Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries
Abstract
1. Introduction
2. Experiment
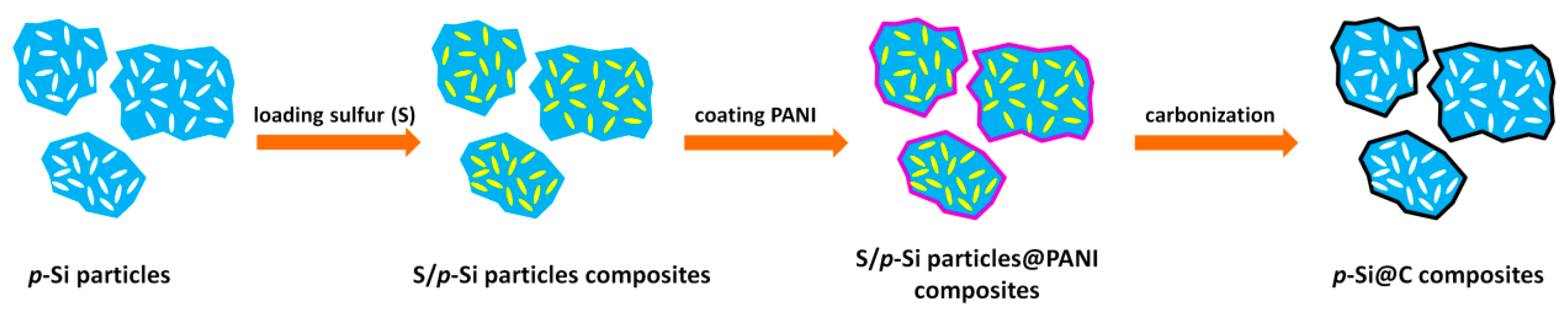
2.1. Preparation of p-Si@C Composites
2.2. Characterization
2.3. Electrochemical Measurements
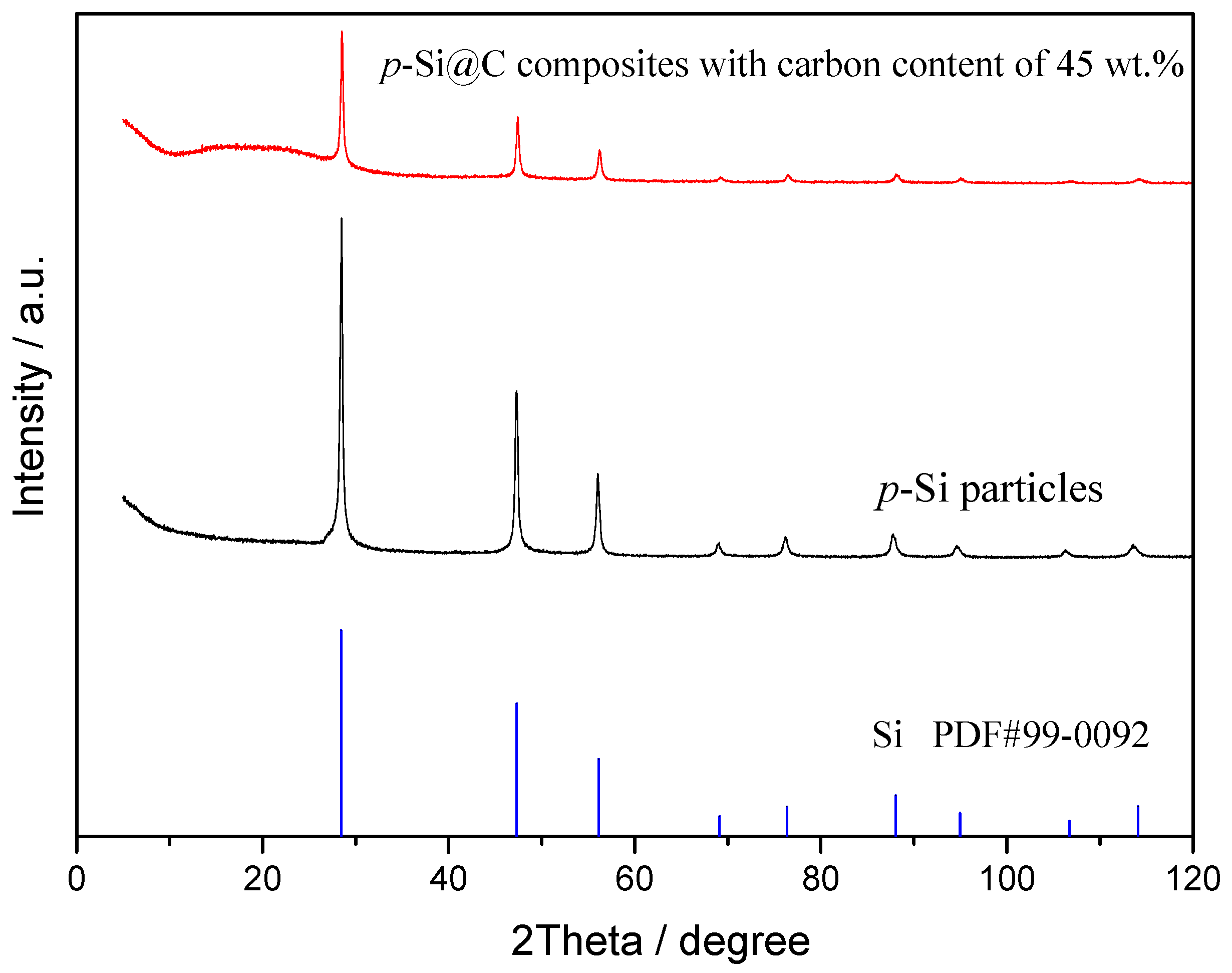
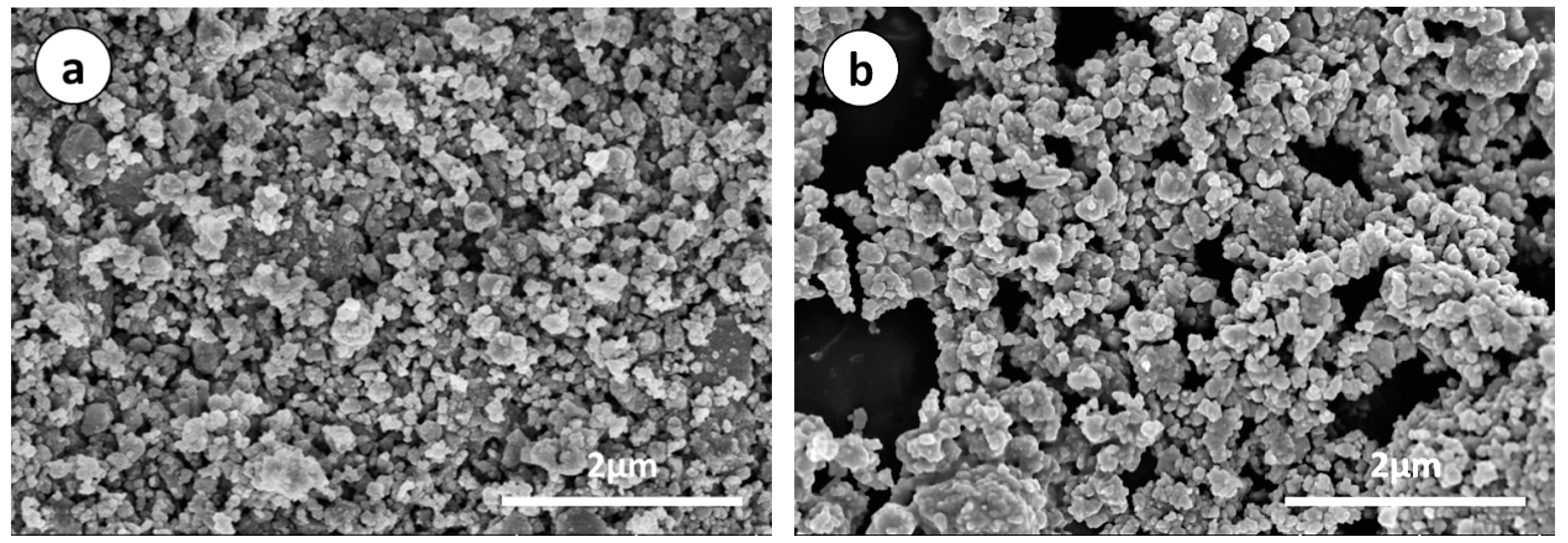
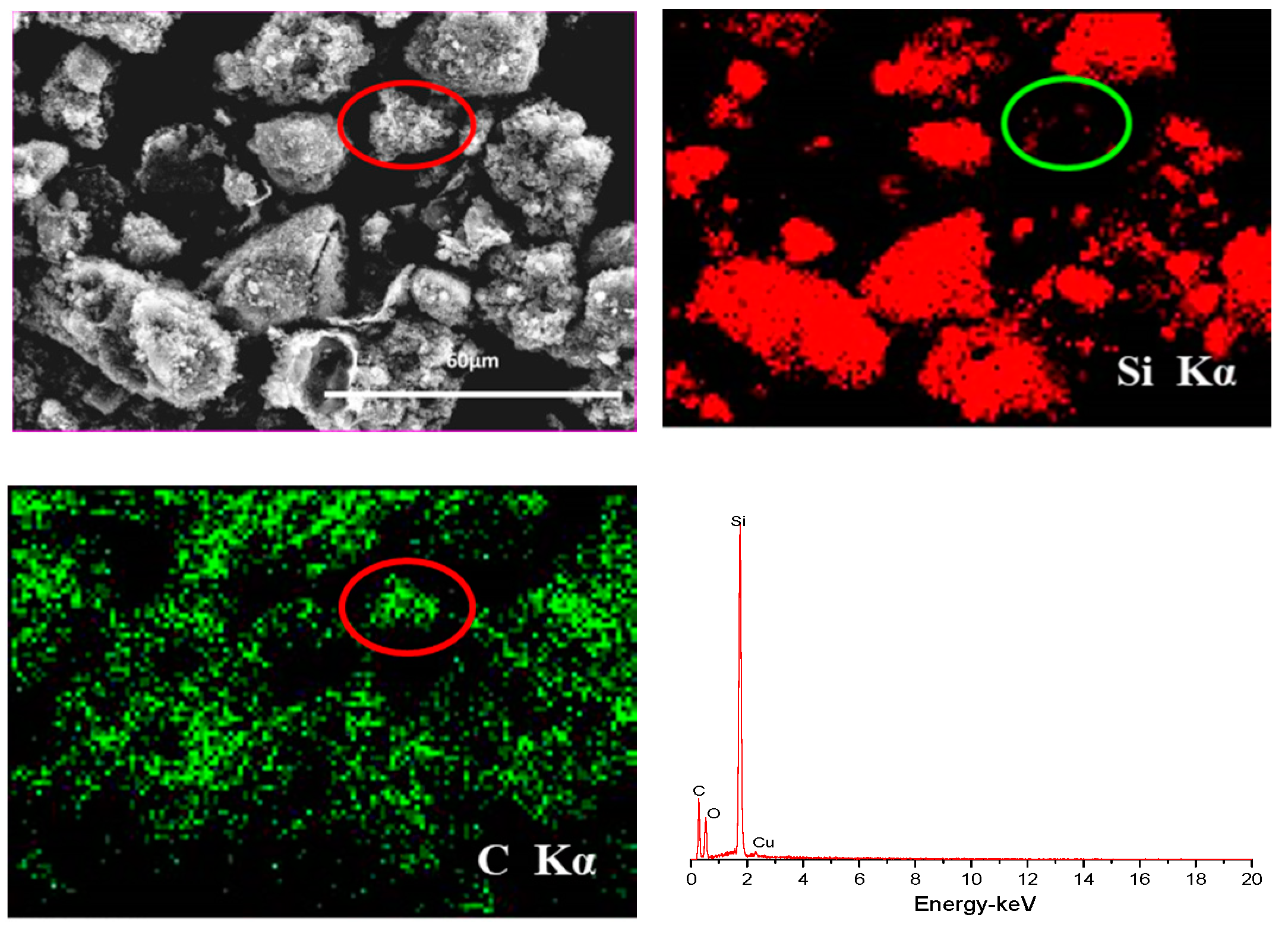
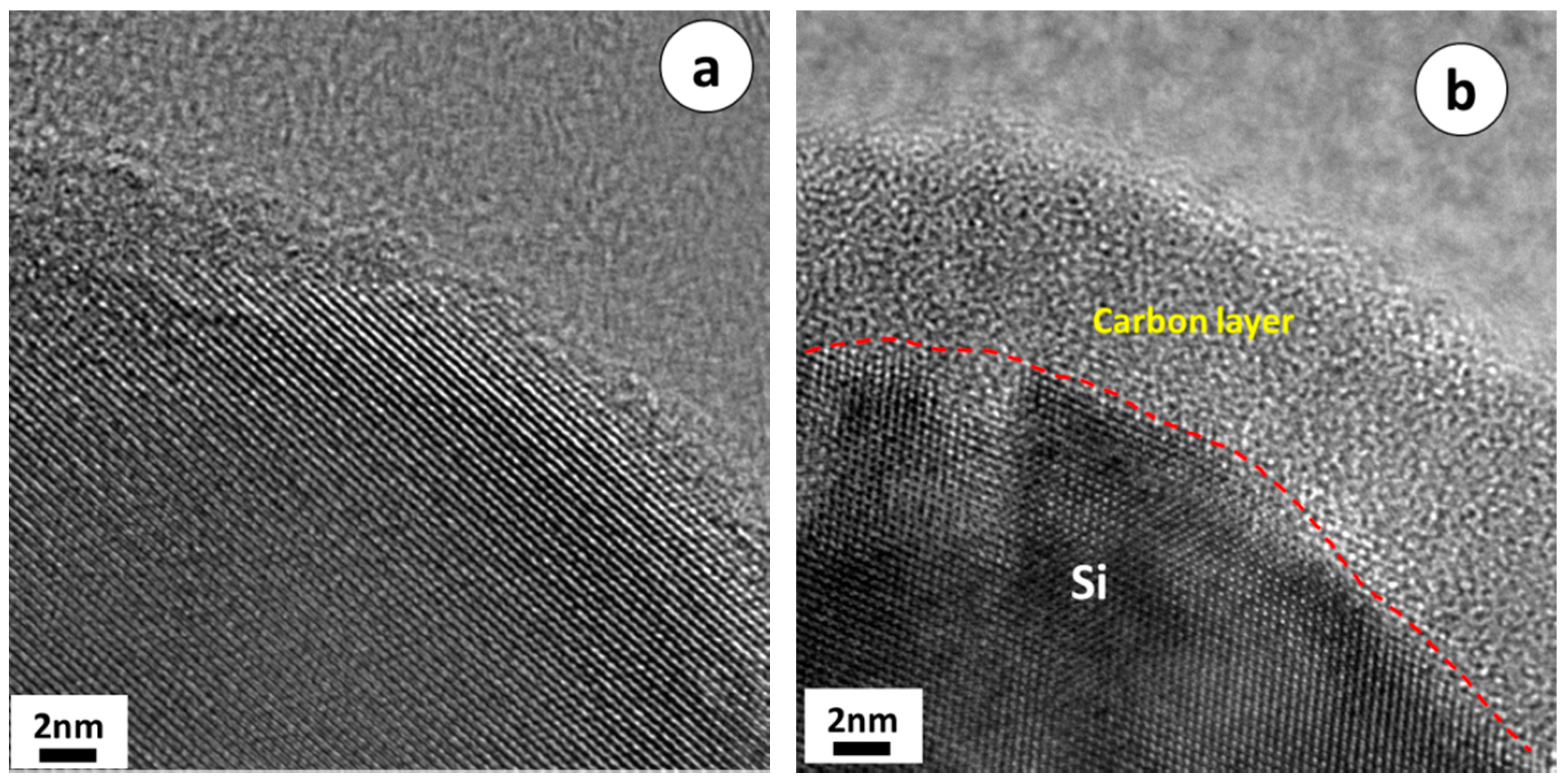
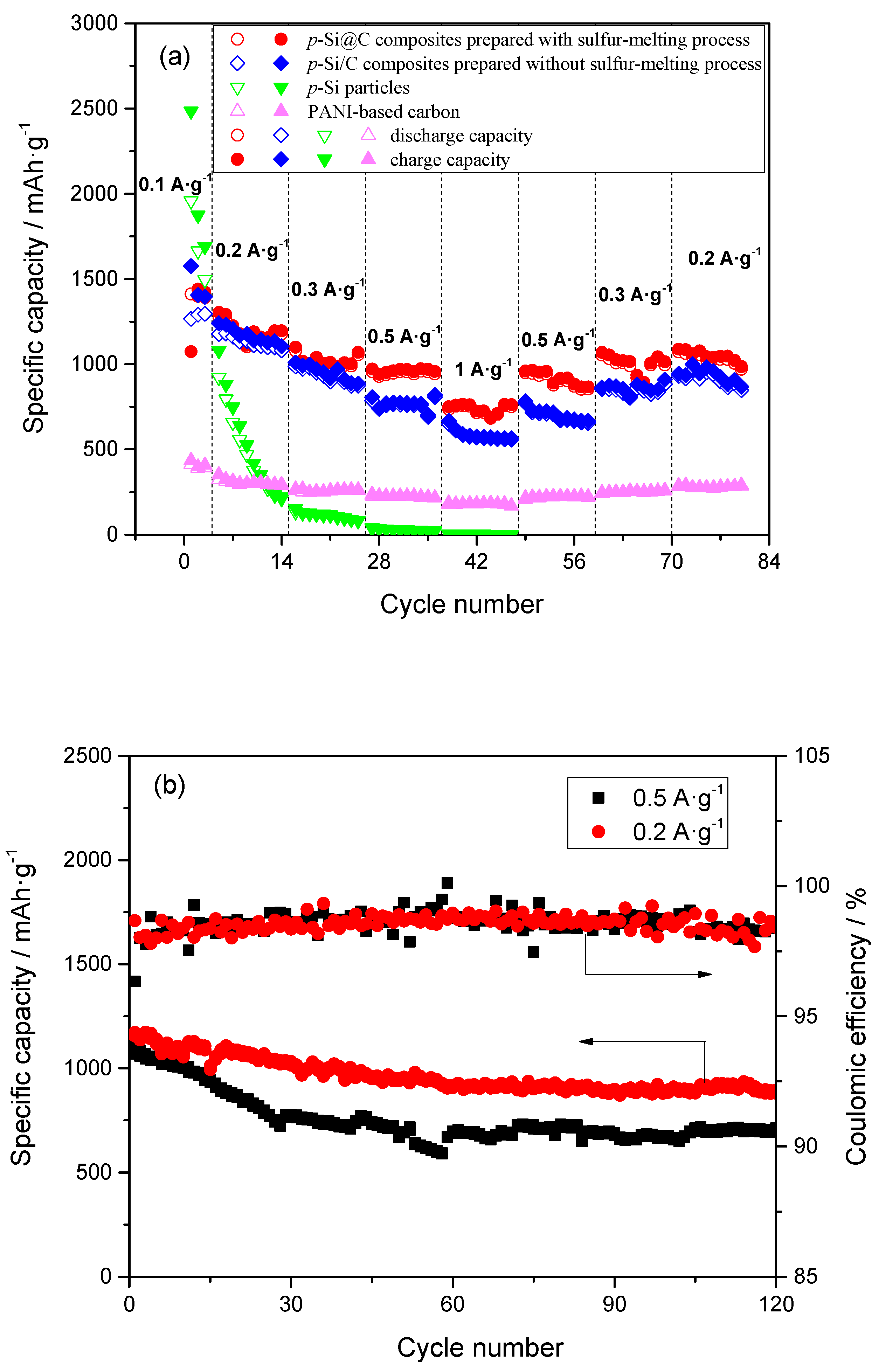
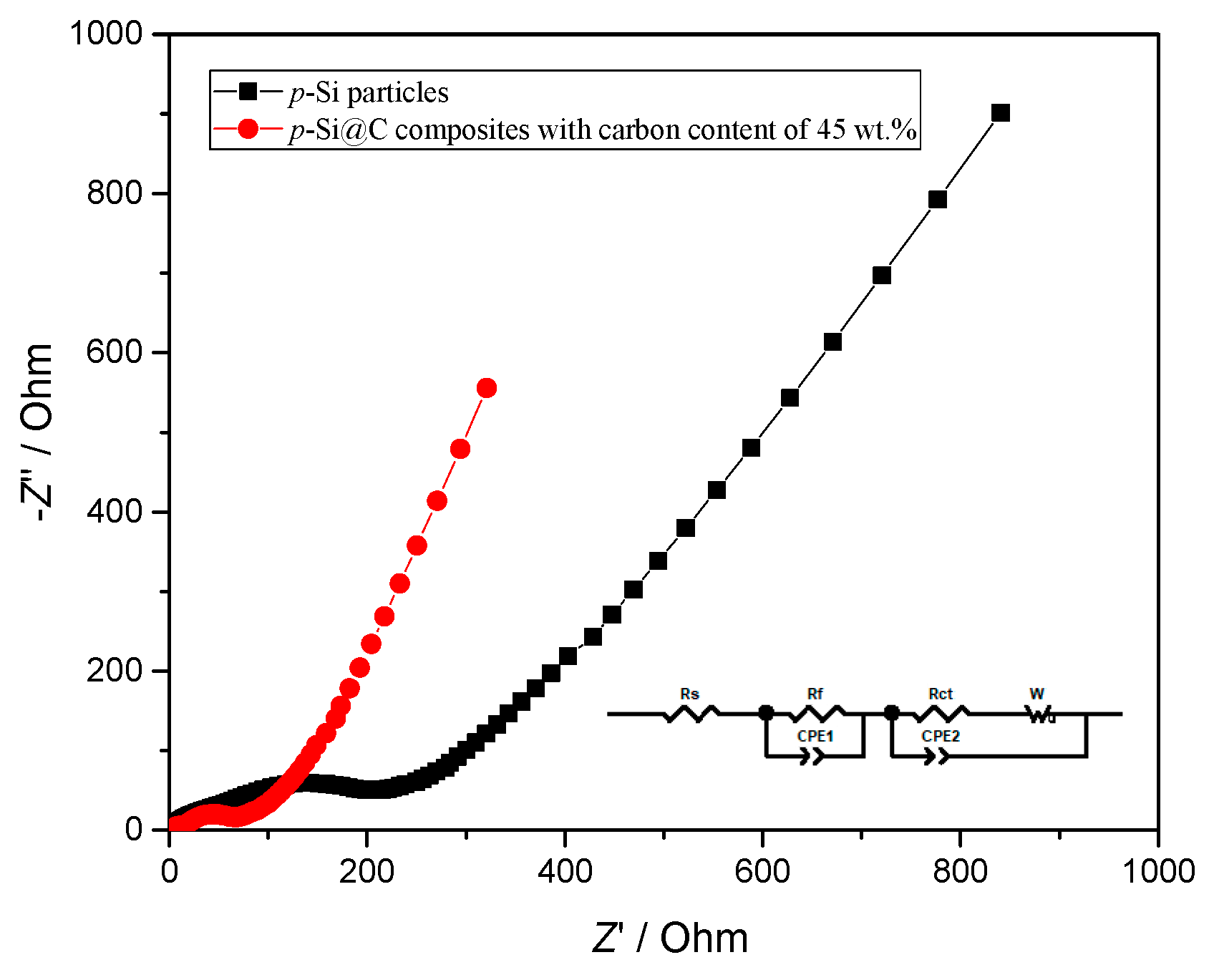
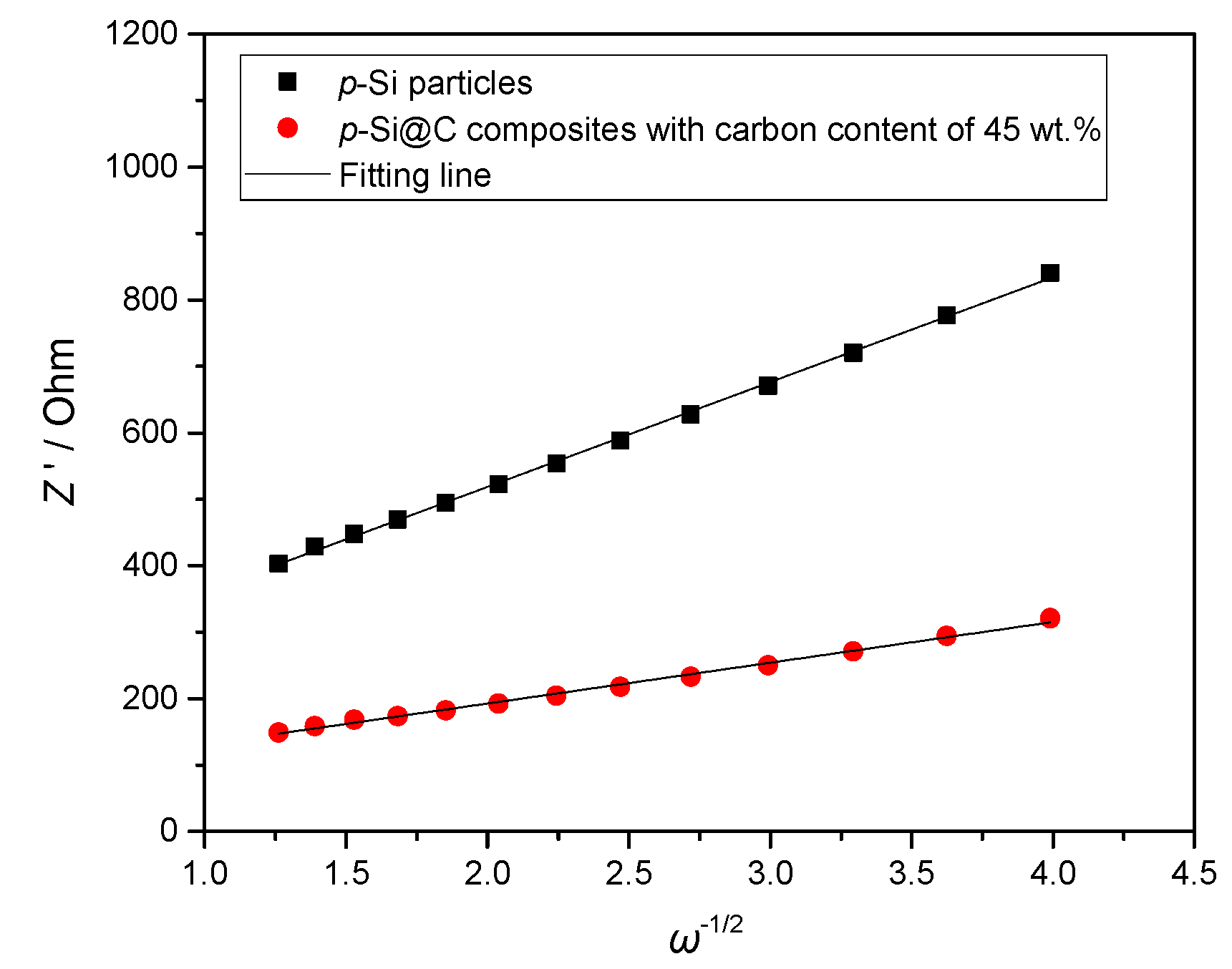
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jang, J.; Kang, I.; Yi, K.-W.; Cho, Y.W. Highly conducting fibrous carbon-coated silicon alloy anode for lithium ion batteries. Appl. Surf. Sci. 2018, 454, 277–283. [Google Scholar] [CrossRef]
- Firnadya, S.A.; Syahrial, A.Z.; Subhan, A. Enhancing battery performance by nano Si addition to Li4Ti5O12 as anode material on lithium-ion battery. Ionics 2018, 24, 1029–1037. [Google Scholar] [CrossRef]
- Yao, M.; Zeng, Z.; Zhang, H.; Yan, J.; Liu, X. Electrophoretic deposition of carbon nanofibers/silicon film with honeycomb structure as integrated anode electrode for lithium-ion batteries. Electrochim. Acta 2018, 281, 312–322. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Zuo, Y.; Song, Z.; Liu, F.; Li, K.; Pan, F. Conductive Binder for Si Anode with Boosted Charge Transfer Capability via n-Type Doping. ACS Appl. Mater. Interfaces 2018, 10, 27795–27800. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zheng, J.; Song, J.; Luo, L.; Yi, R.; Estevez, L.; Zhao, W.; Patel, R.; Li, X.; Zhang, J.-G. A novel approach to synthesize micrometer-sized porous silicon as a high performance anode for lithium-ion batteries. Nano Energy 2018, 50, 589–597. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, C.; Sun, L.; Xian, X. Si/polyaniline-based porous carbon composites with an enhanced electrochemical performance as anode materials for Li-ion batteries. Ionics 2018, 24, 1039–1048. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, M.; Yang, C.; Dai, G.; Huang, J. Fabrication of porous Si/nitrogen doped carbon composite and its enhanced lithium storage capability. Mater. Chem. Phys. 2017, 201, 302–310. [Google Scholar] [CrossRef]
- Holtstiege, F.; Wilken, A.; Winter, M.; Placke, T. Running out of lithium? A route to differentiate between capacity losses and active lithium losses in lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 25905–25918. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Burns, J.C.; Xiong, D.; Dahn, J.R. Interpreting High Precision Coulometry Results on Li-ion Cells. J. Electrochem. Soc. 2011, 158, A1136–A1142. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Du, H.L.; Islam, M.; Lee, J.K.; Sun, Y.-K.; Jung, H.-G. Self-Rearrangement of Silicon Nanoparticles Embedded in Micro-Carbon Sphere Framework for High-Energy and Long-Life Lithium-Ion Batteries. Nano Lett. 2017, 17, 5600–5606. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.-F.; Su, S.-H.; Leu, H.-J.; Wu, C.-Y. Silicon thin film anodes coated on micron carbon-fiber current collectors for lithium ion batteries. Surf. Coat. Technol. 2015, 267, 70–74. [Google Scholar] [CrossRef]
- Yi, Y.; Lee, G.-H.; Kim, J.-C.; Shim, H.-W.; Kim, D.-W. Tailored silicon hollow spheres with Micrococcus for Li ion battery electrodes. Chem. Eng. J. 2017, 327, 297–306. [Google Scholar] [CrossRef]
- Balderas-Valadez, R.F.; Antúnez, E.E.; Olive-Méndez, S.F.; Pacholski, C.; Campos-Alvarez, J.; Bokhimi, X.; Agarwal, V. Porous silicon pillar and bilayer structure as a nucleation center for the formation of aligned vanadium pentoxide nanorods. Ceram. Int. 2017, 43, 8023–8030. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, L.; Chen, J.; Li, X.; Yan, X. Crossed carbon skeleton enhances the electrochemical performance of porous silicon nanowires for lithium ion battery anode. Electrochim. Acta 2018, 280, 86–93. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Guo, H.; Li, X.; Zhou, R.; Zhou, Y. Chitosan: A N-doped carbon source of silicon-based anode material for lithium ion batteries. Ionics 2017, 23, 2311–2318. [Google Scholar] [CrossRef]
- Yi, Z.; Lin, N.; Xu, T.; Qian, Y. TiO2 coated Si/C interconnected microsphere with stable framework and interface for high-rate lithium storage. Chem. Eng. J. 2018, 347, 214–222. [Google Scholar] [CrossRef]
- Carbonari, G.; Maroni, F.; Birrozzi, A.; Tossici, R.; Croce, F.; Nobili, F. Synthesis and characterization of Si nanoparticles wrapped by V2O5 nanosheets as a composite anode material for lithium-ion batteries. Electrochim. Acta 2018, 281, 676–683. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Ossonon, B.D.; Bélanger, D. Graphene nanosheets and polyacrylic acid grafted silicon composite anode for lithium ion batteries. J. Power Sources 2018, 391, 41–50. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Hua, Y.; Dong, P.; Lin, Y.; Xu, M.; Wang, D.; Li, X.; Han, L.; Duan, J. Molten salt electrolytic synthesis of silicon-copper composite nanowires with enhanced performances as lithium ion battery anode. J. Alloys Compd. 2018, 751, 307–315. [Google Scholar] [CrossRef]
- Zhao, X.; Yim, C.-H.; Du, N.; Abu-Lebdeh, Y. Shortly Branched, Linear Dextrans as Efficient Binders for Silicon/Graphite Composite Electrodes in Li-Ion Batteries. Ind. Eng. Chem. Res. 2018, 57, 9062–9074. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, Y.; Hong, K.-S.; Lee, J.K.; Kim, J.-Y.; Bae, J.-S.; Jeong, E.D. Influence of EDTA in poly(acrylic acid) binder for enhancing electrochemical performance and thermal stability of silicon anode. Appl. Surf. Sci. 2018, 447, 442–451. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Chen, Y.; Huang, J.; Zhang, T.; Zeng, H.; Wang, C.; Liu, G.; Deng, Y. A Quadruple-Hydrogen-Bonded Supramolecular Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. Small 2018, 14, 1801189. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kimura, K.; Nishihara, H.; Kasukabe, T.; Kyotani, T.; Kobayashi, H. Fabrication of Si nanopowder from Si swarf and application to high-capacity and low cost Li-ion batteries. J. Alloys Compd. 2017, 720, 529–540. [Google Scholar] [CrossRef]
- Liang, G.; Qin, X.; Zou, J.; Luo, L.; Wang, Y.; Wu, M.; Zhu, H.; Chen, G.; Kang, F.; Li, B. Electrosprayed silicon-embedded porous carbon microspheres as lithium-ion battery anodes with exceptional rate capacities. Carbon 2018, 127, 424–431. [Google Scholar] [CrossRef]
- Ding, X.; Wang, H.; Liu, X.; Gao, Z.; Huang, Y.; Lv, D.; He, P.; Huang, Y. Advanced anodes composed of graphene encapsulated nano-silicon in a carbon nanotube network. RSC Adv. 2017, 7, 15694–15701. [Google Scholar] [CrossRef]
- Ruttert, M.; Holtstiege, F.; Huesker, J.; Boerner, M.; Winter, M.; Placke, T. Hydrothermal-derived carbon as a stabilizing matrix for improved cycling performance of silicon-based anodes for lithium-ion full cells. Beilstein J. Nanotechnol. 2018, 9, 2381–2395. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, C.; Chen, X.; Liu, S.; Huang, T.; Yu, A. Carbon-shell-constrained silicon cluster derived from Al-Si alloy as long-cycling life lithium ion batteries anode. J. Power Sources 2018, 381, 66–71. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Zhou, H.; Tang, J.; Ren, Y.; Bai, T.; Zhang, J.; Yang, J. Enhanced lithium ion battery performance of nano/micro-size Si via combination of metal-assisted chemical etching method and ball-milling. Microporous Mesoporous Mater. 2018, 268, 9–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, N.; Chen, Y.; Lin, Y.; Jiang, J.; He, Y.; Lei, Y.; Yang, D. Carbon dioxide as a green carbon source for the synthesis of carbon cages encapsulating porous silicon as high performance lithium-ion battery anodes. Nanoscale 2018, 10, 5626–5633. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Hu, X.; Hou, Y.; Wen, Z. Tunable Synthesis of Yolk–Shell Porous Silicon@Carbon for Optimizing Si/C-Based Anode of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42084–42092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ka, O.; Xian, X.; Sun, L.; Wang, J. Effect of primary crystallite size on the high-rate performance of Li4Ti5O12 microspheres. Electrochim. Acta 2016, 206, 17–25. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Chen, Y.; Liu, H. Carbon-coated Si/graphite composites with combined electrochemical properties for high-energy-density lithium-ion batteries. Ionics 2016, 22, 1847–1853. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Y.; He, L.; Zhu, Y.; Shi, Q.; Wang, Q.; Song, G.; Yan, X.; Li, L. Strategy to form homogeneously macroporous Si as enhanced anode material of Li-ion batteries. J. Alloys Compd. 2018, 731, 1–9. [Google Scholar] [CrossRef]
- Feng, M.; Tian, J.; Xie, H.; Kang, Y.; Shan, Z. Nano-silicon/polyaniline composites with an enhanced reversible capacity as anode materials for lithium ion batteries. J. Solid State Electrochem. 2015, 19, 1773–1782. [Google Scholar] [CrossRef]
- Kim, N.; Oh, C.; Kim, J.; Kim, J.S.; Jeong, E.D.; Bae, J.S.; Hong, T.E.; Lee, J.K. High-Performance Li-Ion Battery Anodes Based on Silicon-Graphene Self-Assemblies. J. Electrochem. Soc. 2017, 164, A6075–A6083. [Google Scholar] [CrossRef]
- Gómez-Cámer, J.L.; Thuv, H.; Novák, P. Electrochemical study of Si/C composites with particulate and fibrous morphology as negative electrodes for lithium-ion batteries. J. Power Sources 2015, 294, 128–135. [Google Scholar] [CrossRef]
- Wang, J.P.; Wang, C.Y.; Zhu, Y.M.; Wu, N.N.; Tian, W.H. Electrochemical stability of optimized Si/C composites anode for lithium-ion batteries. Ionics 2015, 21, 579–585. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, M.M.; Xian, X.C.; Ka, O.; Wang, Z.H.; Wang, J. Insight into the formation mechanism of Li4Ti5O12 microspheres obtained by a CTAB-assisted synthetic method and their electrochemical performances. J. Mater. Chem. A 2017, 5, 13740–13747. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Xu, Y.; Xian, X.; Liu, N.; Li, W. Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries. Batteries 2019, 5, 27. https://doi.org/10.3390/batteries5010027
Zhao S, Xu Y, Xian X, Liu N, Li W. Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries. Batteries. 2019; 5(1):27. https://doi.org/10.3390/batteries5010027
Chicago/Turabian StyleZhao, Shuo, Yue Xu, Xiaochao Xian, Na Liu, and Wenjing Li. 2019. "Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries" Batteries 5, no. 1: 27. https://doi.org/10.3390/batteries5010027
APA StyleZhao, S., Xu, Y., Xian, X., Liu, N., & Li, W. (2019). Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries. Batteries, 5(1), 27. https://doi.org/10.3390/batteries5010027





