1. Introduction
Redox flow batteries (RFBs) can provide a solution to large scale energy storage, giving a more efficient link between energy production, especially from renewables, and energy demand [
1,
2,
3]. This type of battery system presents the advantage of having a lower cost, rapid response and a low level of self-discharge and is considered to have a much safer operation, as compared to other battery systems such as the sodium sulphur and lithium ion batteries [
4,
5]. Additionally, as with all battery systems, it has the advantage of being more flexible and mobile in comparison to non-electrochemical technologies, such as pumped hydro and compressed air storage. The latter large scale energy management technologies are restrained by the suitability of the terrain whereas batteries, such as RFBs, can be readily installed anywhere [
6] and provide long duration discharge. For rapid response, such as for frequency stabilisation, supercapacitors can be used in combination with electrochemical batteries due to the high-power density capability of these devices [
7,
8,
9].
Aqueous RFBs are among the most developed with numerous flow battery systems having been demonstrated [
10,
11]. Although they have a high-power density, these batteries have low energy densities and depending on the system, relatively high material costs. Despite a number of developments, such as that of the mixed acid electrolyte employed in the all-vanadium redox flow battery to yield higher densities [
12], these systems still struggle to compete with alternative technologies. This has led to the development of a variety of RFB types such as hybrid RFBs and non-aqueous RFBs [
13,
14,
15].
However, the all-vanadium RFB (VRFB) remains the most iconic and commercial of all the RFBs. Developed by M. Skyllas-Kazacos et al. in 1988, the VRFB has seen a lot of development at both the fundamental and industrial level [
16]. This original system was set as a superior alternative to the iron-chromium RFB which was used by National Aeronautics and Space Administration [
13]. One of its advantages is its resilience to membrane crossover by the electroactive species on the negative and positive side of the battery. Since the same elemental species is used on both sides, should species crossover occur, the electrolytes can simply be regenerated through remixing and electrolysis without harm to any of the materials or requirement for the system to undergo complicated separation treatment. However, due to the poor solubility of the vanadium species in pure water, sulphuric acid is commonly added. This is typically referred to as a Generation I—VRFB [
17]. This VRFB gives the following reactions during discharge [
18]:
As indicated above, this gives an overall open circuit voltage of 1.26 V under standard conditions. The energy density is limited by the concentration at which the vanadium ions remain stable within solution. The current operating level is 2 M VOSO
4 in 2 M sulphuric acid. Above this concentration the VO
2+ ions can precipitate out as V
2O
5, especially when temperatures are above 40 °C whereas the V
2+/V
3+ precipitation can also occur at temperatures below 10 °C [
1]. This limits the practical operation of the Generation 1 batteries to 10–40 °C with a concentration less than 2 M. Such concentrations gives an open circuit of 1.6 V when fully charged [
6].
The VRFB has become the most commercially successful RFB due to the systems’ ability to undergo multiple charge-discharge cycles resulting in better levelised cost of electricity (a measure of economic value over the potential lifetime of the technology), despite the vanadium having a relatively high cost. On top of this, the system also has 70–90% energy efficiency due to fast kinetics and can be over-charged or undergo deep discharge with no lasting damage to the system. However, when the cell is overcharged, possible side reactions, such as hydrogen evolution, can occur at the cathode:
This gas evolution is kept to a minimum as it can affect the flow of the electrolyte, create imbalance in the electrolyte, increase the cell resistance, and alter the pH of the solution (affecting the proton-exchange membrane) as well as creating a safety hazard.
Switzerland introduced the “Energy Strategy 2050” strategy, following the Fukushima incident in Japan and this was ratified, via public vote, in 2017. The strategy aims to reduce the nation’s energy consumption, increase energy efficiency and promote the use of renewable energy sources [
19]. It is worth noting that in 2016, Switzerland produced less than 0.2% of their electricity demand from wind energy: producing only 600 GWh per year [
20]. The plan is to increase that capacity to 4000 GWh per year by 2050 as well as that of solar energy to 20% of generated electricity by 2020, compared to the 1% attained in 2013 [
21]. It is nevertheless acknowledged that energy supplied from renewable sources is intermittent and can fluctuate significantly depending on weather conditions and location within Switzerland [
22,
23]. To counter this and achieve the strategic goals, significant interest lies in developing a feasible energy storage strategy to improve the nation’s energy efficiency and security. Better energy management from renewables can be achieved through the use of a feasible storage strategy, which will add to the nation’s energy efficiency and security.
One energy storage system, located at the water treatment plant facility in Martigny, is a 200 kW/400 kWh vanadium flow battery, based on the Generation 1 model using the concentrated 2 M sulphuric acid electrolyte and was provided by Gildemeister in 2014 and operated since by École Polytechnique Fédérale de Lausanne—Laboratory of Physical and Analytical Electrochemistry (EPFL—LEPA). This battery forms the centrepiece of the refuelling station which also hosts an electric vehicle fast charger (50 kW) and two electrolysers which produce hydrogen for the refuelling of the centre’s fuel cell vehicles [
24,
25]. The objective of this demonstration project was to investigate the connections of the battery to the grid and to better understand energy transfer from the grid for transport applications. Additionally, the site can simulate energy productions from intermittent energy sources to determine the battery’s capability to store this excess energy for later use.
The purpose of this work was for the University of Strathclyde, Scotland, to analyse and characterise the 200 kW/400 kWh VRFB and determine its actual capacity, the voltage, coulombic and energy efficiencies, identify and quantify the sources of the energy losses and the self-discharge rate under different scenarios. From this, it should be possible to ascertain the most appropriate application for this energy storage system at the facility in Martigny, Switzerland, viz., intermittent energy storage, power for the 50 kW alkaline electrolyser and/or 50 kW electric vehicle recharging.
2. Methodology
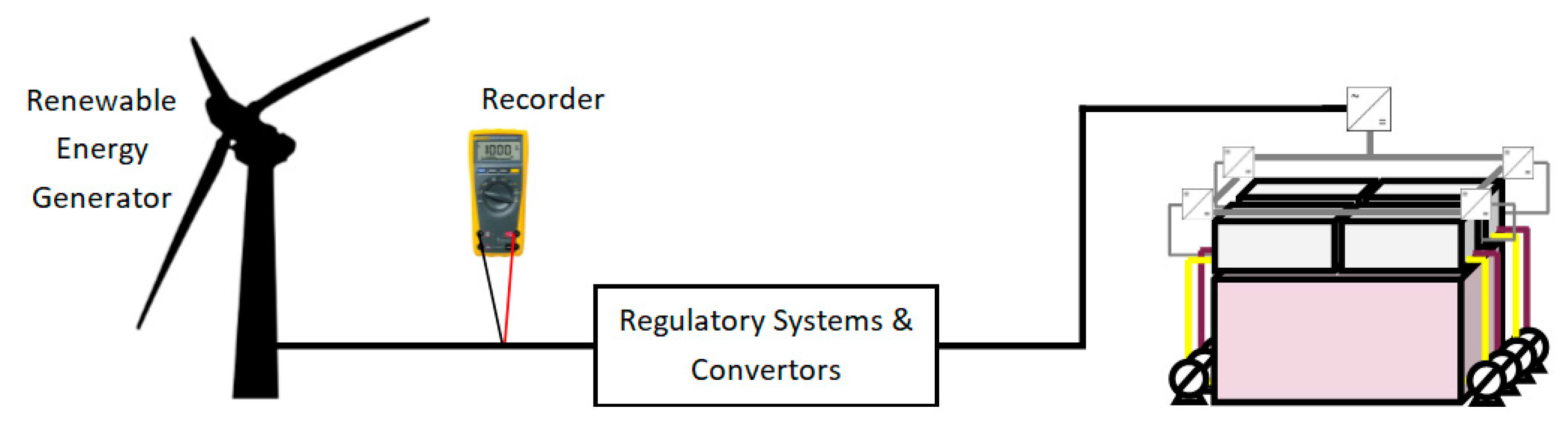
The analysis of the 200 kW/400 kWh vanadium RFB operations required large volumes of data to be recorded in each run. This was achieved through two recording systems: Siemens TIA Portal Program and an APPA 503 multimeter Logger. These recorded the data successfully at specified time allotments during the batteries work sequence. The Siemens TIA Portal Program and the operation of the battery could both be accessed using the TeamViewer (12): software which grants remote access to other computer systems.
The battery work sequences could only be accessed from the battery’s computer directly or through Team Viewer. The programme allowed for work sequences to run and to monitor the battery in real time. From this, recorded values and operational programmes from many cycles were performed at various levels of power to assess this battery. The charge level limits were determined by allowing the battery to charge/discharge at 200 kW until it reached its charge level limitations (0–100%) which were the equivalent to the state of charge (SoC) being 5–85% limits. This data allowed for the stored energy capacity and the charge/discharge profiles to be understood for the individual runs. The cycles from the battery could charge for a theoretical time that would achieve 100% charge level and immediately discharge. This gave the initial evaluation of the system’s coulombic efficiency and, from the multimeter attached to the individual stack, the voltage efficiency.
The battery was then operated using incremental power levels to understand the power requirements for the centrifugal pumps, the AC/DC and DC/DC convertor efficiencies. Finally, the battery was also operated under standby condition with a various numbers of stack groups being kept active. This was measured over a 48-h period to determine the extent of self-discharge in the system.
3. Battery Characteristics
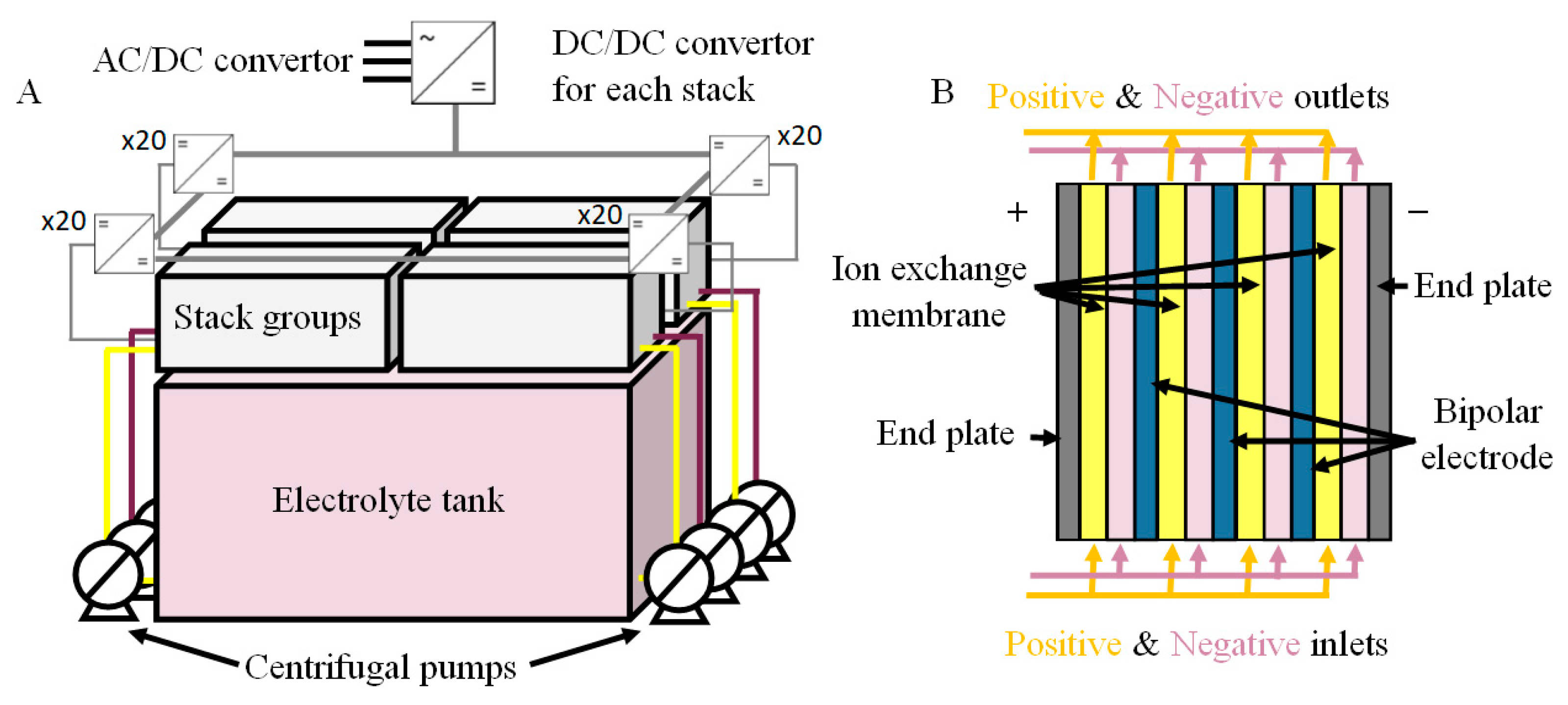
The 200 kW/400 kWh vanadium RFB, shown in
Figure 1A, was comprised of four sections containing the stacks and two electrolyte tanks. Each section had twenty stacks individually connected to the main DC line with a DC/DC convertor. The mains voltage was provided to the battery via the AC/DC convertor (three phase AC). A single stack consisted of 27 bipolar cells with a carbon composite as the bipolar plate and end-of-stack current collector. An example of the stack design which is shown in
Figure 1B. Attached to these current collectors were GFD 4.6 graphitic felts with a dimension of 28 cm × 19.5 cm × 0.46 cm for each felt electrode with a stated surface area of 0.4 m
2·g
−1 [
26]. Therefore, with a sample piece of GFD 4.6 of 20 cm
2 weighing 3.3 g, this would mean that the sample piece had a surface area of roughly 1.32 m
2. Scaling this up to the size of a typical electrode would give a surface area of 36.0 m
2 and the entire battery an area 15.6 × 10
4 m
2. The membrane was unknown, as the information was not provided in the specifications of this battery unit.
For the operation of the battery, the positive and negative electrolytes were pumped into each group of stacks from two centrifugal pumps. The total electrolyte volume was 26,000 L and was composed of 1.6 M vanadium species in concentrated (2 M) sulphuric acid. The battery module also contained numerous sensors to measure and control the ventilation, in particular with respect to hydrogen, the temperature, the electrolyte levels in the tanks, any electrolyte leakage from the tanks into spill containment bund, etc. These sensors thus allowed for the control of the operational temperatures, the balancing of the electrolytes during operation of the battery and to identify and detect problems within this system. For example, two issues identified in this RFB system were that two of the stacks (Stack A07 and Stack C19) or their corresponding DC/DC convertors, were faulty at the moment of the characterisation.
Typically, this battery was operated between nominal charge levels of 0% to 100% as displayed on the human–machine interface (HMI) but the actual states of charge corresponding to these were SoC 5% and SoC 85%, respectively.
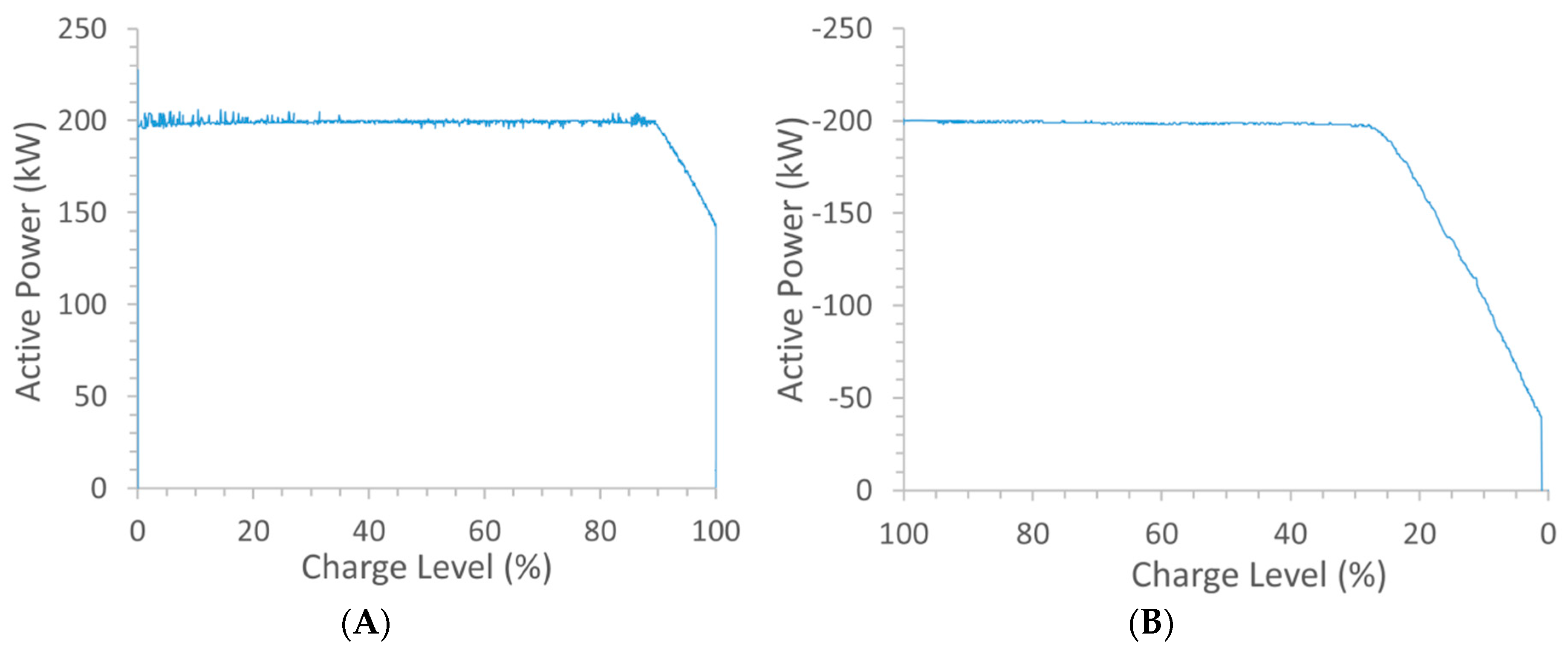
Figure 2A shows the 200 kW of power being applied to charge up the battery. A constant power level was maintained here until a charge level of 90% was indicated on the human-machine interface (HMI). The applied power was then linearly decreased at a rate of 1.8 kW/min until the 100% charge level was achieved, by which time the applied power was only
ca. 140 kW.
The power-charge level profile applied during discharge (
Figure 2B) was overall very similar to that used during charge except that here, when the charge level had reduced to between 25% and 15% (depending on the applied voltage level) on the HMI, the power was decreased along with it. In this system, as the charge level approached 0%, charge pulses were automatically applied so as to prevent the charge level dropping below the 0% level. For both charge and discharge, voltage limits were set so as to minimise the risk of secondary reactions, such as H
2 or O
2 evolution, vanadium precipitation arising from a drop in electrolyte acidity or damage to the electrode materials [
18].
4. System Energy Efficiency
Repeated charge/discharge cycles were conducted in order to determine the system’s energy efficiency at different power set-points. The cycles were operated between 50–200 kW in increments of 50 kW to provide information on the battery’s power capability over this range. In addition, a cycle at 50 kW was also run to simulate its function for integration with the on-site alkaline electrolyser. Both RFB system and 50 kW electrolyser were connected and controlled through the plant facility to a central unit.
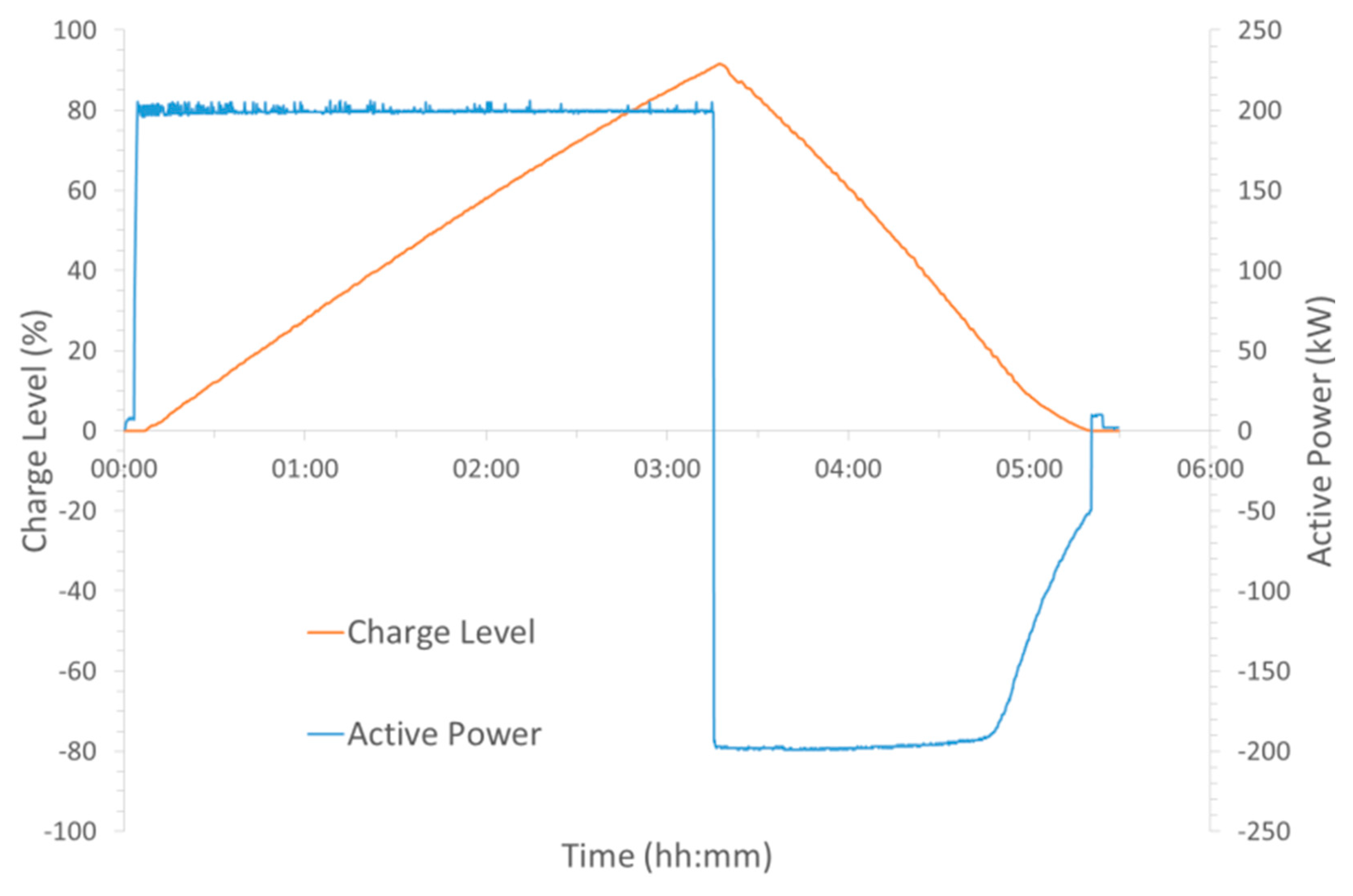
Figure 3 displays a typical data set achieved from these test cycles. It is worth noting here that the charge level for the 200 kW charge was not taken to 100%, so as to avoid the above noted linear reduction in power near the charge level’s upper limit. However, the battery was set to fully discharge to 0% charge level in order to examine how much of the input energy was returned on the discharge part of the cycle.
The system energy efficiency was simply evaluated as the ratio of the total energy returned from what was originally input into the battery.
Table 1 gives the energy efficiencies for each of the cycles carried out and it can be seen that at charging/discharging power ≥60 kW, a value of 56.5 ± 2% was obtained. At 50 kW, the energy efficiency found was 48% and the reason for this lower value is examined below. The impact of electrolyte temperature on the system efficiency is shown by the * data for the 100 kW charge/discharge cycle in the table. Here, the cycle was initiated when the electrolyte in the tank was 44.8 °C and this resulted in a system energy efficiency of only 47% compared to 59% when operating over the normal temperature range (10 °C to 40 °C).
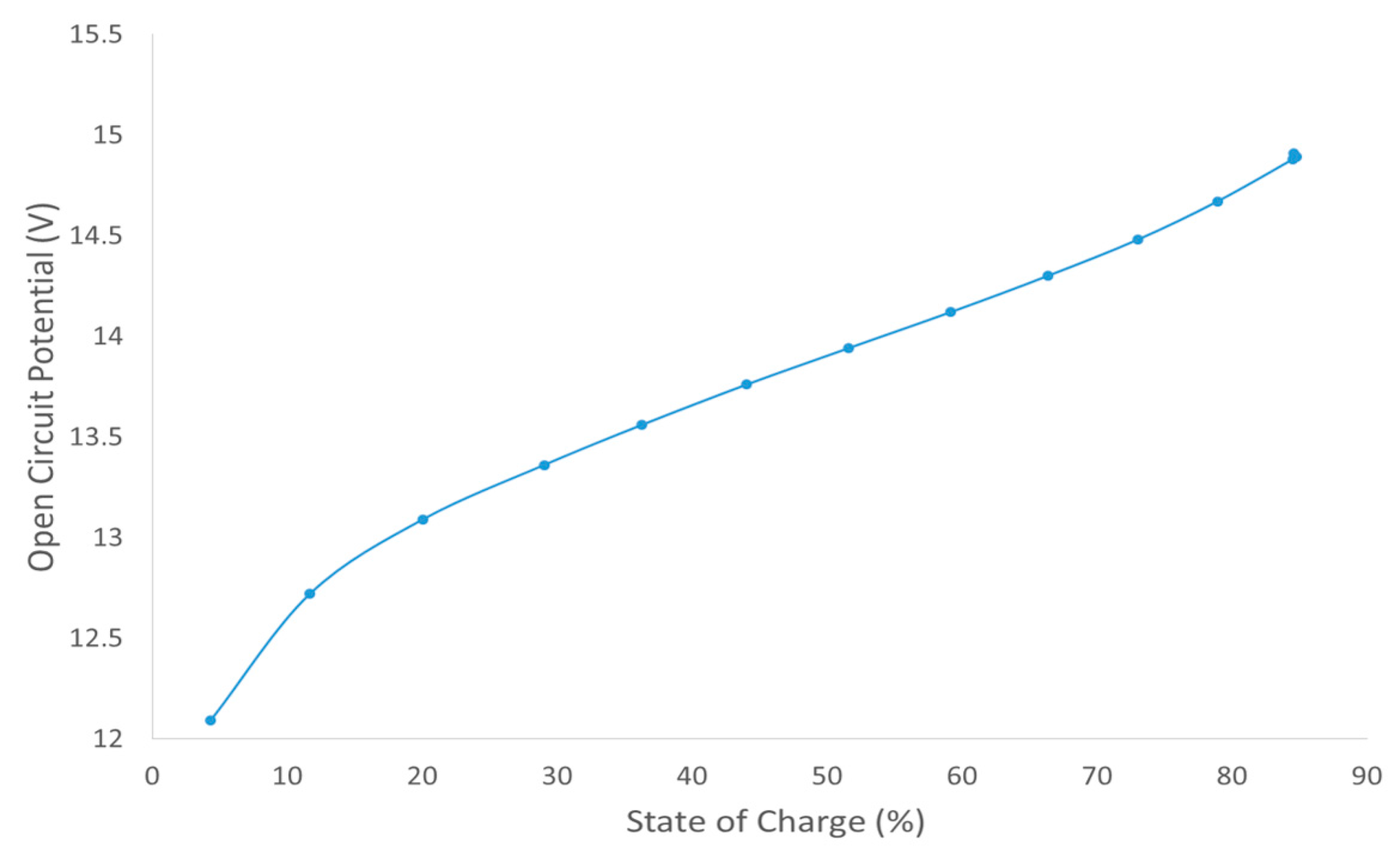
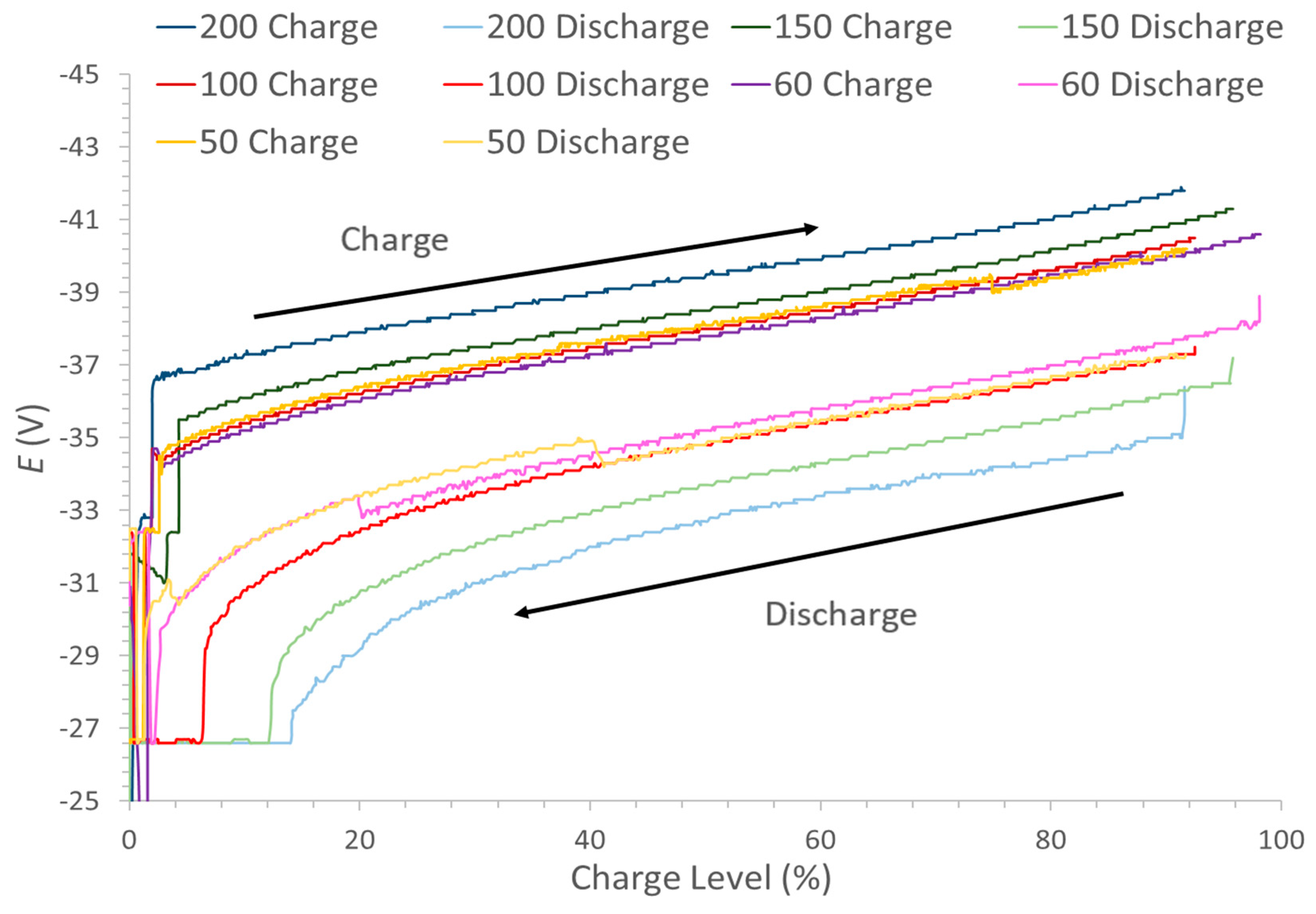
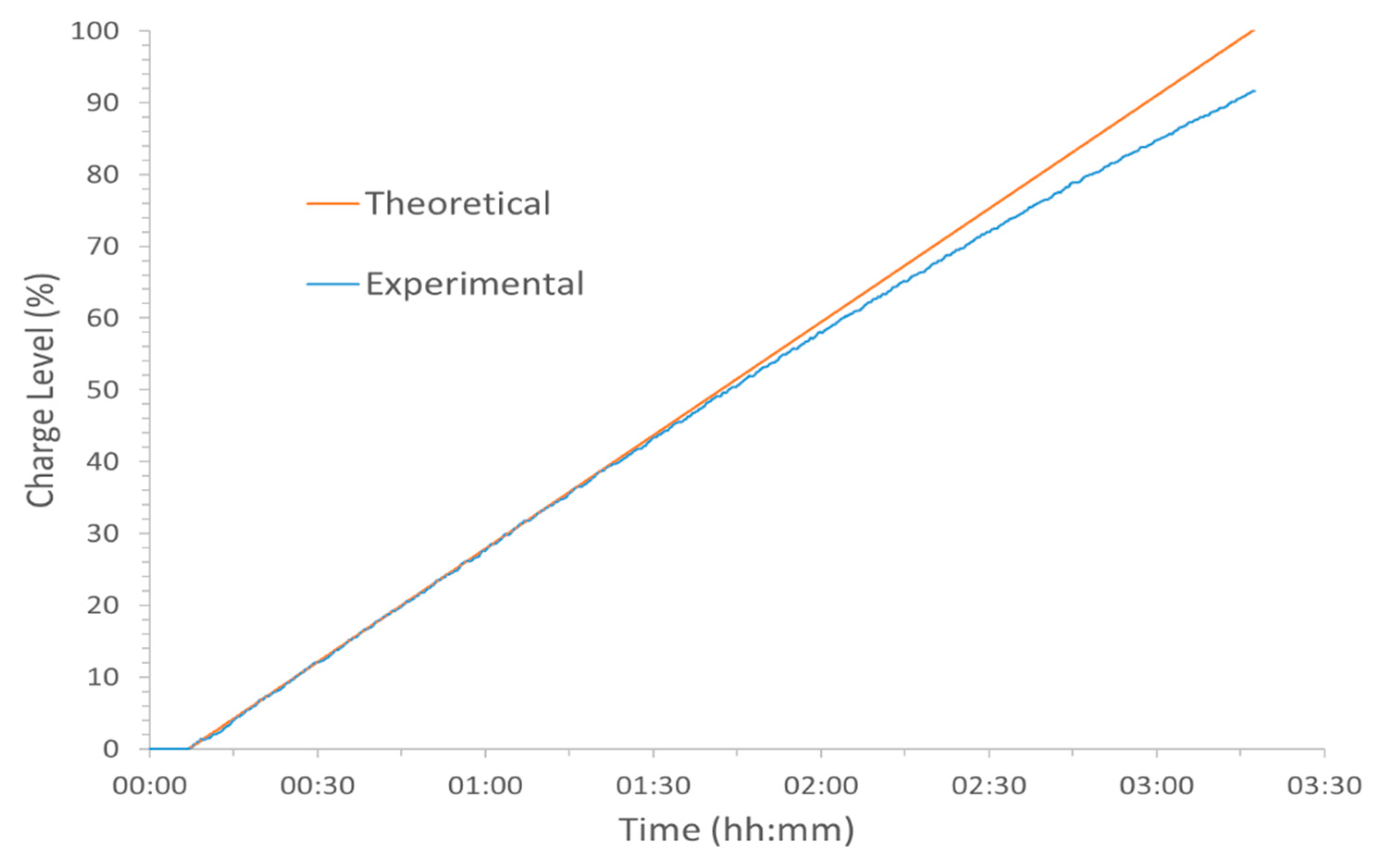
It can also be noted from the table that the charge level at the different powers achieved shows some variation and does not correlate with the actual amount of energy stored in the battery. The 50 kW charge achieved a charge level of 91.8% with 737.8 kWh compared to which 91.6% with 637.8 kWh at 200 kW charge. However, these were determined in the HMI through the open circuit voltage (OCV) value as opposed to simply calculating the theoretical charge level from the power input and time. Therefore, this could be a result of
Figure 4 which shows the relationship between the charge level and the OCV and it illustrates what would be expected from the Nernst equation being applied to the cell reaction. At the lower limit, state of charge 5%, it is observed from the figure that the OCV starts to decrease rapidly. A rapid increase would also have been observed beyond an 85% state of charge (equivalent to 100% charge level), but the battery was never able to be charged beyond these limits, due to the system control which is to prevent secondary reactions primarily.
6. Identification of the Energy Losses
The overall system efficiency represented in
Table 1 shows the system energy efficiency as measured on the AC electrical line. This efficiency includes all the energy consumed by regulatory systems, such as the centrifugal pumps used to circulate the positive and negative electrolytes through the four stacks; sensors used to monitor the temperature, measurement of the state of charge in the external cell, sensors for hydrogen monitoring and electrolyte leakages. Convertor losses between AC/DC links (at the same point as the regulatory systems) and DC/DC links (connected to each stack in the RFB) would also contribute to this low system efficiency as evaluated by the AC
out/AC
in recorder. An illustration for the setup is shown in
Figure 6.
In view of the above, in order to determine the coulombic efficiencies, the method by which energy is stored and returned in this system must be considered in the following:
where
ERec is the actual energy recorded,
EReg is the energy required by the regulatory systems, and
EConv is the energy loss from the convertors during the respective charge and discharge cycles. This expression allows for the energy efficiency of the battery, to be independently determined from that of the whole system. During charge, the convertor losses and the energy for the pumps could be subtracted from the total energy input to the system whereas, during discharge, these would be added on to the energy returned to the load. In this way, a more precise electrochemical energy efficiency could be calculated. However, it is to be noted that Equation (5) does not consider the additional auxiliary systems power requirements (e.g., for the sensors, cooling systems, recording apparatus, etc.) and so, the actual electrochemical current efficiency would still be higher than that evaluated here.
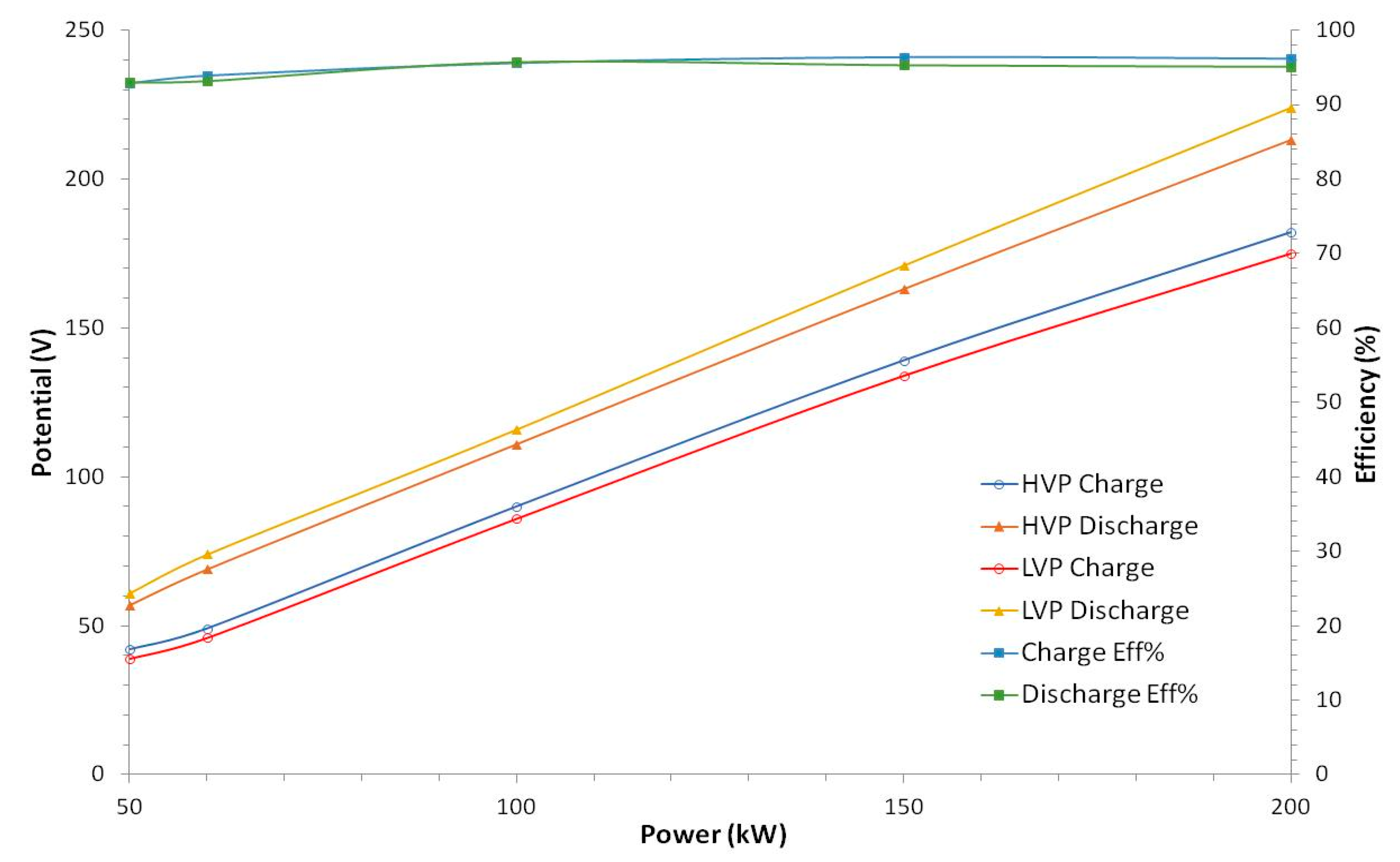
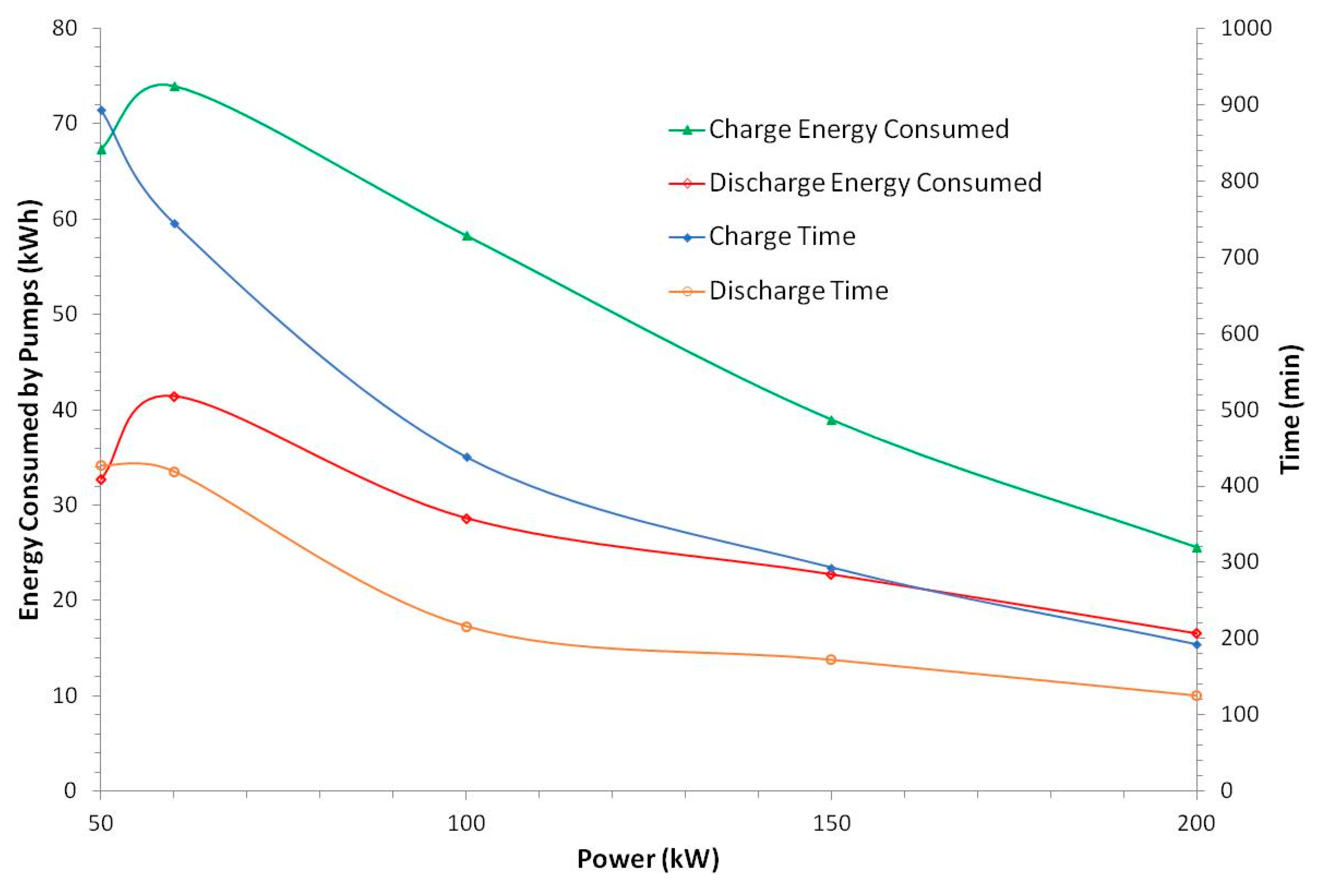
In the following sections, the losses from the AC/DC and DC/DC convertors and by the pumps are evaluated so as to allow the determination of the energy efficiency exclusively of the stacks.
10. Effect of the Electrolyte Temperature
The overall system energy efficiency was also examined as a function of the average temperature of the electrolyte in both reservoirs. The cycles that exhibited the higher efficiencies were found to originate from runs where the electrolyte solution had low temperatures (<40 °C).
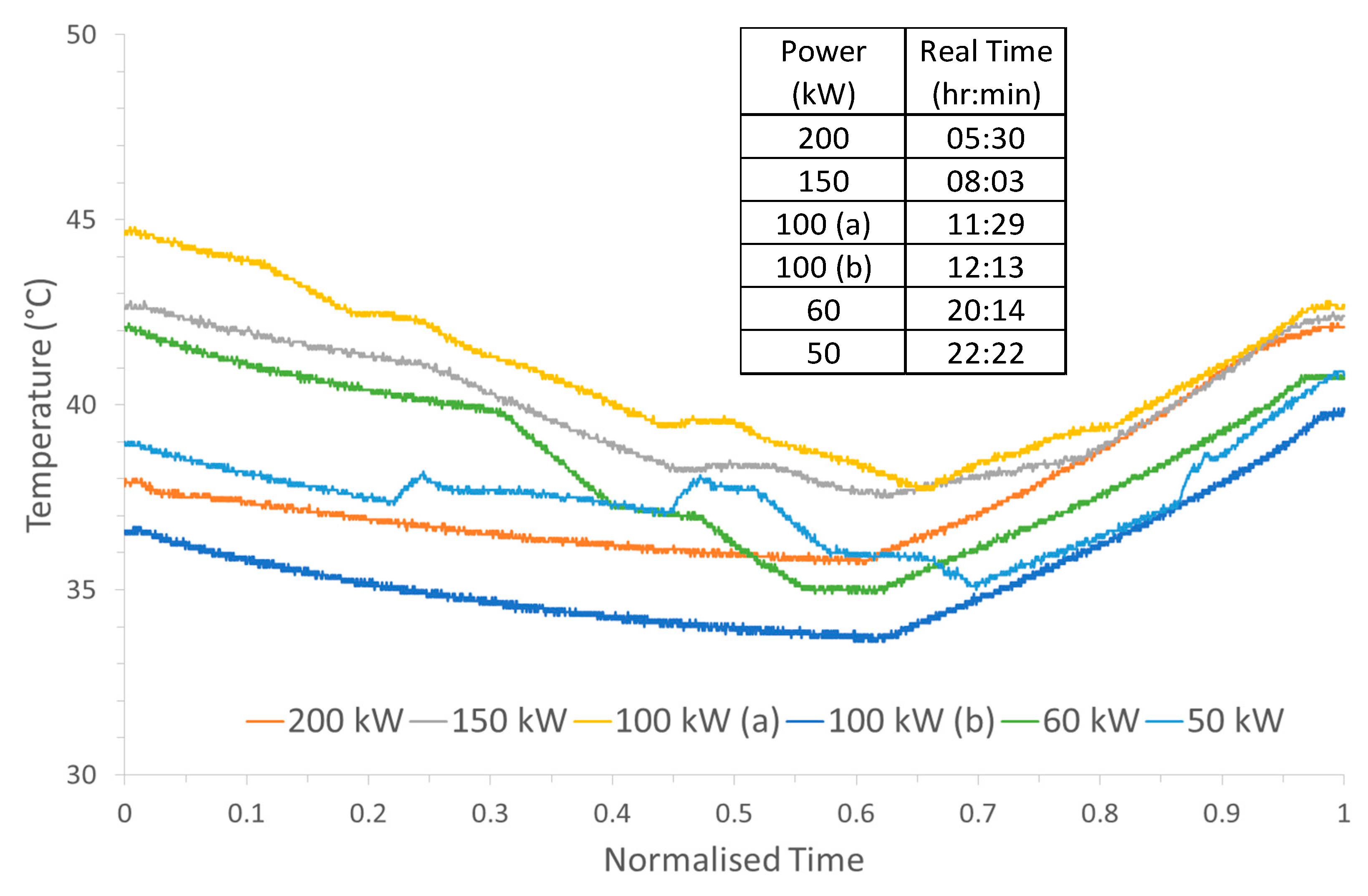
Figure 9 shows the observed variation in temperature for the different charge/discharge cycles carried out at the different power settings. The general trend is that there is a drop in temperature as charging occurs, but during discharge, the temperature increases. The main contributions for these could be from the electrode overpotential requiring heat during the charge step, causing the decrease in temperature, whereas the increase in temperature is from the electrode overpotential generating this heat during the discharge step. This effect was utilised in D Reynard et al. study, where the temperature of the electrolyte was controlled to meet the thermal requirements at both charging and discharging steps to improve the efficiency of the VRFB system [
30]. The data in
Figure 9 show that for the cycles carried out at 200 kW and at 100 kW (b) had the lowest temperatures resulted in the highest energy efficiencies. However, the 60 kW cycle is the outlier to this trend as that power produced one of the highest electrochemical efficiencies, but also had one of the highest electrolyte temperatures. The step-like features during the charge at the lower input powers which results in an increase in temperatures is from the process which rebalances the electrolyte, as the mixing of the V
2+/V
3+ and V
4+/V
5+ results in a release of energy in the form of heat. The drops in temperature could be an effect of the change in the ambient temperature over the course of the charge/discharge cycles which ran through the day, evening and night. The temperature swing in Martigny, Switzerland, where the system is installed could be from 8 to 32 °C in July. Alternatively, these drops in temperature could be from the cooling control system operating when a specific value of charge level, or power applied, is reached at a certain temperature.
In addition, the drop in temperature could originate from the electrolyte retaining the elevated temperature from a previous discharge cycle and slowly returning to the ambient temperature.