Effect of Operating Temperature on Individual Half-Cell Reactions in All-Vanadium Redox Flow Batteries
Abstract
1. Introduction
2. Results
2.1. Full Cell Test
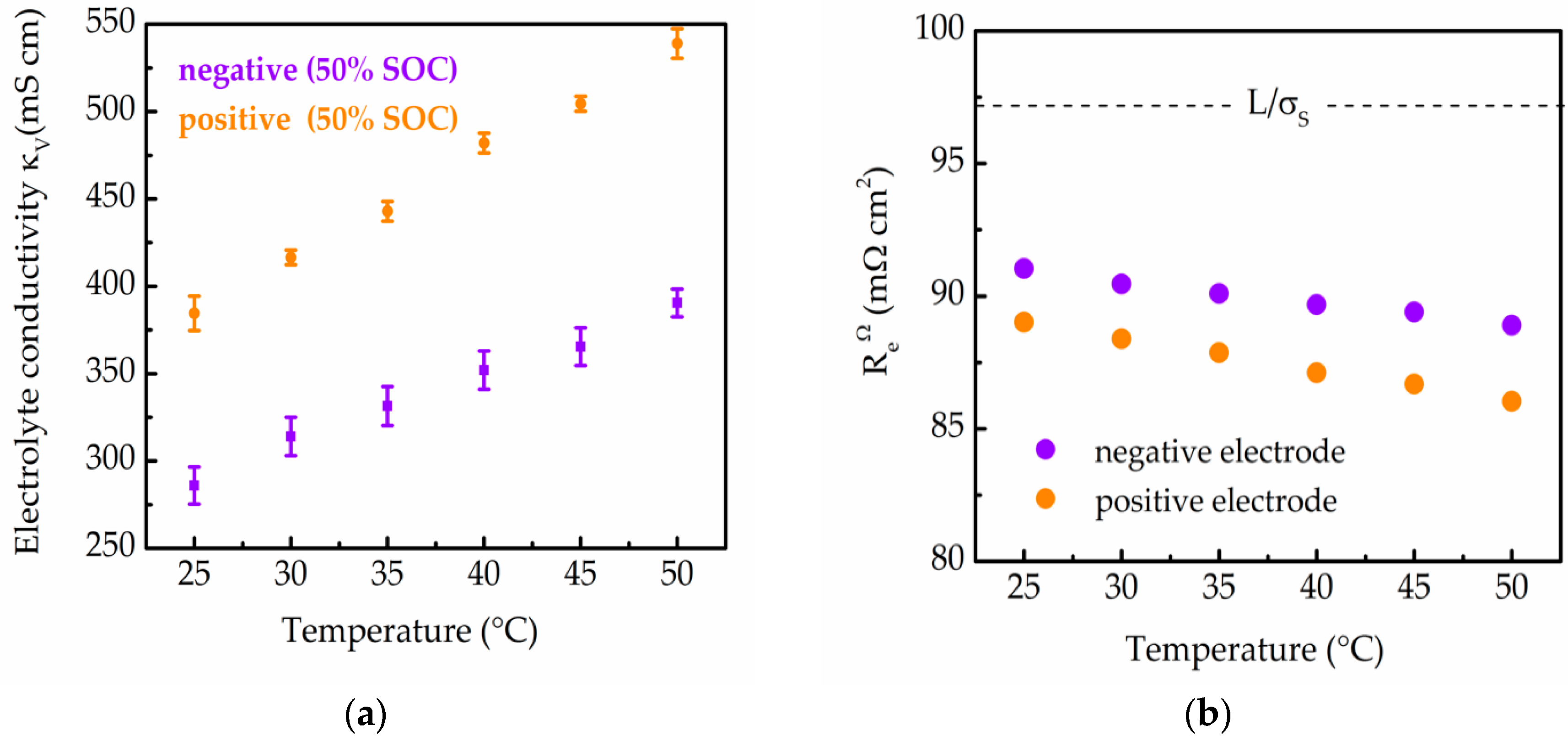
2.2. Effects on Porous Electrode
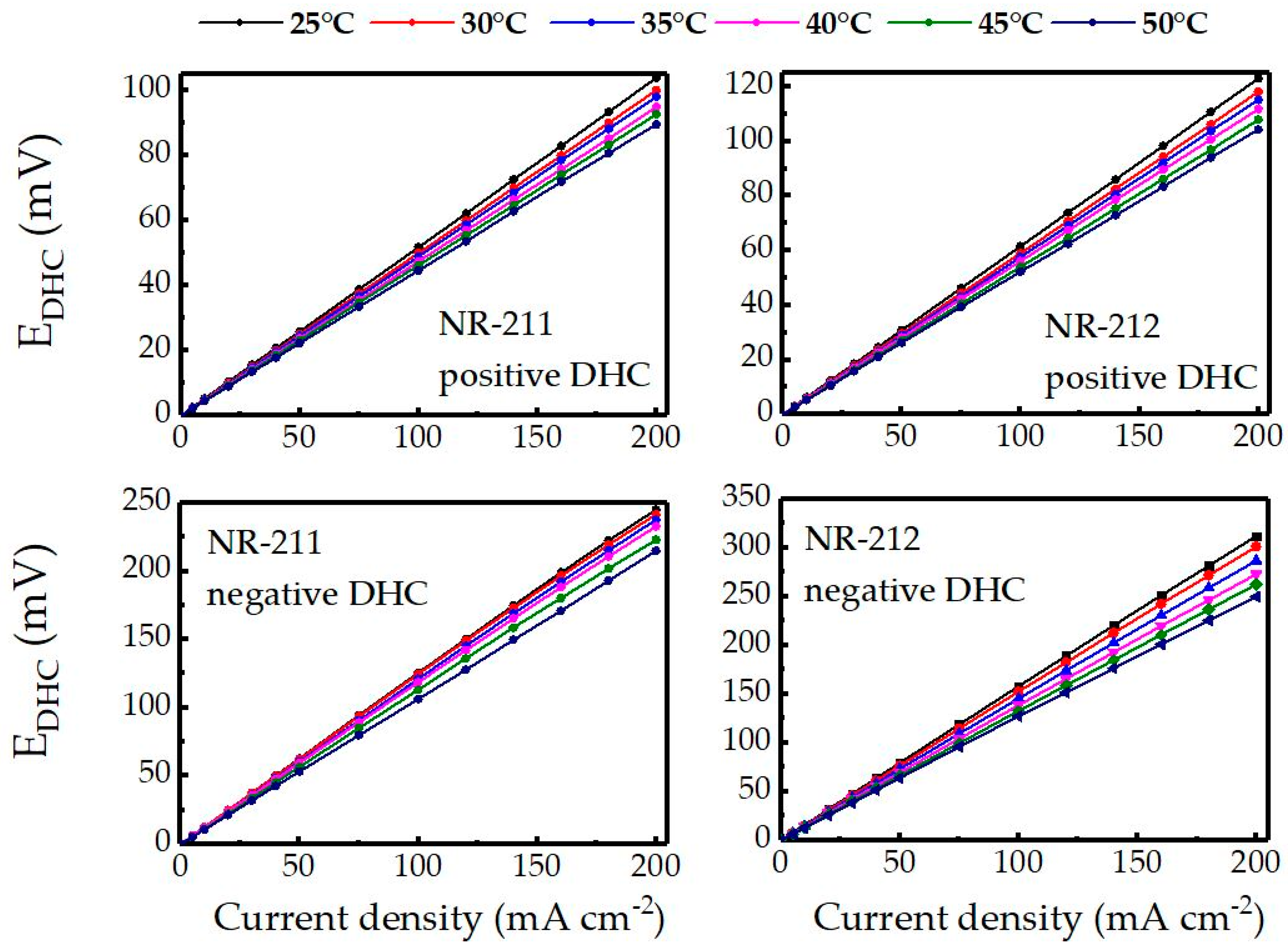
2.3. Double Half-Cell Measurements
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Denotation | Unit |
| Ae | Electrode area (2D) | cm2 |
| BET | BET surface area | m2 g−1 |
| EDHC | Double-half cell potential | mV |
| ΔH≠ | Activation enthalpy | kJ Mol−1 |
| ε0 | Porosity of the uncompressed felt | - |
| ε | Porosity of the uncompressed felt | - |
| F | Faraday’s constant (96486) | As Mol−1 |
| iapp | Applied current density | mA cm−2 |
| j0 | Exchange current density | mA cm−2 |
| κe | Ionic Conductivity of the electrolyte | mS cm−1 |
| κL | Ionic conductivity of the porous electrode | mS cm−1 |
| L0 | Thickness of the uncompressed felt | cm |
| L | Thickness of the compressed felt | cm |
| OCV | Open-circuit voltage | V |
| R | Gas constant (8.3144) | J K−1 Mol−1 |
| Re | Single electrode resistance | mΩ cm2 |
| ReΩ | Ohmic part of the single electrode resistance | mΩ cm2 |
| Rcc | Contact and collector resistance | mΩ cm2 |
| RDHC | DC resistance of double-half cell | mΩ cm2 |
| Rm | Membrane resistance | mΩ cm2 |
| Sa | Volumetric surface area | cm−1 |
| SOC | State of charge | % |
| σS | Electronic conductivity of the solid phase (felt) | mS cm−1 |
| T | Temperature | K |
| y | Dimensionless exchange current | - |
| ZDHC | (High-frequency) AC impedance of double-half cell | mΩ cm2 |
References
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in flow battery research and development. J. Electrochem. Soc. 2011, 58, R55–R79. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow batteries: Current status and trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ji, Y.N.; Qin, L.Y.; Leung, P.K.; Qiao, F.; Li, Y.S.; Su, H.N. Evaluation of redox flow batteries goes beyond round-trip efficiency: A technical review. J. Energy Storage 2018, 16, 108–115. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; Ponce de Leon, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Rabbow, T.J.; Trampert, M.; Pokorny, P. Critical safety features of the vanadium redox flow battery. J. Power Sources 2017, 351, 1–7. [Google Scholar] [CrossRef]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef] [PubMed]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient vanadium redox flow cell. J. Electrochem. Soc. 1987, 134, 2950–2953. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M. Vanadium redox flow batteries. In Encyclopedia of Electrochemical Power Sources, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 444–453. [Google Scholar]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2016, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A high energy density vanadium redox flow battery with 3 M vanadium electrolyte. J. Electrochem. Soc. 2016, 163, A5023–A5028. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Park, M.S.; Kim, Y.J.; Kim, J.H.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, X.; Cheng, Y.; Ning, G.; Xing, F.; Zhang, H. Development and perspective in vanadium flow battery modeling. Appl. Energy 2014, 132, 254–266. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S. Fundamental models for flow batteries. Prog. Energy Combust. Sci. 2015, 49, 40–58. [Google Scholar] [CrossRef]
- Wei, Z.; Tseng, K.J.; Wai, N.; Lim, T.M.; Skyllas-Kazacos, M. Adaptive estimation of state of charge and capacity with online identified battery model for vanadium redox flow battery. J. Power Sources 2016, 332, 389–398. [Google Scholar] [CrossRef]
- Shah, A.; Tangirala, R.; Singh, R.; Wills, R.G.A.; Walsh, F.C. A dynamic unit cell model for the all-vanadium flow battery. J. Electrochem. Soc. 2011, 158, A671–A677. [Google Scholar] [CrossRef]
- Flox, C.; Rubio Garcia, J.; Skoumal, M.; Vázquez-Galván, J.; Ventosa, E.; Ramon Morante, J. Thermally stable positive electrolytes with a superior performance in all-vanadium redox flow batteries. ChemPlusChem 2015, 80, 354–358. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xu, Q.; Yan, C.-W.; Cao, Y.-Z.; Qiao, Y.-L. The effect of temperature on the electrochemical behavior of the V(IV)/V(V) couple on a graphite electrode. Int. J. Electrochem. Sci. 2011, 6, 3483–3496. [Google Scholar]
- Langner, J.; Bruns, M.; Dixon, D.; Nefedov, A.; Wöll, C.; Scheiba, F.; Ehrenberg, H.; Roth, C.; Melke, J. Surface properties and graphitization of polyacrylonitrile based fiber electrodes affecting the negative half-cell reaction in vanadium redox flow batteries. J. Power Sources 2016, 321, 210–218. [Google Scholar] [CrossRef]
- Fetyan, A.; El-Nagar, G.A.; Lauermann, L.; Schnucklake, M.; Schneider, J.; Roth, C. Detrimental role of hydrogen evolution and its temperature-dependent impact on the performance of vanadium redox flow batteries. J. Energy Chem. 2018. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Xu, Q.; Zhou, X.L.; Zhang, Z.H. In-situ investigation of hydrogen evolution behavior in vanadium redox flow batteries. Appl. Energy 2017, 190, 1112–1118. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, T.S.; Xu, Q.; An, L.; Zhao, G. Effects of operating temperature on the performance of vanadium redox flow batteries. Appl. Energy 2015, 155, 349–353. [Google Scholar] [CrossRef]
- Xi, J.; Xiao, S.; Yu, L.; Wu, L.; Liu, L.; Xi, J.; Qiu, X. Broad temperature adaptability of vanadium redox flow battery-Part 2: Cell research. Electrochim. Acta 2016, 191, 695–704. [Google Scholar] [CrossRef]
- Xi, J.; Jiang, B.; Yu, L.; Liu, L. Membrane evaluation for vanadium flow batteries in a temperature range of −20 to 50 °C. J. Membr. Sci. 2017, 522, 45–55. [Google Scholar] [CrossRef]
- Yan, Y.; Skyllas-Kazacos, M.; Bao, J. Effects of battery design, environmental temperature and electrolyte flowrate on thermal behaviour of a vanadium redox flow battery in different applications. J. Energy Storage 2018, 11, 104–118. [Google Scholar] [CrossRef]
- Schweiss, R.; Pritzl, A.; Meiser, C. Parasitic hydrogen evolution at different carbon fiber electrodes in vanadium redox flow batteries. J. Electrochem. Soc. 2016, 163, A2089–A2094. [Google Scholar] [CrossRef]
- Bourke, A.; Miller, M.A.; Lynch, R.P.; Gao, X.; Landon, J.; Wainright, J.S.; Savinell, R.B.; Buckley, D.N. Electrode kinetics of vanadium flow batteries: Contrasting responses of VII-VIII and VIV-VV to electrochemical pretreatment. J. Electrochem. Soc. 2016, 163, A5097–A5105. [Google Scholar] [CrossRef]
- Schweiss, R.; Meiser, C.; Goh, F.W.T. Steady-state measurements of vanadium redox-flow batteries to study particular influences of carbon felt properties. ChemElectroChem 2017, 4, 1969–1974. [Google Scholar] [CrossRef]
- Gattrell, M.; Park, J.; MacDougall, B.; Apte, J.; McCarthy, S.; Wu, C.W. Study of the mechanism of the Vanadium 4+/5+ redox reaction in acidic solutions. J. Electrochem. Soc. 2004, 151, A123–A130. [Google Scholar] [CrossRef]
- Melke, J.; Jakes, P.; Langner, J.; Riekehr, L.; Kunz, U.; Zhao-Karger, Z.; Nefedov, A.; Sezen, H.; Wöll, C.; Ehrenberg, H.; et al. Carbon materials for the positive electrode in all-vanadium redox flow batteries. Carbon 2014, 78, 220–230. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. Half-cell, steady-state flow-battery experiments. ECS Trans. 2013, 53, 31–38. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. The influence of electrode and channel configurations on flow battery performance. J. Electrochem. Soc. 2014, 161, A1381–A1387. [Google Scholar] [CrossRef]
- Pan, J.; Huang, M.; Li, X.; Wang, S.; Li, W.; Ma, T.; Xie, X.; Ramani, V. The performance of all vanadium redox flow batteries at below-ambient temperatures. Energy 2016, 107, 784–790. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, L.; Wu, L.; Liu, L.; Qiu, X.; Xi, J. Broad temperature adaptability of vanadium redox flow battery-Part 1: Electrolyte research. Electrochim. Acta 2016, 187, 525–534. [Google Scholar] [CrossRef]
- Chen, Q.; Gerhardt, M.R.; Aziz, M.J. Dissection of the voltage losses of an acidic quinone redox flow battery. J. Electrochem. Soc. 2017, 164, A1126–A1132. [Google Scholar] [CrossRef]
- Newman, J.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; Wiley & Sons: New York, NY, USA, 2004; pp. 515–534. [Google Scholar]
- Friedl, J.; Stimming, U. Determining electron transfer kinetics at porous electrodes. Electrochim. Acta 2017, 227, 235–245. [Google Scholar] [CrossRef]
- Fink, H.; Friedl, J.; Stimming, U. Composition of the electrode determines which half-cell’s rate constant is higher in a vanadium flow battery. J. Phys. Chem. C 2016, 120, 15893–15901. [Google Scholar] [CrossRef]
- Sun, C.-N.; Delnick, F.M.; Aaron, D.S.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. Probing electrode losses in all-vanadium redox flow batteries with impedance spectroscopy. ECS Electrochem. Lett. 2013, 2, A43–A45. [Google Scholar] [CrossRef]
- Mazúr, P.; Mrlík, J.; Beneš, J.; Pocedič, J.; Vrána, J.; Dundálek, J.; Kosek, J. Performance evaluation of thermally treated graphite felt electrodes for vanadium redox flow battery and their four-point single cell characterization. J. Power Sources 2018, 380, 105–114. [Google Scholar] [CrossRef]
- Sun, C.-N.; Tang, Z.; Belcher, C.; Zawodzinski, T.A.; Fujimoto, C. Evaluation of Diels–Alder poly (phenylene) anion exchange membranes in all-vanadium redox flow batteries. Electrochem. Comm. 2014, 43, 63–66. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, L.; Wang, J.; Liu, L.; Liang, F.; Xi, J. Rational use and reuse of Nafion 212 membrane in vanadium flow batteries. RSC Adv. 2017, 7, 19425–19433. [Google Scholar] [CrossRef]
- Aaron, D.; Sun, C.-N.; Bright, M.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. In situ kinetics studies in all-vanadium redox flow batteries. ECS Electrochem. Lett. 2013, 2, A29–A31. [Google Scholar] [CrossRef]
- Davies, T.J.; Tummino, J.J. High-performance vanadium redox flow batteries with graphite felt electrodes. J. Carbon Res. 2018, 4, 8. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweiss, R.; Meiser, C.; Dan, D. Effect of Operating Temperature on Individual Half-Cell Reactions in All-Vanadium Redox Flow Batteries. Batteries 2018, 4, 55. https://doi.org/10.3390/batteries4040055
Schweiss R, Meiser C, Dan D. Effect of Operating Temperature on Individual Half-Cell Reactions in All-Vanadium Redox Flow Batteries. Batteries. 2018; 4(4):55. https://doi.org/10.3390/batteries4040055
Chicago/Turabian StyleSchweiss, Ruediger, Christian Meiser, and Dana Dan. 2018. "Effect of Operating Temperature on Individual Half-Cell Reactions in All-Vanadium Redox Flow Batteries" Batteries 4, no. 4: 55. https://doi.org/10.3390/batteries4040055
APA StyleSchweiss, R., Meiser, C., & Dan, D. (2018). Effect of Operating Temperature on Individual Half-Cell Reactions in All-Vanadium Redox Flow Batteries. Batteries, 4(4), 55. https://doi.org/10.3390/batteries4040055





