Identification of Degradation Mechanisms by Post-Mortem Analysis for High Power and High Energy Commercial Li-Ion Cells after Electric Vehicle Aging
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Ante Mortem Analysis
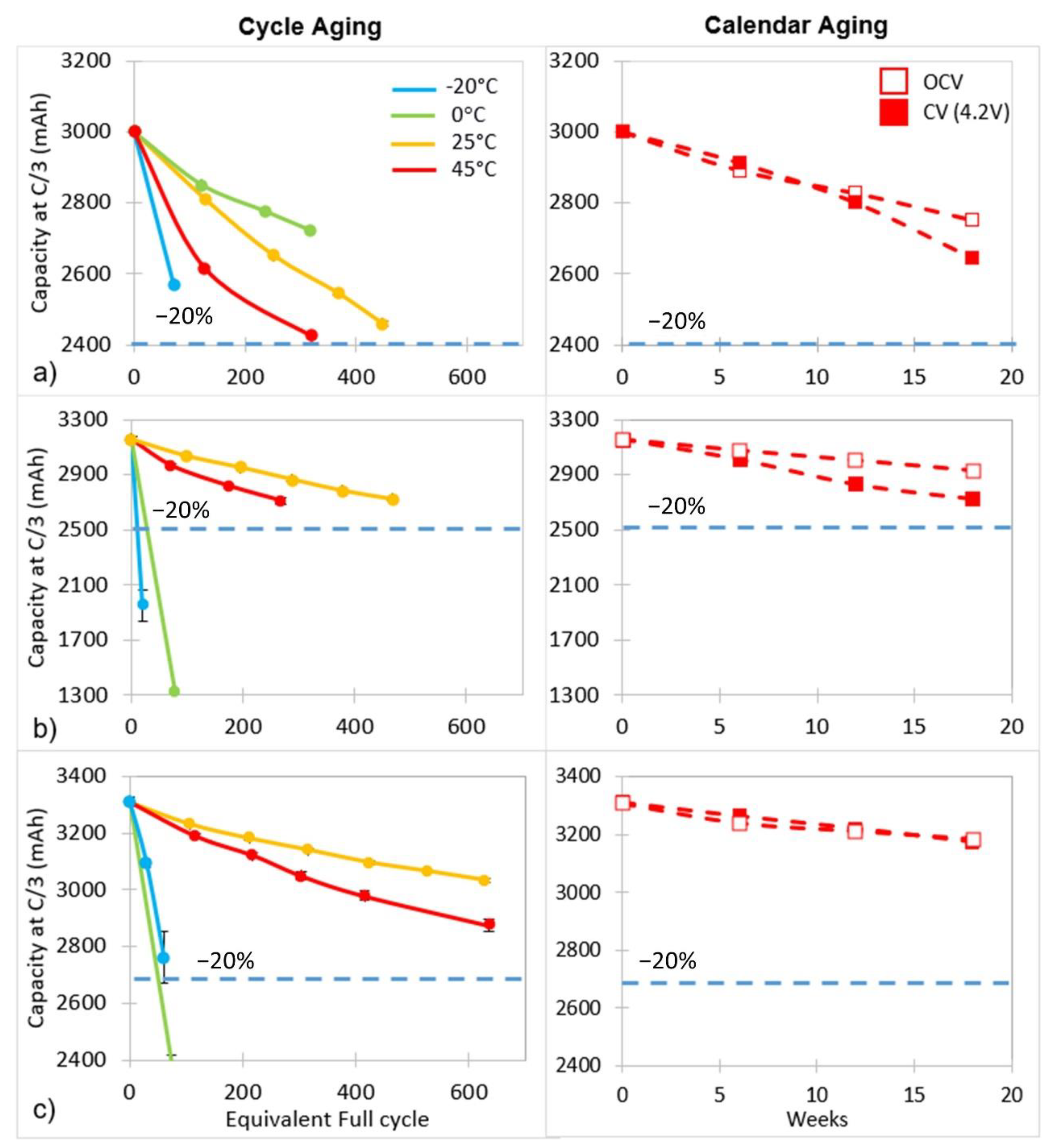
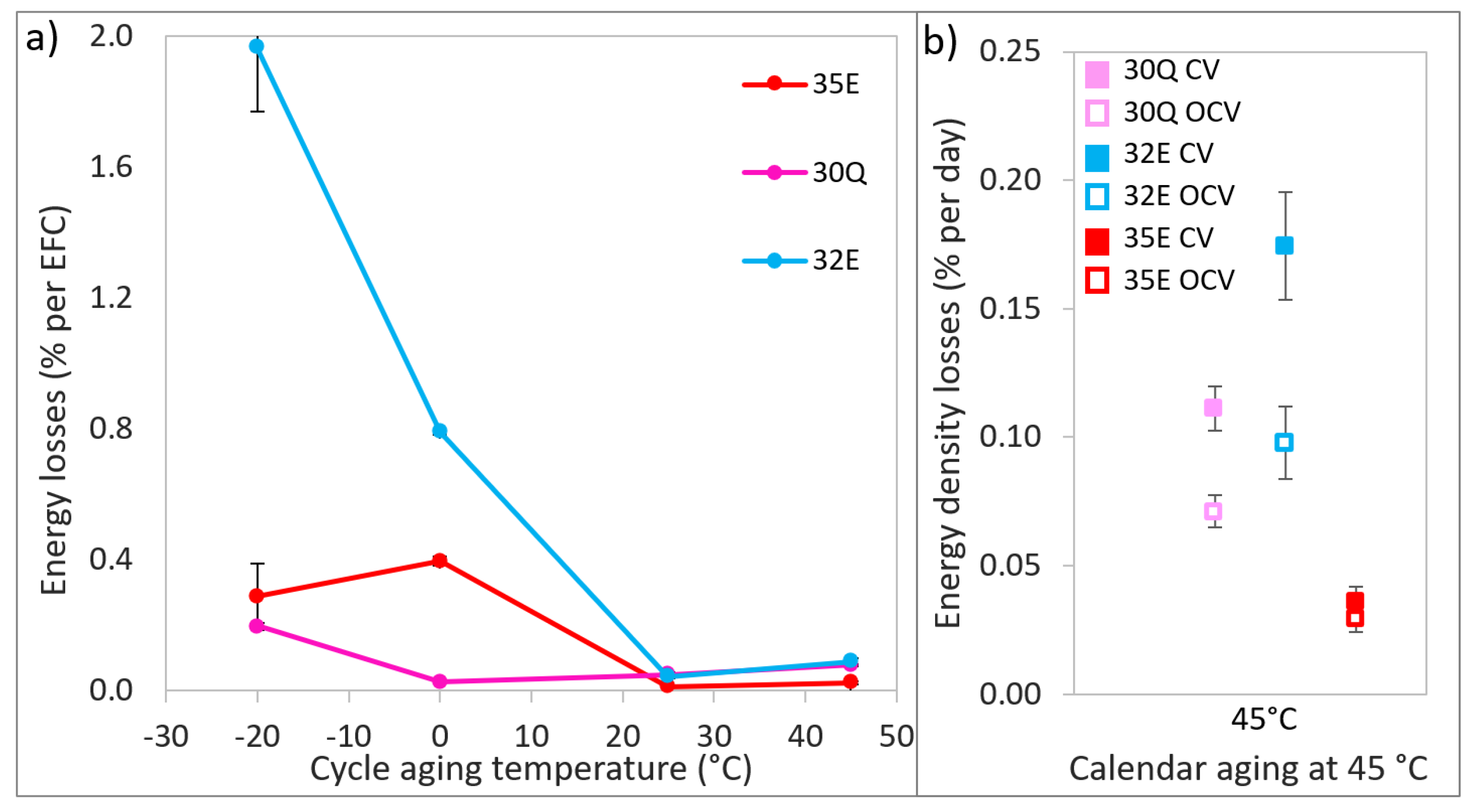
3.2. Aging and Cell Performance Monitoring
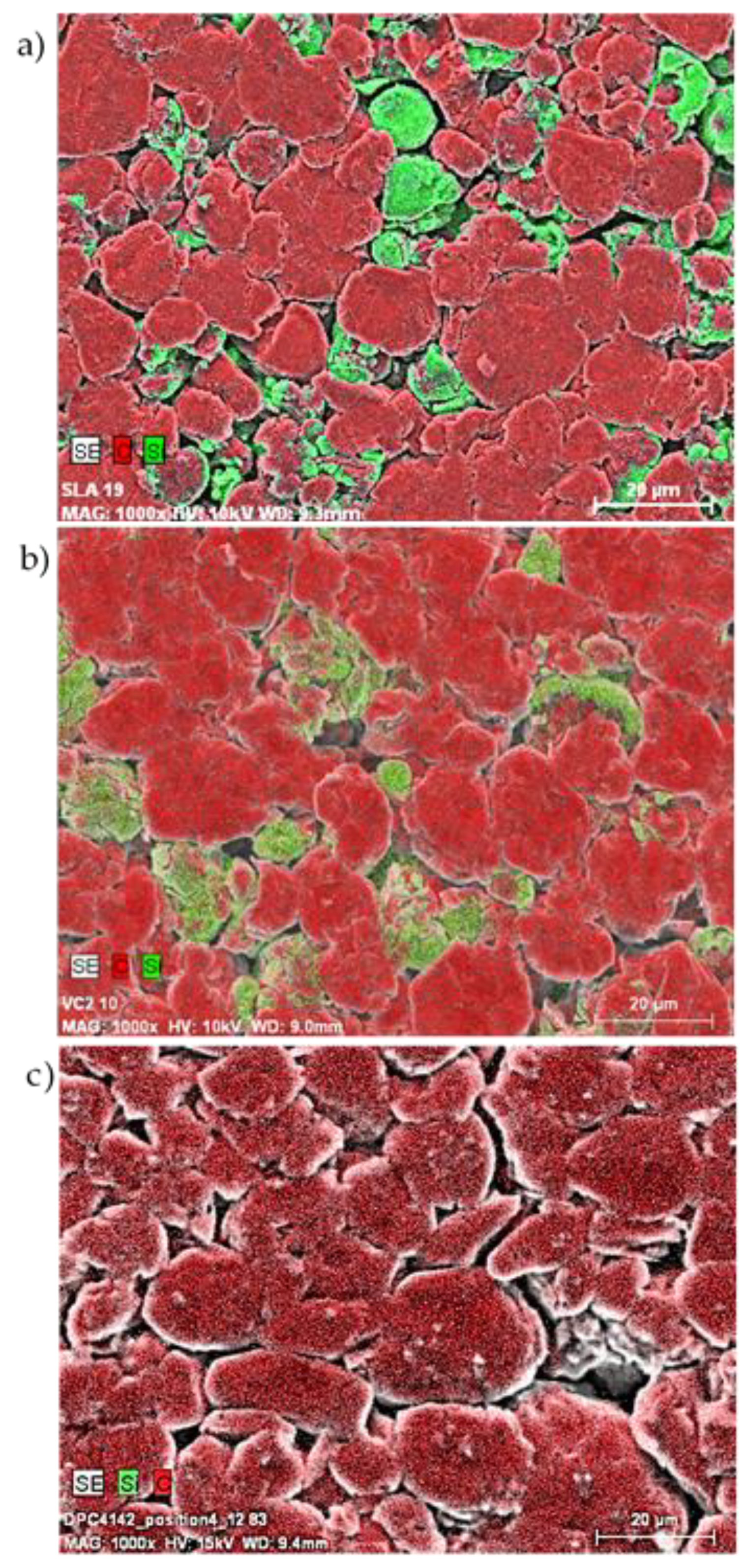
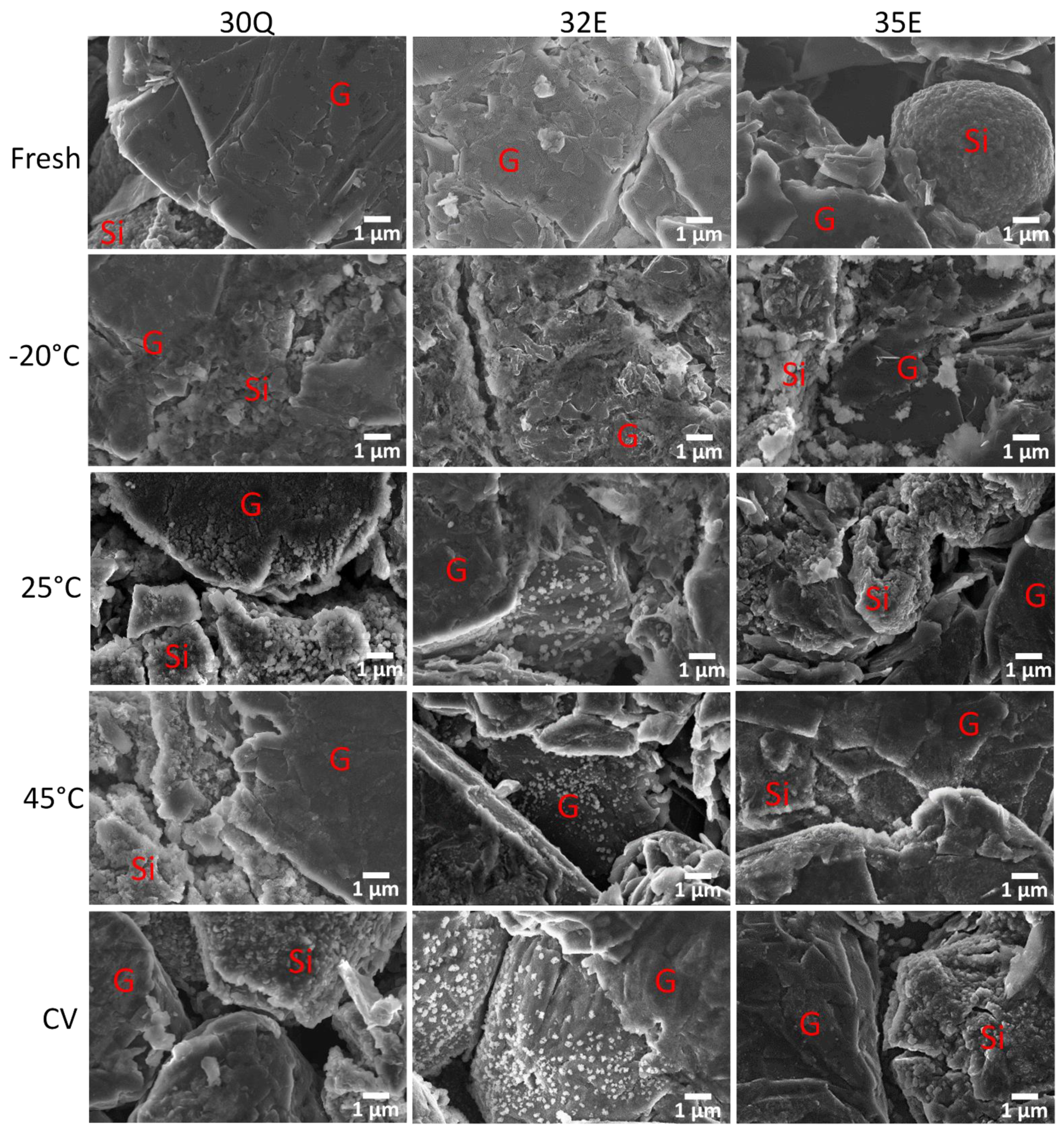
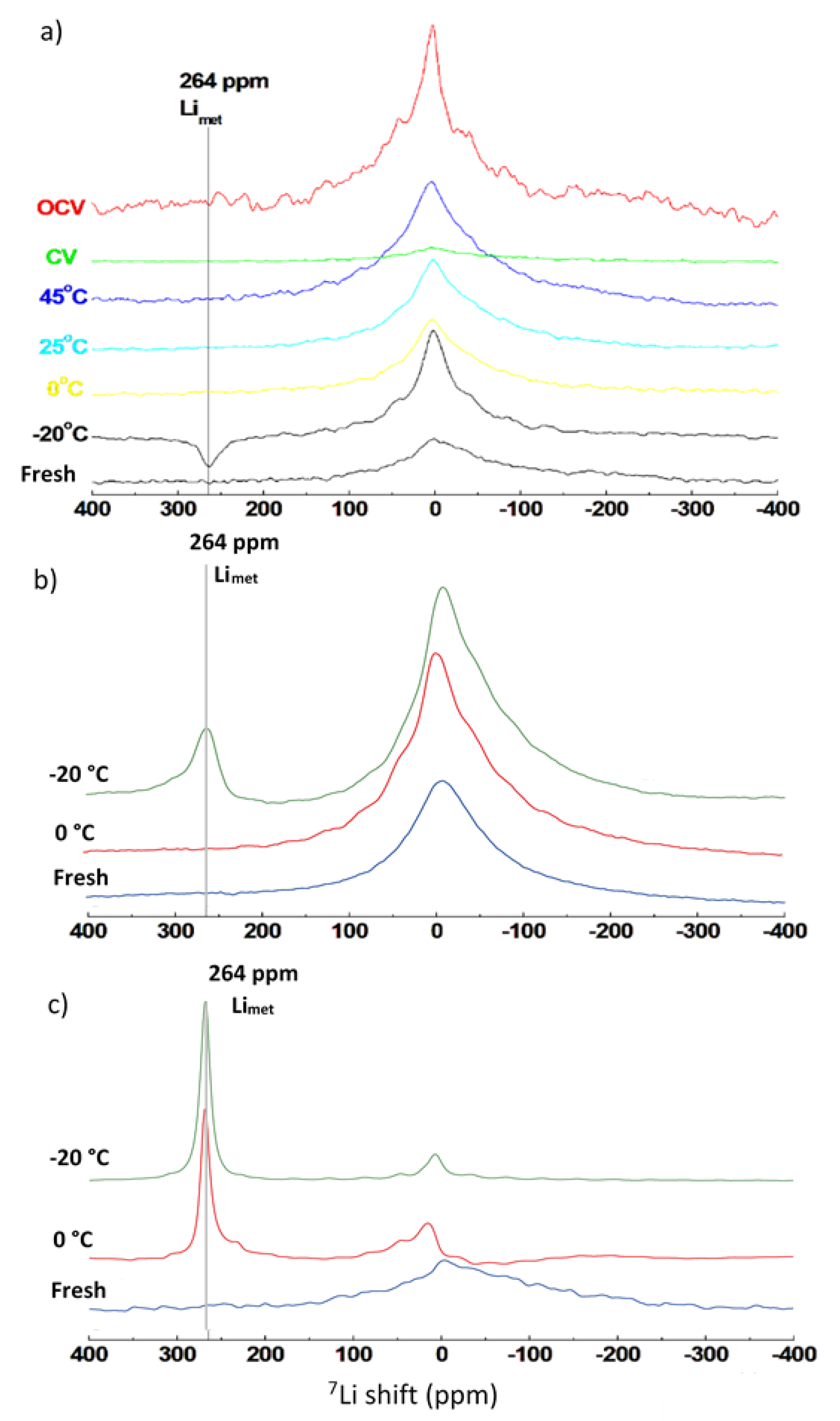
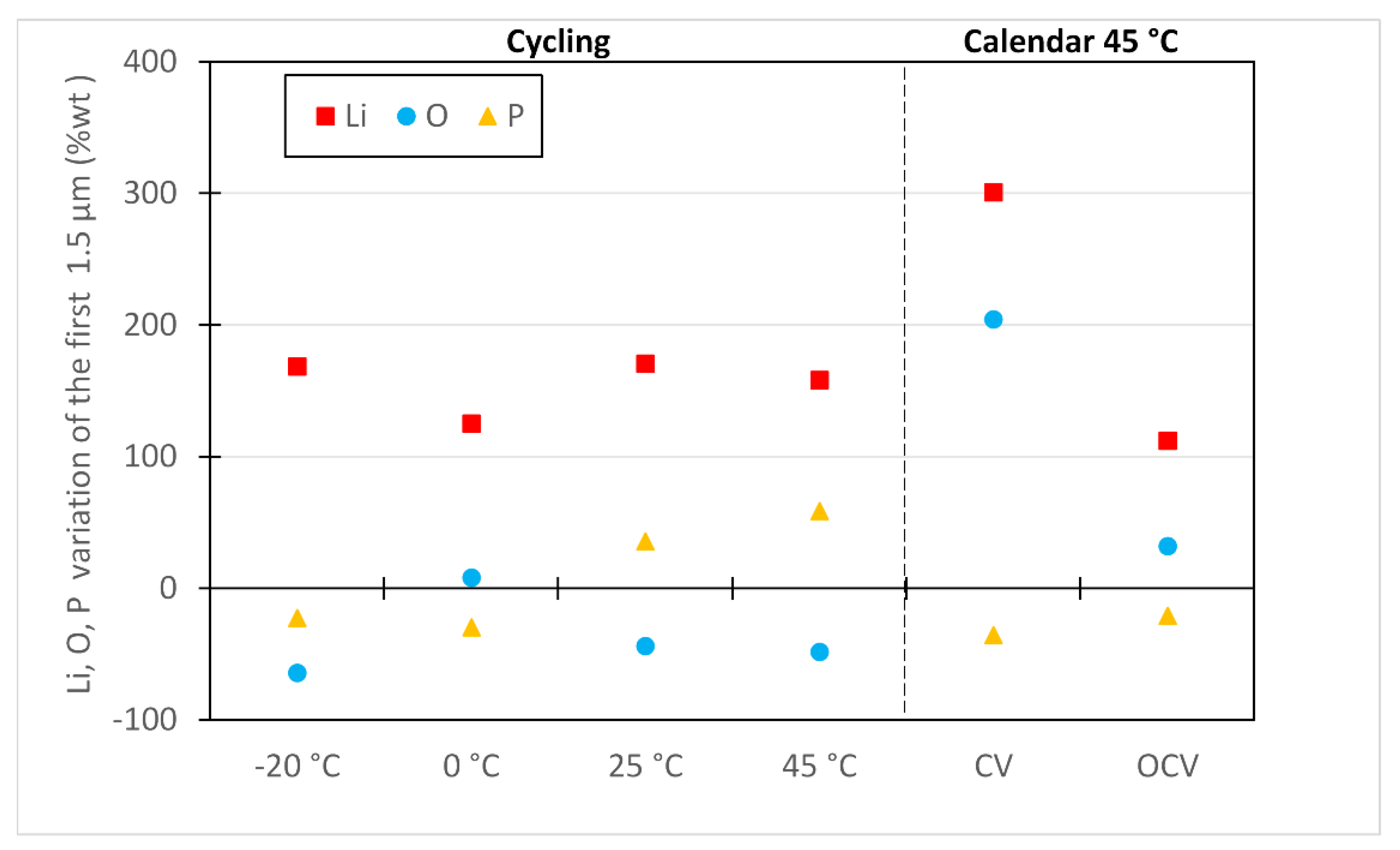
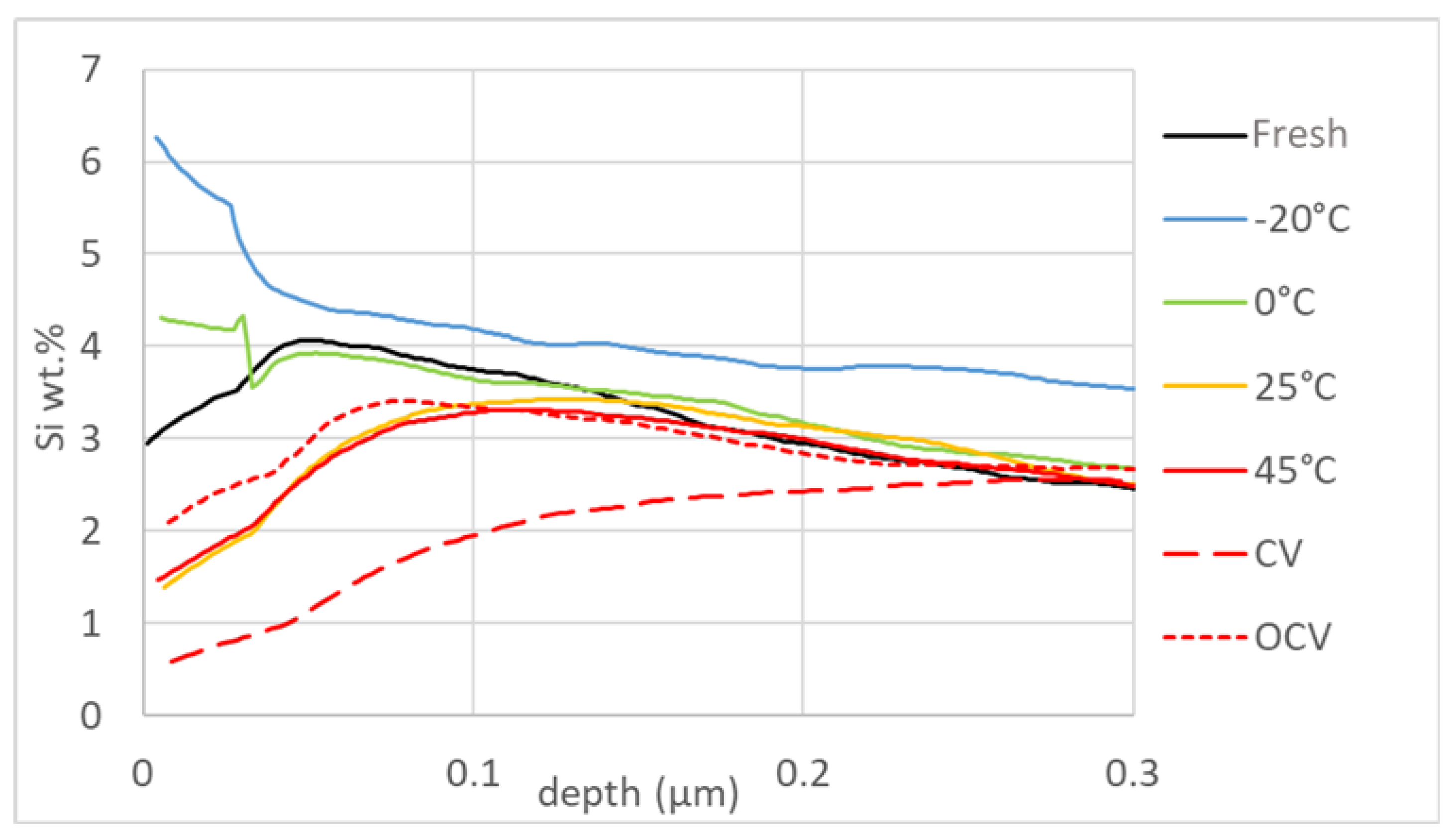
3.3. Post Mortem Analysis
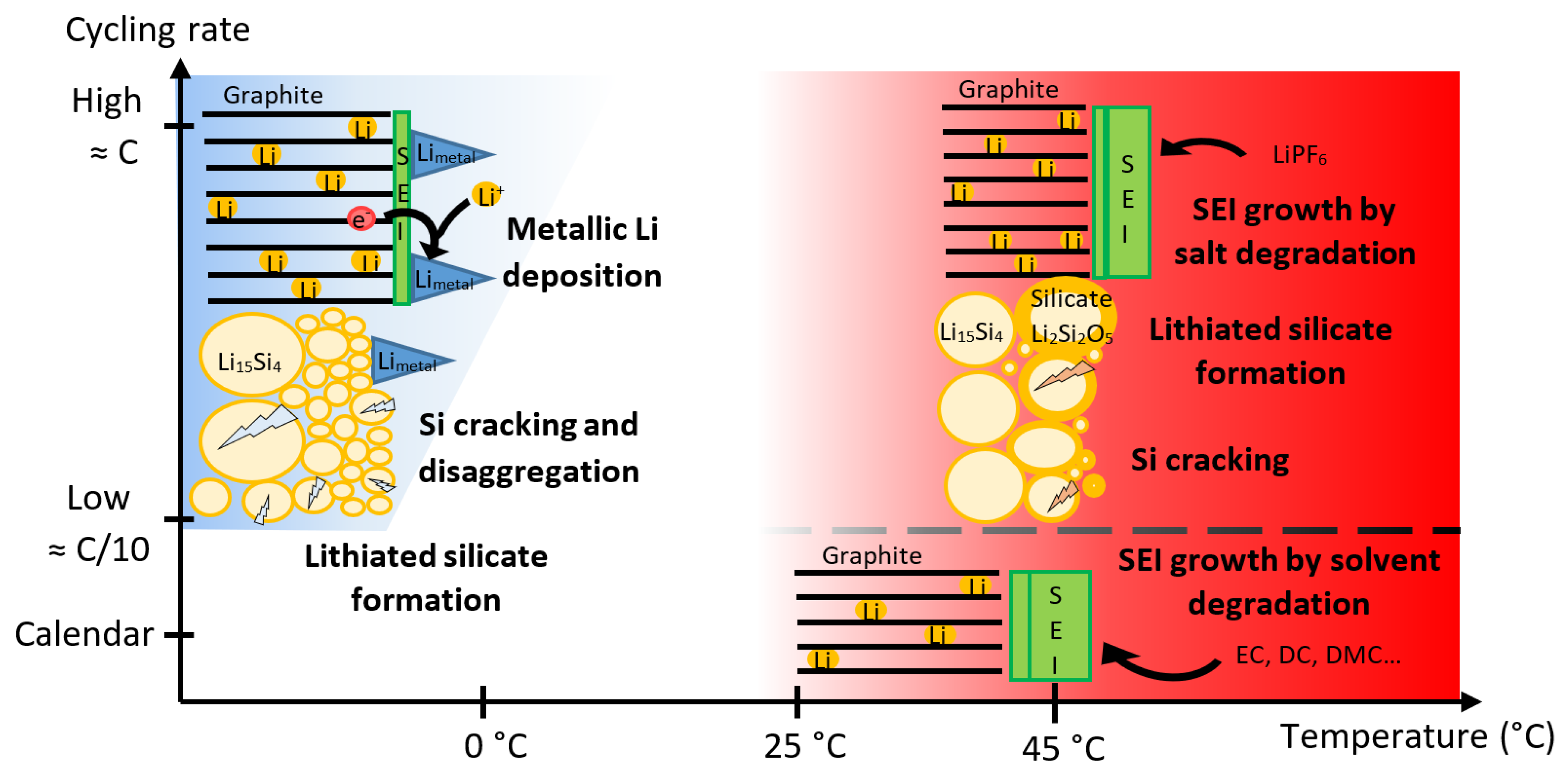
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlasza, C.; Ostertag, P.; Chrenko, D.; Kriesten, R.; Bouquain, D. Review on the aging mechanisms in li-ion batteries for electric vehicles based on the FMEA method. In Proceedings of the 2014 IEEE Transportation Electrification Conference and Expo, Dearborn, MI, USA, 15–18 June 2014. [Google Scholar]
- Waldmann, T.; Iturrondobeita, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Jiménez Gordon, I.W.; Löble, M.; et al. Review-Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Ecker, M.; Shafiei Sabet, P.; Sauer, D.U. Influence of operational condition on lithum plating for commercial lithium-ion batteries —Electrochimical experiments and post-mortem-analysis. Appl. Energy 2017, 206, 934–946. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Rangarajan, S.P.; Barsukov, Y.; Mukherjee, P.P. In operando signature and quantification of lithium plating. J. Mater. Chem. A 2019, 7, 20683–20695. [Google Scholar] [CrossRef]
- Matadi, B.P.; Geniès, S.; Delaille, A.; Chabrol, C.; de Vito, E.; Bardet, M.; Martin, J.-F.; Daniel, L.; Bultel, Y. Irreversible Capacity Loss of Li-Ion Batteries Cycled at Low Temperature Due to an Untypical Layer Hindering Li Diffusion into Graphite Electrode. J. Electrochem. Soc. 2017, 164, A2374–A2389. [Google Scholar] [CrossRef]
- Senyshyn, A.; Mühlbauer, M.J.; Dolotko, O.; Ehrenberg, H. Low-temperature performance of li-ion batteries: The behavior of lithiated graphite. J. Power Sources 2015, 282, 235–240. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review-SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Grolleau, S.; Delaille, A.; Gualous, H.; Gyan, P.; Revel, R.; Julien, B.; Redondo-Iglesias, E.; Peter, J. Calendar aging of commercial graphite/LiFePO4 cell- predicting capacity fade under time depenent storage conditions. J. Power Sources 2013, 255, 450–458. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Vinassa, J.-M. Performance comparison of four lithium-ion battery technologies under calendar aging. Energy 2015, 84, 542–550. [Google Scholar] [CrossRef]
- Klett, M.; Erikson, R.; Groot, J.; Svens, P.; Ciosek Högstrom, K.; Wreland Lindström, R.; Berg, H.; Torbjörn, G.; Lindbergh, G.; Edström, K. Non-uniform aging of cycled commercial LiFePO4//graphite cylindrical cells revealed by post-mortem analysis. J. Power Sources 2014, 257, 126–137. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Piana, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging Analysis of Graphite LiNiMnCoO Cells Using XRD, PGAA, and AC Impedance. J. Electrochem. Soc. 2015, 162. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Bing, J.; Lin, R.; Zhu, X.; Cao, G. Layered tenary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Vogler, C.; Garche, J. Aging mechanisms of tlithium cathode materials. J. Power Sources 2004, 127, 58–64. [Google Scholar] [CrossRef]
- Kabir, M.M.; Demirocak, D.E. Degradation mechanisms in Li-ion batteries: State-of-the art review. Int. J. Energy Res. 2017, 41, 1963–1986. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive application. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-Situ Detection of Lithium Plating Using High Precision Coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Janakiraman, U.; Garrick, T.R.; Fortier, M.E. Review—Lithium Plating Detection Methods in Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 160552. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Schindler, S.; Bauer, M.; Petzl, M.; Danzer, M.A. Voltage relaxation and impedance spectroscopy as in-operando methods for the detection of lithium plating on graphitic anodes in commercial lithium-ion cells. J. Power Sources 2016, 304, 170–180. [Google Scholar] [CrossRef]
- Campbell, I.D.; Marzook, M.; Marinescu, M.; Offer, G.J. How Observable Is Lithium Plating? Differential Voltage Analysis to Identify and Quantify Lithium Plating Following Fast Charging of Cold Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, A725–A739. [Google Scholar] [CrossRef]
- Profatilova, I.; De Vito, E.; Genies, S.; Vincens, C.; Gutel, E.; Fanget, O.; Martin, A.; Chandesris, M.; Tulodziecki, M.; Porcher, W. Impact of Silicon/Graphite Composite Electrode Porosity on the Cycle Life of 18650 Lithium-Ion Cell. ACS Appl. Energy Mater. 2020, 3, 11873–11885. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Morales-Ugarte, J.E.; Bolimowska, E.; Rouault, H.; Santos-Peña, J.; Santini, C.C.; Benayad, A. EIS and XPS Investigation on SEI Layer Formation during First Discharge on Graphite Electrode with a Vinylene Carbonate Doped Imidazolium Based Ionic Liquid Electrolyte. J. Phys. Chem. C 2018, 122, 18223–18230. [Google Scholar] [CrossRef]
- Pastor-Fernandez, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; James, M. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-ion Diagnostic Techniques to identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Smith, A.J.; Burns, J.C.; Zhao, X.; Xiong, D.; Dahn, J.R. A High Precision Coulometry Study of the SEI Growth in Li/Graphite Cells. J. Electrochem. Soc. 2011, 158, A447. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4-Li(Ni0.5Mn0.3Co0.2)/graphite lithium ion batteries. Part I: Cycle aging. J. Power Sources 2014, 251, 439–450. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Zhang, M.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4-Li(Ni0.5Mn0.3Co0.2)/graphite lithium ion batteries. Part II: Calendar aging. J. Power Sources 2014, 258, 61–75. [Google Scholar] [CrossRef]
- Dollé, M.; Grugeon, S.; Beaudoin, B.; Dupont, L.; Tarascon, J.-M. In Situ TEM study of the interface carbon/electrolyte. J. Power Sources 2001, 97–98, 104–106. [Google Scholar] [CrossRef]
- Nie, M.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Silicon Solid Electrolyte Interphase (SEI) of Lithium Ion Battery Characterized by Microscopy and Spectroscopy. J. Phys. Chem. C 2013, 117, 13403–13412. [Google Scholar] [CrossRef]
- Luchkin, S.Y.; Lipovskikh, S.A.; Katorova, N.S.; Savina, A.A.; Abakumov, A.M.; Stevenson, K.J. Solid-electrolyte interphase nucleation and growth on carbonaceous negative electrodes for Li-ion batteries visualized with in situ atomic force microscopy. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 05, 1350040. [Google Scholar] [CrossRef]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Liu, F.; Chen, Y.; Ye, Y.; Huang, Y.; Wu, F.; Li, L. An investigation of functionalized electrolyte using succinonitrile additive for high voltage lithium-ion batteries. J. Power Sources 2016, 306, 70–77. [Google Scholar] [CrossRef]
- Smart, M.; Tomcsi, M.R.; Whitcanack, L.D.; Ratnakumar, B.V.; Nagata, M.; Visco, V. The Use of Methyl Propionate-Based Electrolytes with Additives to Improve the Low Temperature Performance of LiNiCoAlO2-Based Li-Ion Cells. Meet. Abstr. 2013, MA2013-02, 1135. [Google Scholar]
- Samsung. Specification Sheet INR 18650-30Q 2014. Available online: https://datasheetspdf.com/pdf-file/951041/Samsung/INR18650-30Q/1 (accessed on 15 July 2021).
- Radin, M.D.; Hy, S.; Sina, M.; Fang, C.; Liu, H.; Vinckeviciute, J.; Zhang, M.; Whittingham, M.S.; Meng, Y.S.; Van der Ven, A. Narrowing the Gap between Theoretical and Practical Capacities in Li-Ion Layered Oxide Cathode Materials. Adv. Energy Mater. 2017, 7, 1602888. [Google Scholar] [CrossRef] [Green Version]
- Shu, Z.X.; Mc Millan, R.S.; Murray, J.J. Electrochemical Intercalation of Lithium into Graphite. J. Electrochem. Soc. 1993, 140, 922–927. [Google Scholar] [CrossRef]
- Son, S.; Cao, L.; Yoon, T.; Cresce, A.; Hafner, S.E.; Liu, J.; Groner, M.; Xu, K.; Ban, C. Interfacially Induced Cascading Failure in Graphite-Silicon Composite Anodes. Adv. Sci. 2019, 6, 1801007. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.; Besenhard, J.O.; Spahe, M.E.; Novak, P. Insertion Electrode Materials for rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725. [Google Scholar] [CrossRef]
- Klett, M.; Gilbert, J.A.; Pupek, K.Z.; Trask, S.E.; Abraham, D.P. Layered Oxide, Graphite and Silicon-Graphite Electrodes for Lithium-Ion Cells: Effect of Electrolyte Composition and Cycling Windows. J. Electrochem. Soc. 2017, 164, A6095–A6102. [Google Scholar] [CrossRef]
- Kuntz, P.; Azais, P.; Genies, S.; Raccurt, O. Evolution of the safety behavior of Li-ion cells after aging. In Proceedings of the 32nd Electric Vehicle Symposium EVS32, Lyon, France, 20–22 May 2019. [Google Scholar]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Li, Q.; Wang, Y.; Zheng, J.; Yu, X.; Li, H. Dynamic evolution of cathode electrolyte interphase (CEI) on high voltage LiCoO2 cathode and its interraction with Li anode. Energy Storage Mater. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Vetter, J.; Novak, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Richter, K.; Waldmann, T.; Kasper, M.; Pfeifer, C.; Memm, M.; Axmann, P.; Wohlfahrt-Mehrens, M. Surface Film Formation and Dissolution in Si/C Anodes of Li-ion Batteries: A Glow Discharge Optical Emission Spectroscopy Depth Profiling Study. J. Phys. Chem. 2019, 18795–18803. [Google Scholar] [CrossRef]
- Andersson, A. Surface Phenomena in Li-Ion Batteries. Compr. Summ. Upps. Diss. Fac. Sci. Technol. 2001, 656, 11–12. [Google Scholar]
- Aurbach, D.; Markovsky, B.; Shechter, A.; Ein-Eli, Y.; Cohen, H. A Comparative Study of Synthetic Graphite and Li Electrodes in Electrolyte Solutions Based on Ethylene Carbonate-Dimethyl Carbonate Mixtures. J. Electrochem. Soc. 1996, 143, 3809–3820. [Google Scholar] [CrossRef]
- Nakagawa, A.; Kuwata, N.; Matsuda, Y.; Kawamura, J. Characterization of Stable Solid Electrolyte Lithium Silicate for Thin Film Lithium Battery. J. Phys. Soc. Jpn. 2010, 79, 98–101. [Google Scholar] [CrossRef]
- Klinsmann, M.; Rosato, D.; Kamlah, M.; McMeeking, R.M. Modeling Crack Growth during Li Extraction in Storage Particles Using a Fracture Phase Field Approach. J. Electrochem. Soc. 2016, 163, A102–A118. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in Lithium-ion batteries—A Post-Mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]

| 30Q Cell | 32E Cell | 35E Cell | |
|---|---|---|---|
| Manufacturer data sheet | |||
| Voltage limits (V) | 2.5–4.2 | 2.5–4.2 | 2.65–4.2 |
| Maximal current discharge-charge (A) | 15–4 | 6.4–3.2 | 8–2 |
| Temperature range (°C) | 0–50 | 0–45 | 0–45 |
| Lifetime (cycles) | 250 | 300 | 500 |
| Measured data | |||
| Batch of cells average (σ = standard deviation) | |||
| Weight (g) | 45.83 (σ = 0.04) | 47.93 (σ = 0.06) | 47.88 (σ = 0.04) |
| Capacity at C/3 (mAh) | 3025 (σ = 9) | 3158 (σ = 25) | 3328 (σ = 19) |
| Nominal voltage at C/3 (V) | 3.63 (σ = 0.01) | 3.65 (σ = 0.01) | 3.61 (σ = 0.01) |
| Rint (1 kHz) at 50% SOC (mΩ) | 13.73 (σ = 0.13) | 21.71 (σ = 0.10) | 22.28 (σ = 0.22) |
| Rint (pulse) at 50% SOC 5A-5 ms (mΩ) | 15.38 (σ = 0.34) | 26.89 (σ = 0.44) | 26.39 (σ = 0.45) |
| Energy at C/3 (Wh) | 10.92 (σ = 0.01) | 11.48 (σ = 0.17) | 11.98 (σ = 0.11) |
| Positive electrode | |||
| Dimensions | 790 × 58 mm | 610 × 58 mm | 560 × 59 mm |
| Thickness | 45 µm | 65 µm | 73 µm |
| Chemistry | NCA | NCA | NCA |
| Specific capacity (mAh/cm2) | 3.14 | 4.62 | 4.86 |
| Negative electrode | |||
| Dimensions | 810 × 59 mm | 660 × 59, 5 mm | 610 × 60 mm |
| Thickness | 44 µm | 87 µm | 75 µm |
| Chemistry | Graphite 4.5%w SiOx | Graphite | Graphite 1.5%w SiOx |
| Specific capacity (mAh/cm2) | 3.10 | 4.81 | 4.94 |
| Electrolyte | |||
| Solvent | 10EC:7.5DMC: 7.5FEC | 10FEC:7DMC: 4EC:4PC | 10DMC:7EC: 3EMC:2FEC |
| Additive | Succinonitrile | Methyl propionate Succinonitrile | Succinonitrile |
| Separator | |||
| Matrix | PE | PE | PE |
| Coated layer | AlOOH | AlOOH | AlOOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuntz, P.; Raccurt, O.; Azaïs, P.; Richter, K.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Bardet, M.; Buzlukov, A.; Genies, S. Identification of Degradation Mechanisms by Post-Mortem Analysis for High Power and High Energy Commercial Li-Ion Cells after Electric Vehicle Aging. Batteries 2021, 7, 48. https://doi.org/10.3390/batteries7030048
Kuntz P, Raccurt O, Azaïs P, Richter K, Waldmann T, Wohlfahrt-Mehrens M, Bardet M, Buzlukov A, Genies S. Identification of Degradation Mechanisms by Post-Mortem Analysis for High Power and High Energy Commercial Li-Ion Cells after Electric Vehicle Aging. Batteries. 2021; 7(3):48. https://doi.org/10.3390/batteries7030048
Chicago/Turabian StyleKuntz, Pierre, Olivier Raccurt, Philippe Azaïs, Karsten Richter, Thomas Waldmann, Margret Wohlfahrt-Mehrens, Michel Bardet, Anton Buzlukov, and Sylvie Genies. 2021. "Identification of Degradation Mechanisms by Post-Mortem Analysis for High Power and High Energy Commercial Li-Ion Cells after Electric Vehicle Aging" Batteries 7, no. 3: 48. https://doi.org/10.3390/batteries7030048
APA StyleKuntz, P., Raccurt, O., Azaïs, P., Richter, K., Waldmann, T., Wohlfahrt-Mehrens, M., Bardet, M., Buzlukov, A., & Genies, S. (2021). Identification of Degradation Mechanisms by Post-Mortem Analysis for High Power and High Energy Commercial Li-Ion Cells after Electric Vehicle Aging. Batteries, 7(3), 48. https://doi.org/10.3390/batteries7030048







