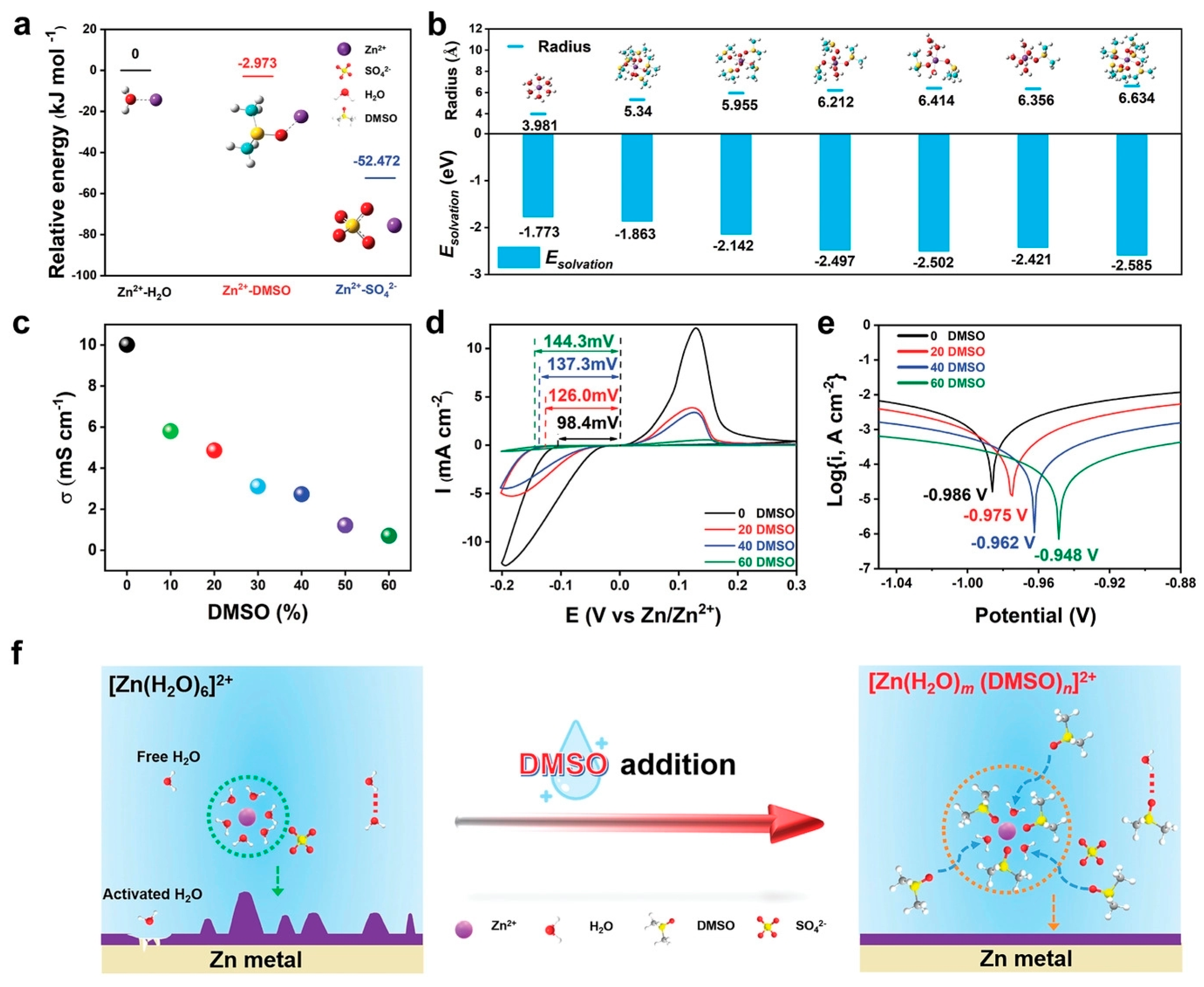
- Wang, L.; Yan, S.; Quilty, C.D.; Kuang, J.; Dunkin, M.R.; Ehrlich, S.N.; Ma, L.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C. Achieving Stable Molybdenum Oxide Cathodes for Aqueous Zinc-Ion Batteries in Water-in-Salt Electrolyte. Adv. Mater. Interfaces 2021, 8, 2002080. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Qin, B.; Passerini, S. Electrochemical intercalation of anions in graphite for high-voltage aqueous zinc battery. J. Power Sources 2020, 449, 227594. [Google Scholar] [CrossRef]
- Olbasa, B.W.; Fenta, F.W.; Chiu, S.-F.; Tsai, M.-C.; Huang, C.-J.; Jote, B.A.; Beyene, T.T.; Liao, Y.-F.; Wang, C.-H.; Su, W.-N.; et al. High-Rate and Long-Cycle Stability with a Dendrite-Free Zinc Anode in an Aqueous Zn-Ion Battery Using Concentrated Electrolytes. ACS Appl. Energy Mater. 2020, 3, 4499–4508. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, P.; Qin, A.; Cui, G.; Mai, W. Metal-coordination chemistry guiding preferred crystallographic orientation for reversible zinc anode. Energy Storage Mater. 2022, 49, 463–470. [Google Scholar] [CrossRef]
- Han, D.; Wang, Z.; Lu, H.; Li, H.; Cui, C.; Zhang, Z.; Sun, R.; Geng, C.; Liang, Q.; Guo, X.; et al. A Self-Regulated Interface toward Highly Reversible Aqueous Zinc Batteries. Adv. Energy Mater. 2022, 12, 2102982. [Google Scholar] [CrossRef]
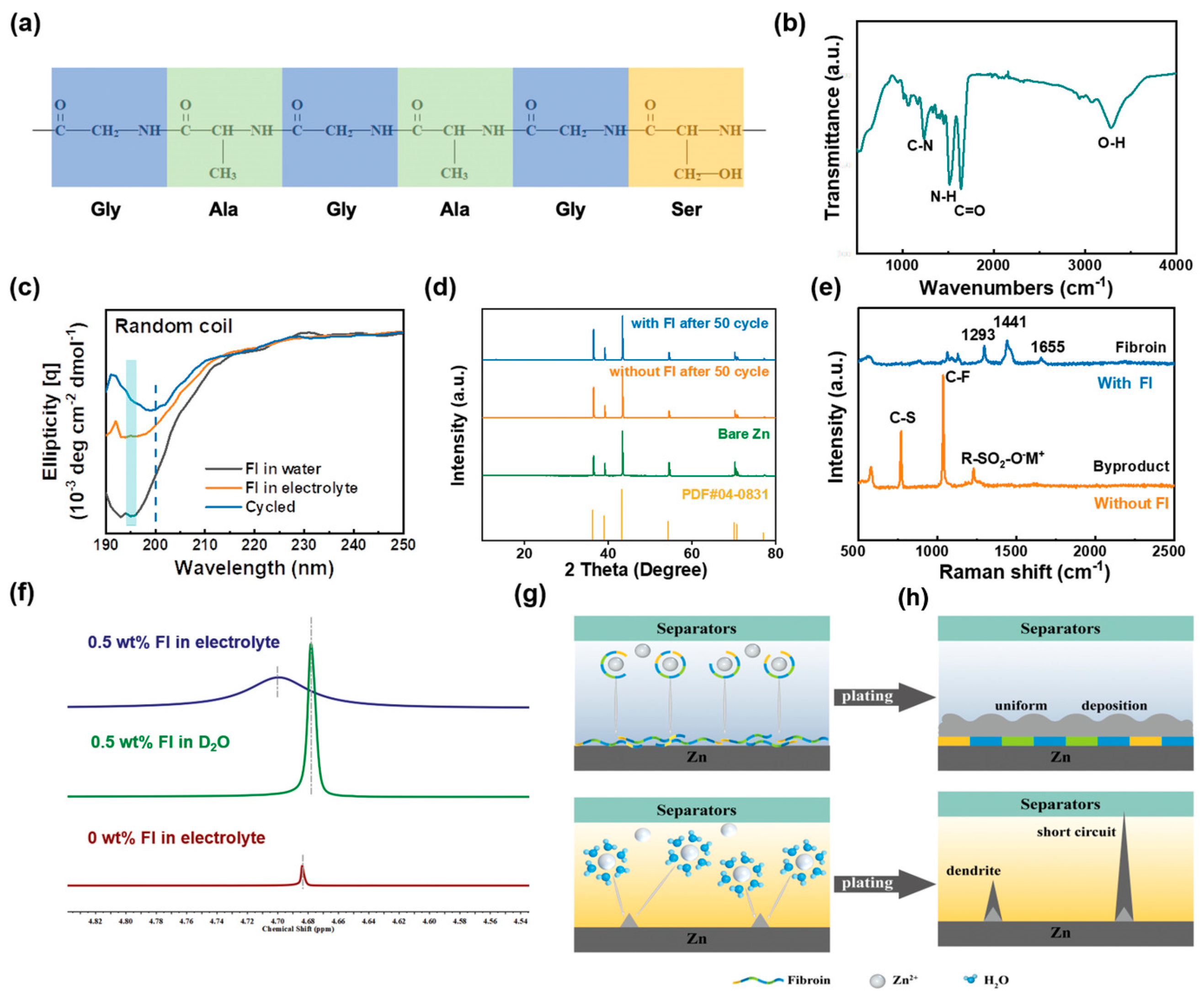
- Zhu, J.; Deng, W.; Yang, N.; Xu, X.; Huang, C.; Zhou, Y.; Zhang, M.; Yuan, X.; Hu, J.; Li, C.; et al. Biomolecular Regulation of Zinc Deposition to Achieve Ultra-Long Life and High-Rate Zn Metal Anodes. Small 2022, 18, 2202509. [Google Scholar] [CrossRef]
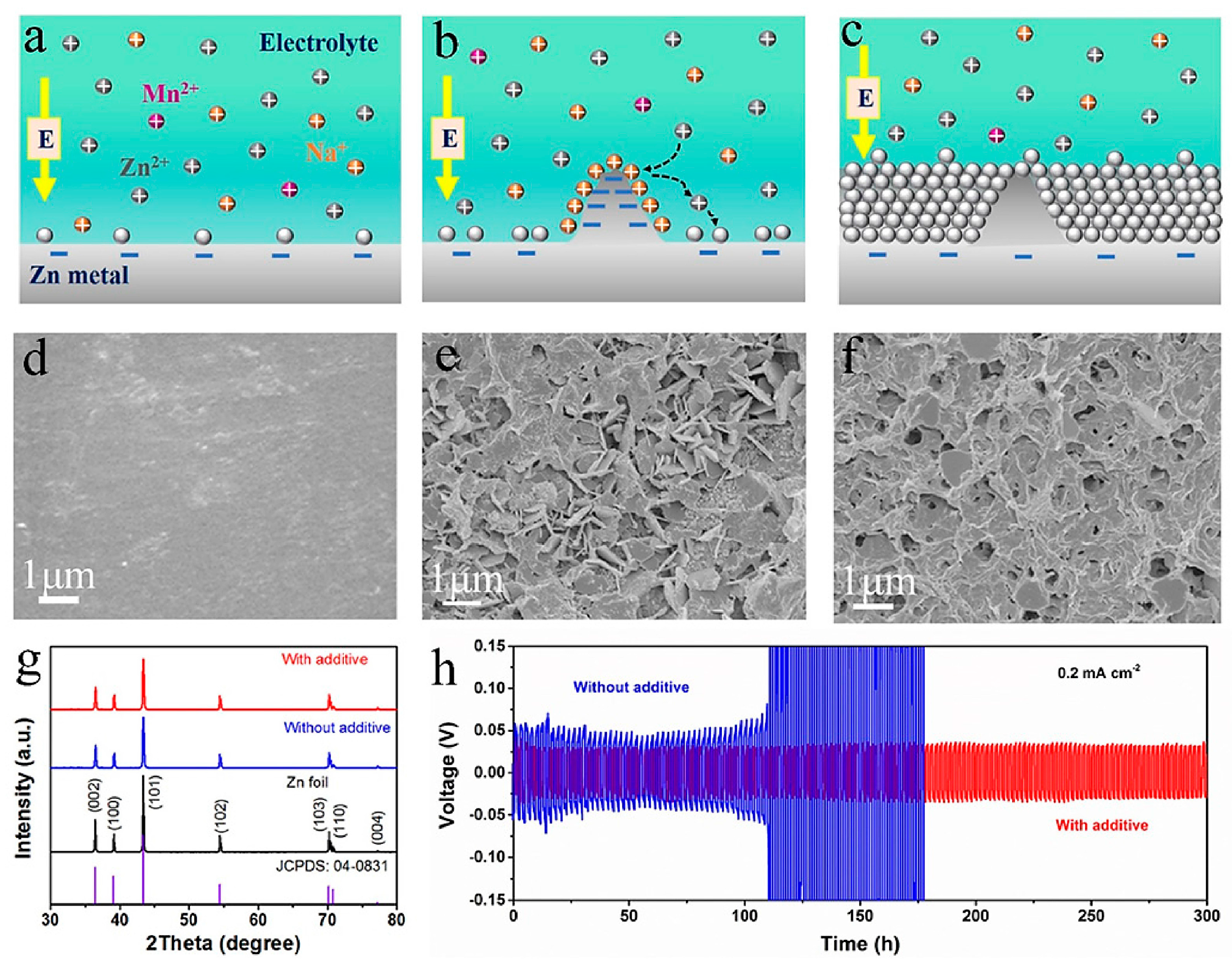
- Chang, G.; Liu, S.; Fu, Y.; Hao, X.; Jin, W.; Ji, X.; Hu, J. Inhibition Role of Trace Metal Ion Additives on Zinc Dendrites during Plating and Striping Processes. Adv. Mater. Interfaces 2019, 6, 1901358. [Google Scholar] [CrossRef]
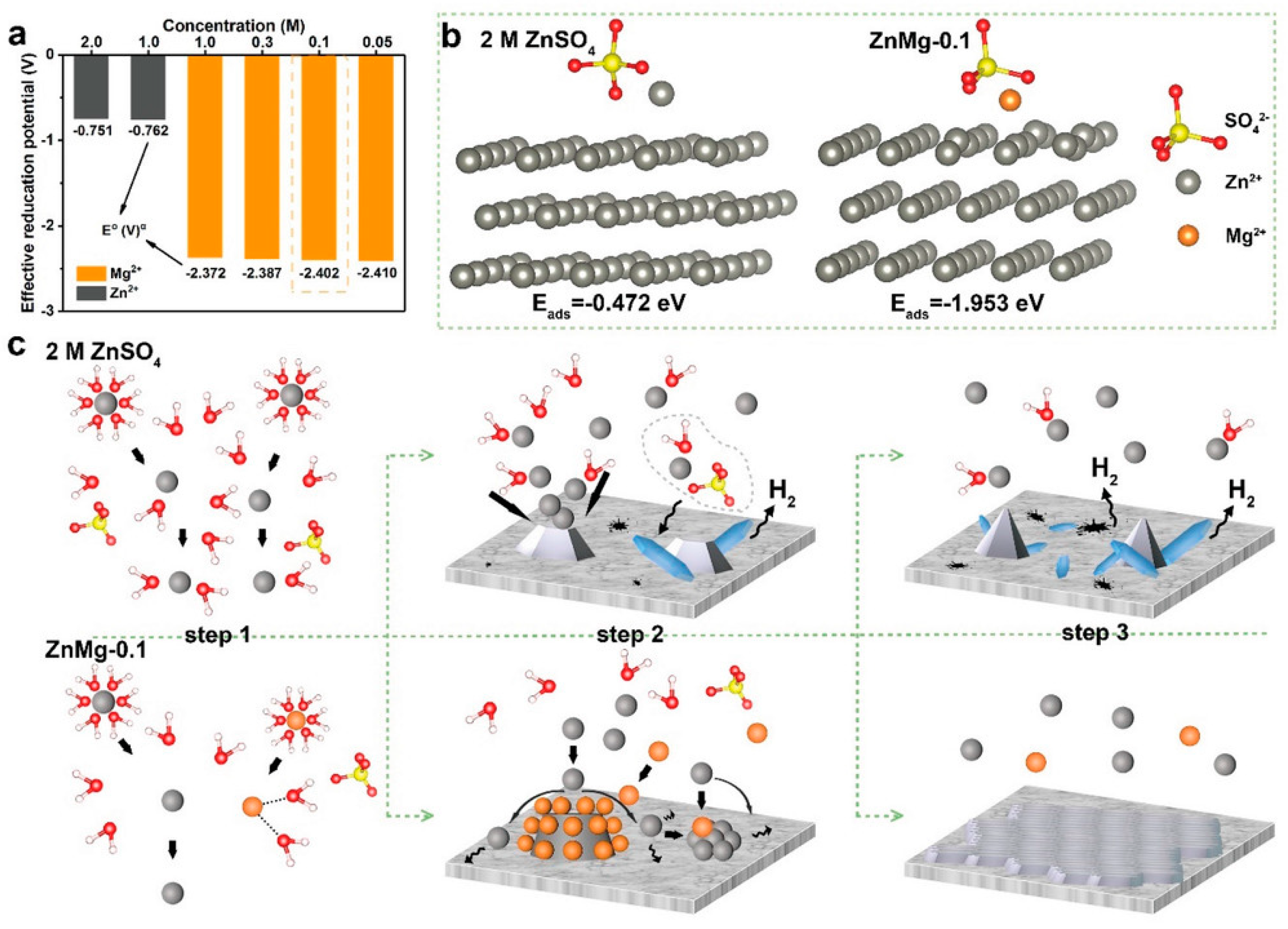
- Wang, P.; Xie, X.; Xing, Z.; Chen, X.; Fang, G.; Lu, B.; Zhou, J.; Liang, S.; Fan, H.J. Mechanistic Insights of Mg2+-Electrolyte Additive for High-Energy and Long-Life Zinc-Ion Hybrid Capacitors. Adv. Energy Mater. 2021, 11, 2101158. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Du, H.; Yang, Y.; Gao, Z.; Qie, L.; Huang, Y. Lanthanum nitrate as aqueous electrolyte additive for favourable zinc metal electrodeposition. Nat. Commun. 2022, 13, 3252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Q.; Wu, Y.; Wang, Q.; Huang, J.; Chen, P. Highly efficient dendrite suppressor and corrosion inhibitor based on gelatin/Mn2+ Co-additives for aqueous rechargeable zinc-manganese dioxide battery. Chem. Eng. J. 2021, 407, 127189. [Google Scholar] [CrossRef]
- Nie, X.; Miao, L.; Yuan, W.; Ma, G.; Di, S.; Wang, Y.; Shen, S.; Zhang, N. Cholinium Cations Enable Highly Compact and Dendrite-Free Zn Metal Anodes in Aqueous Electrolytes. Adv. Funct. Mater. 2022, 32, 2203905. [Google Scholar] [CrossRef]
- Yao, R.; Qian, L.; Sui, Y.; Zhao, G.; Guo, R.; Hu, S.; Liu, P.; Zhu, H.; Wang, F.; Zhi, C.; et al. A Versatile Cation Additive Enabled Highly Reversible Zinc Metal Anode. Adv. Energy Mater. 2022, 12, 2102780. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, J.; Luo, N.; Chai, G.-L.; Miller, T.S.; Lai, F.; Shearing, P.; Brett, D.J.L.; Han, D.; et al. Alleviation of Dendrite Formation on Zinc Anodes via Electrolyte Additives. ACS Energy Lett. 2021, 6, 395–403. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, J.; Wang, Y.; Wu, X.; Ma, P.; Zhang, X.; Wang, G.; Yan, X.J.E.S.M. A rechargeable aqueous zinc/sodium manganese oxides battery with robust performance enabled by Na2SO4 electrolyte additive. Energy Storage Mater. 2021, 38, 299–308. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, K.; Zhang, Q.; Yang, G.; Wang, C. Highly stable aqueous zinc-ion batteries enabled by suppressing the dendrite and by-product formation in multifunctional Al3+ electrolyte additive. Nano Res. 2022, 15, 8039–8047. [Google Scholar] [CrossRef]
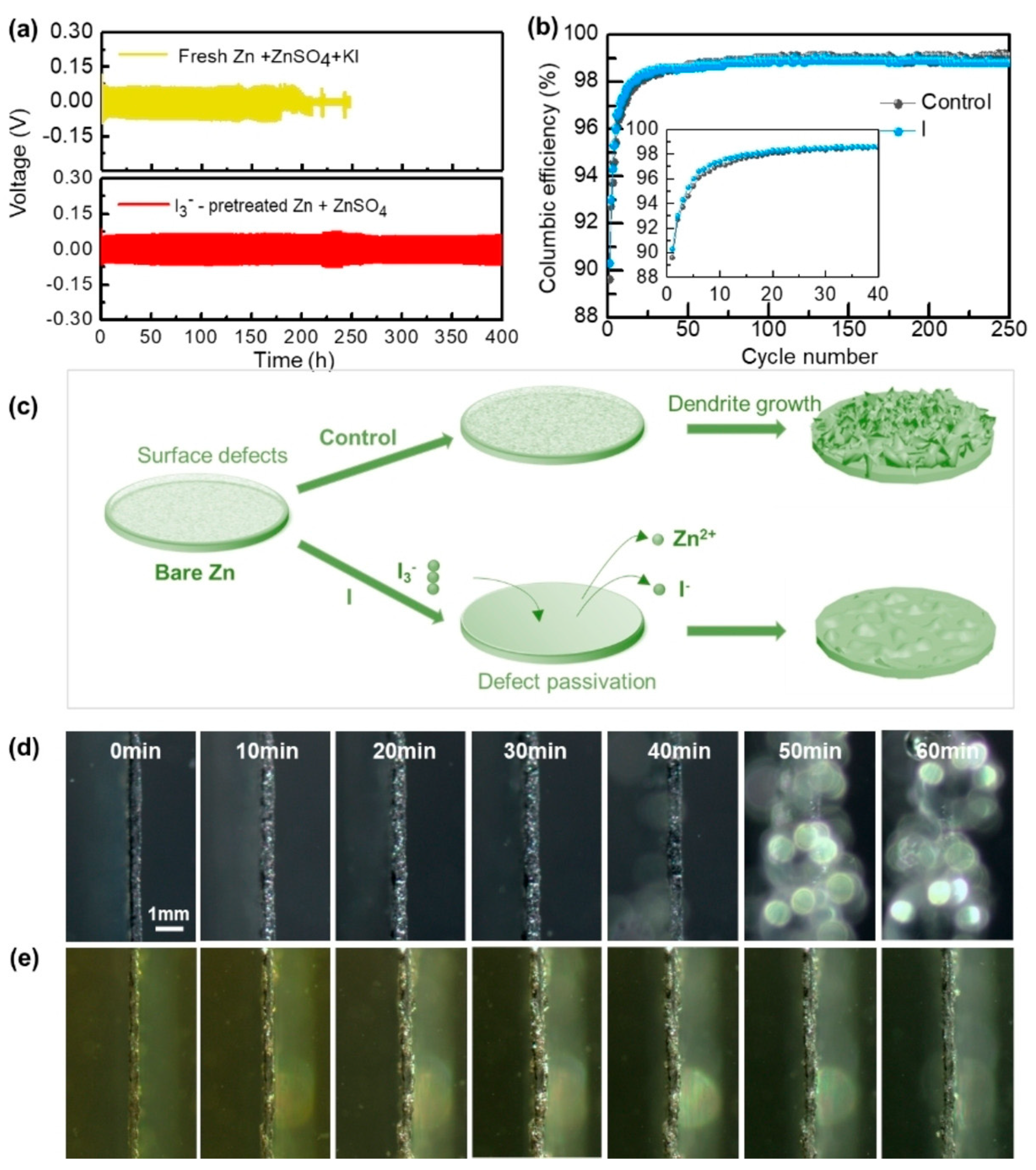
- Liu, S.; Shang, W.; Yang, Y.; Kang, D.; Li, C.; Sun, B.; Kang, L.; Yun, S.; Jiang, F. Effects of I3− Electrolyte Additive on the Electrochemical Performance of Zn Anodes and Zn/MnO2 Batteries. Batter. Supercaps 2022, 5, e202100221. [Google Scholar] [CrossRef]
- Otani, T.; Fukunaka, Y.; Homma, T. Effect of lead and tin additives on surface morphology evolution of electrodeposited zinc. Electrochim. Acta 2017, 242, 364–372. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, R.; Gong, Y.; Wang, M.; Chen, Y.; Chu, M.; Chen, L. Effects of doping Al on the structure and electrochemical performances of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials. Ionics 2018, 24, 967–976. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B.; Manthiram, A. An in-depth understanding of the effect of aluminum doping in high-nickel cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, Y.; Cheng, Y.; Qi, R.; Peng, H.; Lin, H.; Huang, R. Understanding the Effect of Al Doping on the Electrochemical Performance Improvement of the LiMn2O4 Cathode Material. ACS Appl. Mater. Interfaces 2021, 13, 45446–45454. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, H.; Li, J.; Zhang, X.; Yang, Z.; Xu, M.; Pan, L. A novel redox bromide-ion additive hydrogel electrolyte for flexible Zn-ion hybrid supercapacitors with boosted energy density and controllable zinc deposition. J. Mater. Chem. A 2020, 8, 15042–15050. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Yin, Y.; Chang, N.; Zhang, H.; Li, X. High-energy-density aqueous zinc-based hybrid supercapacitor-battery with uniform zinc deposition achieved by multifunctional decoupled additive. Nano Energy 2022, 96, 107120. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Xu, Y.; An, Y.; Wu, L.; Sun, Y.; Liao, H.; Zheng, K.; Zhang, X. High-Energy Density Aqueous Zinc–Iodine Batteries with Ultra-long Cycle Life Enabled by the ZnI2 Additive. ACS Sustain. Chem. Eng. 2021, 9, 13268–13276. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic Surfactant-Type Electrolyte Additive Enables Three-Dimensional Dendrite-Free Zinc Anode for Stable Zinc-Ion Batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Dong, N.; Yan, M.; Zhang, F.; Mochizuki, K.; Pan, H. Advanced Buffering Acidic Aqueous Electrolytes for Ultra-Long Life Aqueous Zinc-Ion Batteries. Small 2022, 18, 2200742. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Tang, J.; Chang, G.; Fu, Y.; Jin, W.; Ji, X.; Hu, J. Effective inhibition of zinc dendrites during electrodeposition using thiourea derivatives as additives. J. Mater. Sci. 2019, 54, 3536–3546. [Google Scholar] [CrossRef]
- Banik, S.J.; Akolkar, R. Suppressing Dendritic Growth during Alkaline Zinc Electrodeposition using Polyethylenimine Additive. Electrochim. Acta 2015, 179, 475–481. [Google Scholar] [CrossRef]
- Dong, Y.; Miao, L.; Ma, G.; Di, S.; Wang, Y.; Wang, L.; Xu, J.; Zhang, N. Non-concentrated aqueous electrolytes with organic solvent additives for stable zinc batteries. Chem. Sci. 2021, 12, 5843–5852. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, R.; Di, S.; Qian, Z.; Zhang, L.; Xin, W.; Liu, M.; Zhu, Z.; Chu, S.; Du, Y.; et al. Aqueous Electrolytes with Hydrophobic Organic Cosolvents for Stabilizing Zinc Metal Anodes. ACS Nano 2022, 16, 9667–9678. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yang, X.; Xiong, P.; Lin, H.; He, L.; Yao, Q.; Wei, M.; Qian, Q.; Chen, Q.; Zeng, L. High-Rate, Large Capacity, and Long Life Dendrite-Free Zn Metal Anode Enabled by Trifunctional Electrolyte Additive with a Wide Temperature Range. Adv. Sci. 2022, 9, 2201433. [Google Scholar] [CrossRef] [PubMed]
- Wijitrat, A.; Qin, J.; Kasemchainan, J.; Tantavichet, N. Ethylene carbonate as an organic electrolyte additive for high-performance aqueous rechargeable zinc-ion batteries. J. Ind. Eng. Chem. 2022, 112, 96–105. [Google Scholar] [CrossRef]
- Miao, Z.; Liu, Q.; Wei, W.; Zhao, X.; Du, M.; Li, H.; Zhang, F.; Hao, M.; Cui, Z.; Sang, Y.; et al. Unveiling unique steric effect of threonine additive for highly reversible Zn anode. Nano Energy 2022, 97, 107145. [Google Scholar] [CrossRef]
- Meng, Q.; Zhao, R.; Cao, P.; Bai, Q.; Tang, J.; Liu, G.; Zhou, X.; Yang, J. Stabilization of Zn anode via a multifunctional cysteine additive. Chem. Eng. J. 2022, 447, 137471. [Google Scholar] [CrossRef]
- Shi, J.; Xia, K.; Liu, L.; Liu, C.; Zhang, Q.; Li, L.; Zhou, X.; Liang, J.; Tao, Z. Ultrahigh coulombic efficiency and long-life aqueous Zn anodes enabled by electrolyte additive of acetonitrile. Electrochim. Acta 2020, 358, 136937. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Shao, Y.; Yang, J.; Yanna, N.; Liu, J.; Shi, S.; Zhang, L.; Ye, A.; He, B.; et al. A Highly Reversible Zn Anode with Intrinsically Safe Organic Electrolyte for Long-Cycle-Life Batteries. Adv. Mater. 2019, 31, 1900668. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, H.; Chen, Z.; Hu, Z.; Wang, N.; Jin, Y.; Yu, X.; Meng, H. Nonionic Surfactant Coconut Diethanol Amide Inhibits the Growth of Zinc Dendrites for More Stable Zinc-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 7590–7599. [Google Scholar] [CrossRef]
- Xia, S.; Lopez, J.; Liang, C.; Zhang, Z.; Bao, Z.; Cui, Y.; Liu, W. High-Rate and Large-Capacity Lithium Metal Anode Enabled by Volume Conformal and Self-Healable Composite Polymer Electrolyte. Adv. Sci. 2019, 6, 1802353. [Google Scholar] [CrossRef]
- Li, C.; Shyamsunder, A.; Hoane, A.G.; Long, D.M.; Kwok, C.Y.; Kotula, P.G.; Zavadil, K.R.; Gewirth, A.A.; Nazar, L.F. Highly reversible Zn anode with a practical areal capacity enabled by a sustainable electrolyte and superacid interfacial chemistry. Joule 2022, 6, 1103–1120. [Google Scholar] [CrossRef]
- Chen, A.; Zhao, C.; Guo, Z.; Lu, X.; Zhang, J.; Liu, N.; Zhang, Y.; Zhang, N. Stabilized Zn Anode Based on SO42– Trapping Ability and High Hydrogen Evolution Barrier. Adv. Funct. Mater. 2022, 32, 2203595. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Liu, S.; Hao, Y.; Tang, Q.; Hu, A.; Liu, Z.; Chen, X. Stabilizing Zinc Anodes by Regulating the Electrical Double Layer with Saccharin Anions. Adv. Mater. 2021, 33, 2100445. [Google Scholar] [CrossRef] [PubMed]
- Bani Hashemi, A.; Kasiri, G.; La Mantia, F. The effect of polyethyleneimine as an electrolyte additive on zinc electrodeposition mechanism in aqueous zinc-ion batteries. Electrochim. Acta 2017, 258, 703–708. [Google Scholar] [CrossRef]
- Zhou, T.; Mu, Y.; Chen, L.; Li, D.; Liu, W.; Yang, C.; Zhang, S.; Wang, Q.; Jiang, P.; Ge, G.; et al. Toward stable zinc aqueous rechargeable batteries by anode morphology modulation via polyaspartic acid additive. Energy Storage Mater. 2022, 45, 777–785. [Google Scholar] [CrossRef]
- Feng, D.; Cao, F.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Immunizing Aqueous Zn Batteries against Dendrite Formation and Side Reactions at Various Temperatures via Electrolyte Additives. Small 2021, 17, 2103195. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Gao, S.; Xu, D.; Wang, Z.; Ye, Z.; Wang, L.; Chen, B.; Li, L.; Ye, M.; et al. Ultrastable Zinc Anode by Simultaneously Manipulating Solvation Sheath and Inducing Oriented Deposition with PEG Stability Promoter. Small 2022, 18, 2103345. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, P.; Musso, T.; Mittal, U.; Du, Q.; Liang, X.; Kundu, D. Long-Life Zn Anode Enabled by Low Volume Concentration of a Benign Electrolyte Additive. Adv. Funct. Mater. 2022, 32, 2200606. [Google Scholar] [CrossRef]
- Zhao, K.; Fan, G.; Liu, J.; Liu, F.; Li, J.; Zhou, X.; Ni, Y.; Yu, M.; Zhang, Y.-M.; Su, H.; et al. Boosting the Kinetics and Stability of Zn Anodes in Aqueous Electrolytes with Supramolecular Cyclodextrin Additives. J. Am. Chem. Soc. 2022, 144, 11129–11137. [Google Scholar] [CrossRef]
- Lin, X.-S.; Wang, Z.-R.; Ge, L.-H.; Xu, J.-W.; Ma, W.-Q.; Ren, M.-M.; Liu, W.-L.; Yao, J.-S.; Zhang, C.-B. Electrolyte Modification for Long-Life Zn Ion Batteries: Achieved by Methanol Additive. ChemElectroChem 2022, 9, e202101724. [Google Scholar] [CrossRef]
- Ma, L.; Vatamanu, J.; Hahn, N.T.; Pollard, T.P.; Borodin, O.; Petkov, V.; Schroeder, M.A.; Ren, Y.; Ding, M.S.; Luo, C.; et al. Highly reversible Zn metal anode enabled by sustainable hydroxyl chemistry. Proc. Natl. Acad. Sci. USA 2022, 119, e2121138119. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Han, Z.; Lin, Y.; Yu, B.; Wu, D.; Zhao, L.; Wang, M.; Chen, J.; Ma, Z.; Guo, B.; et al. Stabilizing zinc anode for high-performance aqueous zinc ion batteries via employing a novel inositol additive. J. Alloy Compd. 2022, 914, 165231. [Google Scholar] [CrossRef]
- Wu, F.; Chen, Y.; Chen, Y.; Yin, R.; Feng, Y.; Zheng, D.; Xu, X.; Shi, W.; Liu, W.; Cao, X. Achieving Highly Reversible Zinc Anodes via N, N-Dimethylacetamide Enabled Zn-Ion Solvation Regulation. Small 2022, 18, 2202363. [Google Scholar] [CrossRef]
- Sun, K.E.K.; Hoang, T.K.A.; Doan, T.N.L.; Yu, Y.; Chen, P. Highly Sustainable Zinc Anodes for a Rechargeable Hybrid Aqueous Battery. Chem. Eur. J. 2018, 24, 1667–1673. [Google Scholar] [CrossRef]
- Lee, C.W.; Sathiyanarayanan, K.; Eom, S.W.; Kim, H.S.; Yun, M.S. Novel electrochemical behavior of zinc anodes in zinc/air batteries in the presence of additives. J. Power Sources 2006, 159, 1474–1477. [Google Scholar] [CrossRef]
- Hamilton, S.T.; Feric, T.G.; Gładysiak, A.; Cantillo, N.M.; Zawodzinski, T.A.; Park, A.-H.A. Mechanistic Study of Controlled Zinc Electrodeposition Behaviors Facilitated by Nanoscale Electrolyte Additives at the Electrode Interface. ACS Appl. Mater. Interfaces 2022, 14, 22016–22029. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Hao, Y.; Yang, Y.; Qian, Y.; Chang, G.; Zhang, Y.; Tang, Q.; Hu, A.; Chen, X. Self-Healing SeO2 Additives Enable Zinc Metal Reversibility in Aqueous ZnSO4 Electrolytes. Adv. Funct. Mater. 2022, 32, 2112091. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Wu, K.; Yu, F.; Zhu, M.; Wang, G.; Xu, G.; Wu, M.; Liu, H.-K.; Dou, S.-X.; et al. 2D anionic nanosheet additive for stable Zn metal anodes in aqueous electrolyte. Chem. Eng. J. 2022, 430, 133042. [Google Scholar] [CrossRef]
- Sun, C.; Wu, C.; Gu, X.; Wang, C.; Wang, Q. Interface Engineering via Ti3C2Tx MXene Electrolyte Additive toward Dendrite-Free Zinc Deposition. Nano-Micro Lett. 2021, 13, 89. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.; Li, S.; Liu, C.; Li, H.; Zou, G.; Hu, J.; Hou, H.; Ji, X. Graphene quantum dots enable dendrite-free zinc ion battery. Nano Energy 2022, 92, 106752. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, D.; Li, P.; Guo, X.; Sun, B.; Liu, H.; Yan, K.; Gogotsi, Y.; Wang, G. MXene-Based Dendrite-Free Potassium Metal Batteries. Adv. Mater. 2020, 32, 1906739. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shi, X.; Zhang, Y.; Liang, J.; Qu, J.; Lai, C. Ti3C2Tx MXene Interface Layer Driving Ultra-Stable Lithium-Iodine Batteries with Both High Iodine Content and Mass Loading. ACS Nano 2020, 14, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, J.; Cao, J.; Zhang, D.; Zhang, X.; Sriprachuabwong, C.; Kheawhom, S.; Wangyao, P.; Qin, J. Elimination of Zinc Dendrites by Graphene Oxide Electrolyte Additive for Zinc-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4602–4609. [Google Scholar] [CrossRef]



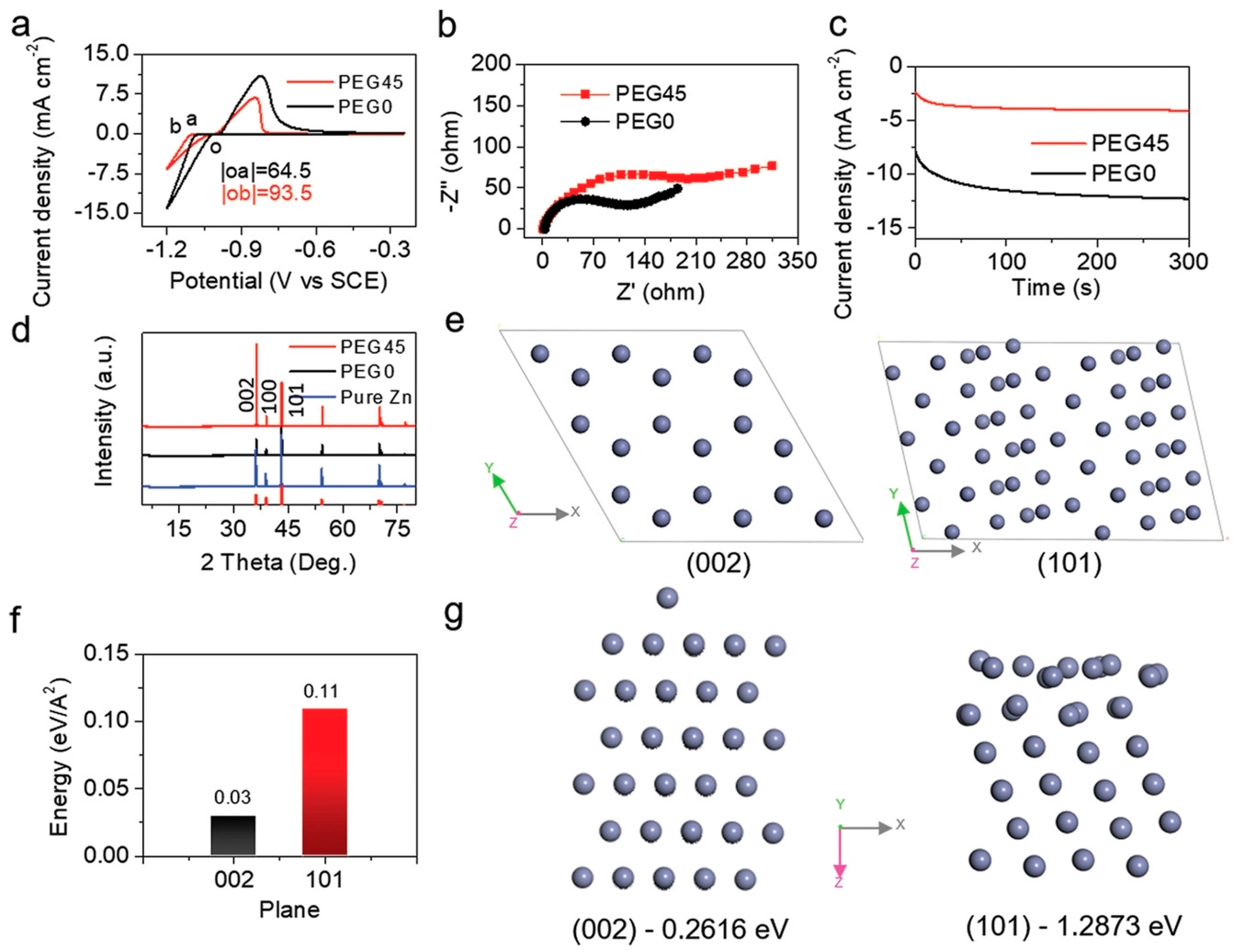
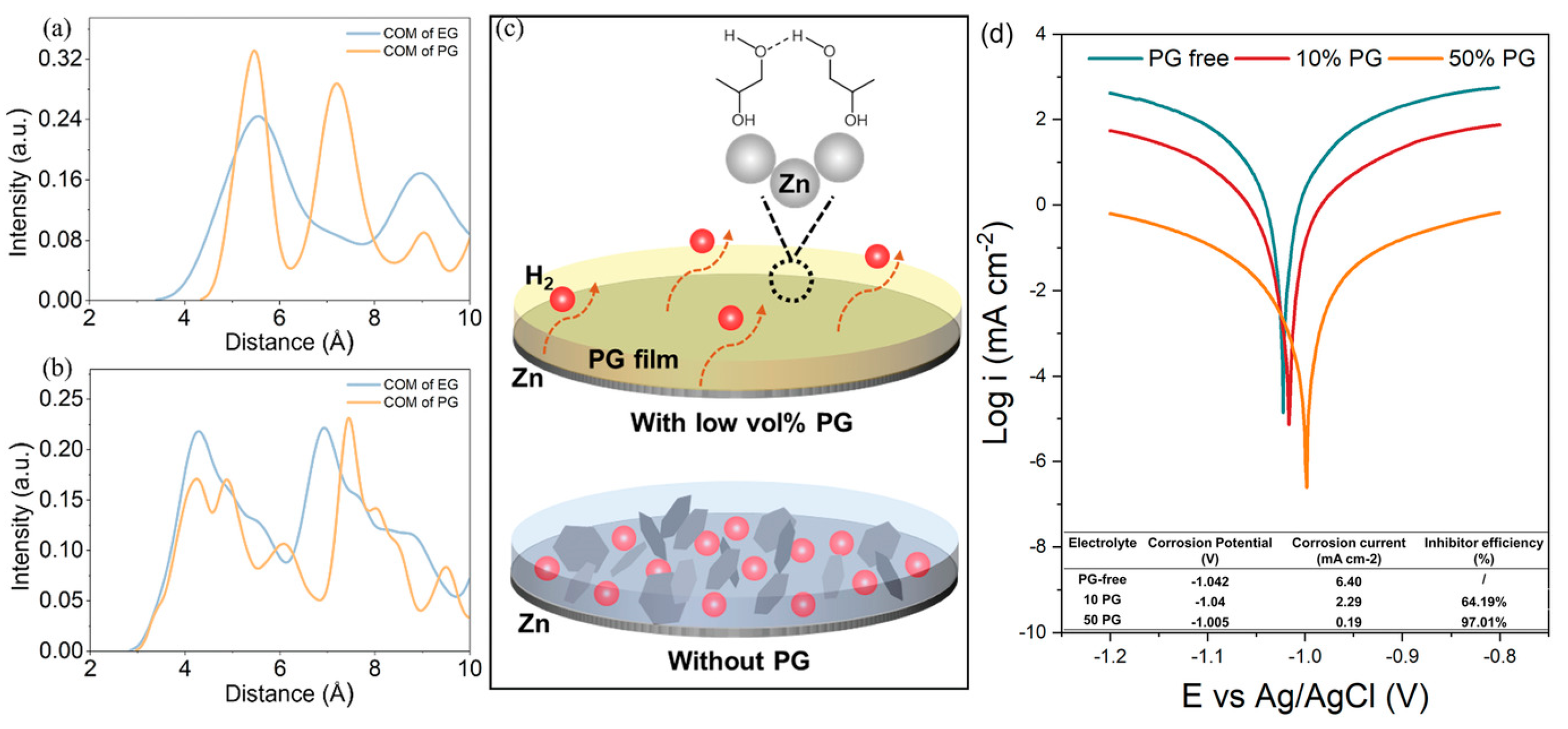
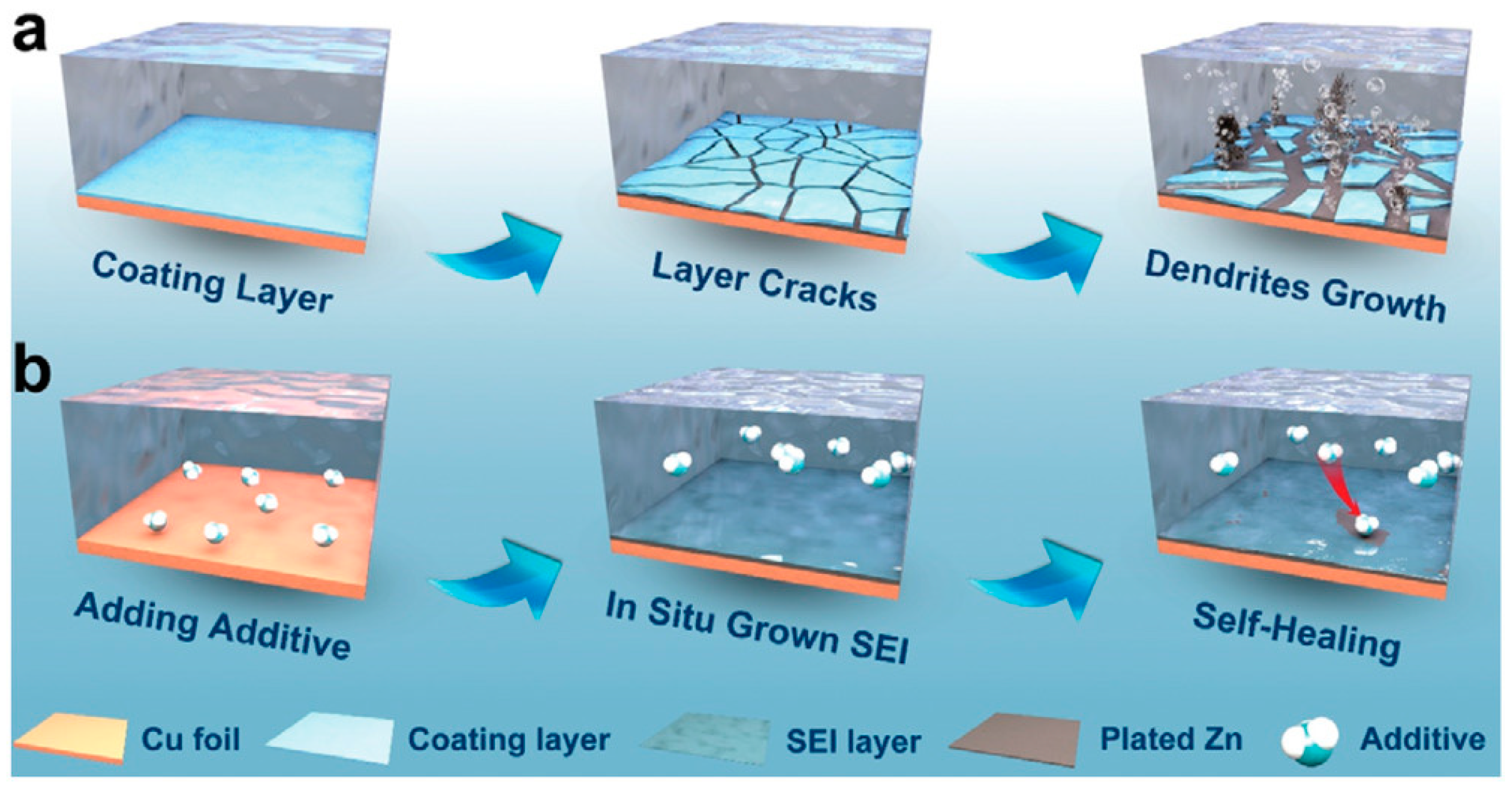
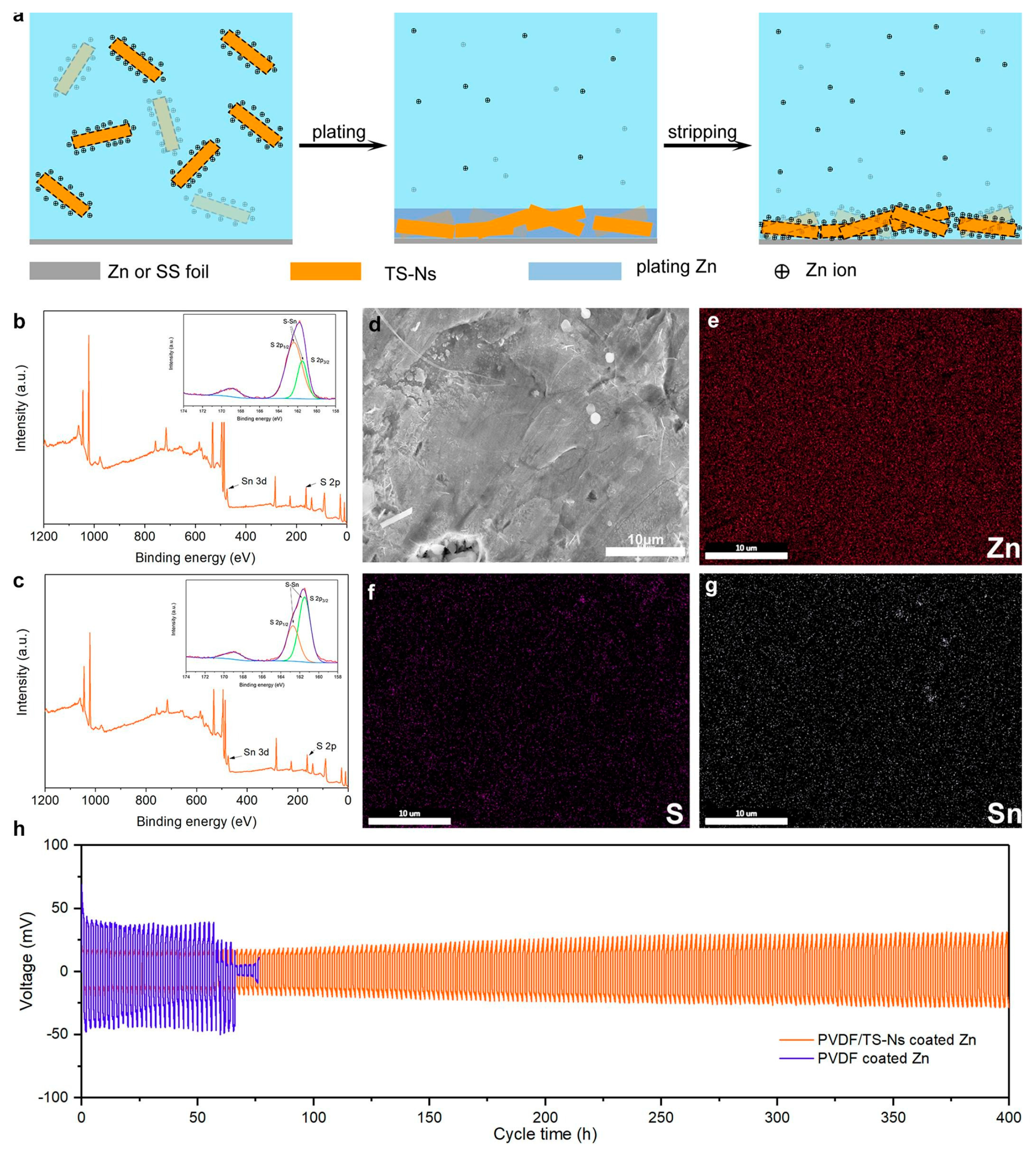
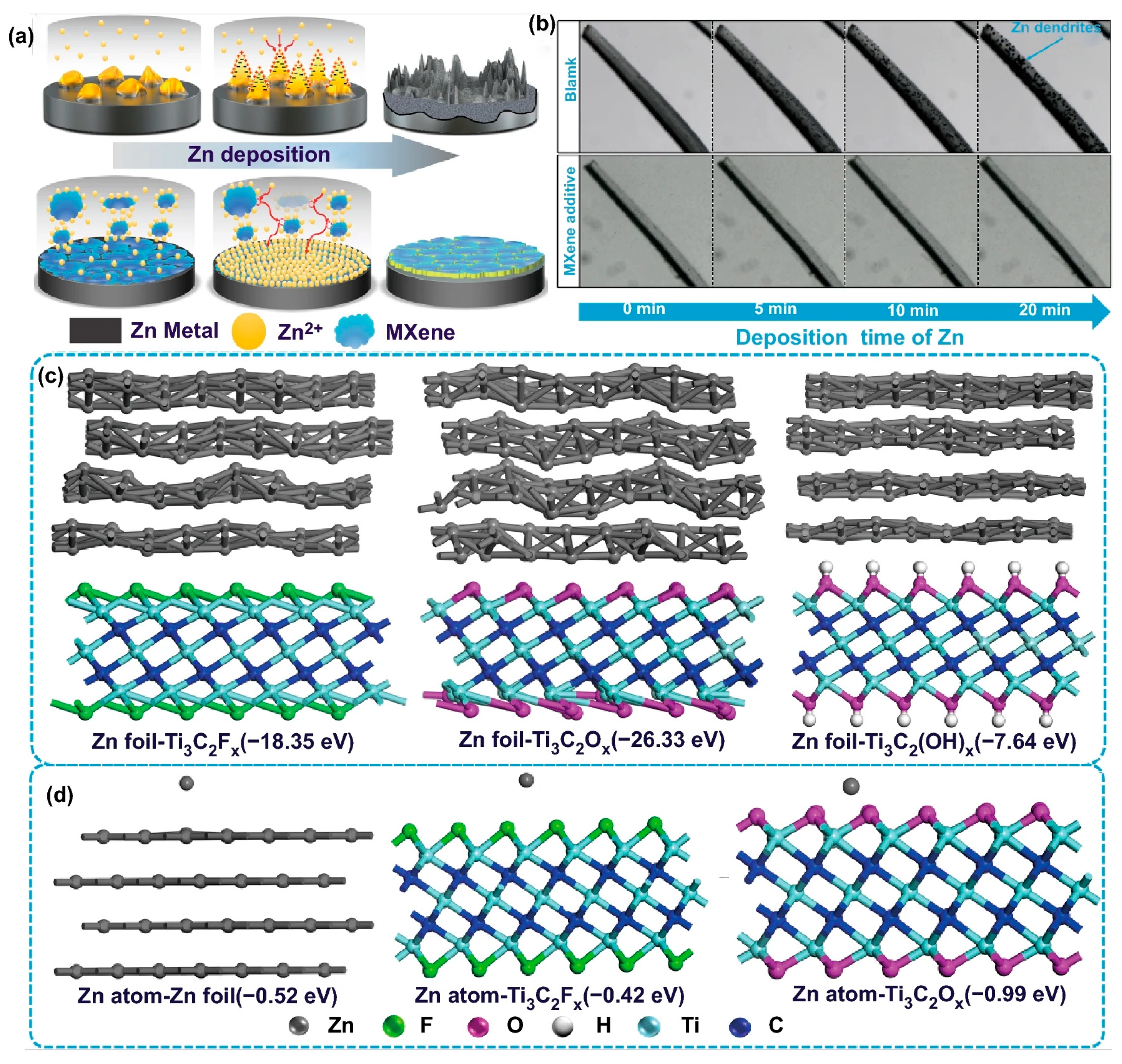
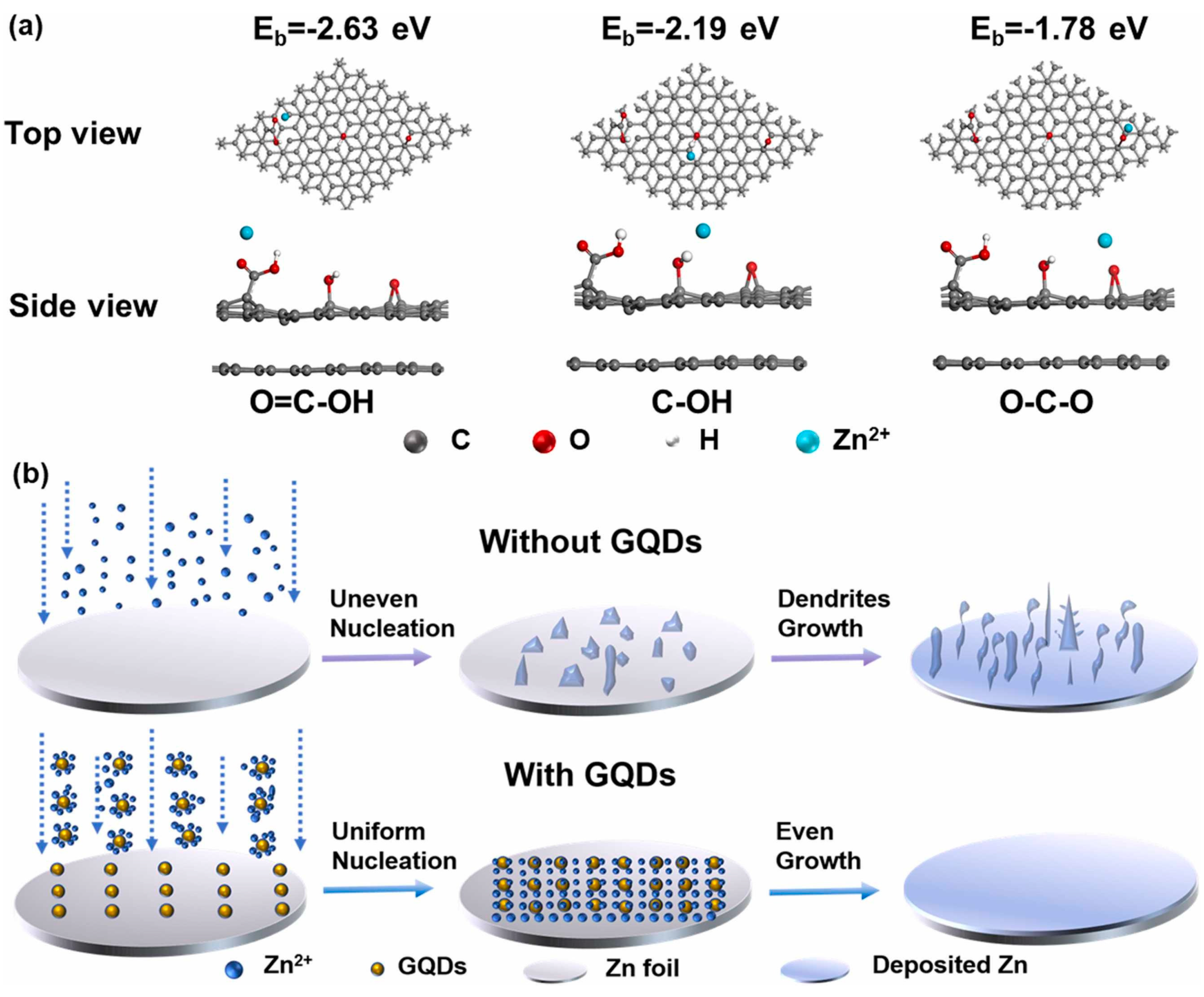
| Solution with Additives | Effects | Operation Parameters and Results | Ref. |
|---|---|---|---|
| 1.25 M (NH4)2SO4 + 2 M NH3·H2O + 0.5 M ZnSO4 + 1.67 mM PbSO4 | Inhibit zinc dendrites Improve formation of homogeneous zinc deposits | - | [60] |
| 3 M ZnSO4 + 2 M LiCl | Inhibit the formation of dendrites | 0.2 mA cm−2 @ 0.0333 mAh cm−2, 170 h | [66] |
| 2 M ZnSO4 + 0.1 M MnSO4 + 0.5 M Na2SO4 | Promote the uniform deposition Prevent further dissolution of Mn Improve the structure stability | 0.2 mA cm−2 @ 0.2 mAh cm−2, 300 h | [67] |
| 2 M ZnSO4 + 0.1 M MgSO4 | Inhibit the HER Enable uniform Zn nucleation/deposition Suppress the growth of Zn dendrites | 1 mA cm−2 @ 0.25 mAh cm−2, 600 h | [61] |
| 2 M ZnSO4 + 0.2 M MnSO4 + 0.5% gelatin | Mitigate both dendrite growth and corrosion | 0.25 mA cm−2 @ 0.05 mAh cm−2, 500 h | [63] |
| 1 M KCF3SO3 + 0.1 M Zn(CF3SO3)2 + 0.1 M Al(CF3SO3)3 | Improve the reversibility Inhibit the formation of byproduct Improved the reversible deposition of zinc ions | 3 mA cm−2, 1500 h | [68] |
| 2 M ZnSO4 + 8.5 mM La(NO3)3 | Weaken the EDL repulsive force Favor dense metallic zinc deposits Regulate the charge distribution | 1.0 mA cm−2 @ 1.0 mAh cm−2, 1200 h | [62] |
| 1 M ZnSO4 with 4 M cholinium | Preferential adsorption of Ch+ cations Create “leveling effect” to homogenize Zn deposition Weaken water activity Promote the desolvation of hydrated Zn cations | 1.0 mA cm−2 @ 1.0 mAh cm−2, 2000 h | [64] |
| 2 M ZnSO4 + 0.2 M MnSO4 + 25 mM KI + 25 mM I2 | Extend the strip/plating stability of zinc Passivate zinc dendrite growth hotspots Reduce decomposition rate of H2O and corrosion rate of Zn | 1.75 mA cm−2 @ 0.585 mAh cm−2, 1430 h | [69] |
| Solution with Additives | Effects | Operation Parameters and Results | Ref. |
|---|---|---|---|
| 2 M NH3·H2O + 4 M NH4Cl + 20 g L−1 Zn2+ + 5 mM methylthiourea | Inhibit the growth of zinc dendrites Get smooth deposits Proceed with a homogenous dissolution | - | [79] |
| 0.5 M ZnSO4 + 30 ppm polyethyleneimine | Improve the zinc deposition kinetics and morphology | - | [94] |
| 2 M Zn(OTf)2 in molar ratio of H2O:dimethyl carbonate = 4:1 | Form a SEI layer on the Zn electrode | 5 mA cm−2 @ 2.5 mAh cm−2, 800 h | [81] |
| 2 M Zn(OTf)2 + 7 M diethyl carbonate | Hydrophobic organic cosolvent Reduce the solvating H2O number Weak water activity Suppress the interfacial side reactions | 5 mA cm−2 @ 2.5 mAh cm−2, 3500 h | [82] |
| 1 M ZnSO4 + 75 mM Na4 EDTA | Dominate active sites for H2 generation Inhibit water electrolysis Promotes desolvation of Zn(H2O)62+ | 5 mA cm−2 @ 2 mAh cm−2, 2500 cycles | [32] |
| 1 M ZnSO4 + 0.5 M sorbitol | Improve the solvation structure Restrict all kinds of side reactions Induce the final exposure of the crystal plane (002) with lowest growth rate | 1 mA cm−2 @ 1 mAh cm−2, 1000 h | [57] |
| 2 M ZnSO4 + 0.2 M CH3COONH4 | Promote the homogenization of zinc deposition Inhibit the formation of by-products Restrains the increase in local pH | 2 mA cm−2, 2400 h | [83] |
| 2 M ZnSO4 + 0.5 M MnSO4 + 6% (w/v) ethylene carbonate | Suppress dendrite formation Inhibit the parasitic reactions on the Zn anode | 0.2 mA cm−2 @ 0.035 mAh cm−2, 280 h | [84] |
| 0.5 M ZnCl2 + 1 M triethylmethyl-ammonium chloride | Homogenize zinc deposition via adsorbing onto the zinc metal surface Inhibit the formation of by-products by participating in the constitution of contact ion pairs | 1 mA cm−2 @ 0.5 mAh cm−2, 2145 h | [65] |
| 3 M ZnSO4 + 0.1 M threonine | Restrict the 2D diffusion of Zn2+ ions Facilitate the homogeneous deposition of Zn Suppress HER Inhibit the dendritic growth of Zn | 1 mA cm−2 @ 1 mAh cm−2, 580 h | [85] |
| ZnSO4 + 0.1 M cysteine | Exhibit strong interactions with Zn metal Facilitate the reconfiguration of solvation structures | 0.5 mA cm−2 @ 0.5 mAh cm−2, 2300 h | [86] |
| 1 M Zn(CF3SO3)2 in H2O:acetonitrile solution with volume ratio of 3:1 | Accumulate on the Zn surface to shield free water Suppress hydrogen evolution | 1 mA cm−2 @ 1 mAh cm−2, 1300h | [87] |
| 0.5 M Zn(OTf)2 + trimethyl phosphate-DMC (1:1 by volume) | Improve the homogeneity of Zn substrate Formation of Zn3(PO4)2 | 1 mA cm−2, 5000h | [88] |
| 3 M Zn(CF3SO3)2 + 0.5 wt% FI | Anchor on the surface of the Zn electrode to create an interface protective layer Regulate the Zn2+ solvation shell in the electrolyte | 1 mA cm−2 @ 0.5 mAh cm−2, 4000 h | [59] |
| 2 M ZnSO4 + 8mg ml−1 polyaspartic acid | Adjust the morphology of the deposited metal zinc | 0.5 mA cm−2 @ 0.5 mAh cm−2, 3200 h | [95] |
| 2 M ZnSO4 in 20 vol% dimethyl sulfoxide | Alleviate the side reactions Inducing the fine-grained deposition manner Resist side reactions and dendrite formation | 1 mA cm−2 @ 1 mA h cm−2, 2100 h | [96] |
| 1 M Zn(CF3SO3)2 with weight ratio of polyethylene glycol 45% | Tailor the solvation sheath of Zn2+ and the intensity of hydrogen bonding Favor oriented deposition (002) plane with the low surface energy | 0.25 mA cm−2 @ 0.125 mAh cm−2, 1500 h | [97] |
| 1 M ZnSO4 with propylene glycol volume fraction 10% | Regulate the Zn deposition morphology Self-organize into a hydrophobic film Facilitate H2 removal from the Zn surface | 1 mA cm−2 @ 1 mAh cm−2, 1025 h | [98] |
| Solution with Additives | Effects | Operation Parameters and Results | Ref. |
|---|---|---|---|
| 2 M ZnSO4 + 2 mM SeO2 | Form ZnSe protective layer Promote the nucleation and growth Self-healing | 2 mA cm−2 @ 2 mAh cm−2, 2100 h | [107] |
| 3 M ZnSO4 + 0.1 M MnSO4 + 0.5 mg ml−1 TS-Ns | attract plenty of Zn ions from electrolyte Increase local Zn2+ ion Reduce Zn nucleation overpotential | 1 mA cm−2 @ 1 mAh cm−2, 480 h | [108] |
| 2 M ZnSO4 + 0.05 M Ti3C2Tx Mxene | Uniform initial Zn deposition via providing abundant zincophilic-O groups Form robust solid-electrolyte interface | 1 mA cm−2 @ 1 mAh cm−2, 500 cycles | [109] |
| 2 M ZnSO4 + 0.4 g L−1 graphene quantum dots | Strong coordination interactions between GQDs and Zn2+ ions Promote homogeneous Zn2+ ions distribution Accelerate the Zn2+ deposition kinetics | 0.8 mA cm−2 @ 0.2 mAh cm−2, 2200 h | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; Zhao, D.; He, Y.; Huang, H.; Chen, B.; Wang, X.; Guo, Z. Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries. Batteries 2022, 8, 153. https://doi.org/10.3390/batteries8100153
Zhai C, Zhao D, He Y, Huang H, Chen B, Wang X, Guo Z. Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries. Batteries. 2022; 8(10):153. https://doi.org/10.3390/batteries8100153
Chicago/Turabian StyleZhai, Chongyuan, Dandi Zhao, Yapeng He, Hui Huang, Buming Chen, Xue Wang, and Zhongcheng Guo. 2022. "Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries" Batteries 8, no. 10: 153. https://doi.org/10.3390/batteries8100153
APA StyleZhai, C., Zhao, D., He, Y., Huang, H., Chen, B., Wang, X., & Guo, Z. (2022). Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries. Batteries, 8(10), 153. https://doi.org/10.3390/batteries8100153







