Temperature-Induced Precipitation of V2O5 in Vanadium Flow Batteries—Revisited
Abstract
:1. Introduction
2. Experimental
2.1. Titration
2.2. Vanadium Solutions for Batch Experiments
- 1 L of the pristine vanadium electrolyte solution was charged (experimental setup described in Section 2.4) to 100% SoC by constant current followed by constant voltage at 1.6 V for about 24 h. After 24 h, the current density was less than 1 mA/cm2 and the SoC was estimated to be >99%. The SoC was also verified by titration, considering the experimental resolution.
- Three vanadium solutions at 84%, 93%, and 100% SoC were prepared. The first two were obtained by dilution of 300 mL of catholyte (100% SoC) with the corresponding amount of pristine vanadium solution. SoC was verified by reductive titration.
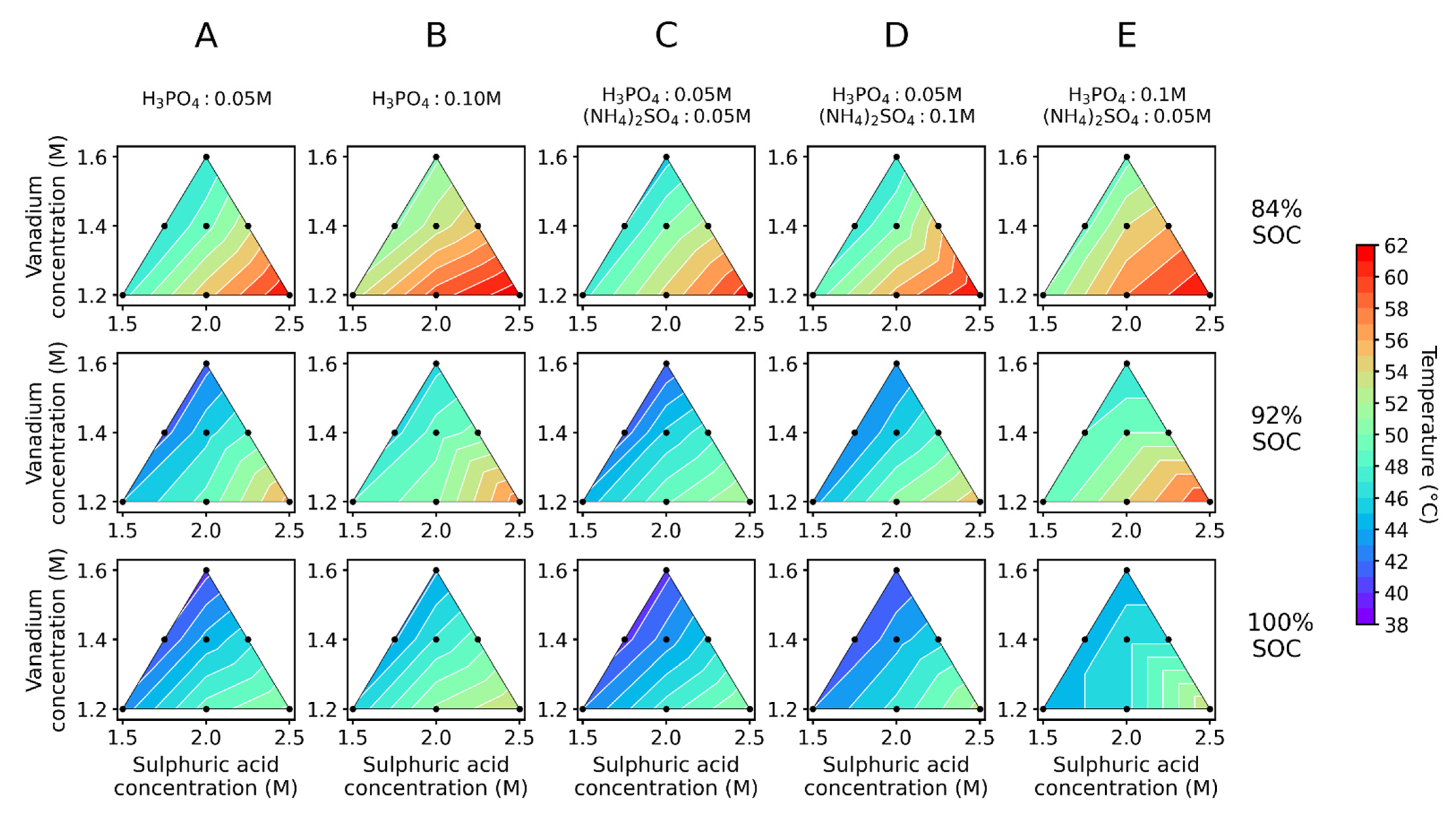
- For each of the three above solutions, six master solutions, with different concentrations of additives (Sigma-Aldrich (Burlington, NJ, USA), 85 wt.%) and (Sigma-Aldrich (Burlington, NJ, USA), ≥99% purity) were prepared. They are indicated as A–F (Table 1). It was noted that a white precipitate appeared with the addition of phosphoric acid in master solution F (0.15 M H3PO4) (see Supporting Information S2). It was reported in previous studies that the precipitation of VOPO4 was observed whenever the concentration of phosphate exceeded 0.1 M in the presence of vanadium [23]. Hence, no further experiments were conducted with master solution F.
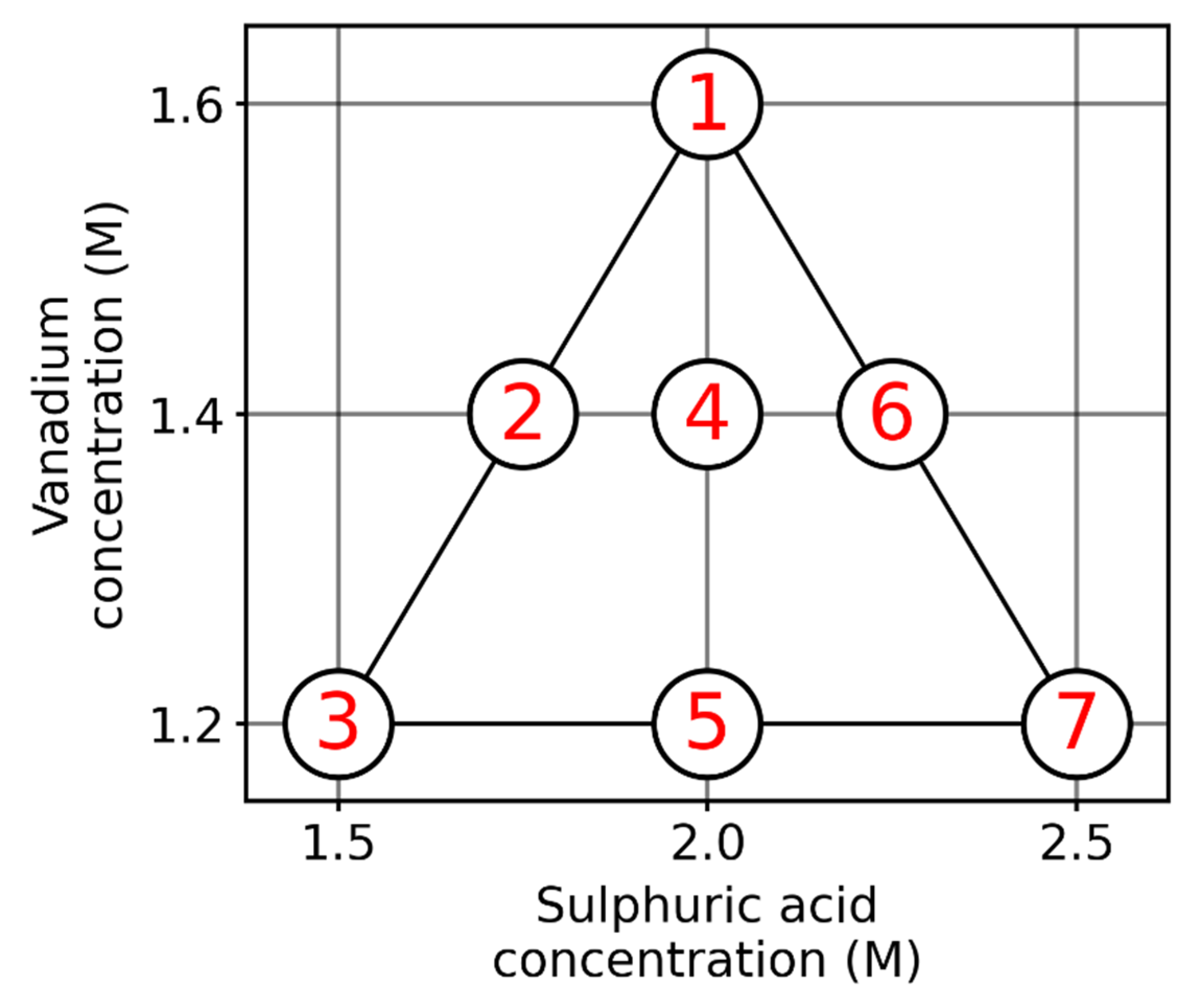
- From the five different master solutions (at different SoCs) a dilution series following the scheme in Figure 2 was made. Sample 1 is the undiluted master solution, samples 2 and 3 are diluted with water, samples 4 and 5 with 2 M , and samples 6 and 7 with 4 M .
2.3. Batch Experiments
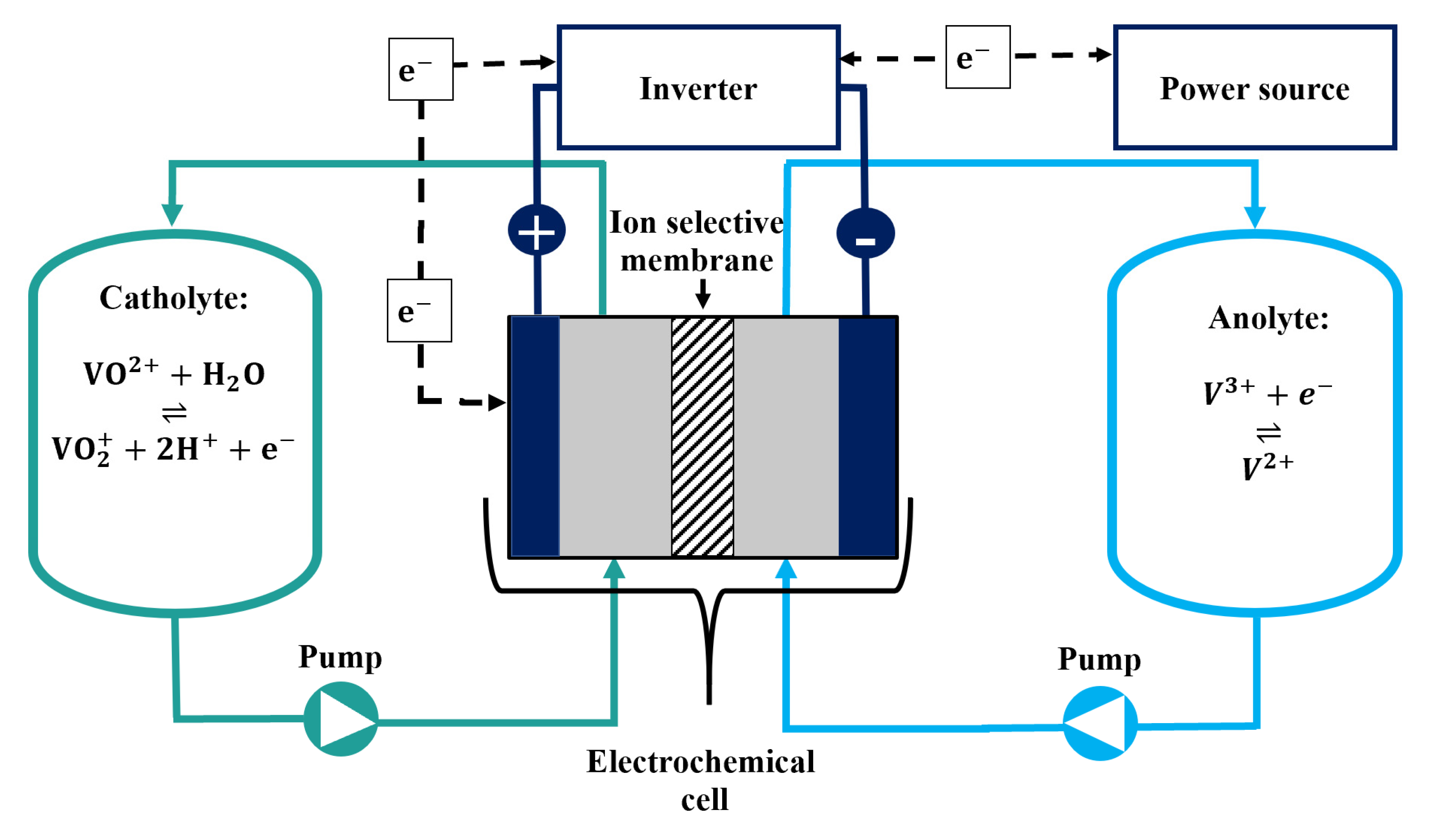
2.4. Electrochemical Cell
2.5. Battery Test
2.6. In Operando Temperature Control
3. Results and Discussion
3.1. Batch Precipitation Experiments
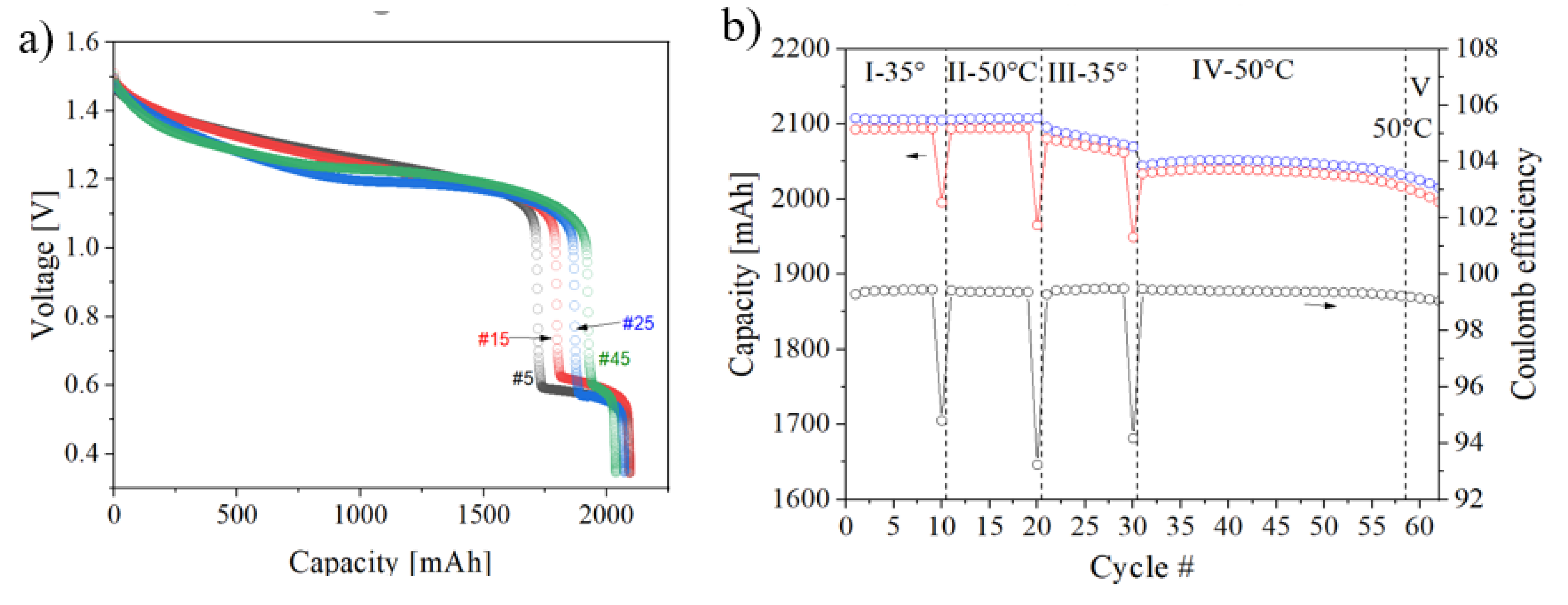
3.2. Influence of Air and Cycling Parameters on Battery Performance
3.3. In Operando Assessment of the Temperature Stability of Vanadium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IRENA. Renewable Power Generation Costs in 2017; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2018; p. 160. [Google Scholar]
- Ralon, P.; Taylor, M.; Ilas, A.; Diaz-Bone, H.; Kairies, K. Electricity Storage and Renewables: Costs and Markets to 2030; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2017. [Google Scholar]
- EU SET Plan. Available online: https://setis.ec.europa.eu/system/files/integrated_set-plan/action7_declaration_of_intent_0.pdf) (accessed on 10 November 2020).
- Department of Energy, USA, Grid Energy Storage, December 2013. Available online: https://www.energy.gov/sites/default/files/2014/09/f18/Grid%20Energy%20Storage%20December%202013.pdf (accessed on 10 November 2020).
- Akhil, A.A.; Huff, G.; Currier, A.B.; Kaun, B.C.; Rastler, D.M.; Chen, S.B.; Cotter, A.L.; Bradshaw, D.T.; Gauntlett, W.D. Electricity Storage Handbook in Collaboration with NRECA; DOE/EPRI 2013; Sandia National Laboratories: Albuquerque, NM, USA, 2013; p. 340.
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3) x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Schafner, K.; Becker, M.; Turek, T. Capacity balancing for vanadium redox flow batteries through electrolyte overflow. J. Appl. Electrochem. 2018, 48, 639–649. [Google Scholar] [CrossRef]
- Catalano, J.; Bentien, A.; Østedgaard-Munck, D.N.; Kjelstrup, S. Efficiency of electrochemical gas compression, pumping and power generation in membranes. J. Membr. Sci. 2015, 478, 37–48. [Google Scholar] [CrossRef]
- Knehr, K.; Kumbur, E. Role of convection and related effects on species crossover and capacity loss in vanadium redox flow batteries. Electrochem. Commun. 2012, 23, 76–79. [Google Scholar] [CrossRef]
- Wang, K.; Liu, L.; Xi, J.; Wu, Z.; Qiu, X. Reduction of capacity decay in vanadium flow batteries by an electrolyte-reflow method. J. Power Sources 2017, 338, 17–25. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—a review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Yu, L.; Wu, L.; Liu, L.; Qiu, X.; Xi, J. Broad temperature adaptability of vanadium redox flow battery—Part 1: Electrolyte research. Electrochim. Acta 2016, 187, 525–534. [Google Scholar] [CrossRef]
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A high energy density vanadium redox flow battery with 3 M vanadium electrolyte. J. Electrochem. Soc. 2015, 163, A5023. [Google Scholar] [CrossRef]
- Kausar, N.; Mousa, A.; Skyllas-Kazacos, M. The effect of additives on the high-temperature stability of the vanadium redox flow battery positive electrolytes. ChemElectroChem 2016, 3, 276–282. [Google Scholar] [CrossRef]
- Rahman, F.; Skyllas-Kazacos, M. Vanadium redox battery: Positive half-cell electrolyte studies. J. Power Sources 2009, 189, 1212–1219. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Liu, L.; Xi, J.; Wu, Z.; Qiu, X. Broad temperature adaptability of vanadium redox flow battery-Part 3: The effects of total vanadium concentration and sulfuric acid concentration. Electrochim. Acta 2018, 259, 11–19. [Google Scholar] [CrossRef]
- Carvalho Jr, W.M.; Cassayre, L.; Quaranta, D.; Chauvet, F.; El-Hage, R.; Tzedakis, T.; Biscans, B. Stability of highly supersaturated vanadium electrolyte solution and characterization of precipitated phases for Vanadium Redox Flow Battery. J. Energy Chem. 2021, 61, 436–445. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Leng, F.; Tan, C.M.; Pecht, M. Effect of temperature on the aging rate of Li ion battery operating above room temperature. Sci. Rep. 2015, 5, 1–12. [Google Scholar]
- Xi, J.; Xiao, S.; Yu, L.; Wu, L.; Liu, L.; Qiu, X. Broad temperature adaptability of vanadium redox flow battery—Part 2: Cell research. Electrochim. Acta 2016, 191, 695–704. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Li, L.; Graff, G.; Liu, J.; Zhang, H.; Yang, Z.; Hu, J.Z. Towards understanding the poor thermal stability of V5+ electrolyte solution in vanadium redox flow batteries. J. Power Sources 2011, 196, 3669–3672. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Song, C.; Platt, A.; Zhao, N.; Wang, H.; Li, H.; Fatih, K.; Jang, D. A review of all-vanadium redox flow battery durability: Degradation mechanisms and mitigation strategies. Int. J. Energy Res. 2019, 43, 6599–6638. [Google Scholar] [CrossRef]
- Roznyatovskaya, N.V.; Roznyatovsky, V.A.; Höhne, C.-C.; Fühl, M.; Gerber, T.; Küttinger, M.; Noack, J.; Fischer, P.; Pinkwart, K.; Tübke, J. The role of phosphate additive in stabilization of sulphuric-acid-based vanadium (V) electrolyte for all-vanadium redox-flow batteries. J. Power Sources 2017, 363, 234–243. [Google Scholar] [CrossRef]
- Oldenburg, F.J.; Bon, M.; Perego, D.; Polino, D.; Laino, T.; Gubler, L.; Schmidt, T.J. Revealing the role of phosphoric acid in all-vanadium redox flow batteries with DFT calculations and in situ analysis. Phys. Chem. Chem. Phys. 2018, 20, 23664–23673. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Skyllas-Kazacos, M. Evaluation of additive formulations to inhibit precipitation of positive electrolyte in vanadium battery. J. Power Sources 2017, 340, 139–149. [Google Scholar] [CrossRef]
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G. A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Zhang, J.; Chen, J.; Zhu, S.; Liu, X.; Wang, R. Effect of different additives with–NH2 or–NH4+ functional groups on V (V) electrolytes for a vanadium redox flow battery. J. Electroanal. Chem. 2016, 768, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Ngamsai, K.; Arpornwichanop, A. Analysis and measurement of the electrolyte imbalance in a vanadium redox flow battery. J. Power Sources 2015, 282, 534–543. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Yan, C.; Qiao, Y. Corrosion behavior of a positive graphite electrode in vanadium redox flow battery. Electrochim. Acta 2011, 56, 8783–8790. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Yan, C. On-line mass spectrometry study of electrochemical corrosion of the graphite electrode for vanadium redox flow battery. Electrochem. Commun. 2013, 28, 58–62. [Google Scholar] [CrossRef]

| Content of Electrolyte Solution | ||||
|---|---|---|---|---|
| Master solution | Vanadium (M) | H2SO4 (M) | H3PO4 (M) | (NH4)2SO4 (M) |
| A | 1.6 | 2.0 | 0.05 | 0 |
| B | 1.6 | 2.0 | 0.10 | 0 |
| C | 1.6 | 2.0 | 0.05 | 0.05 |
| D | 1.6 | 2.0 | 0.05 | 0.10 |
| E | 1.6 | 2.0 | 0.10 | 0.05 |
| F | 1.6 | 2.0 | 0.15 | 0 |
| Period | Temperature | Conditions | Notes |
|---|---|---|---|
| I | 35 °C |
| No precipitation observed during cycling or potential hold. |
| II | 50 °C |
| No precipitation observed during cycling or potential hold. |
| III | 35 °C |
| No precipitation observed during cycling or potential hold. |
| IV | 50 °C |
| No precipitation observed during cycling. However, the capacity was observed to decrease. Voltage–capacity plot suggests the carbon felt was oxidized. |
| V | 50 °C |
| No precipitation observed during cycling. However, the capacity was observed to decrease. Voltage–capacity plot suggests oxidation/corrosion of the carbon felt electrodes. The selectivity of the membrane started to deteriorate. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirk, E.H.; Fenini, F.; Oreiro, S.N.; Bentien, A. Temperature-Induced Precipitation of V2O5 in Vanadium Flow Batteries—Revisited. Batteries 2021, 7, 87. https://doi.org/10.3390/batteries7040087
Kirk EH, Fenini F, Oreiro SN, Bentien A. Temperature-Induced Precipitation of V2O5 in Vanadium Flow Batteries—Revisited. Batteries. 2021; 7(4):87. https://doi.org/10.3390/batteries7040087
Chicago/Turabian StyleKirk, Emil Holm, Filippo Fenini, Sara Noriega Oreiro, and Anders Bentien. 2021. "Temperature-Induced Precipitation of V2O5 in Vanadium Flow Batteries—Revisited" Batteries 7, no. 4: 87. https://doi.org/10.3390/batteries7040087
APA StyleKirk, E. H., Fenini, F., Oreiro, S. N., & Bentien, A. (2021). Temperature-Induced Precipitation of V2O5 in Vanadium Flow Batteries—Revisited. Batteries, 7(4), 87. https://doi.org/10.3390/batteries7040087






