Symmetric Aqueous Batteries of Titanium Hexacyanoferrate in Na+, K+, and Mg2+ Media
Abstract
:1. Introduction
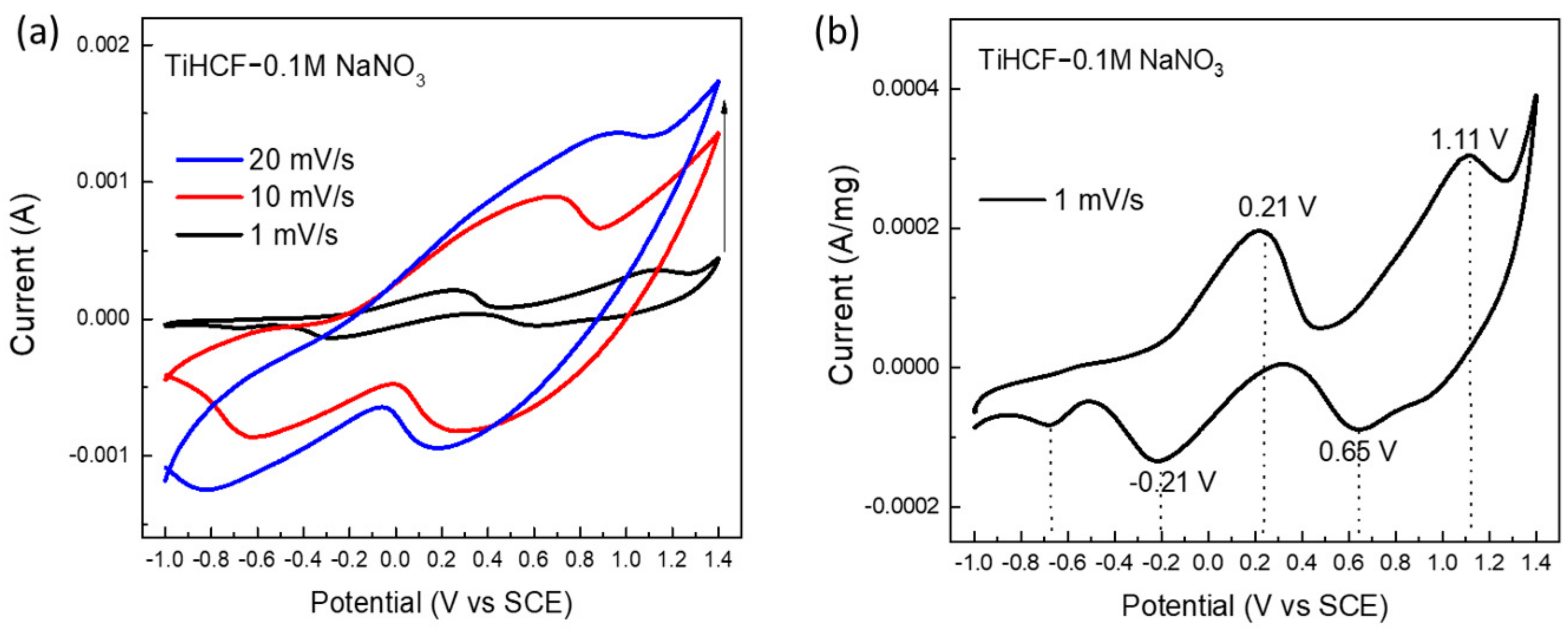
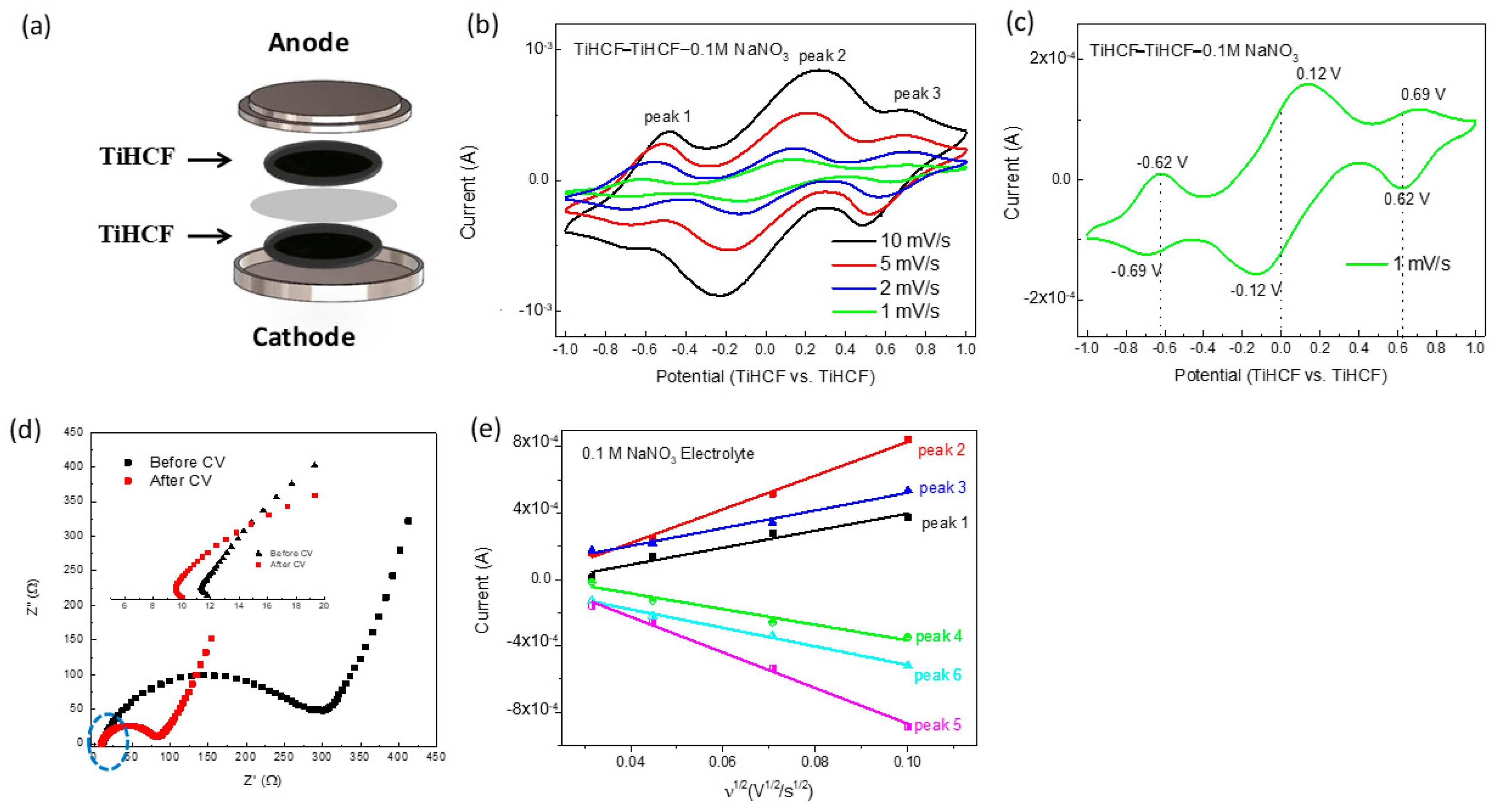
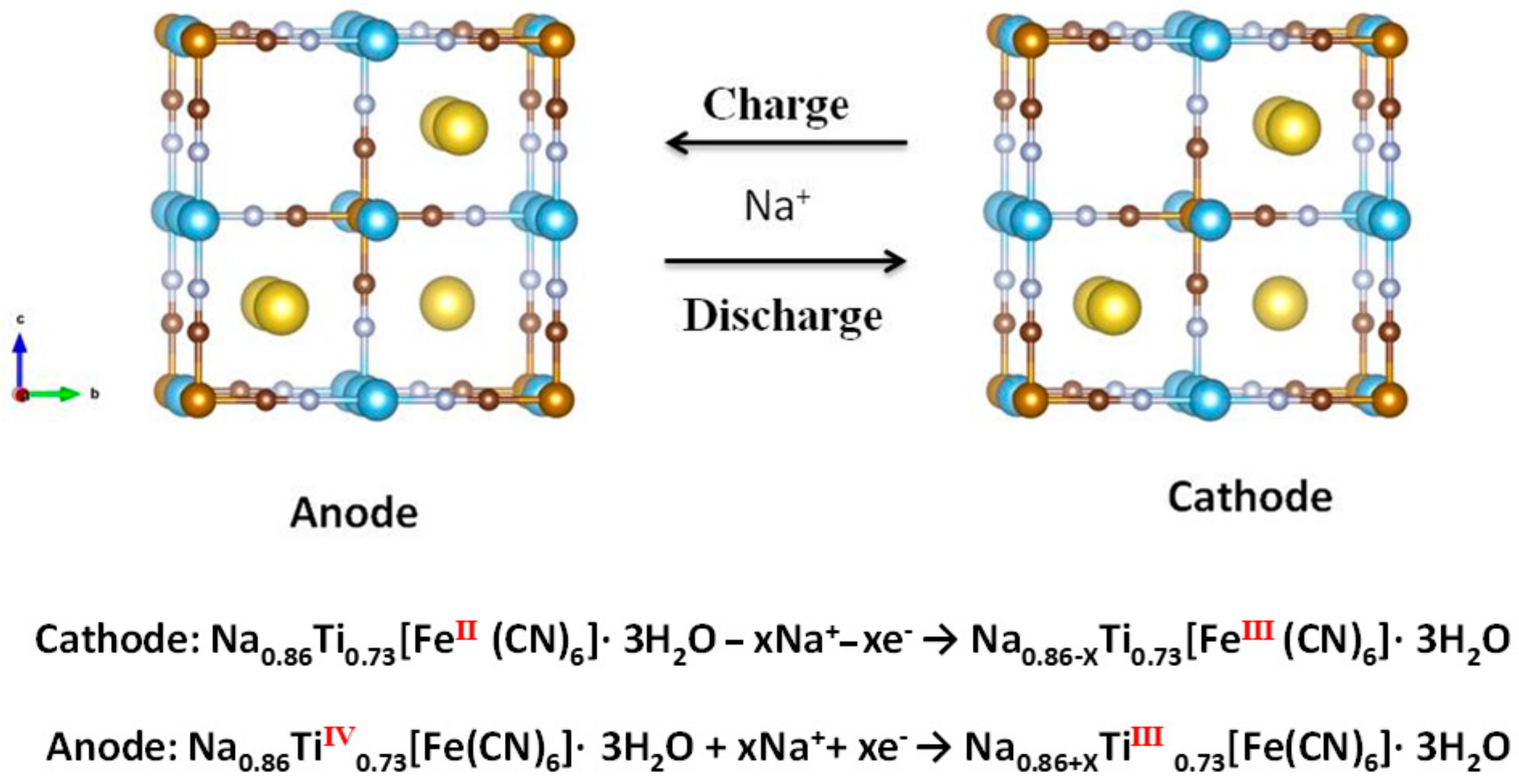
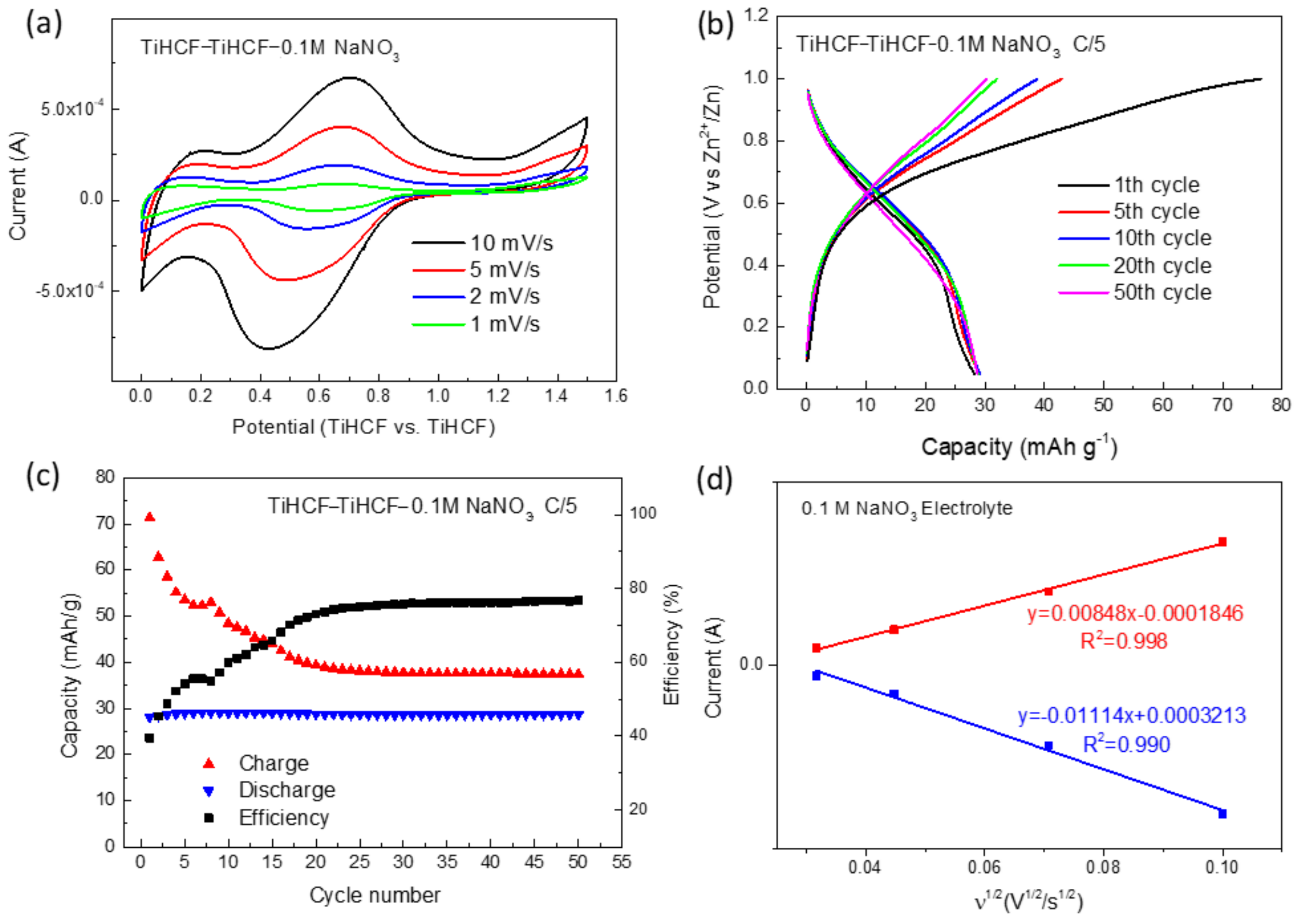
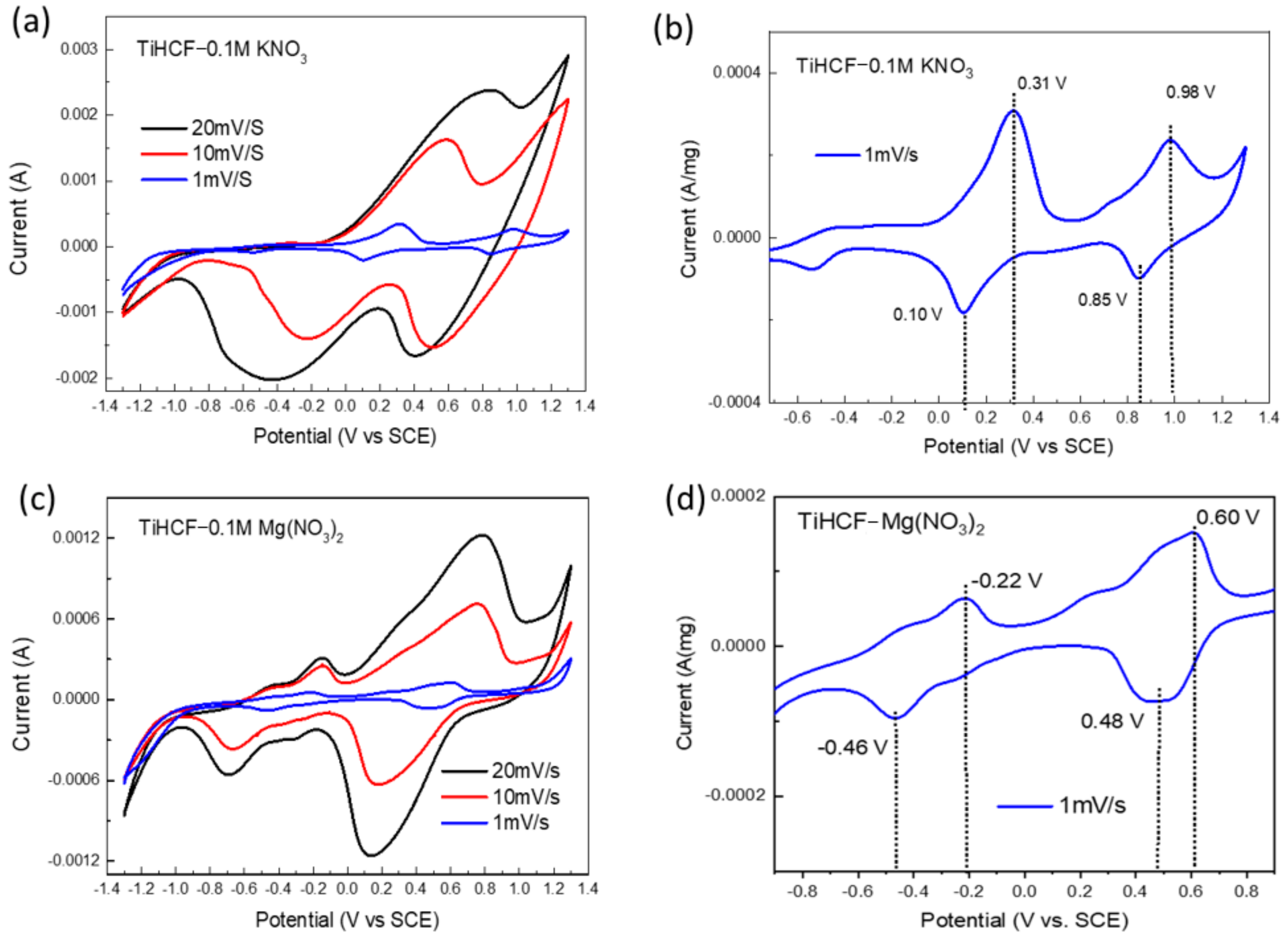
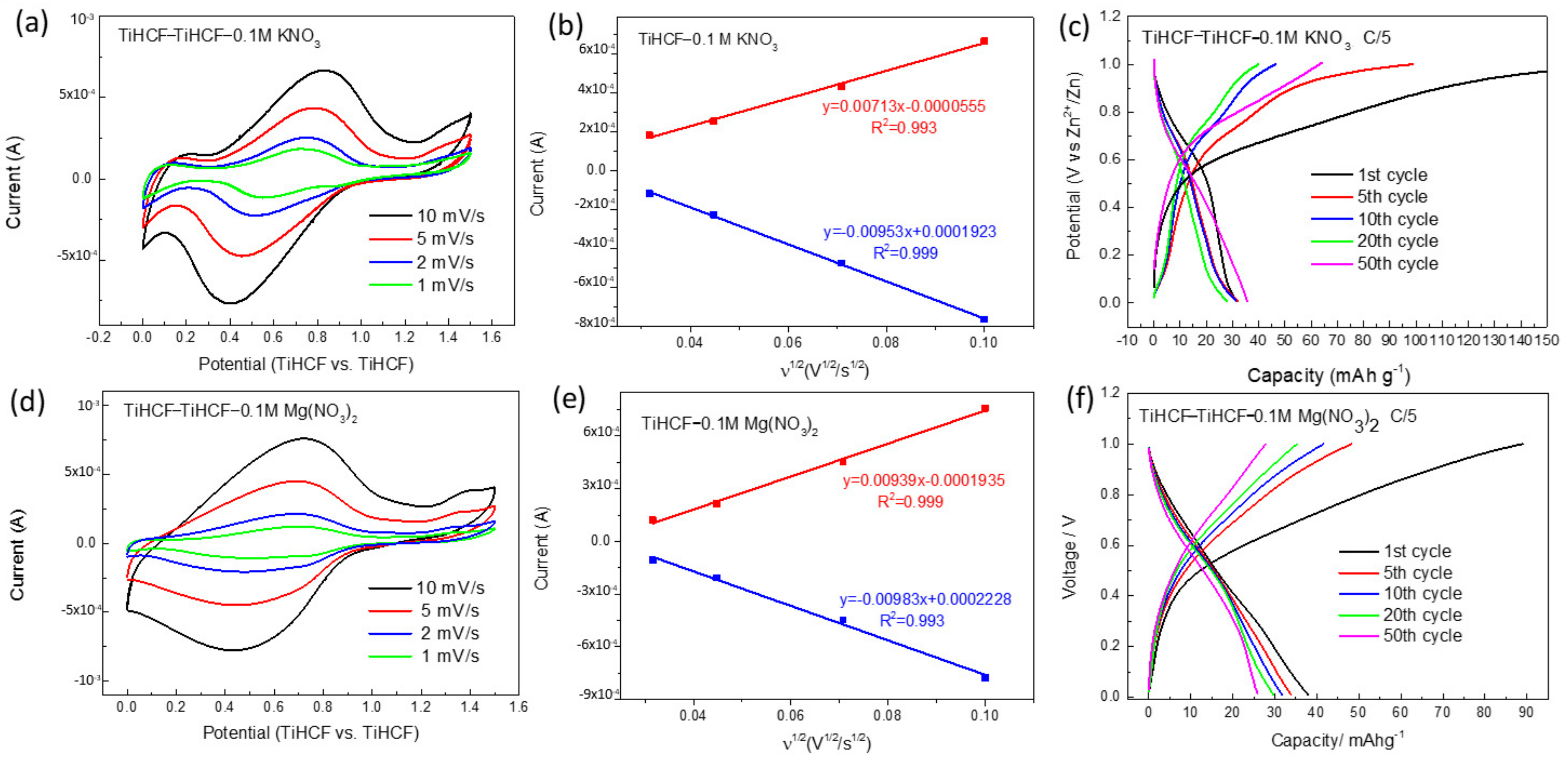
2. Results and Discussion
3. Materials and Methods
3.1. Material Synthesis
3.2. Electrode Preparation and Electrochemical Tests
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Mehta, A.; Basu, S.; Malode, S.J.; Shetti, N.P.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Electrode materials for lithium-ion batteries. Mater. Sci. Energy Technol. 2018, 1, 182–187. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4404–4419. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Recent Progress in Rechargeable Potassium Batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Rasul, S.; Suzuki, S.; Yamaguchi, S.; Miyayama, M. High capacity positive electrodes for secondary Mg-ion batteries. Electrochim. Acta 2012, 82, 243–249. [Google Scholar] [CrossRef]
- Aurbach, D.; Suresh, G.S.; Levi, E.; Mitelman, A.; Mizrahi, O.; Chusid, O.; Brunelli, M. Progress in rechargeable magnesium battery technology. Adv. Mater. 2007, 19, 4260–4267. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, S.X.; Liu, H.K.; Huang, Y.H.; Hu, X.L. Symmetric electrodes for electrochemical energy-storage devices. Adv. Sci. 2016, 3, 1600115. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Plashnitsa, L.S.; Doi, T.; Okada, S.; Yamaki, J.I. Electrochemical properties of Li symmetric solid-state cell with NASICON-type solid electrolyte and electrodes. Electrochem. Commun. 2010, 12, 894–896. [Google Scholar] [CrossRef]
- Noguchi, Y.; Kobayashi, E.; Plashnitsa, L.S.; Okada, S.; Yamaki, J.I. Fabrication and performances of all solid-state symmetric sodium battery based on NASICON-related compounds. Electrochim. Acta 2013, 101, 59–65. [Google Scholar] [CrossRef]
- Plashnitsa, L.S.; Kobayashi, E.; Noguchi, Y.; Okada, S.; Yamaki, J. Performance of NASICON Symmetric Cell with Ionic Liquid Electrolyte. J. Electrochem. Soc. 2010, 157, A536. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Wang, C.; Dou, Y.; Zhang, Q.; Liu, Y.; Gao, H.; Al-Mamun, M.; Pang, W.K.; Guo, Z.; et al. Constructing the best symmetric full K-ion battery with the NASICON-type K3V2(PO4)3. Nano Energy 2019, 60, 432–439. [Google Scholar] [CrossRef]
- Kosova, N.V.; Osintsev, D.I.; Uvarov, N.F.; Devyatkina, E.T. Lithium Titanium Phosphate as Cathode, Anode and Electrolyte for Lithium Rechargeable Batteries. Chem. Sustain. Dev. 2005, 13, 253–260. [Google Scholar]
- Senguttuvan, P.; Rousse, G.; Arroyo, Y.; De Dompablo, M.E.; Vezin, H.; Tarascon, J.M.; Palacín, M.R. Low-potential sodium insertion in a nasicon-type structure through the Ti(III)/Ti(II) redox couple. J. Am. Chem. Soc. 2013, 135, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Goodenough, J.B. An Aqueous Symmetric Sodium-Ion Battery with NASICON-Structured Na3MnTi(PO4)3. Angew. Chem. 2016, 128, 12960–12964. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, X.; Lam, K.H.; Zheng, Y.; Zhao, L.; Wang, K.; Zhao, J.; Chen, F.; Hou, X. Symmetric Sodium-Ion Battery Based on Dual-Electron Reactions of NASICON-Structured Na3MnTi(PO4)3 Material. ACS Appl. Mater. Interfaces 2020, 12, 30328–30335. [Google Scholar] [CrossRef]
- Inoishi, A.; Nishio, A.; Yoshioka, Y.; Kitajou, A.; Okada, S. A single-phase all-solid-state lithium battery based on Li1.5Cr0.5Ti1.5(PO4)3 for high rate capability and low temperature operation. Chem. Commun. 2018, 54, 3178–3181. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, C.; Zhai, T.; Li, H. A High Rate 1.2V Aqueous Sodium-ion Battery Based on All NASICON Structured NaTi2(PO4)3 and Na3V2(PO4)3. Electrochim. Acta 2016, 196, 470–478. [Google Scholar] [CrossRef]
- Mullaliu, A.; Asenbauer, J.; Aquilanti, G.; Passerini, S.; Giorgetti, M. Highlighting the Reversible Manganese Electroactivity in Na-Rich Manganese Hexacyanoferrate Material for Li- and Na-Ion Storage. Small Methods 2020, 4, 1900529. [Google Scholar] [CrossRef] [Green Version]
- Mullaliu, A.; Gaboardi, M.; Plaisier, J.R.; Passerini, S.; Giorgetti, M. Lattice Compensation to Jahn-Teller Distortion in Na-Rich Manganese Hexacyanoferrate for Li-Ion Storage: An Operando Study. ACS Appl. Energy Mater. 2020, 3, 5728–5733. [Google Scholar] [CrossRef]
- Neff, V.D. Some Performance Characteristics of a Prussian Blue Battery. J. Electrochem. Soc. 1985, 132, 1382–1384. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Niu, F.; Li, X.; Wang, C.; Yang, J. FeFe(CN)6 Nanocubes as a Bipolar Electrode Material in Aqueous Symmetric Sodium-Ion Batteries. Chempluschem 2017, 82, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yu, H.; Liu, P.; Ren, Y.; Zhang, T.; Chen, M.; Ishida, M.; Zhou, H. High-performance symmetric sodium-ion batteries using a new, bipolar O3-type material, Na0.8Ni0.4Ti0.6O2. Energy Environ. Sci. 2015, 8, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Liu, P.; Sun, Y.; Zhu, K.; Yi, J.; Chen, M.; Ishida, M.; Zhou, H. A High-Voltage and Ultralong-Life Sodium Full Cell for Stationary Energy Storage. Angew. Chem.-Int. Ed. 2015, 54, 11701–11705. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mullaliu, A.; Passerini, S.; Giorgetti, M. Titanium activation in prussian blue based electrodes for na-ion batteries: A synthesis and electrochemical study. Batteries 2021, 7, 5. [Google Scholar] [CrossRef]
- Paulitsch, B.; Yun, J.; Bandarenka, A.S. Electrodeposited Na2VOx[Fe(CN)6] films As a Cathode Material for Aqueous Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 8107–8112. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, B.; Guo, B.; Hu, L.; Xu, Q.; Zhan, R.; Zhang, Y.; Bao, S.; Xu, M. Potassium titanium hexacyanoferrate as a cathode material for potassium-ion batteries. J. Phys. Chem. Solids 2018, 122, 31–35. [Google Scholar] [CrossRef]
- Makowski, O.; Stroka, J.; Kulesza, P.J.; Malik, M.A.; Galus, Z. Electrochemical identity of copper hexacyanoferrate in the solid-state: Evidence for the presence and redox activity of both iron and copper ionic sites. J. Electroanal. Chem. 2002, 532, 157–164. [Google Scholar] [CrossRef]
- Kulesza, P.J. Solid-state electrochemistry of iron hexacyanoferrate (Prussian Blue type) powders Evidence for redox transitions in mixed-valence ionically conducting microstructures. J. Electroanal. Chem. Interfacial Electrochem. 1990, 289, 103–116. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Li, X.; Zhu, Y.; Liang, J.; Qian, Y. Surfactant widens the electrochemical window of an aqueous electrolyte for better rechargeable aqueous sodium/zinc battery. J. Mater. Chem. A 2017, 5, 730–738. [Google Scholar] [CrossRef]
- Scavetta, E.; Tonelli, D.; Giorgetti, M.; Nobili, F.; Marassi, R.; Berrettoni, M. AC impedance study of a synthetic hydrotalcite-like compound modified electrode in aqueous solution. Electrochim. Acta 2003, 48, 1347–1355. [Google Scholar] [CrossRef]
- Shoparwe, N.F.; Makhtar, M.M.Z.; Sata, S.A.; Kew, W.S.; Mohamad, M.; Shukor, H. Cyclic voltammetry studies of bioanode microbial fuel fells from batch culture of Geobacter sulfurreducens. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Bina, A.; Maisuradze, M.; Giorgetti, M. Symmetric Aqueous Batteries of Titanium Hexacyanoferrate in Na+, K+, and Mg2+ Media. Batteries 2022, 8, 1. https://doi.org/10.3390/batteries8010001
Li M, Bina A, Maisuradze M, Giorgetti M. Symmetric Aqueous Batteries of Titanium Hexacyanoferrate in Na+, K+, and Mg2+ Media. Batteries. 2022; 8(1):1. https://doi.org/10.3390/batteries8010001
Chicago/Turabian StyleLi, Min, Alessandro Bina, Mariam Maisuradze, and Marco Giorgetti. 2022. "Symmetric Aqueous Batteries of Titanium Hexacyanoferrate in Na+, K+, and Mg2+ Media" Batteries 8, no. 1: 1. https://doi.org/10.3390/batteries8010001
APA StyleLi, M., Bina, A., Maisuradze, M., & Giorgetti, M. (2022). Symmetric Aqueous Batteries of Titanium Hexacyanoferrate in Na+, K+, and Mg2+ Media. Batteries, 8(1), 1. https://doi.org/10.3390/batteries8010001






