The Battery Life Estimation of a Battery under Different Stress Conditions
Abstract
:1. Introduction
2. Battery Aging and Modeling
- Loss of conductivity (CL);
- Loss of active material (LAM), due to the degradation processes in which the use of the active electrode material is reduced;
- Loss of the lithium supply (LLI), caused by side reactions that irreversibly consume a portion of the lithium available in the cell.
3. Experimental Set Up and Modeling
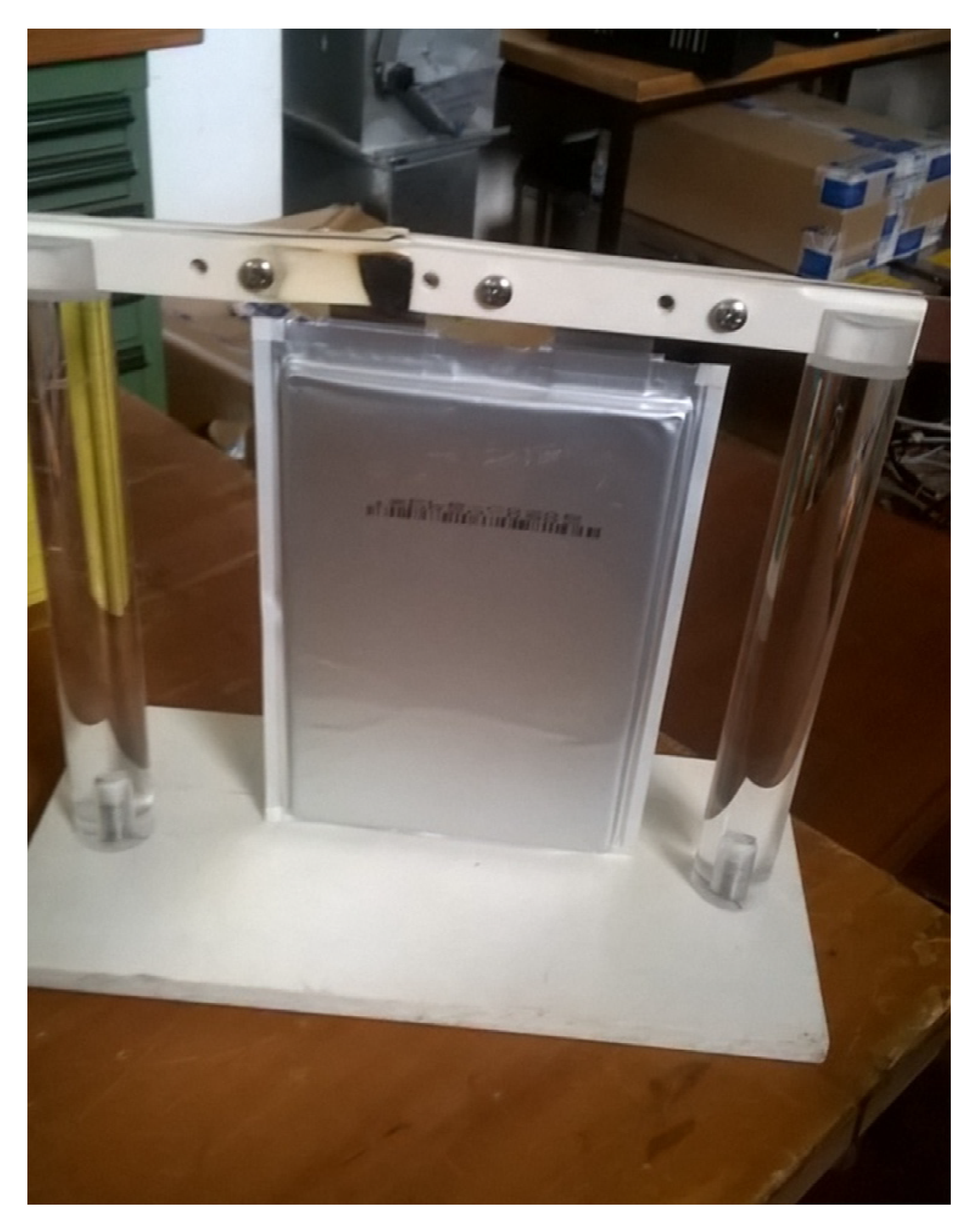
3.1. Life Tests for Lithium-Ion Cells
- (1)
- Initial inspection, to verify the absence of damage to the cell visible from the outside and the correspondence of the physical characteristics with those provided by the manufacturer. In our case, none of the tested cells were found to have any damage or discrepancies from the physical characteristics provided in the datasheet.
- (2)
- Electrical formation: consisting of a sequence of standard cycles (discharge at constant current (CC) at C/2, pause 1 h, charge at constant current-constant voltage (CC-CV) with current at C/2, and pause 1 h), which ends when the discharge capacity relating to 2 consecutive discharges does not vary more than 3% of the value of the nominal capacity. The procedure ensures that the cells have reached an adequate stabilization of performance, before starting the actual test sequence.
- (3)
- Life cycles and periodic tests (see Section 3.1.1).
- (4)
- Final inspection, to verify the presence of any damage and deformations that occurred during the tests.
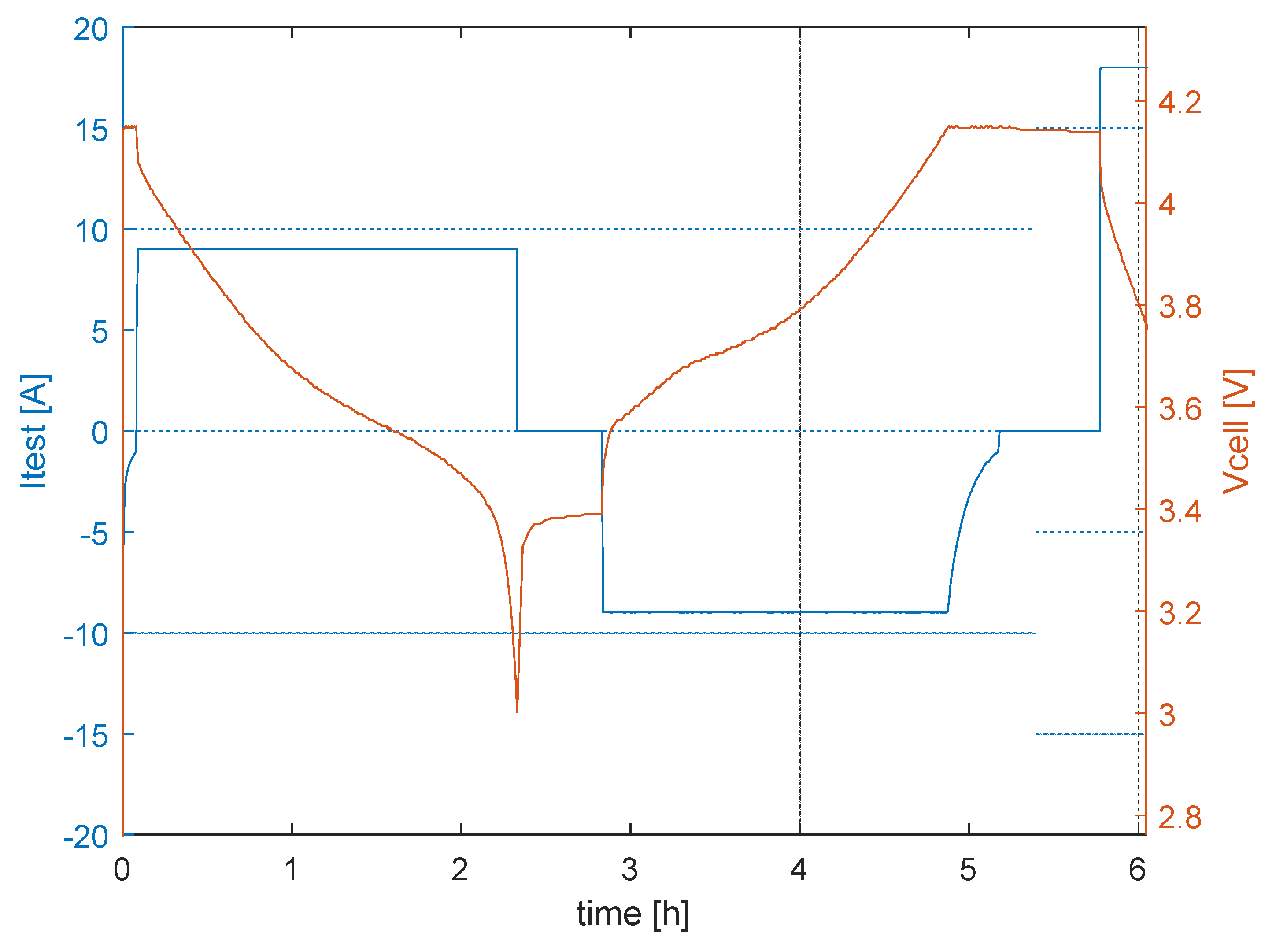
3.1.1. Life Test Procedures
3.1.2. Control Test Procedure
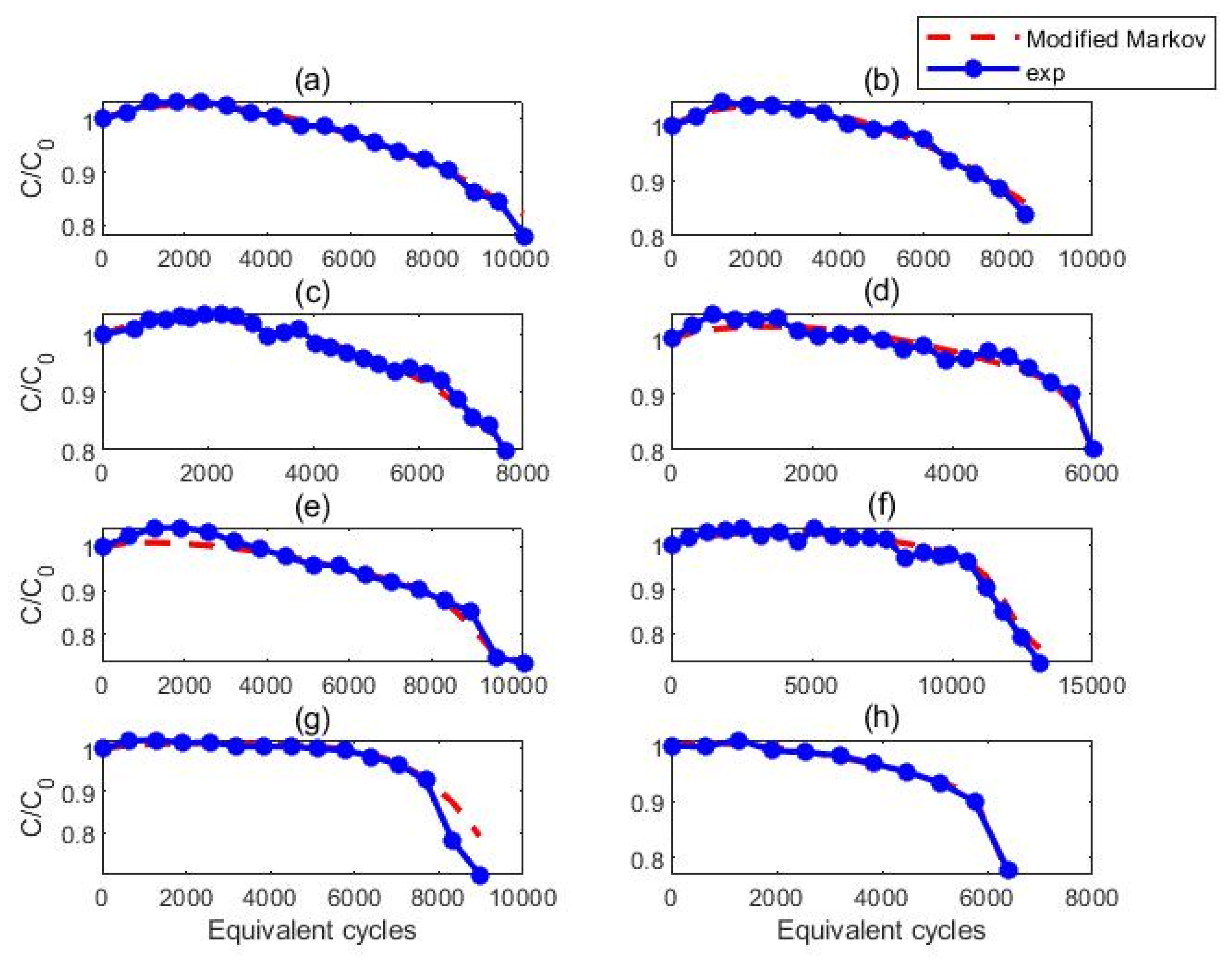
3.2. Modeling of Cell Behavior
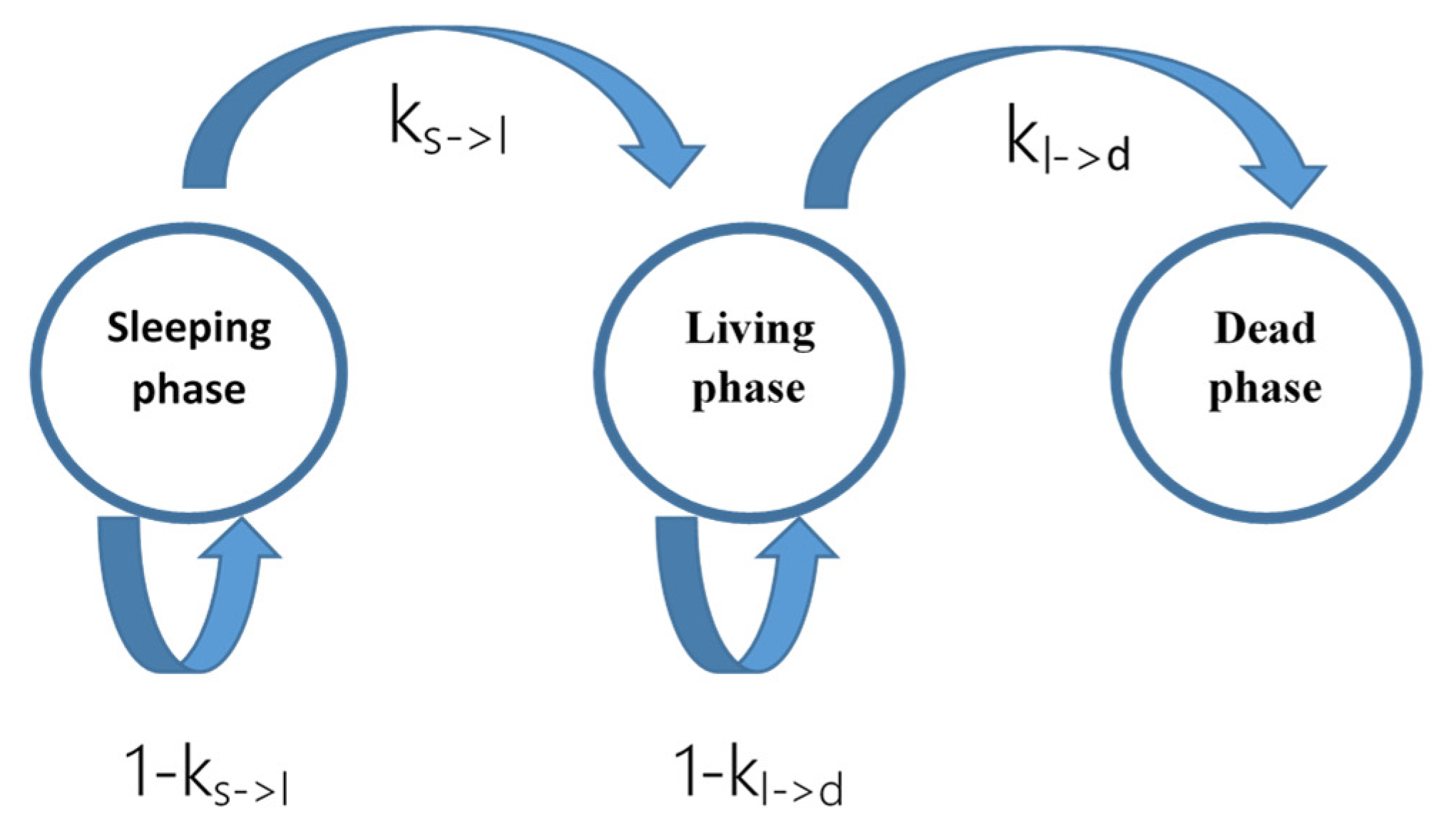
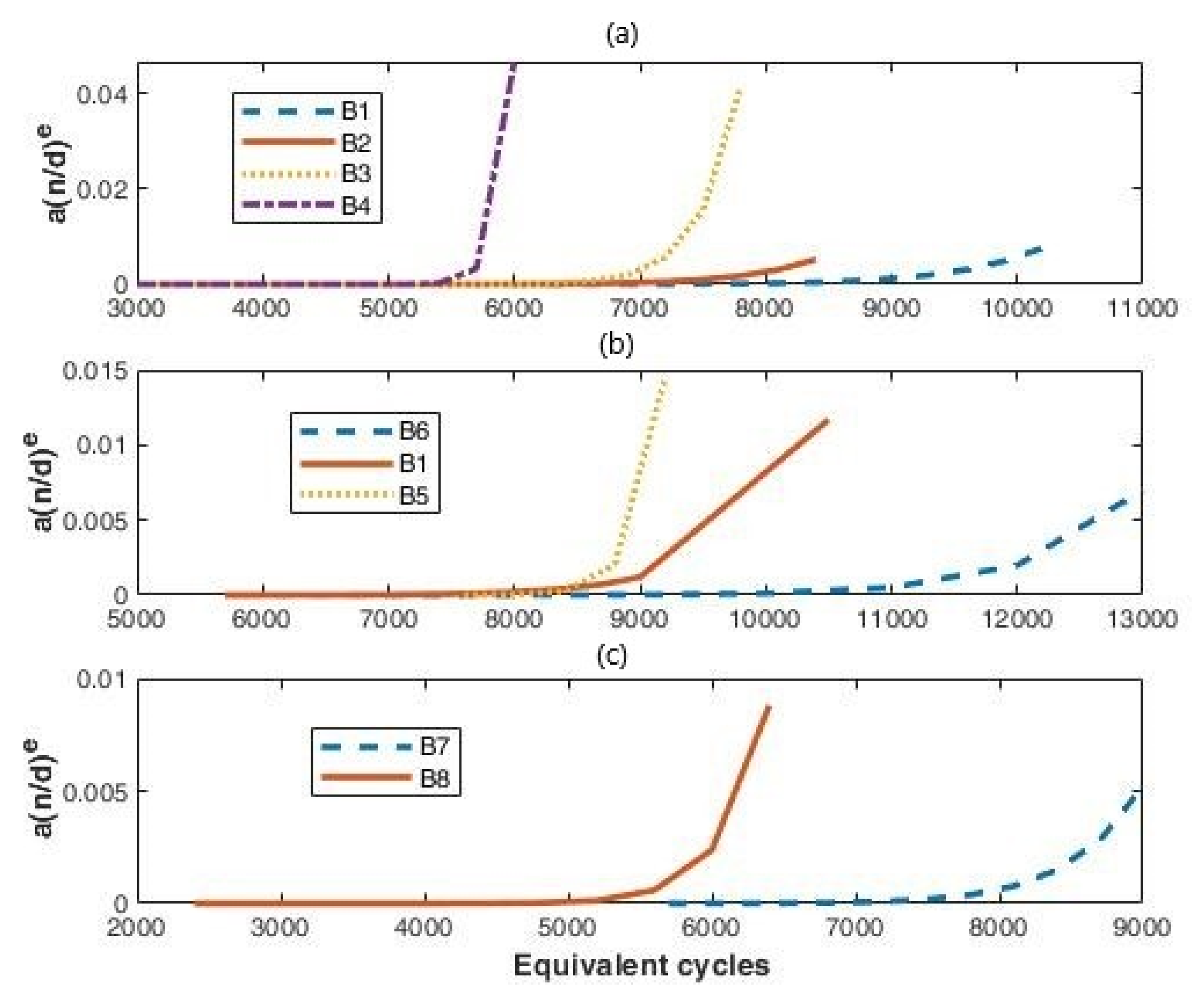
3.2.1. Proposed Model
- is the transition probability between the living and dead phase;
- is the transition probability between the sleeping and living phase.
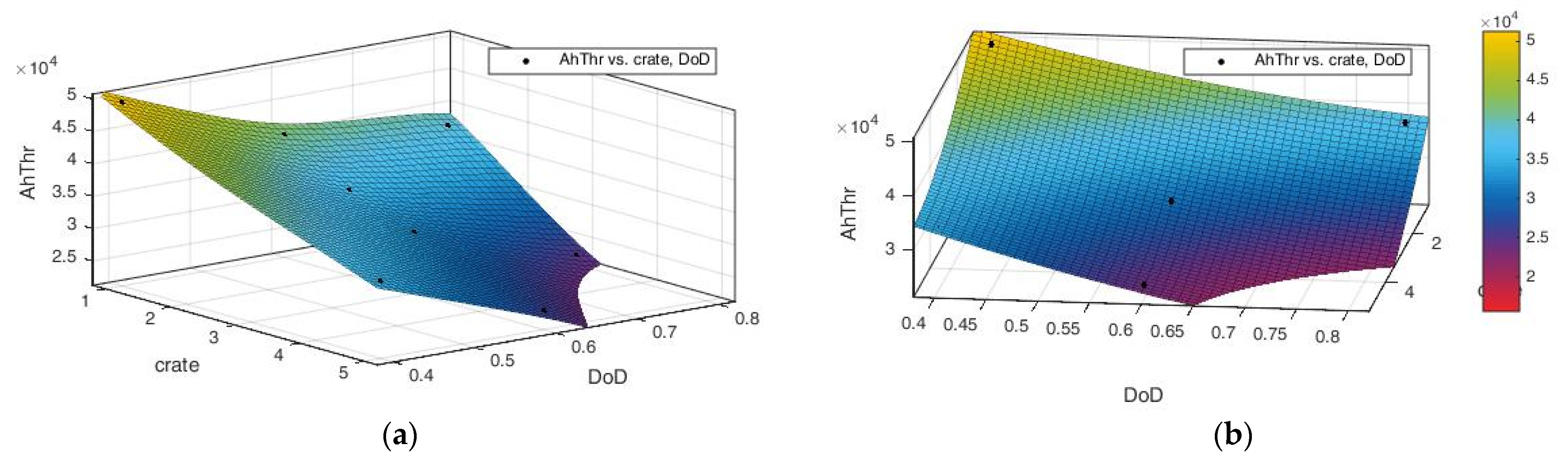
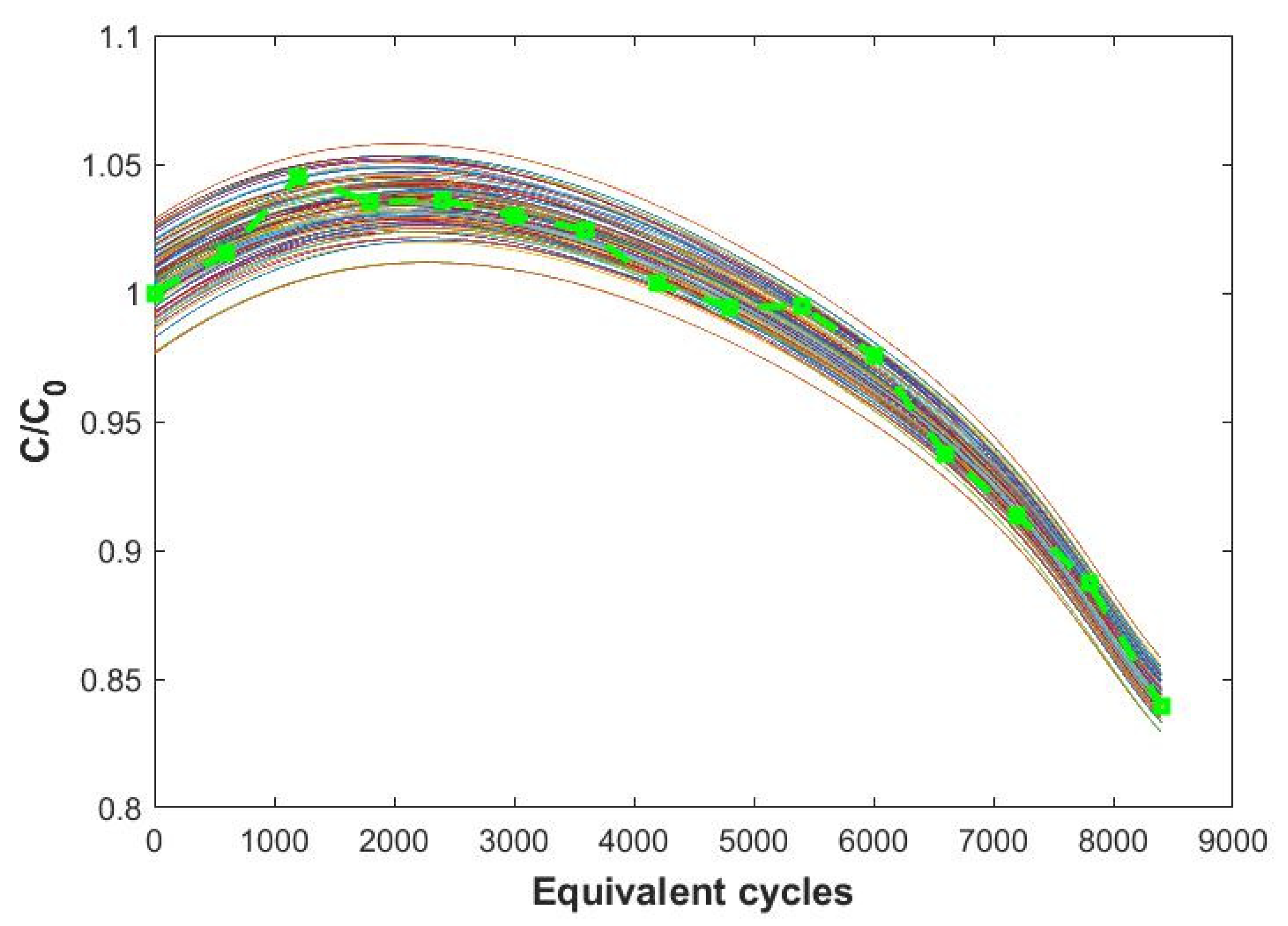
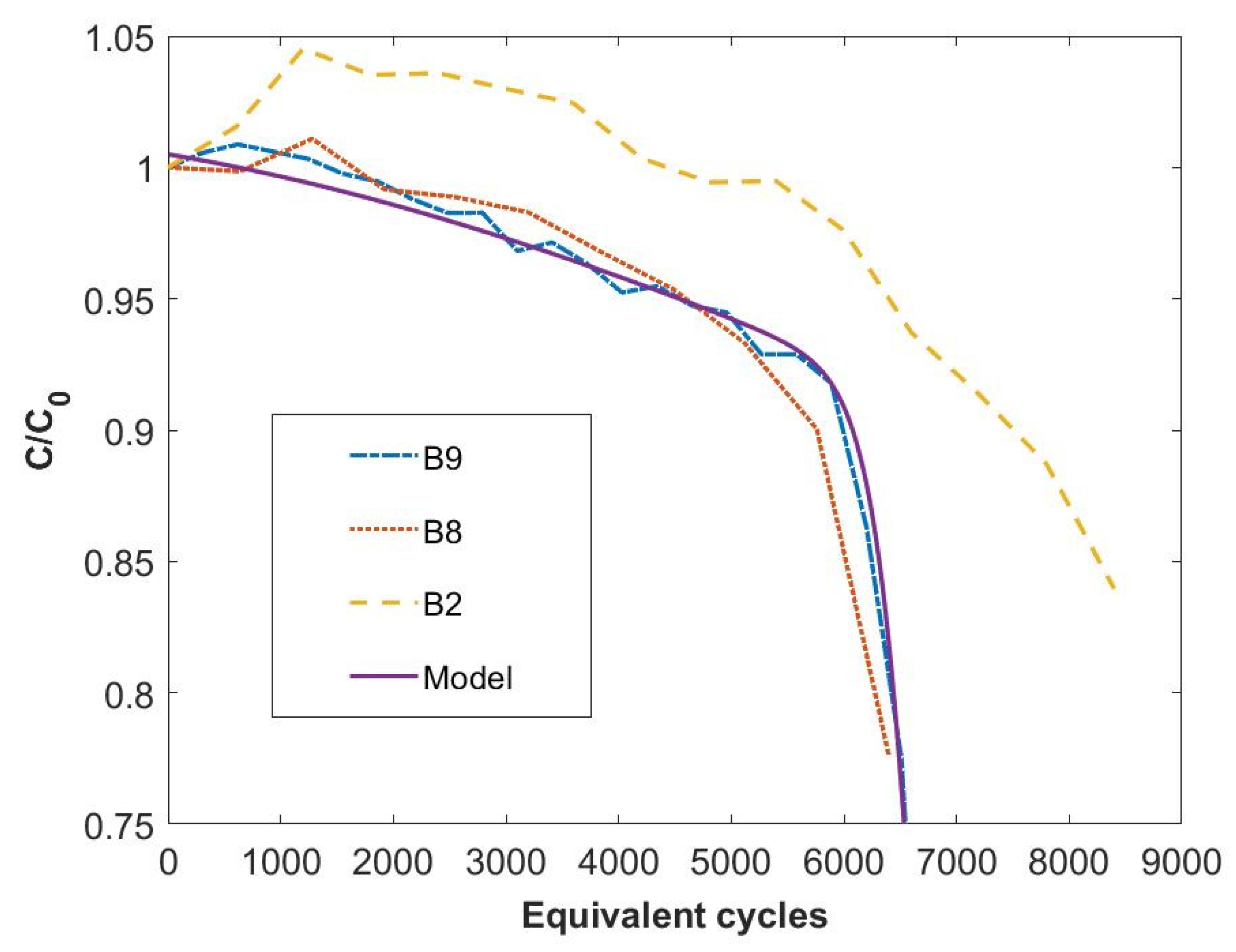
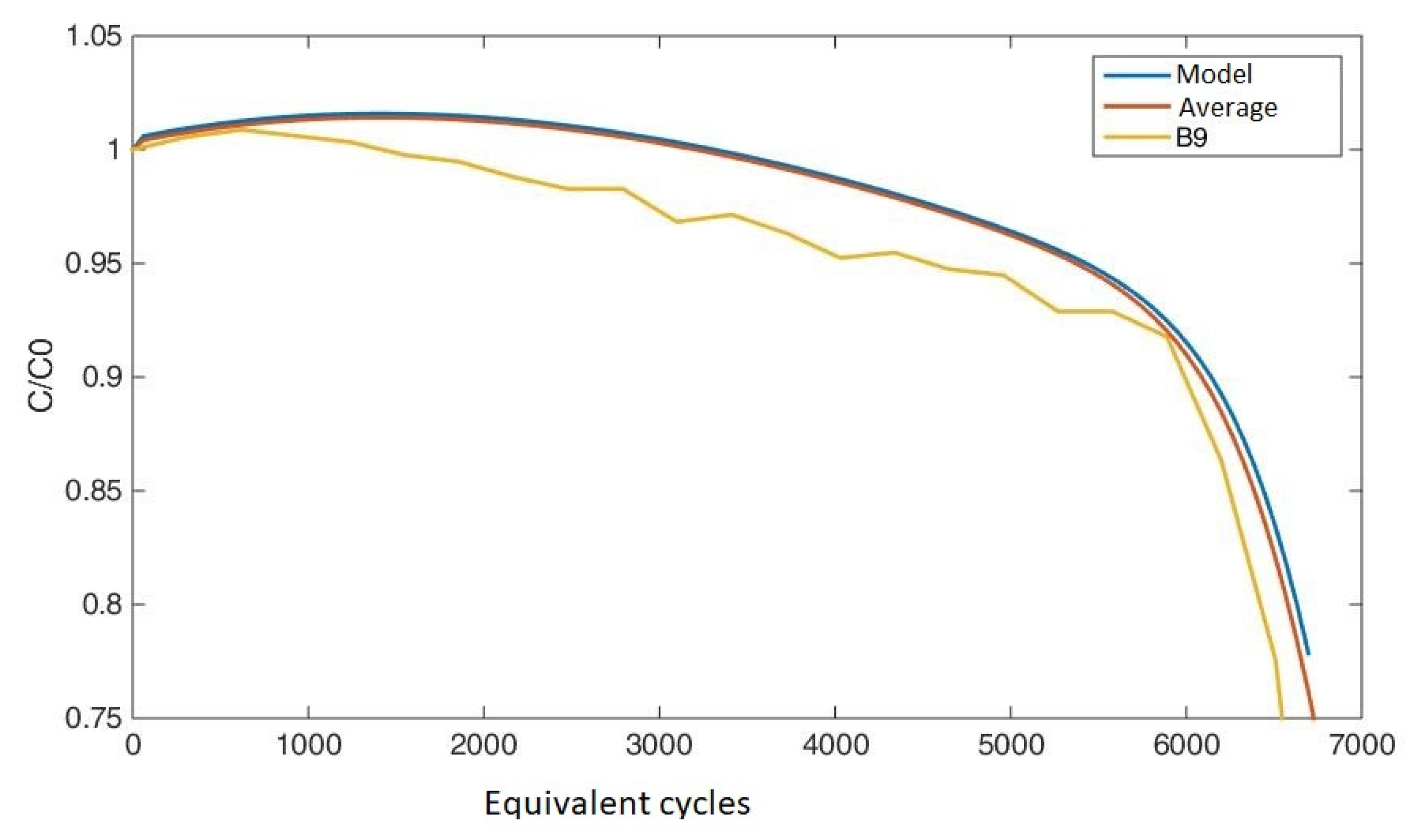
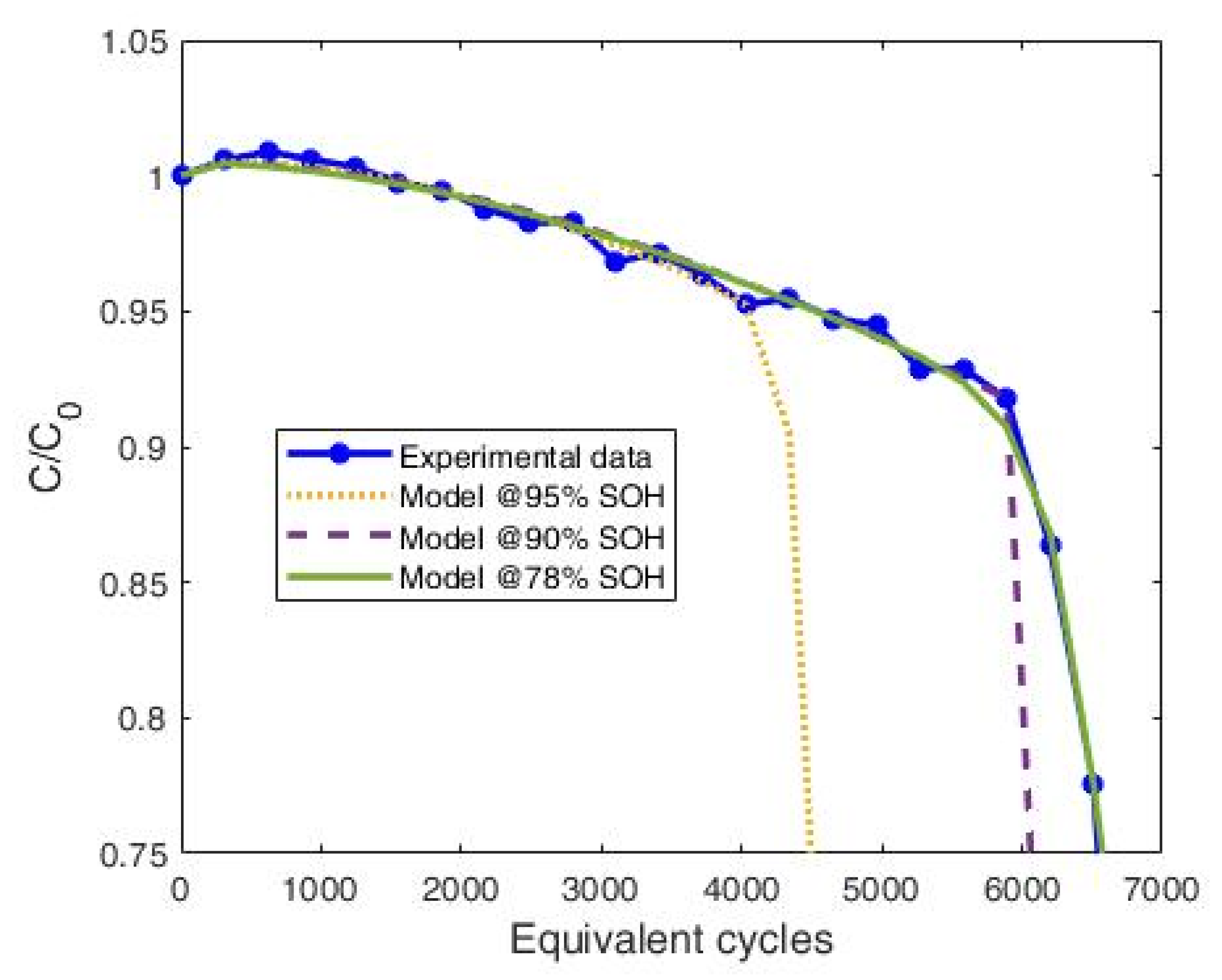
3.2.2. Predictive Efficacy of the Model
- (a)
- 8 cycles at a current rate of C-rate = 3 C and ΔSOC = 80%;
- (b)
- 10 cycles at a current rate of C-rate = 2 C and ΔSOC = 60%.
- Inherent system uncertainties: due to the uncertainties in the manufacturing assembly and material properties, batteries can have different initial capacities. Each battery can also be individually affected by impurities or defects, which can lead to different aging rates [30].
- Measurement uncertainties: uncertainties are likely to arise from the background noise of measurement devices.
- Uncertainties in the operating environment: the rate of capacity fade can be affected by conditions of use, such as a shorter or longer shelf life before testing.
- Modeling uncertainties: the model is an approximation of battery degradation, which will lead to some modeling errors.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venet, P.; Redondo-Iglesias, E. Batteries and Supercapacitors Aging. Batteries 2020, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zou, C.; Yao, K.; Lu, J.; Xia, Y.; Gao, F. Aging trajectory prediction for lithium-ion batteries via model migration and Bayesian Monte Carlo method. Appl. Energy 2019, 254, 113591. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, K.; Foley, A.M.; Zülke, A.; Berecibar, M.; Nanini-Maury, E.; Van Mierlo, J.; Hoster, H.E. Data-driven health estimation and lifetime prediction of lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2019, 113, 109254. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, M.; Lu, L.; Li, J. Understanding aging mechanisms in lithium-ion battery packs: From cell capacity loss to pack capacity evolution. J. Power Sources 2015, 278, 287–295. [Google Scholar] [CrossRef]
- Jafari, M.; Khan, K.; Gauchia, L. Deterministic models of Li-ion battery aging: It is a matter of scale. J. Energy Storage 2018, 20, 67–77. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Werner, D.; Paarmann, S.; Wetzel, T. Calendar Aging of Li-Ion Cells—Experimental Investigation and Empirical Correlation. Batteries 2021, 7, 28. [Google Scholar] [CrossRef]
- Redondo-Iglesias, E.; Venet, P.; Pelissier, S. Modelling Lithium-Ion Battery Ageing in Electric Vehicle Applications—Calendar and Cycling Ageing Combination Effects. Batteries 2020, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Leng, F.; Tan, C.; Pecht, M. Effect of Temperature on the Aging rate of Li Ion Battery Operating above Room Temperature. Sci. Rep. 2015, 5, 12967. [Google Scholar] [CrossRef] [Green Version]
- Alipour, M.; Ziebert, C.; Conte, F.V.; Kizilel, R. A Review on Temperature-Dependent Electrochemical Properties, Aging, and Performance of Lithium-Ion Cells. Batteries 2020, 6, 35. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium Ion Battery Anode Aging Mechanisms. Materials 2013, 6, 1310. [Google Scholar] [CrossRef] [Green Version]
- Bank, T.; Feldmann, J.; Klamor, S.; Bihn, S.; Sauer, D.U. Extensive aging analysis of high-power lithium titanate oxide batteries: Impact of the passive electrode effect. J. Power Sources 2020, 473, 228566. [Google Scholar] [CrossRef]
- Yu, R.; Banis, M.N.; Wang, C.; Wu, B.; Huang, Y.; Cao, S.; Li, J.; Jamil, S.; Lin, X.; Zhao, F.; et al. Tailoring bulk Li+ ion diffusion kinetics and surface lattice oxygen activity for high-performance lithium-rich manganese-based layered oxides. Energy Storage Mater. 2021, 37, 509–520. [Google Scholar] [CrossRef]
- International Organization for Standardization. Electrically Propelled Road Vehicles—Test Specification for Lithium-Ion Traction Battery Packs and Systems—Part 4: Performance Testing. Available online: https://www.iso.org/standard/71407.html (accessed on 26 May 2021).
- Ning, G.; Popov, B.N. Cycle life modeling of lithium-ion batteries. J. Electrochem. Soc. 2005, 151, A1584. [Google Scholar] [CrossRef] [Green Version]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J. Analysis of capacity–rate data for lithium batteries using simplified models of the discharge process. J. Appl. Electrochem. 1997, 27, 846–856. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Relaxation phenomena in lithium-ion-insertion cells. J. Electrochem. Soc. 1994, 141, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Madani, S.S.; Schaltz, E.; Kær, S.K. A Review of Different Electric Equivalent Circuit Models and Parameter Identification Methods of Lithium-Ion Batteries. ECS Trans. 2018, 87, 23–37. [Google Scholar] [CrossRef]
- Delacourt, C.; Safari, M. Mathematical Modeling of Aging of Li-Ion Batteries. In Physical Multiscale Modeling and Numerical Simulation of Electrochemical Devices for Energy Conversion and Storage. Green Energy and Technology; Franco, A., Doublet, M., Bessler, W., Eds.; Springer: London, UK, 2016; pp. 151–190. [Google Scholar]
- Andrenacci, N.; Sglavo, V.; Vellucci, F.; State of the Art of Aging Models for Lithium-Ion Cells. Application to the Case Study of Aged NMC cells in ENEA. Report RDS/ PAR2016/163. Available online: https://www.enea.it/it/Ricerca_sviluppo/lenergia/ricerca-di-sistema-elettrico/accordo-di-programma-MiSE-ENEA-2015-2017/trasmissione-e-distribuzione-dellenergia-elettrica/sistemi-di-accumulo-di-energia/report-2016 (accessed on 25 May 2021). (In Italian).
- Tamilselvi, S.; Gunasundari, S.; Karuppiah, N.; Abdul Razak, R.K.; Madhusudan, S.; Nagarajan, V.M.; Sathish, T.; Shamim, M.Z.M.; Saleel, C.A.; Afzal, A. A Review on Battery Modelling Techniques. Sustainability 2021, 13, 10042. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Tulpule, P.; Rizzoni, G.; Zhang, W.; Du, X. A probabilistic approach for prognosis of battery pack aging. J. Power Sources 2017, 347, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Xian, W.M.; Long, B.; Wang, H.J. Analysis of Data-Driven Prediction Algorithms for Lithium-Ion Batteries Remaining Useful Life. Adv. Mater. Res. 2013, 717, 390–395. [Google Scholar] [CrossRef]
- Chiodo, E.; Lauria, D.; Mottola, F.; Andrenacci, N. Online Bayes Estimation of Capacity Fading for Battery Lifetime Assessment. In Proceedings of the 2019 International Conference on Clean Electrical Power (ICCEP), Otranto, Italy, 2–4 July 2019; pp. 599–604. [Google Scholar]
- Risse, S.; Angioletti-Uberti, S.; Dzubiella, J.; Ballauff, M. Capacity fading in lithium/sulfur batteries: A linear four-state model. J. Power Sources 2014, 267, 648–654. [Google Scholar] [CrossRef]
- Bacaloni, A.; Insogna, S.; Di Bari, C.; Andrenacci, N.; Mazzaro, M.; Navarra, M.A. Characterization of Li-ion batteries for safety and health protection. Ital. J. Occup. Environ. Hyg. 2019, 10, 40–52. [Google Scholar]
- Harris, S.J.; Harris, D.J.; Li, C. Failure statistics for commercial lithium ion batteries: A study of 24 pouch cells. J. Power Sources 2017, 342, 589–597. [Google Scholar] [CrossRef] [Green Version]


| Cell Characteristic | Value |
|---|---|
| Nominal voltage | 3.65 V |
| Maximum voltage | 4.15 V |
| Minimum voltage | 2.5 V |
| Nominal capacity | 20 Ah |
| Maximum discharge current (continuous) | 5 C |
| Maximum discharge current (peak < 10 s) | 10 C |
| Operating temperature | −30 °C/+55 °C |
| Charging temperature | 0 °C/+40 °C |
| Storage temperature | −30 °C/+55 °C |
| Specific energy | 174 Wh/Kg |
| Volumetric energy density | 370 Wh/L |
| Specific power (DoD 50%, 10 s) | 2300 W/kg |
| Volumetric power density (DoD 50%, 10 s) | 4600 W/L |
| Storage temperature | −30 °C/+55 °C |
| Specific energy | 174 Wh/Kg |
| Volumetric energy density | 370 Wh/L |
| Equipment | Name | Rating |
|---|---|---|
| Cycler | ELTRA E-8094 (double field) | V = 0–36 V; I = 280 A; Vmax = 36–52 V; I = 400 A |
| Cycler | ELTRA E-8376 (double field) | V = 0–35 V; I = 400 A; V = 36–350 V; I = 600 A |
| Cycler | ELTRA E-8325 | V = 0–20 V; I = 80 A (charge)–150A (discharge) |
| Cycler | DIGATRON 80V 8 channels | V = 0–100 V; I = 50 A |
| Cycler | Maccor Series 4000 48 channels | V = 0–5 V; I = 5 A |
| Cycler | Maccor Series 4000 8 channels | V = 0–80 V; I = 50 A |
| Climatic chamber | Angelantoni EOS 1000 | −40 °C, +180 °C; U.R. 15–98% |
| Climatic chamber | Angelantoni UY 2250 SP | −40 °C, +180 °C; U.R. 15–98% |
| Climatic chamber | Angelantoni DY 1200C EX | −60 °C, +150 °C; U.R. 15–98% |
| Test Number | Discharge Current (C-Rate) | ΔSOC = SOCin − SOCfin | Cycle between Control Tests |
|---|---|---|---|
| 1 | 1 C | 60 = 80−20 | 200 |
| 2 | 2 C | 60 = 80−20 | 200 |
| 3 | 3 C | 60 = 80−20 | 100 1 |
| 4 | 5 C | 60 = 80−20 | 100 |
| 5 | 1 C | 80 = 90−10 | 160 |
| 6 | 1 C | 40 = 70−30 | 320 |
| 7 | 5 C | 40 = 70−30 | 320 |
| 8 | 3 C | 80 = 90−10 | 160 |
| 9 | 8 × 3C@80%DOD + 10 × 2C@60%DOD | 80 = 90−10, 40 = 70−30 | 90 |
| Model Parameters | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| fl | 1.01 | 1.008 | 1.009 | 1.04 |
| fs | 1.114 | 1.016 | 1.036 | 1.185 |
| a | 0.0001846 | 0.0003431 | 0.000104 | 0.0001705 |
| b | 8.733 × 10−5 | 9.388 × 10−5 | 0.000107 | 9.571 × 10−5 |
| c | 9.667 × 10−5 | 0.0001235 | 0.0001035 | 8.01 × 10−5 |
| d | 1.003 × 104 | 9200 | 7138 | 5609 |
| e | 14.86 | 10.98 | 15.88 | 26.54 |
| R2 | 0.994 | 0.9873 | 0.9842 | 0.9496 |
| Model Parameters | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| a | 0.0001713 | 0.0003379 | 0.0001123 | 0.0001705 |
| b | 8.847 × 10−5 | 9.762 × 10−5 | 0.000112 | 0.0001189 |
| c | 0.0001018 | 0.0001183 | 0.0001298 | 0.0001331 |
| d | 9970 | 9175 | 7172 | 5669 |
| e | 16.43 | 10.19 | 16.74 | 36.66 |
| R2 | 0.994 | 0.9873 | 0.9842 | 0.9496 |
| Model Parameters | B6 | B1 | B5 |
|---|---|---|---|
| a | 0.0001013 | 0.0001713 | 0.0001379 |
| b | 4.993 × 10−5 | 8.847 × 10−5 | 9.817 × 10−5 |
| c | 5.802 × 10−5 | 0.0001018 | 0.000112 |
| d | 1.139 × 104 | 9970 | 9844 |
| e | 8.65 | 16.43 | 44.15 |
| R2 | 0.9777 | 0.994 | 0.9844 |
| Model Parameters | B7 | B8 |
|---|---|---|
| a | 9.021 × 10−5 | 0.0002348 |
| b | 4.396 × 10−5 | 9.183 × 10−5 |
| c | 4.583 × 10−5 | 8.713 × 10−5 |
| d | 7202 | 6086 |
| e | 18.203 | 17.31 |
| R2 | 0.9818 | 0.9964 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrenacci, N.; Vellucci, F.; Sglavo, V. The Battery Life Estimation of a Battery under Different Stress Conditions. Batteries 2021, 7, 88. https://doi.org/10.3390/batteries7040088
Andrenacci N, Vellucci F, Sglavo V. The Battery Life Estimation of a Battery under Different Stress Conditions. Batteries. 2021; 7(4):88. https://doi.org/10.3390/batteries7040088
Chicago/Turabian StyleAndrenacci, Natascia, Francesco Vellucci, and Vincenzo Sglavo. 2021. "The Battery Life Estimation of a Battery under Different Stress Conditions" Batteries 7, no. 4: 88. https://doi.org/10.3390/batteries7040088
APA StyleAndrenacci, N., Vellucci, F., & Sglavo, V. (2021). The Battery Life Estimation of a Battery under Different Stress Conditions. Batteries, 7(4), 88. https://doi.org/10.3390/batteries7040088







